Mercury Toxicity and Detection Using Chromo-Fluorogenic Chemosensors
Abstract
1. Introduction
2. Mercury Toxicity and Intoxication
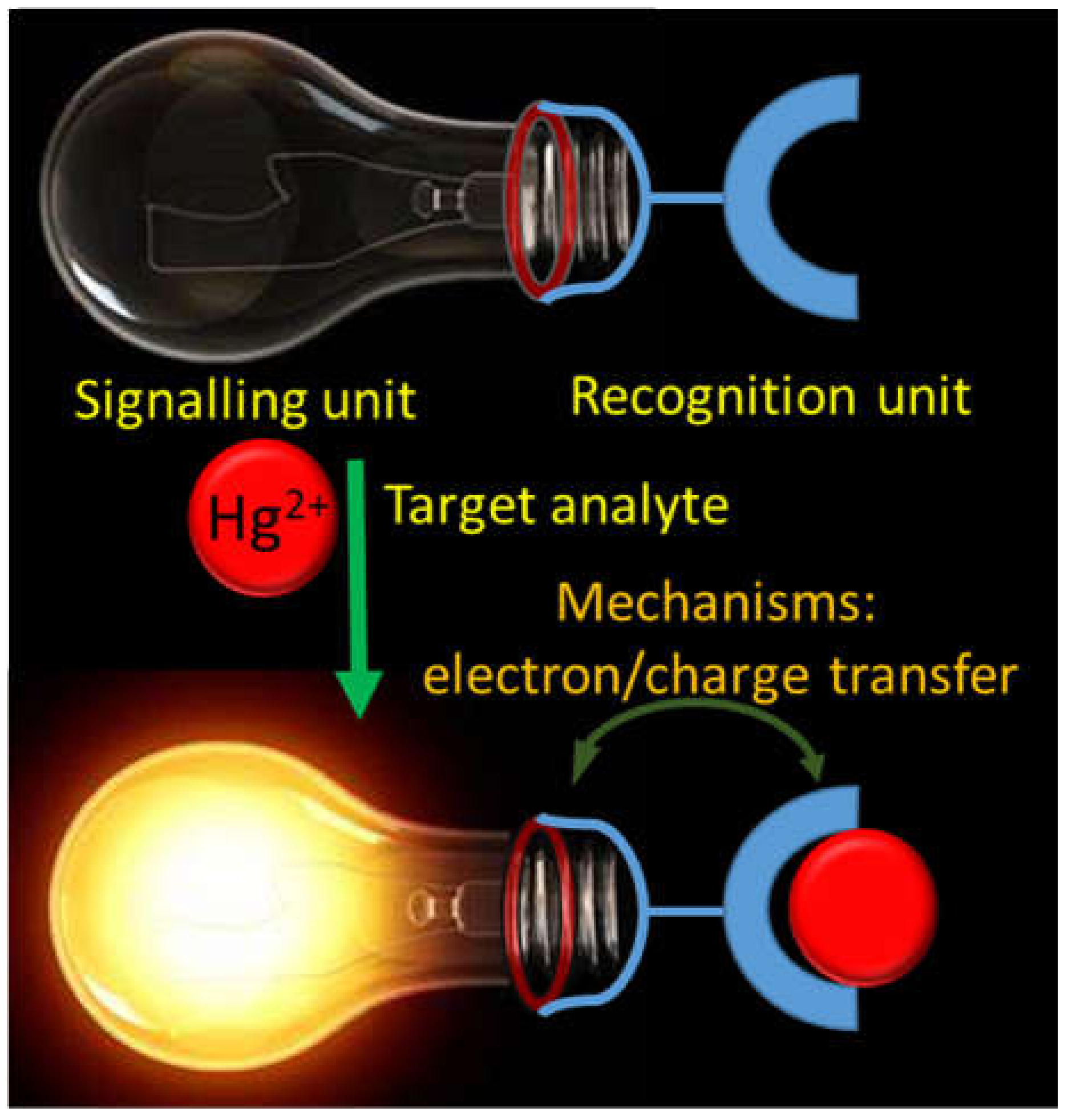
3. Chromo-Fluorogenic Chemosensors
3.1. Fluorescent Chemosensors
3.1.1. Fluorescent Turn-Off Chemosensors
3.1.2. Fluorescent Turn-On and Ratiometric Chemosensors
3.1.3. Reaction-Based Chemosensors
3.2. Colorimetric Sensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric Metal Ion Sensors—A Comprehensive Review of the Years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Patil, A.; Salunke-Gawali, S. Overview of the Chemosensor Ligands Used for Selective Detection of Anions and Metal Ions (Zn2+, Cu2+, Ni2+, Co2+, Fe2+, Hg2+). Inorg. Chim. Acta 2018, 482, 99–112. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Crisponi, G. Recent Advances on Iron (III) Selective Fluorescent Probes with Possible Applications in Bioimaging. Molecules 2019, 24, 3267. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponi, G.; Callan, J.F. Iron (III) Selective Molecular and Supramolecular Fluorescent Probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef]
- Houston, M.C. Role of Mercury Toxicity in Hypertension, Cardiovascular Disease, and Stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Dufault, R.; Schnoll, R.; Lukiw, W.J.; LeBlanc, B.; Cornett, C.; Patrick, L.; Wallinga, D.; Gilbert, S.G.; Crider, R. Mercury Exposure, Nutritional Deficiencies and Metabolic Disruptions may Affect Learning in Children. Behav. Brain Funct. 2009, 5, 44. [Google Scholar] [CrossRef]
- Jayadevimanoranjitham, J.; Narayanan, S.S. 2,4,6-Trimercaptotriazine Incorporated Gold Nanoparticle Modifiedelectrode for Anodic Stripping Voltammetric Determination of Hg (II). Appl. Surf. Sci. 2018, 448, 444–454. [Google Scholar] [CrossRef]
- Aderinto, S.O. Fluorescent, Colourimetric, and Ratiometric Probes Based on Diverse Fluorophore Motifs for Mercuric (II) ion (Hg2+) Sensing: Highlights from 2011 to 2019. Chem. Pap. 2020, 74, 3195–3232. [Google Scholar] [CrossRef]
- Culzoni, M.J.; de La Peña, A.M.; Machuca, A.; Goicoechea, H.C.; Babiano, R. Rhodamine and BODIPY Chemodosimeters and Chemosensors for the Detection of Hg2+, Based on Fluorescence Enhancement Effects. Anal. Methods 2013, 5, 30–49. [Google Scholar] [CrossRef]
- Yan, Z.; Yuen, M.F.; Hu, L.; Sun, P.; Lee, C.S. Advances for the Colorimetric Detection of Hg2+ in Aqueous Solution. RSC Adv. 2014, 4, 48373–48388. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Zong, B.; Mao, S. Recent Advances in Sensitive and Rapid Mercury Determination with Graphene-Based Sensors. J. Mater. Chem. 2019, 7, 6616–6630. [Google Scholar] [CrossRef]
- Mahato, P.; Saha, S.; Das, P.; Agarwalla, H.; Das, A. An Overview of the Recent Developments on Hg2+ Recognition. RSC Adv. 2014, 4, 36140–36174. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and Colorimetric Sensors for Environmental Mercury Detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. 500 Years of Mercury Production: Global Annual Inventory by Region until 2000 and Associated Emissions. Sci. Total Environ. 2003, 304, 13–27. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M. Metal ion toxicity. Encycl. Inorg. Bioinorg. Chem. 2011, 1–14. [Google Scholar] [CrossRef]
- Andrade, V.; Aschner, M.; Dos Santos, A.M. Neurotoxicity of Metal Mixtures. In Neurotoxicity of Metals; Springer: Berlin/Heidelberg, Germany, 2017; pp. 227–265. [Google Scholar]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The Toxicology of Mercury: Current Research and Emerging Trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, G.; La Porta, C.A. Molecular Mechanisms Triggered by Mercury. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Buha Djordjevic, A.; Aaseth, J. A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef] [PubMed]
- Vas, J.; Monestier, M. Immunology of Mercury. Ann. N. Y. Acad. Sci. 2008, 1143, 240–267. [Google Scholar] [CrossRef] [PubMed]
- Crisponi, G.; Nurchi, V.M.; Crespo-Alonso, M.; Toso, L. Chelating Agents for Metal Intoxication. Curr. Med. Chem. 2012, 19, 2794–2815. [Google Scholar] [CrossRef] [PubMed]
- Bakka, A.; Aaseth, J.; Rugstad, H.E. Influence of Certain Chelating Agents on Egress of Cadmium from Cultured Epithelial Cells Containing High Amounts of Metallothionein: A Screening of Cd-Releasing and Toxic Effects. Acta Pharmacol. Toxicol. 1981, 49, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Walker Jr, E.M.; Stone, A.; Milligan, L.B.; Gale, G.R.; Atkins, L.M.; Smith, A.B.; Jones, M.M.; Singh, P.K.; Basinger, M.A. Mobilization of Lead in Mice by Administration of Monoalkyl Esters of Meso-2, 3-Dimercaptosuccinic Acid. Toxicology 1992, 76, 79–87. [Google Scholar] [CrossRef]
- Andersen, O. Principles and Recent Developments in Chelation Treatment of Metal Intoxication. Chem. Rev. 1999, 99, 2683–2710. [Google Scholar] [CrossRef]
- Gersl, V.; Hrdina, R.; Vávrová, J.; Policka, V.; Voglova, J.; Mazurova, Y.; Balgar, J. Effects of Repeated Administration of Dithiol Chelating Agent-Sodium 2, 3-Dimercapto-1-Propanesulphonate (DMPS) on Biochemical and Haematological Parameters in Rabbits. Acta Med. Hradec Kral. 1997, 40, 3–8. [Google Scholar]
- Hurlbut, K.M.; Maiorino, R.M.; Mayersohn, M.; Dart, R.C.; Bruce, D.C.; Aposhian, H.V. Determination and Metabolism of Dithiol Chelating Agents. XVI: Pharmacokinetics of 2,3-Dimercapto-1-Propanesulfonate After Intravenous Administration to Human Volunteers. J. Pharmacol. Exp. Ther. 1994, 268, 662–668. [Google Scholar]
- Aaseth, J.; Jacobsen, D.; Andersen, O.; Wickstrøm, E. Treatment of Mercury and Lead Poisonings with Dimercaptosuccinic Acid and Sodium Dimercaptopropanesulfonate. A Review. Analyst 1995, 120, 853–854. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V. DMSA and DMPS-Water Soluble Antidotes for Heavy Metal Poisoning. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 193–215. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Crisponi, G.; Rahman, M.M.; Chirumbolo, S. Insights on Alpha Lipoic and Dihydrolipoic Acids as Promising Scavengers of Oxidative Stress and Possible Chelators in Mercury Toxicology. J. Inorg. Biochem. 2019, 195, 111–119. [Google Scholar] [CrossRef]
- Bozkurt, E.; Gul, H.I. Selective Fluorometric “Turn-off” Sensing for Hg2+ with Pyrazoline Compound and Its Application in Real Water Sample Analysis. Inorg. Chim. Acta 2020, 502, 119288. [Google Scholar] [CrossRef]
- Ergun, E.G.C.; Ertas, G.; Eroglu, D. A Benzimidazole-Based Turn-Off Fluorescent Sensor for Selective Detection of Mercury (II). J. Photochem. Photobiol. 2020, 394, 112469. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Liu, J.; Zhou, Y.; Zhao, D.; Li, C. An Acid-Base Resistant Zn-Based Metal-Organic Framework as a Luminescent Sensor for Mercury (II). J. Solid State Chem. 2020, 283, 121153. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Wang, K.; Wang, L. A Triarylamine-Based Fluorescent Covalent Organic Framework for Efficient Detection and Removal of Mercury (II) Ion. Dye. Pigment. 2020, 173, 107880. [Google Scholar] [CrossRef]
- Wang, L.; Lou, C.; Duan, S.; Cheng, D.; Wang, A.; Zhao, B.; Zhao, H.; Yin, G.; Zhao, M. Piperazine-tuned Benzimidazole-based Multifunctional Fluorescent Sensor for the Detection of Mercury (II) Ion and pH. Inorg. Chem. Commun. 2020, 119, 108096. [Google Scholar] [CrossRef]
- Mondal, S.; Patra, N.; Nayek, H.P.; Hira, S.K.; Chatterjee, S.; Dey, S. Unusual Absence of FRET in Triazole Bridged Coumarin–Hydroxyquinoline, an Active Sensor for Hg2+ Detection. Photochem. Photobiol. Sci. 2020, 19, 1211–1221. [Google Scholar] [CrossRef]
- Sethupathi, M.; Muthusankar, G.; Thamilarasan, V.; Sengottuvelan, N.; Gopu, G.; Vinita, N.M.; Kumar, P.; Perdih, F. Macrocyclic “Tet a” Derived Colorimetric Sensor for the Detection of Mercury Cations and Hydrogen Sulphate Anions and Its Bio-Imaging in Living Cells. J. Photochem. Photobiol. 2020, 203, 111739. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Chatterjee, S.; Bhayani, K.; Mishra, S. A Natural Cyanobacterial Protein C-Phycoerythrin as an Hg2+ Selective Fluorescent Probe in Aqueous Systems. New J. Chem. 2020, 44, 6601–6609. [Google Scholar] [CrossRef]
- Nunes, M.C.; dos Santos Carlos, F.; Fuganti, O.; da Silva, L.A.; Ribas, H.T.; Winnischofer, S.M.B.; Nunes, F.S. A Facile Preparation of a New Water-Soluble Acridine Derivative and Application as a Turn-off Fluorescence Chemosensor for Selective Detection of Hg2+. J. Fluoresc. 2020, 30, 235–247. [Google Scholar] [CrossRef]
- Xue, S.; Wang, P.; Chen, K. A Novel Fluorescent Chemosensor For Detection of Mercury (II) Ions Based on Dansyl-Peptide and Its Application in Real Water Samples and Living LNcap Cells. Spectrochim. Acta Part A 2020, 226, 117616. [Google Scholar] [CrossRef]
- Bhaskar, R.; Sarveswari, S. Thiocarbohydrazide Based Schiff Base as a Selective Colorimetric and Fluorescent Chemosensor for Hg2+ with “Turn-Off” Fluorescence Responses. ChemistrySelect 2020, 5, 4050–4057. [Google Scholar] [CrossRef]
- Yu, Y.; Li, G.; Liu, J.; Yuan, D. A Recyclable Fluorescent Covalent Organic Framework for Exclusive Detection and Removal of Mercury (II). Chem. Eng. J. 2020, 401, 126139. [Google Scholar] [CrossRef]
- Cui, W.R.; Jiang, W.; Zhang, C.R.; Liang, R.P.; Liu, J.; Qiu, J.D. Regenerable Carbohydrazide-Linked Fluorescent Covalent Organic Frameworks for Ultrasensitive Detection and Removal of Mercury. ACS Sustain. Chem. Eng. 2019, 8, 445–451. [Google Scholar] [CrossRef]
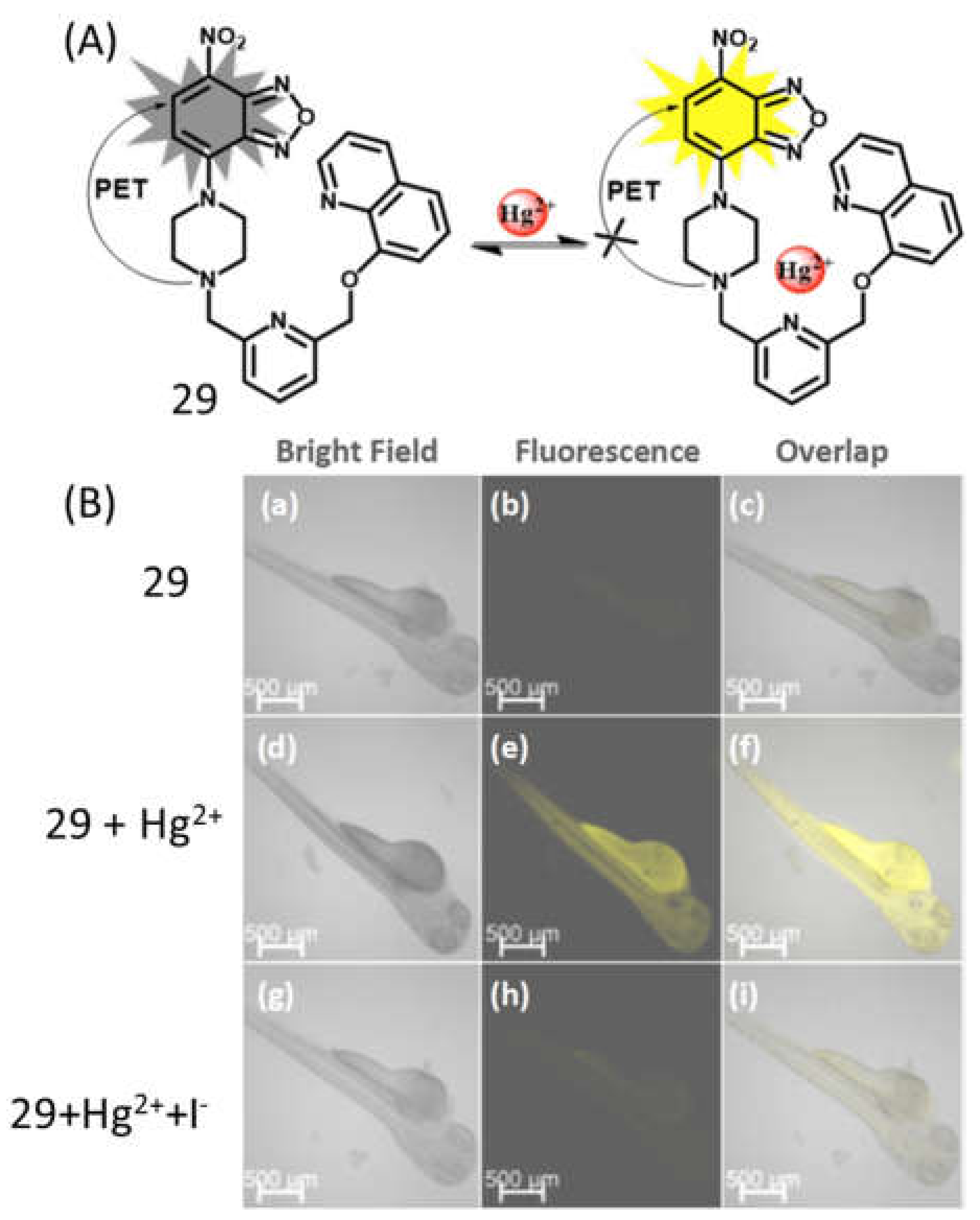
- Moradi, E.; Rahimi, R.; Safarifard, V. Porphyrinic Zirconium-based MOF with Exposed Pyrrole Lewis Base Site as an Efficient Fluorescence Sensing for Hg2+ ions, DMF small Molecule, and Adsorption of Hg2+ ions from Water Solution. J. Solid State Chem. 2020, 286, 121277. [Google Scholar] [CrossRef]
- Rathod, R.V.; Bera, S.; Maity, P.; Mondal, D. Mechanochemical Synthesis of a Fluorescein-Based Sensor for the Selective Detection and Removal of Hg2+ Ions in Industrial Effluents. ACS Omega 2020, 5, 4982–4990. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Mohamad, S. Curcumin/Beta-Cyclodextrin Inclusion Complex as a New “Turn-Off” Fluorescent Sensor System for Sensitive Recognition of Mercury Ion. J. Mol. Struct. 2020, 1204, 127528. [Google Scholar] [CrossRef]
- Gharami, S.; Aich, K.; Ghosh, P.; Patra, L.; Murmu, N.; Mondal, T.K. A Fluorescent “ON–OFF–ON” Switch for the Selective and Sequential Detection of Hg2+ and I− with Applications in Imaging using Human AGS Gastric Cancer Cells. Dalton Trans. 2020, 49, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Chae, P.S. A Novel Anthrapyridone Diamine-Based Probe for Selective and Distinctive Cu2+ and Hg2+ Sensing in Aqueous Solution; Utility as Molecular Logic Gates. Dye. Pigment. 2020, 181, 108522. [Google Scholar] [CrossRef]
- Mandegani, F.; Zali-Boeini, H.; Khayat, Z.; Braun, J.D.; Herbert, D.E. Low-Molecular-Weight Gelators as Dual-Responsive Chemosensors for the Naked-Eye Detection of Mercury (II) and Copper (II) Ions and Molecular Logic Gates. ChemistrySelect 2020, 5, 886–893. [Google Scholar] [CrossRef]
- Kumar, P.S.; Elango, K.P. A Simple Organic Probe for Ratiometric Fluorescent Detection of Zn(II), Cd(II) and Hg(II) Ions in Aqueous Solution via Varying Emission Colours to Distinguish One Another. Spectrochim. Acta Part A 2020, 241, 118610. [Google Scholar] [CrossRef]
- Choudhury, N.; Ruidas, B.; Saha, B.; Srikanth, K.; Mukhopadhyay, C.D.; De, P. Multifunctional Tryptophan-Based Fluorescent Polymeric Probes for Sensing, Bioimaging and Removal of Cu2+ and Hg2+ Ions. Polym. Chem. 2020, 11, 2015–2026. [Google Scholar] [CrossRef]
- Elmas, S.N.K.; Dincer, Z.E.; Erturk, A.S.; Bostanci, A.; Karagoz, A.; Koca, M.; Sadi, G.; Yilmaz, I. A Novel Fluorescent Probe Based on Isocoumarin for Hg2+ and Fe3+ Ions and Its Application in live-Cell Imaging. Spectrochim. Acta 2020, 224, 117402. [Google Scholar] [CrossRef] [PubMed]
- Germani, R.; Anastasio, P.; Chiodini, M.; del Giacco, T.; Tiecco, M.; Belpassi, L. Fluorescent Signal Transduction in a Self-Assembled Hg2+ Chemosensor Tuned by Various Interactions in Micellar Aqueous Environment. J. Photochem. Photobiol. 2020, 389, 112276. [Google Scholar] [CrossRef]
- Ngororabanga, J.M.V.; Moyo, C.B.; Tshentu, Z.R. A Novel Multidentate Pyridyl Ligand: A Turn-on Fluorescent Chemosensor for Hg2+ and Its Potential Application in Real Sample Analysis. Spectrochim. Acta Part A 2020, 242, 118651. [Google Scholar] [CrossRef] [PubMed]
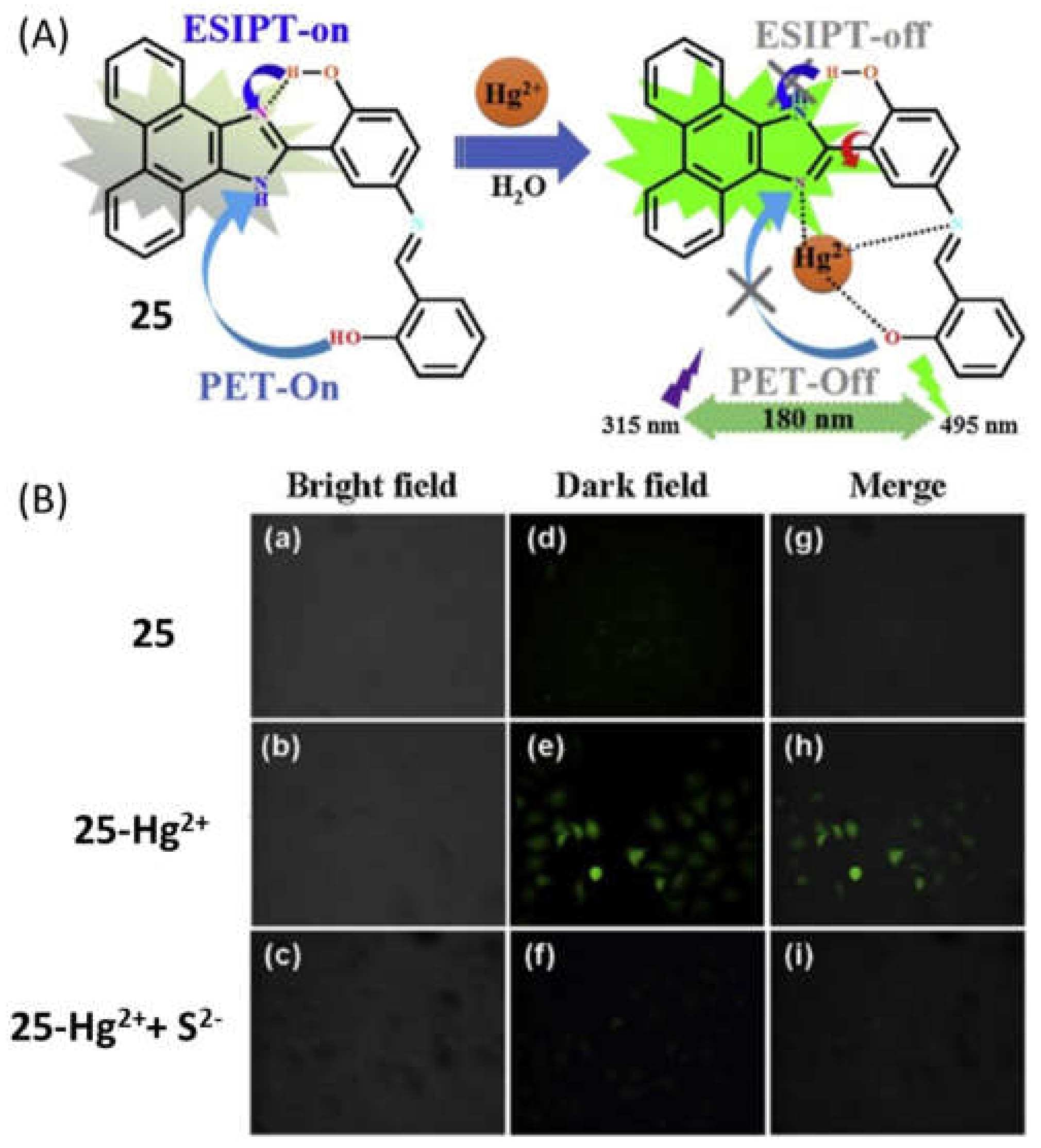
- Bu, F.; Zhao, B.; Kan, W.; Ding, L.; Liu, T.; Wang, L.; Song, B.; Wang, W.; Deng, Q. An ESIPT characteristic “Turn-On” Fluorescence Sensor for Hg2+ with Large Stokes Shift and Sequential “Turn-Off” Detection of S2– as well as the Application in Living Cells. J. Photochem. Photobiol. 2020, 387, 112165. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Feng, T.; Li, Y. Synthesis, Structure–Fluorescence Relationships and Density Functional Theory Studies of Novel Naphthalimide–Piperazine–Pyridine-Based Polystyrene Sensors for Hg (II) Detection. RSC Adv. 2020, 10, 25281–25289. [Google Scholar] [CrossRef]
- He, H.; Meng, X.; Deng, L.; Sun, Q.; Huang, X.; Lan, N.; Zhao, F. A Novel Benzothiadiazole-Based and NIR-Emissive Fluorescent Sensor for Detection of Hg2+ and Its Application in Living Cell and Zebrafish Imaging. Org. Biomol. Chem. 2020, 18, 6357–6363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Liu, Y.M.; Dong, Z.M.; Chao, J.B.; Wang, H.; Wang, Y.; Shuang, S. New Colorimetric and Fluorometric Chemosensor for Selective Hg2+ Sensing in a Near-Perfect Aqueous Solution and Bio-Imaging. J. Hazard. Mater. 2020, 382, 121056. [Google Scholar] [CrossRef]
- Kraithong, S.; Panchan, W.; Charoenpanich, A.; Sirirak, J.; Sahasithiwat, S.; Swanglap, P.; Promarak, V.; Thamyongkit, P.; Wanichacheva, N. A Method to Detect Hg2+ in Vegetable via a “Turn–ON” Hg2+–Fluorescent Sensor with a Nanomolar Sensitivity. J. Photochem. Photobiol. 2020, 389, 112224. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Wen, J.; Geng, Z.; Wang, Z. A Novel Bimacrocyclic Polyamine-Based Fluorescent Probe for Sensitive Detection of Hg2+ and Glutathione in Human Serum. Talanta 2020, 207, 120311. [Google Scholar] [CrossRef]
- Tripathy, M.; Subuddhi, U.; Patel, S. A Styrylpyridinium Dye as Chromogenic and Fluorogenic Dual Mode Chemosensor for Selective Detection of Mercuric Ion: Application in Bacterial Cell Imaging and Molecular Logic Gate. Dye. Pigment. 2020, 174, 108054. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, X.; Ni, X.L. Tuning the Amphiphilicity of Terpyridine-Based Fluorescent Probe in Water: Assembly and Disassembly-Controlled Hg2+ Sensing, Removal, and Adsorption of H2S. J. Hazard. Mater. 2020, 384, 121474. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yan, L.; Fan, Z. A novel AIE Active NIR Fluorophore Based Triphenylamine for Sensing of Hg2+ and CN− and Its Multiple Application. Spectrochim. Acta 2020, 241, 118664. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Li, R.T.; Wu, K.Y.; Hu, P.P.; Zhang, Z.; Huang, N.H.; Zhang, W.H.; Chen, J.X. Experimental and Theoretical Validations of a One-Pot Sequential Sensing of Hg2+ and Biothiols by a 3D Cu-Based Zwitterionic Metal−Organic Framework. Talanta 2020, 210, 120596. [Google Scholar] [CrossRef]
- Inal, E.K. A Fluorescent Chemosensor Based on Schiff Base for the Determination of Zn2+, Cd 2+ and Hg2+. J. Fluoresc. 2020, 30, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, D.; Li, Z.; Li, J.; Liu, H.; Meng, Q.; Han, Q.; Liu, X. A Dual-Channel Chemosensor Based on 8-Hydroxyquinoline for Fluorescent Detection of Hg2+ and Colorimetric Recognition of Cu2+. Spectrochim. Acta 2020, 243, 118784. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, Q.; Zhao, Q.; Cheng, X. Highly Sensitive and Selective Fluorescent Monomer/Polymer Probes for Hg2+ and Ag+ Recognition and Imaging of Hg2+ in Living Cells. Anal. Bioanal. Chem. 2020, 412, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.G.; Shin, J.S.; Dige, N.C.; Vanjare, B.D.; Han, Y.; Choi, N.G.; Kim, S.J.; Seo, S.Y.; Lee, K.H. Chelation Enhanced Fluorescence of Rhodamine Based Novel Organic Nanoparticles for Selective Detection of Mercury Ions in Aqueous Medium and Intracellular Cell Imaging. J. Photochem. Photobiol. 2020, 397, 112579. [Google Scholar] [CrossRef]
- Wei, G.; Yan, Z.; Tian, J.; Zhao, G.; Guang, S.; Xu, H. Efficient Polymer Pendant Approach toward High Stable Organic Fluorophore for Sensing Ultratrace Hg2+ with Improved Biological Compatibility and Cell Permeability. Anal. Chem. 2020, 92, 3293–3301. [Google Scholar] [CrossRef]
- Wang, K.; Kong, Q.; Chen, X.; Yoon, J.; Swamy, K.; Wang, F. A Bifunctional Rhodamine Derivative as Chemosensor for Recognizing Cu2+ and Hg2+ Ions via Different Spectra. Chin. Chem. Lett. 2020, 31, 1087–1090. [Google Scholar] [CrossRef]
- Zhao, H.; Kang, H.; Fan, C.; Liu, G.; Pu, S. A New Multi-Functional Fluorescent Mercuric Ion Sensor Based on Diarylethene with Triazole-Linked Rhodamine B Unit. Tetrahedron 2020, 76, 131393. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. A Novel Near-Infrared Fluorescent Probe with an Improved Stokes Shift for Specific Detection of Hg2+ in Mitochondria. Org. Biomol. Chem. 2020, 18, 5238–5244. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Ruhan, A.; Han, Y. A New Rhodamine Probe with Large Stokes Shift for Hg2+ Detection and Its Application in Real Sample Analysis. Tetrahedron Lett. 2020, 61, 152077. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Oguz, M.; Yilmaz, M. New water soluble p-Sulphonatocalix [4] Arene Chemosensor Appended with Rhodamine for Selective Detection of Hg2+ ion. J. Mol. Struct. 2020, 1203, 127436. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, H.; Wang, Y.; Fan, C.; Tu, Y.; Liu, G.; Pu, S. A Ratiometric and Colorimetric Fluorescent Probe for the Detection of Mercury Ion Based on Rhodamine and Quinoline–Benzothiazole Conjugated Dyad. J. Photochem. Photobiol. 2020, 400, 112657. [Google Scholar] [CrossRef]
- Roy, S.G.; Mondal, S.; Ghosh, K. Copillar [5] Arene-Rhodamine Conjugate as a Selective Sensor for Hg2+ Ions. New J. Chem. 2020, 44, 5921–5928. [Google Scholar] [CrossRef]
- Hu, J.P.; He, J.X.; Fang, H.; Yang, H.H.; Zhang, Q.; Lin, Q.; Yao, H.; Zhang, Y.M.; Wei, T.B.; Qu, W.J. A Novel Pillar [5] Arene-Based Emission Enhanced Supramolecular Sensor for Dual-Channel Selective Detection and Separation of Hg2+. New J. Chem. 2020, 44, 13157–13162. [Google Scholar] [CrossRef]
- Hu, J.; Yu, X.; Zhang, X.; Jing, C.; Liu, T.; Hu, X.; Lu, S.; Uvdal, K.; Gao, H.W.; Hu, Z. Rapid Detection of Mercury(II) Ions and Water Content by a New Rhodamine B-Based Fluorescent Chemosensor. Spectrochim. Acta 2020, 241, 118657. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.K.; Das, D. A novel Rhodamine-Based Optical Probe for Mercury (II) Ion in Aqueous Medium: A Nanomolar Detection, wide pH Range and Real Water Sample Application. Spectrochim. Acta 2020, 225, 117504. [Google Scholar] [CrossRef]
- Qu, Z.; Meng, X.; Duan, H.; Qin, D.; Wang, L. Rhodamine-Immobilized Optical Hydrogels with Shape Deformation and Hg2+-Sensitive Fluorescence Behaviors. Sci. Rep. 2020, 10, 7723. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, H.; Zhu, Z.; Fan, C.; Tu, Y.; Liu, G.; Pu, S. Selective Rhodamine–Based Probe for Detecting Hg2+ and its Application as Test Strips and Cell Staining. J. Photochem. Photobiol. 2020, 390, 112302. [Google Scholar] [CrossRef]
- Ding, G.; Wu, L.; Feng, H.; Liu, Y.; Li, J.; Si, H.; Yao, X.; He, M.; He, W. The Specific Binding of a New 1, 2, 3-Triazole to Three Blood Proteins and it’s Appended Rhodamine Complex for Selective Detection of Hg2+. Spectrochim. Acta 2020, 228, 117728. [Google Scholar] [CrossRef]
- Zhao, M.; Shao, G.K.; Guo, Y.S.; Tang, Y.L.; Liu, J.B.; Guo, D.S. A Reaction-Type Receptor for the Multi-Feature Detection of Hg2+ in Water and Living Cells. New J. Chem. 2020, 44, 12538–12545. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, L.; Qin, D.; Zhou, J.; Duan, H. Two Novel Fluorescent Probes as Systematic Sensors for Multiple Metal Ions: Focus on Detection of Hg2+. ACS Omega 2020, 5, 24285–24295. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, L.; Fang, S.; Qin, D.; Zhou, J.; Yang, G.; Duan, H. Two Novel Colorimetric Fluorescent Probes: Hg2+ and Al3+ in the Visual Colorimetric Recognition Environment. RSC Adv. 2020, 10, 3048–3059. [Google Scholar] [CrossRef]
- Li, W.; Jiang, C.; Lu, S.; Wang, F.; Zhang, Z.; Wei, T.; Chen, Y.; Qiang, J.; Yu, Z.; Chen, X. A Hydrogel Microsphere-Based Sensor for Dual and Highly Selective Detection of Al3+ and Hg2+. Sens. Actuators 2020, 321, 128490. [Google Scholar] [CrossRef]
- Pang, X.; Dong, J.; Gao, L.; Wang, L.; Yu, S.; Kong, J.; Li, L. Dansyl-Peptide Dual-Functional Fluorescent Chemosensor for Hg2+ and Biothiols. Dye. Pigment. 2020, 173, 107888. [Google Scholar] [CrossRef]
- Lv, P.; Cao, Y.; Liu, Z.; Wang, R.; Ye, B.; Li, G. Dual Luminescent Lanthanide Coordination Polymers for Ratiometric Sensing and Efficient Removal of Hg2+. Anal. Methods 2020, 12, 91–96. [Google Scholar] [CrossRef]
- Wang, P.; An, Y.; Wu, J. Highly Sensitive Turn-On Detection of Mercury (II) in Aqueous Solutions and Live Cells with a Chemosensor Based on Tyrosine. Spectrochim. Acta 2020, 230, 118004. [Google Scholar] [CrossRef]
- Muzey, B.; Naseem, A. An AIEE active 1, 8-Naphthalimide-Sulfamethizole Probe for Ratiometric Fuorescent Detection of Hg2+ Ions in Aqueous Media. J. Photochem. Photobiol. 2020, 391, 112354. [Google Scholar] [CrossRef]
- Mondal, T.; Mondal, I.; Biswas, S.; Mane, M.V.; Panja, S.S. Mechanistic Insight into Selective Sensing of Hazardous Hg2+ and Explosive Picric Acid by Using a Pyrene-Azine-Hydroxyquinoline Framework in Differential Media. ChemistrySelect 2020, 5, 9336–9349. [Google Scholar] [CrossRef]
- Rodríguez-Lavado, J.; Lorente, A.; Flores, E.; Ochoa, A.; Godoy, F.; Jaque, P.; Saitz, C. Elucidating Sensing Mechanisms of a Pyrene Excimer-Based Calix [4] Arene for Ratiometric Detection of Hg (II) and Ag(I) and Chemosensor Behaviour as INHIBITION or IMPLICATION Logic Gates. RSC Adv. 2020, 10, 21963–21973. [Google Scholar] [CrossRef]
- Li, X.; Duan, Q.; Yu, Y.; Wang, K.; Zhu, H.; Zhang, X.; Liu, C.; Jia, P.; Li, Z.; Sheng, W. A Coumarin-Based Fluorescent Probe for Hg2+ and Its Application in Living Cells and Zebrafish. Luminescence 2020, 35, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Yang, S.; Tian, H.; Sun, B. A Multiple-Detection-Point Fluorescent Probe for the Rapid Detection of Mercury (II), Hydrazine and Hydrogen Sulphide. Dye. Pigment. 2020, 174, 108056. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Yang, S.; Tian, H.; Sun, B. Discriminative Detection of Mercury (II) and Hydrazine Using a Dual-Function Fluorescent Probe. Luminescence 2020, 35, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Xu, Z.; Chen, J.; Hu, L.; Li, H.; Zhang, X.; Gao, X.; Wang, M.; Zhang, J. Coumarin Thiourea-Based Fluorescent Turn-On Hg2+ Probe that can be Utilized in a Broad pH Range 1–11. J. Fluoresc. 2020, 30, 505–514. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, C.; Tian, B.; Cai, X.; Zhu, H.; Jia, P.; Li, Z.; Zhang, X.; Sheng, W.; Zhu, B. A Novel Highly Selective Ratiometric Fluorescent Probe with Large Emission Shift for Detecting Mercury Ions in Living Cells and Zebrafish. Dye. Pigment. 2020, 177, 108290. [Google Scholar] [CrossRef]
- Wu, X.; Duan, N.; Li, Y.; Yang, S.; Tian, H.; Sun, B. A Dual-Mode Fluorescent Probe for the Separate Detection of Mercury (II) and Hydrogen Sulfide. J. Photochem. Photobiol. 2020, 388, 112209. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, K.; Kaur, N.; Kaur, S.; Kaur, S. Perylene Diimide Dye Threaded with Dual-React able Sites for Detection of H2S and Hg2+: Diagnostic Kit and Cell Imaging. Dye. Pigment. 2020, 180, 108448. [Google Scholar] [CrossRef]
- Aliaga, M.E.; Gazitua, M.; Rojas-Bolaños, A.; Fuentes-Estrada, M.; Durango, D.; García-Beltrán, O. A Selective Thioxothiazolidin-Coumarin Probe for Hg2+ Based on Its Desulfurization Reaction. Exploring its Potential for Live Cell Imaging. Spectrochim. Acta 2020, 224, 117372. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, L.; Zhou, J.; Qin, D.; Duan, H. A New Phenothiazine-Based Fluorescence Sensor for Imaging Hg2+ in Living Cells. Appl. Organomet. Chem. 2020, 34, e5945. [Google Scholar] [CrossRef]
- Huang, S.; Gao, T.; Bi, A.; Cao, X.; Feng, B.; Liu, M.; Du, T.; Feng, X.; Zeng, W. Revealing Aggregation-Induced Emission Effect of Imidazolium Derivatives and Application for Detection of Hg2+. Dye. Pigment. 2020, 172, 107830. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Yin, J.; Yang, Y.; Luo, H.; Song, J.; Xu, X.; Wang, S. A Novel Camphor-Based “Turn-On” Fluorescent Probe with High Specificity and Sensitivity for Sensing Mercury (II) in Aqueous Medium and Its Bioimaging Application. ACS Sustain. Chem. Eng. 2020, 8, 12348–12359. [Google Scholar] [CrossRef]
- Li, C.; Niu, Q.; Wang, J.; Wei, T.; Li, T.; Chen, J.; Qin, X.; Yang, Q. Bithiophene-Based Fluorescent Sensor for Highly Sensitive and Ultrarapid Detection of Hg2+ in Water, Seafood, Urine and Live Cells. Spectrochim. Acta 2020, 233, 118208. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, C.; Ma, Q.; Bai, Y.; Sun, J.; Ding, C. A Highly Selective Fluorescent Probe for Hg2+ Based on a 1, 8-Naphthalimide Derivative. ACS Omega 2020, 5, 18176–18184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Z.; Liu, Y.; Wei, J.; Liu, Q.; Ran, P.; Li, X. Fibrous Strips Decorated with Cleavable Aggregation-Induced Emission Probes for Visual Detection of Hg2+. J. Hazard. Mater. 2020, 385, 121556. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, A.; Chakraborty, T.; Purkait, S.; Samanta, D.; Maity, S.; Das, D. A Chemodosimetric Approach for Fluorimetric Detection of Hg2+ Ions by Trinuclear Zn (II)/Cd (II) Schiff Base Complex: First Case of Intermediate Trapping in a Chemodosimetric Approach. Inorg. Chem. 2020, 59, 9014–9028. [Google Scholar] [CrossRef]
- Li, C.; Xiao, L.; Zhang, Q.; Cheng, X. Reaction-Based Highly Selective and Sensitive Monomer/Polymer Probes with Schiff Base Groups for the Detection of Hg2+ and Fe3+ Ions. Spectrochim. Acta 2020, 243, 118763. [Google Scholar] [CrossRef]
- Li, X.; Du, K.; Xie, C.; Wu, Y.; Zhang, B.; Tang, D. A Highly Sensitive and Selective Colorimetric Probe Based on a Cycloruthenated Complex: An Hg2+-Promoted Switch of Thiophene Coordination. Dalton Trans. 2020, 49, 2024–2032. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, X.; Xie, C.; Du, K.; Li, X.; Tang, D. A Polymer Membrane Tethered with a Cycloruthenated Complex for Colorimetric Detection of Hg2+ Ions. Spectrochim. Acta 2020, 228, 117541. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sahoo, P.R.; Kumar, S. A Light Controlled, Reversible, Sensitive and Highly Selective Colorimetric Sensor for Mercuric Ions in Water. J. Mol. Struct. 2020, 1206, 127702. [Google Scholar] [CrossRef]
- Karuppusamy, P.; Senthilvelan, J.; Vijayakumar, V.; Sarveswari, S. A Pyrazole-Based Highly Selective Colorimetric Chemosensor for Hg2+ Ion in Semi-Aqueous Medium. ChemistrySelect 2020, 5, 49–53. [Google Scholar] [CrossRef]
- Tripathy, M.; Subuddhi, U.; Patel, S. An Azo Dye Based D-π-A Chromogenic Probe for Selective Naked-Eye Detection of Hg2+ Ion: Application in Logic Gate Operation. ChemistrySelect 2020, 5, 4803–4815. [Google Scholar] [CrossRef]
- Kim, A.; Kim, S.; Kim, C. A Conjugated Schiff Base-Based Chemosensor for Selectively Detecting Mercury Ion. J. Chem. Sci. 2020, 132, 82. [Google Scholar] [CrossRef]
- Madhesan, T.; Mohan, A.M. Porous Silica and Polymer Monolith Architectures as Solid-State Optical Chemosensors for Hg2+ Ions. Anal. Bioanal. Chem. 2020, 412, 7357–7370. [Google Scholar] [CrossRef] [PubMed]
- Kongasseri, A.; Sompalli, N.K.; Rao, B.C.; Nagarajan, S.; Mohan, A.M.; Deivasigamani, P. Solid-State Optical Sensing of Ultra-Trace Hg2+ Ions Using Chromoionophoric Probe Anchored Silica Monolithic Architectures. Sens. Actuators 2020, 321, 128558. [Google Scholar] [CrossRef]
- Jeon, H.; Ryu, H.; Nam, I.; Noh, D.Y. Heteroleptic Pt (II)-Dithiolene-Based Colorimetric Chemosensors: Selectivity Control for Hg (II) Ion Sensing. Materials 2020, 13, 1385. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, A.; Kaur, N. A Novel, Anthracene-Based Naked Eye Probe for Detecting Hg2+ Ions in Aqueous as well as Solid State Media. Microchem. J. 2020, 153, 104508. [Google Scholar] [CrossRef]
- Lv, H.; Yuan, G.; Zhang, G.; Ren, Z.; He, H.; Sun, Q.; Zhang, X.; Wang, S. A Novel Benzopyran-Based Colorimetric and Near-Infrared Fluorescent Sensor for Hg2+ and Its Imaging in Living Cell and Zebrafish. Dye. Pigment. 2020, 172, 107658. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Z.; Hou, L.; Qu, Y.; Deng, Y.; Zhang, C.; Xie, C.; Wu, Z. Selective Mercury (II) Detection in Aqueous Solutions Upon the Absorption Changes Corresponding to the Transition Moments Polarized Along the Short Axis of an Azobenzene Chemosensor. Analyst 2020, 145, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Sankar, M. A Dual Colorimetric Chemosensor for Hg (II) and Cyanide Ions in Aqueous Media Based on a Nitrobenzoxadiazole (NBD)–Antipyrine Conjugate with INHIBIT Logic Gate Behaviour. Anal. Methods 2020, 12, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Divya, D.; Thennarasu, S. A Novel Isatin-Based Probe for Ratiometric and Selective Detection of Hg2+ and Cu2+ Ions Present in Aqueous and Environmental Samples. Spectrochim. Acta 2020, 243, 118796. [Google Scholar] [CrossRef] [PubMed]
- Hosseinjani-Pirdehi, H.; Mahmoodi, N.O.; Nadamani, M.P.; Taheri, A. Novel Synthesized Azo-Benzylidene-Thiourea as Dual Naked-Eye Chemosensor for Selective Detection of Hg2+ and CN¯ Ions. J. Photochem. Photobiol. 2020, 391, 112365. [Google Scholar] [CrossRef]
- Dhaka, G.; Jindal, G.; Kaur, R.; Rana, S.; Gupta, A.; Kaur, N. Multianalyte Azo Dye as an On-Site Assay Kit for Colorimetric Detection of Hg2+ Ions and Electrochemical Sensing of Zn2+ Ions. Spectrochim. Acta 2020, 229, 117869. [Google Scholar] [CrossRef] [PubMed]
- Raju, V.; Kumar, R.S.; Kumar, S.A.; Madhu, G.; Bothra, S.; Sahoo, S.K. A Ninhydrin-Thiosemicarbazone Based Highly Selective and Sensitive Chromogenic Sensor for Hg2+ and F− Ions. J. Chem. Sci. 2020, 132, 89. [Google Scholar] [CrossRef]
- Singh, G.; Satija, P.; Singh, A.; Sharma, G. First Report on the Synthesis of Antipyrine Crowned Siloxy Framework: Optical Recognition of Fe2+ and Hg2+ Ions. ChemistrySelect 2020, 5, 8823–8830. [Google Scholar] [CrossRef]

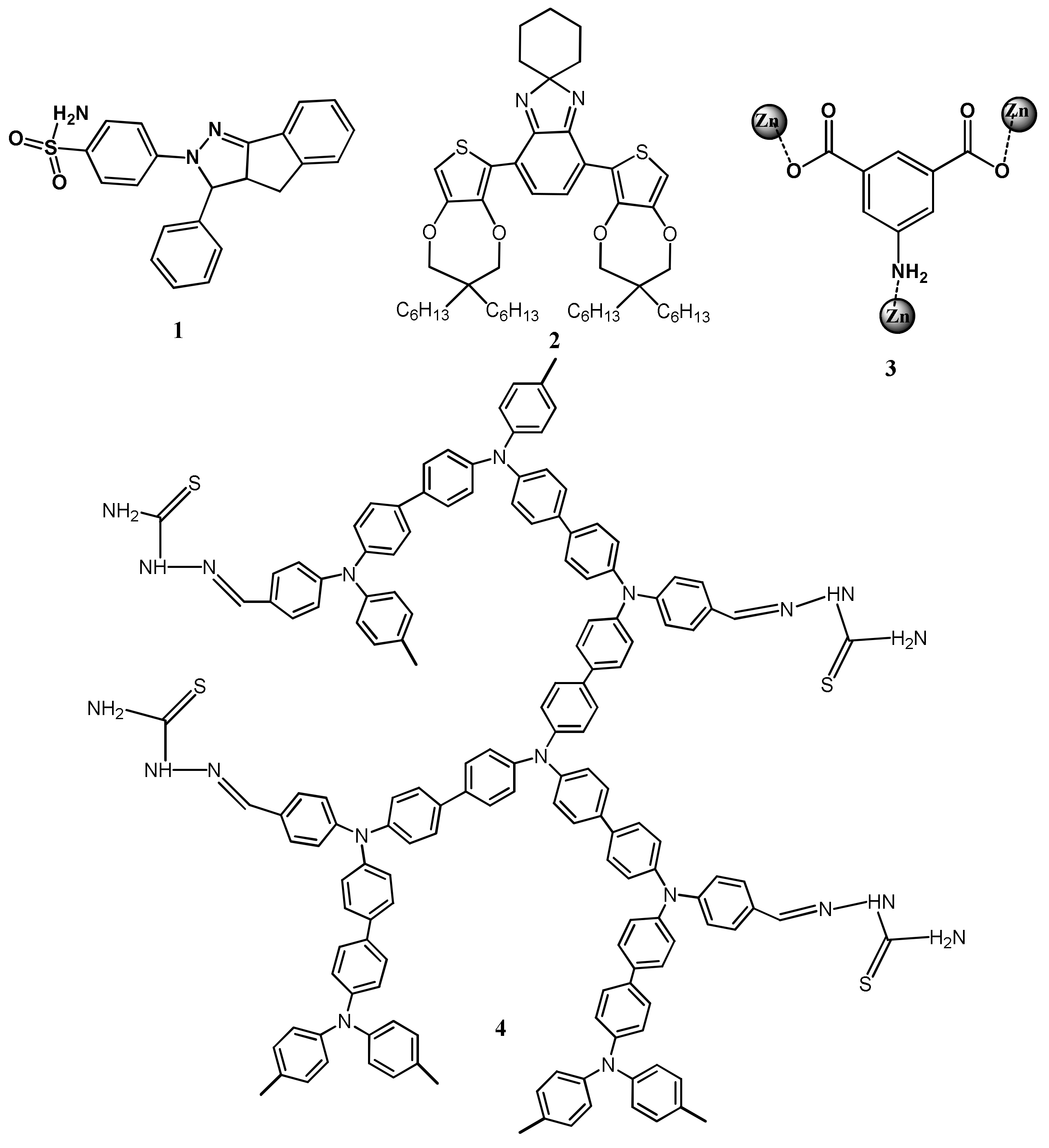
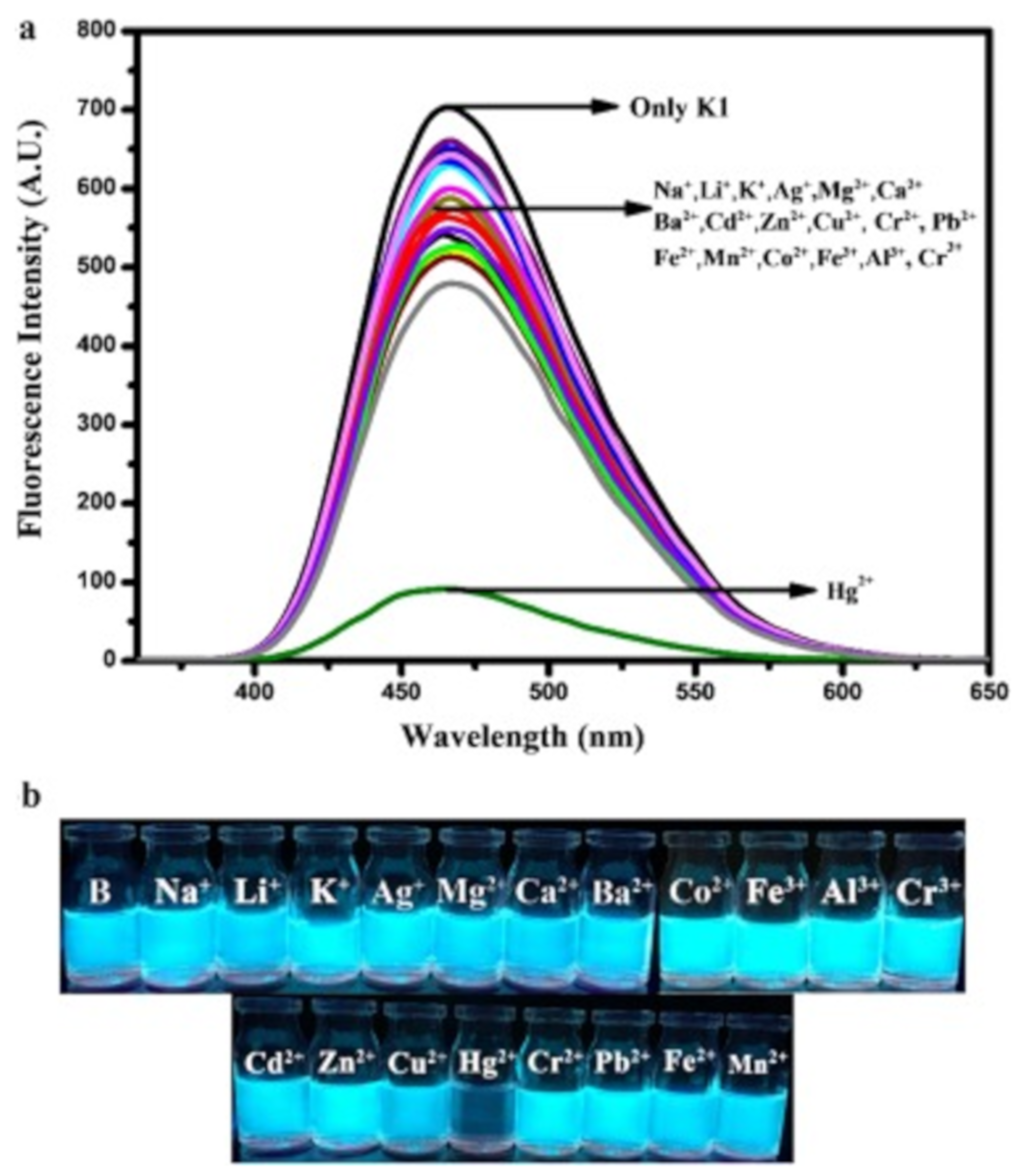
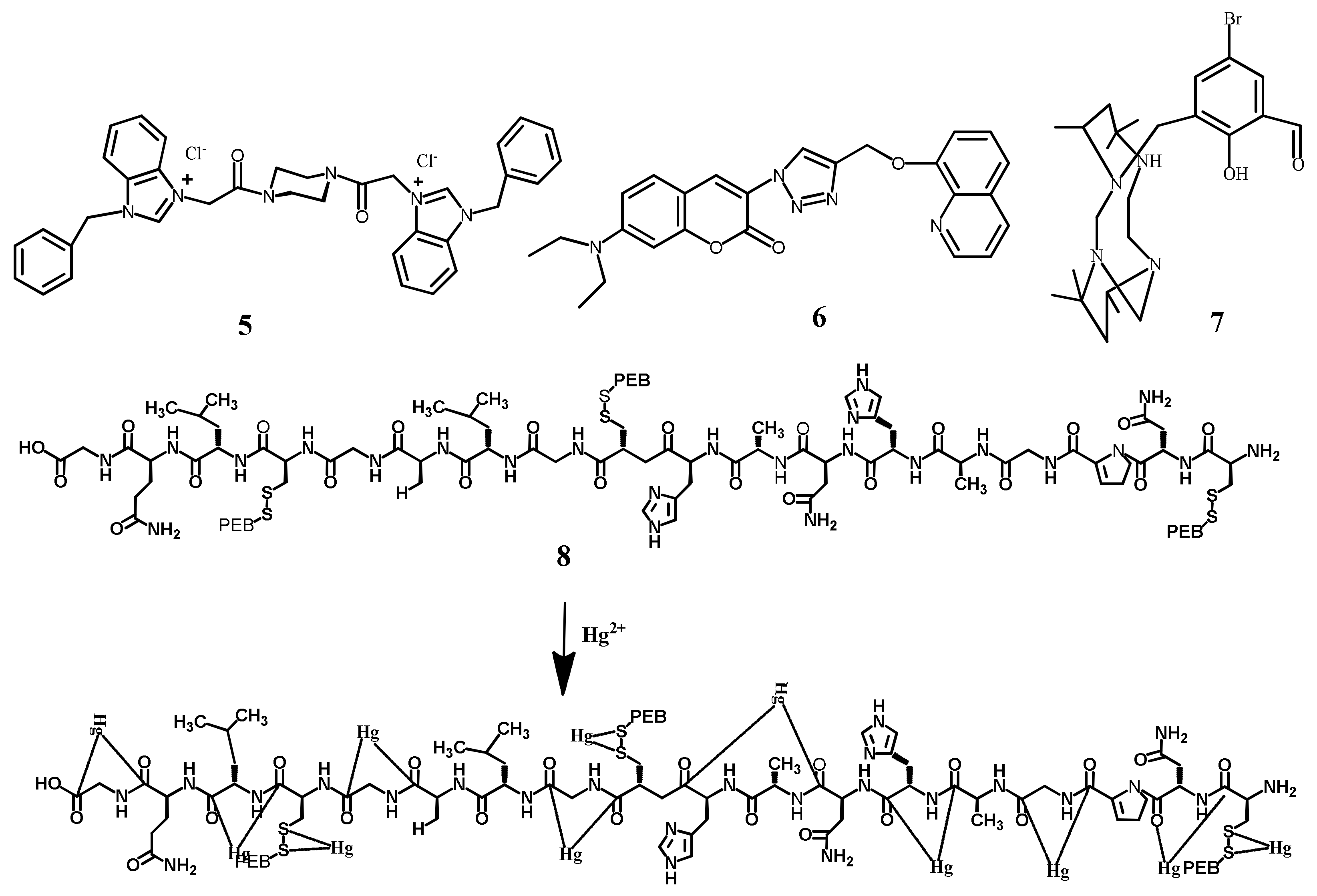
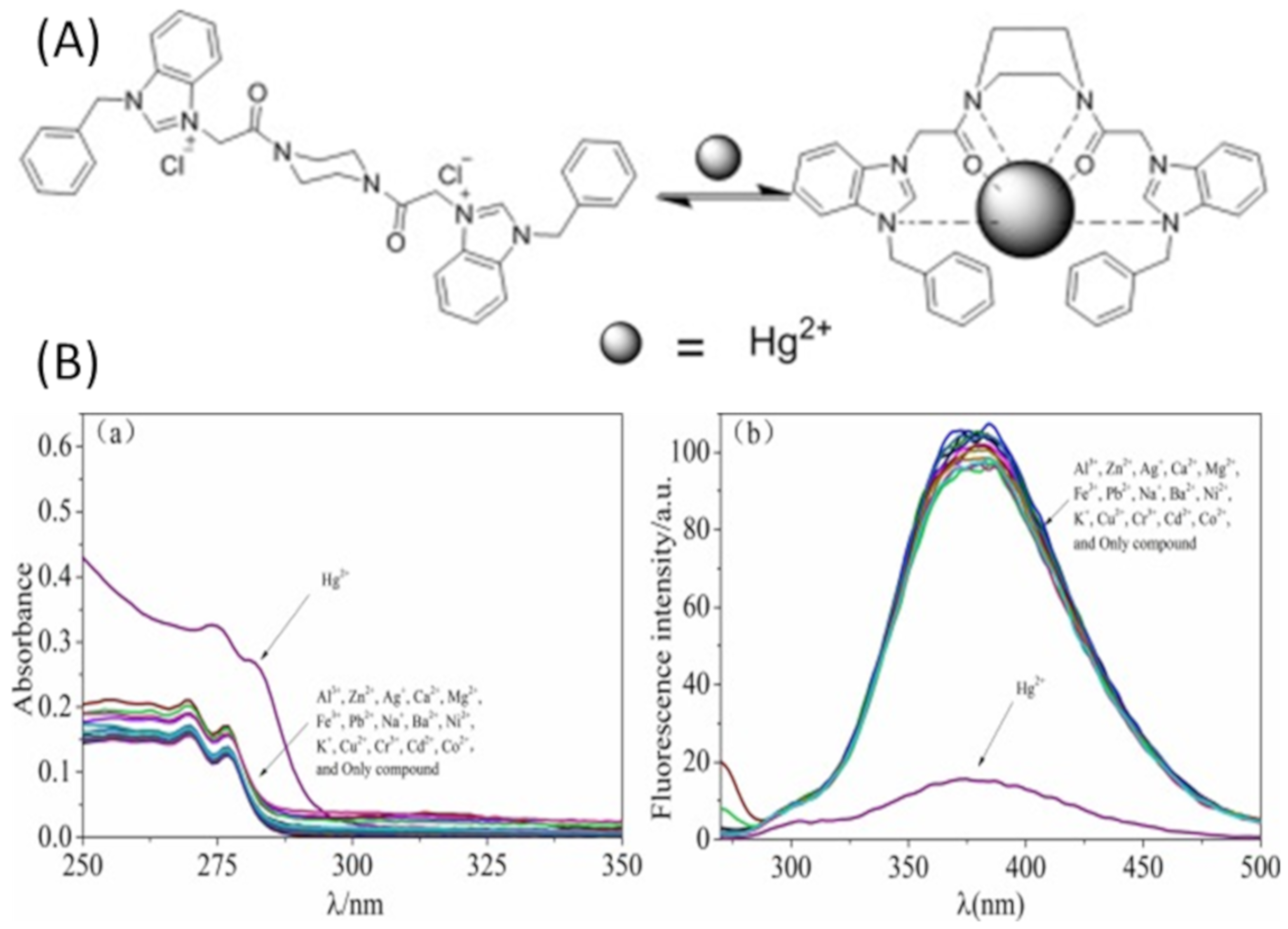
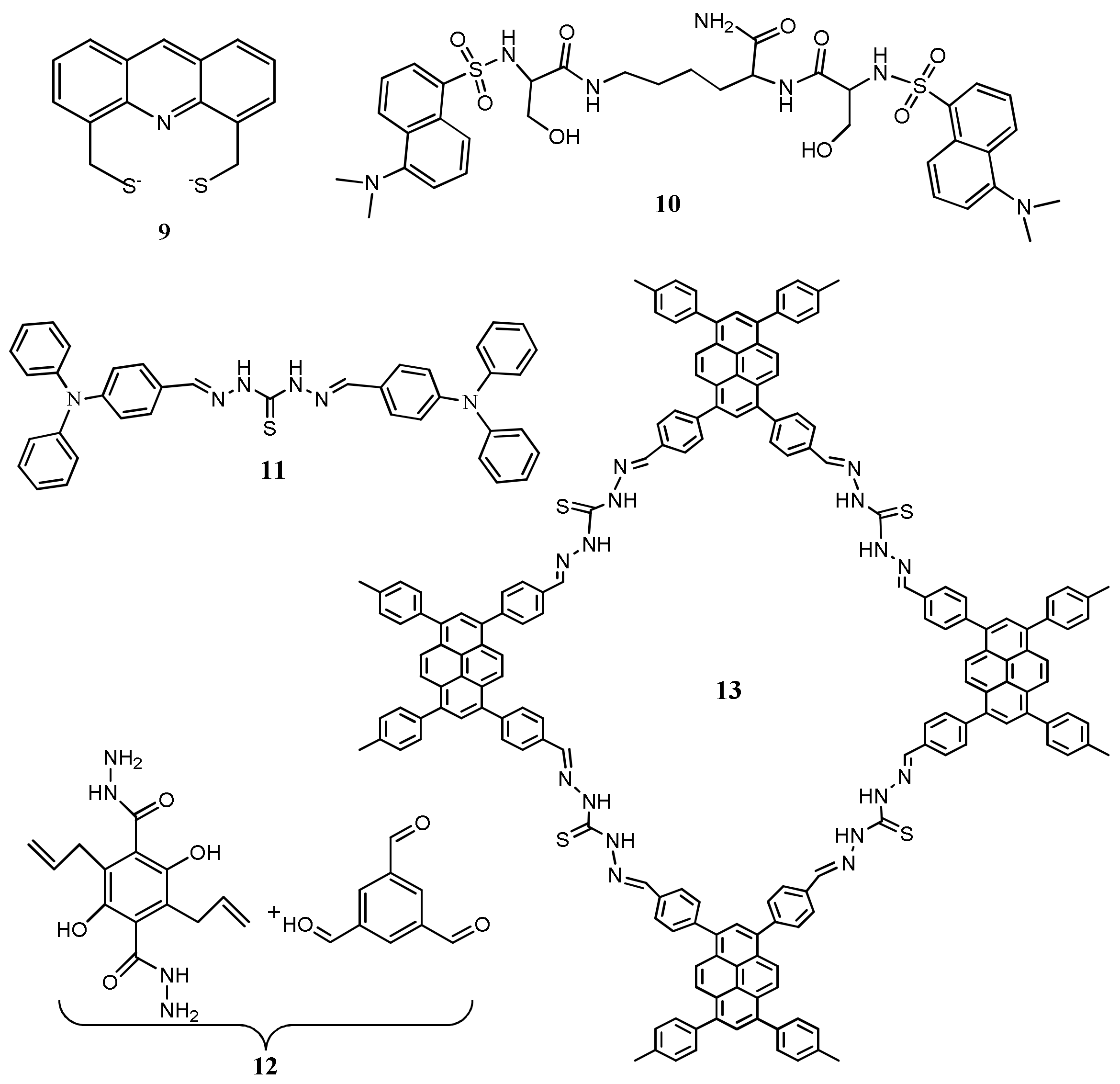
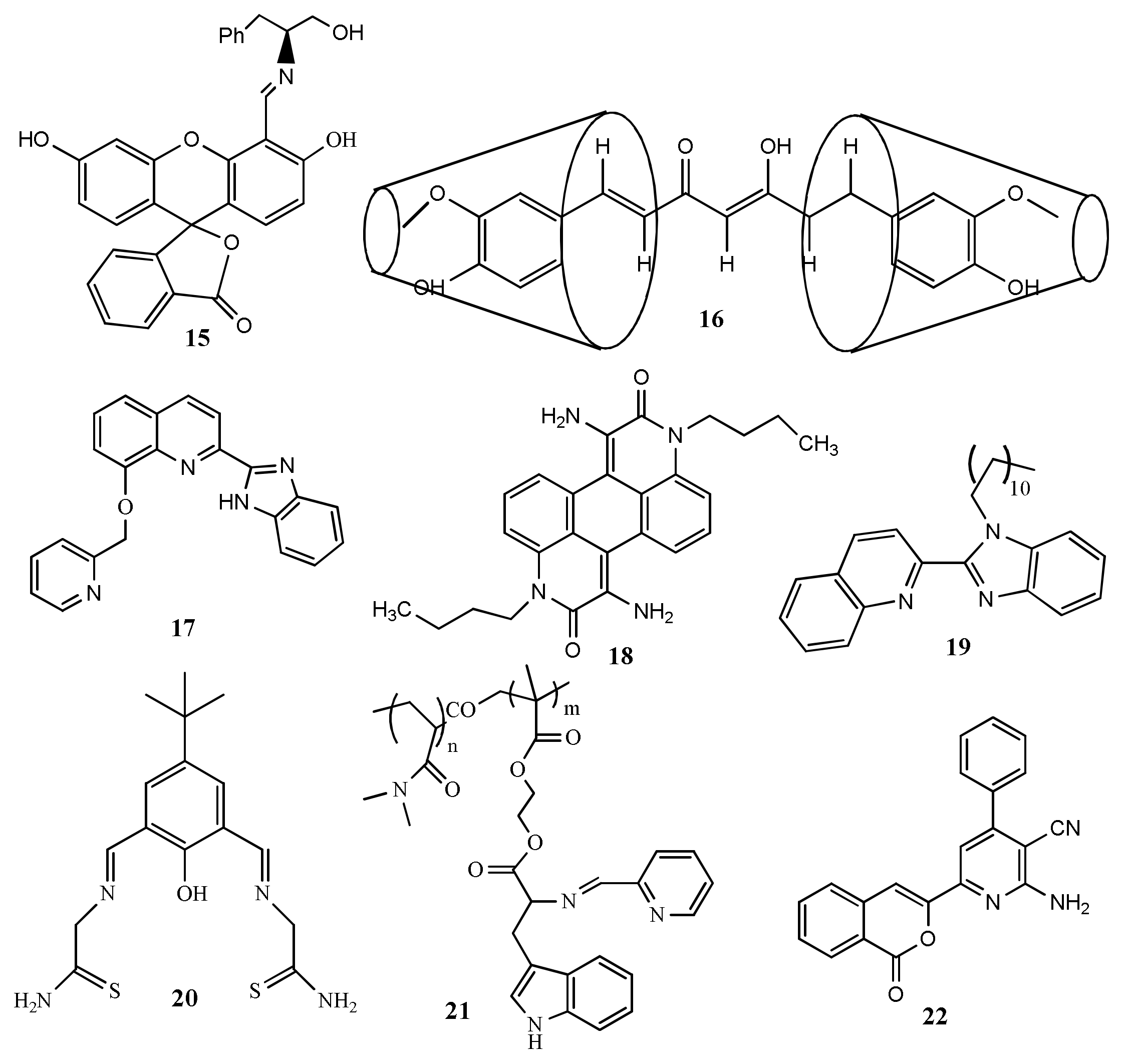
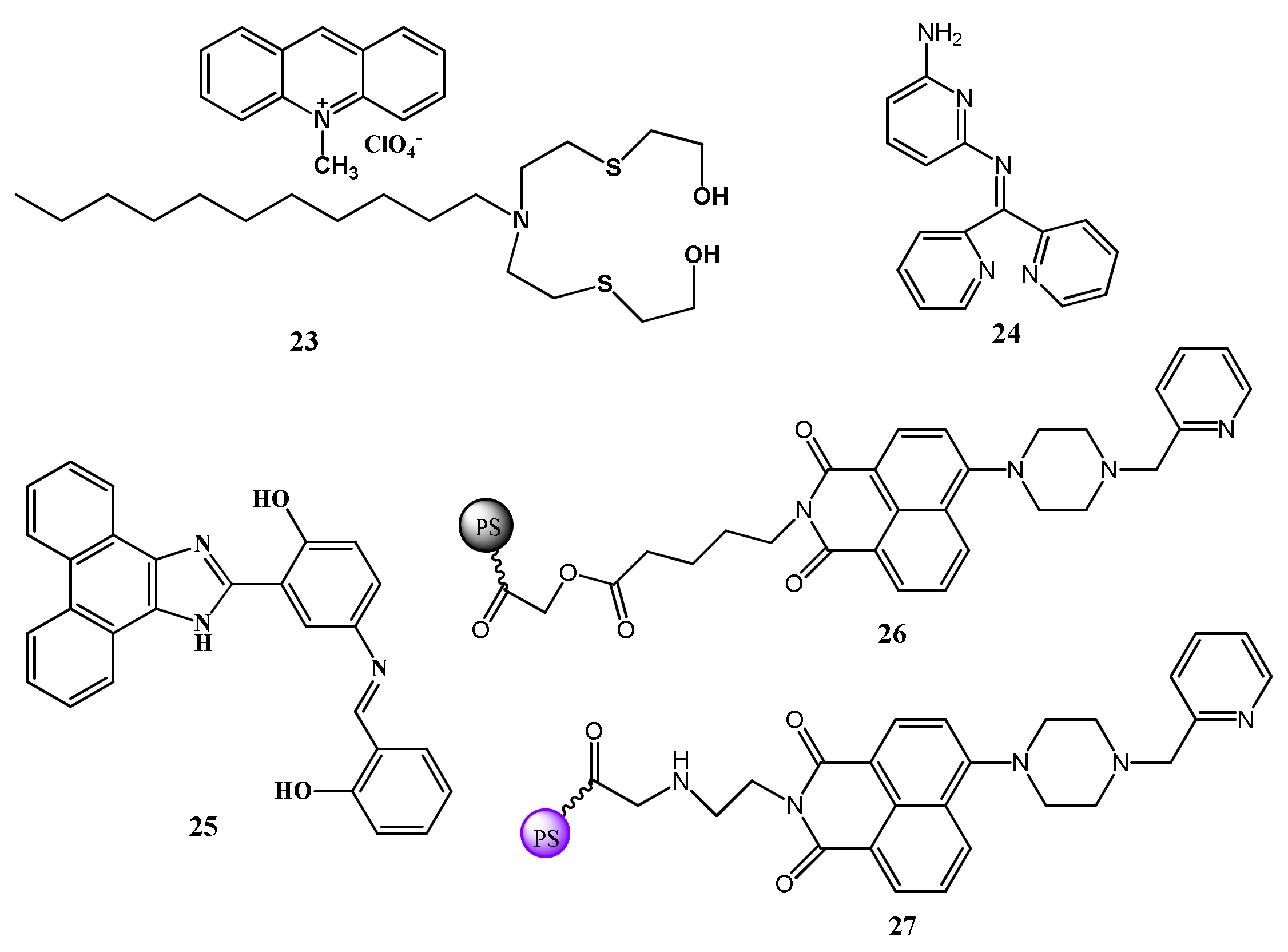
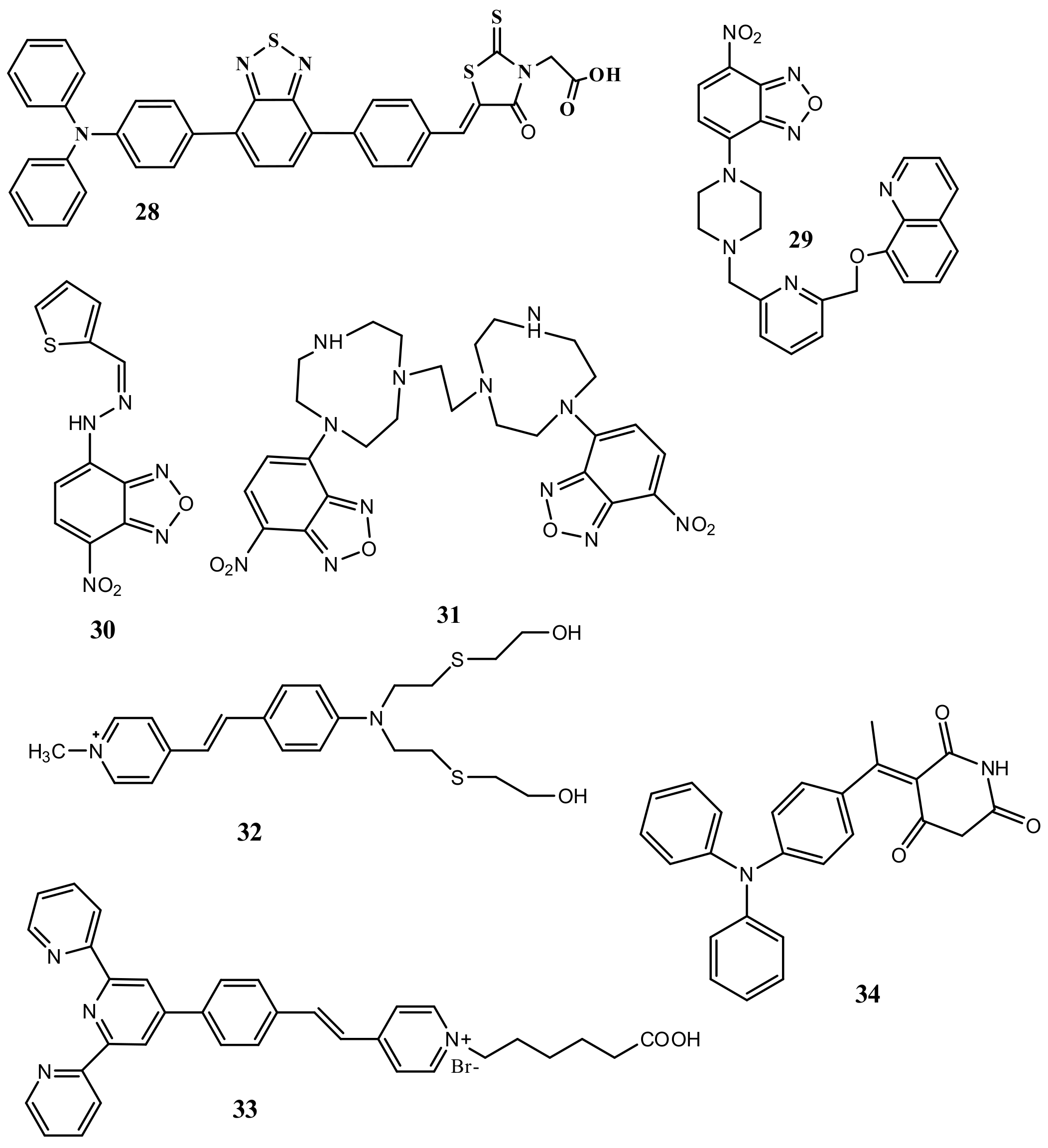
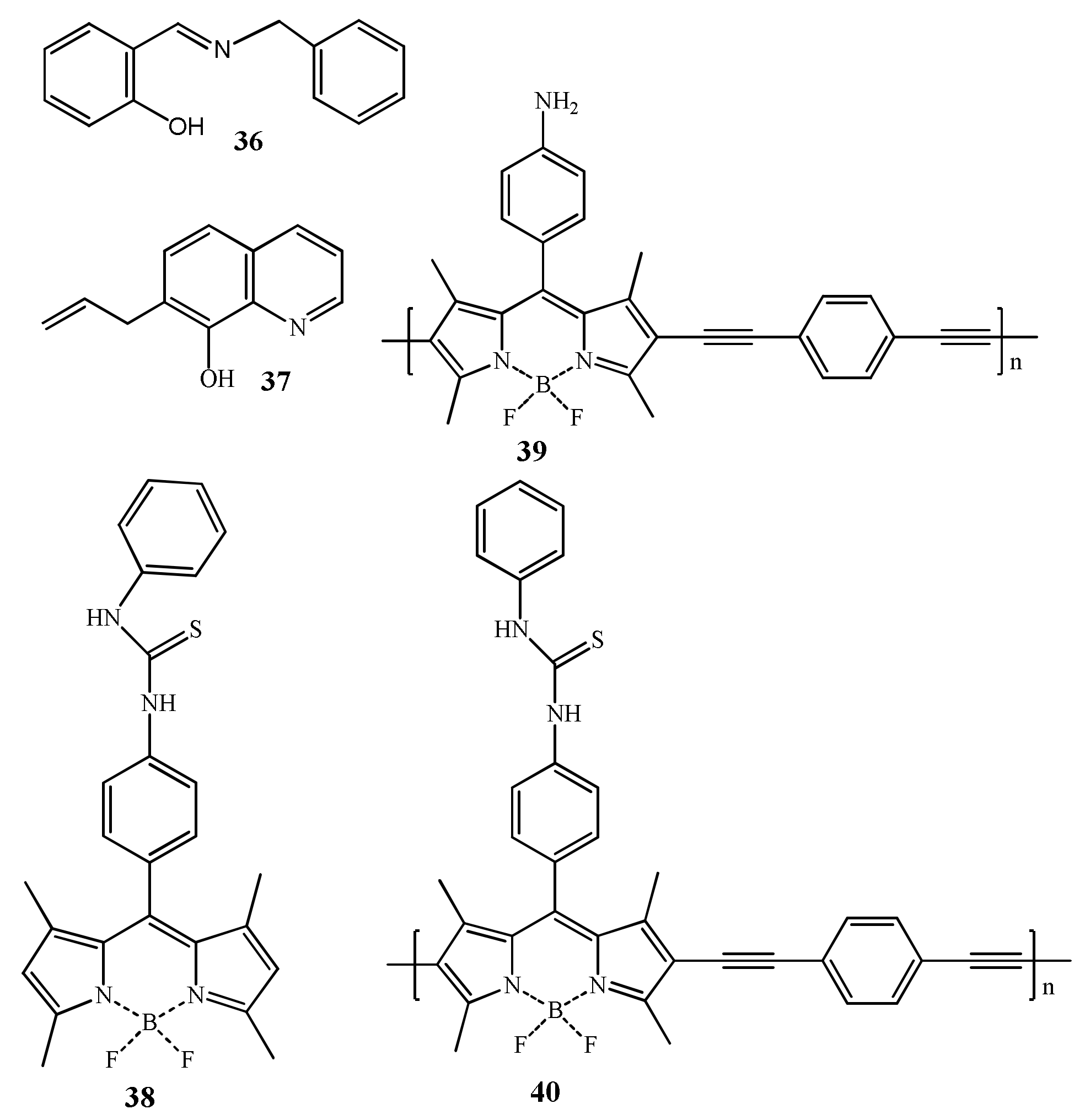
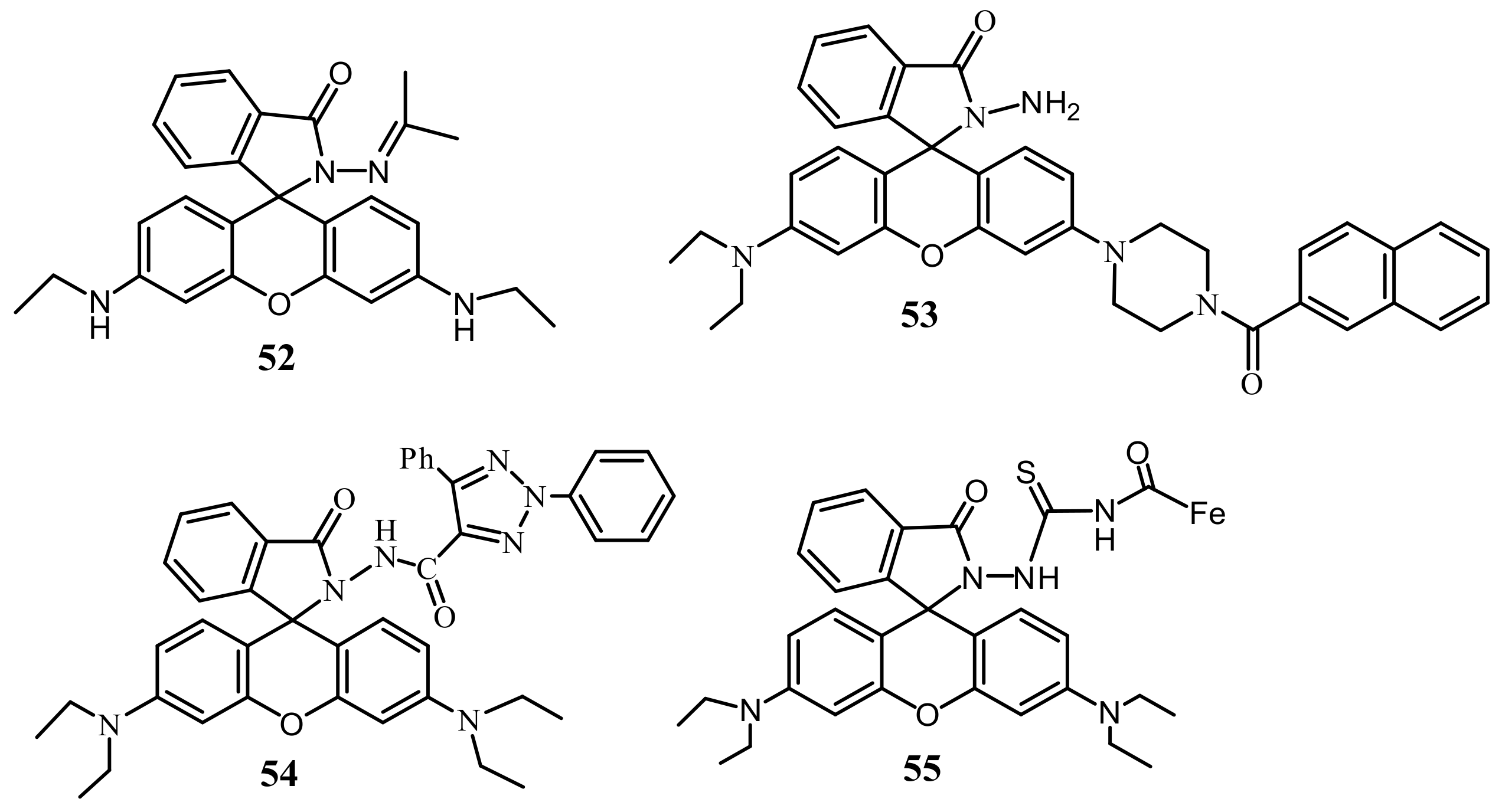
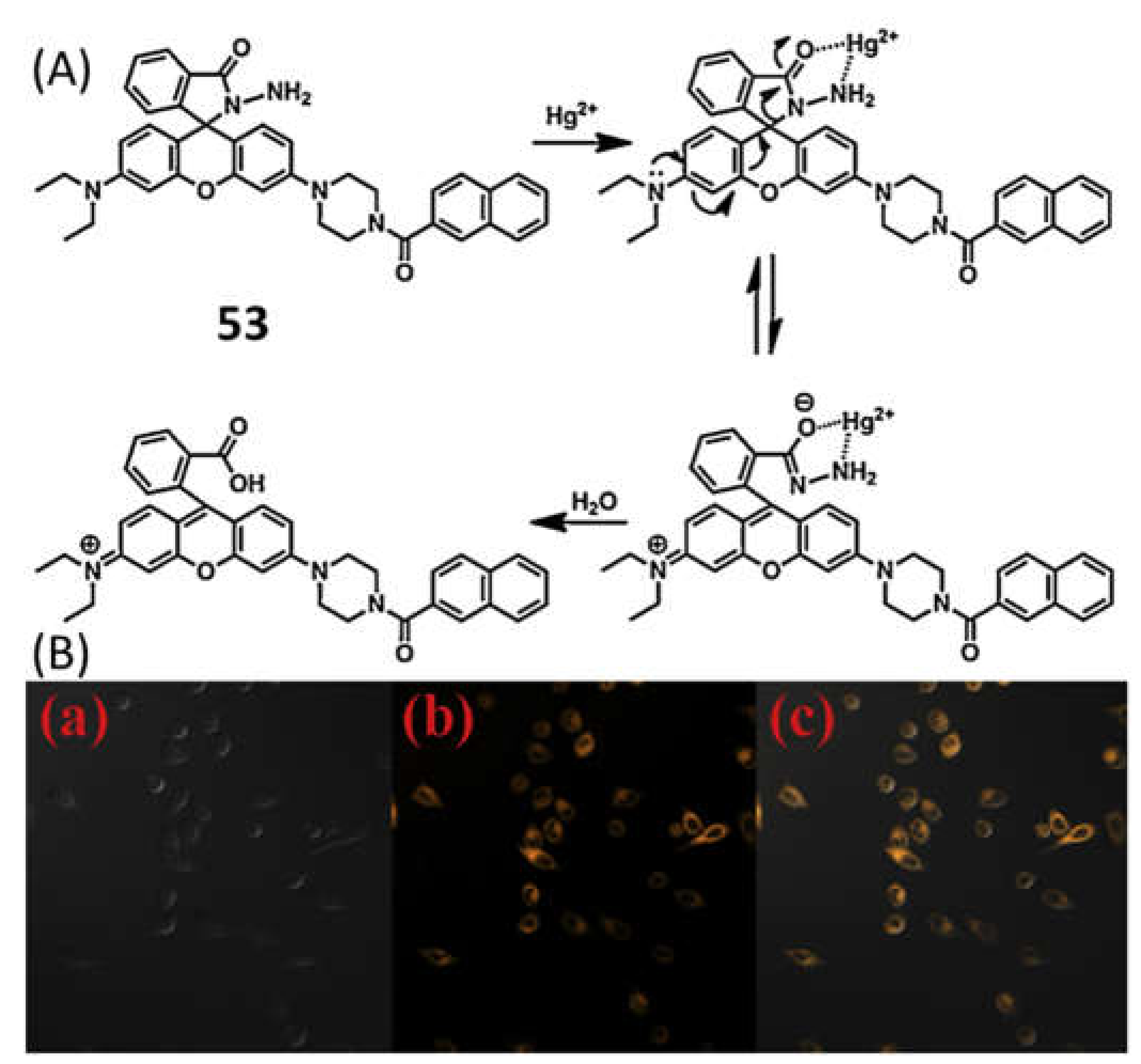
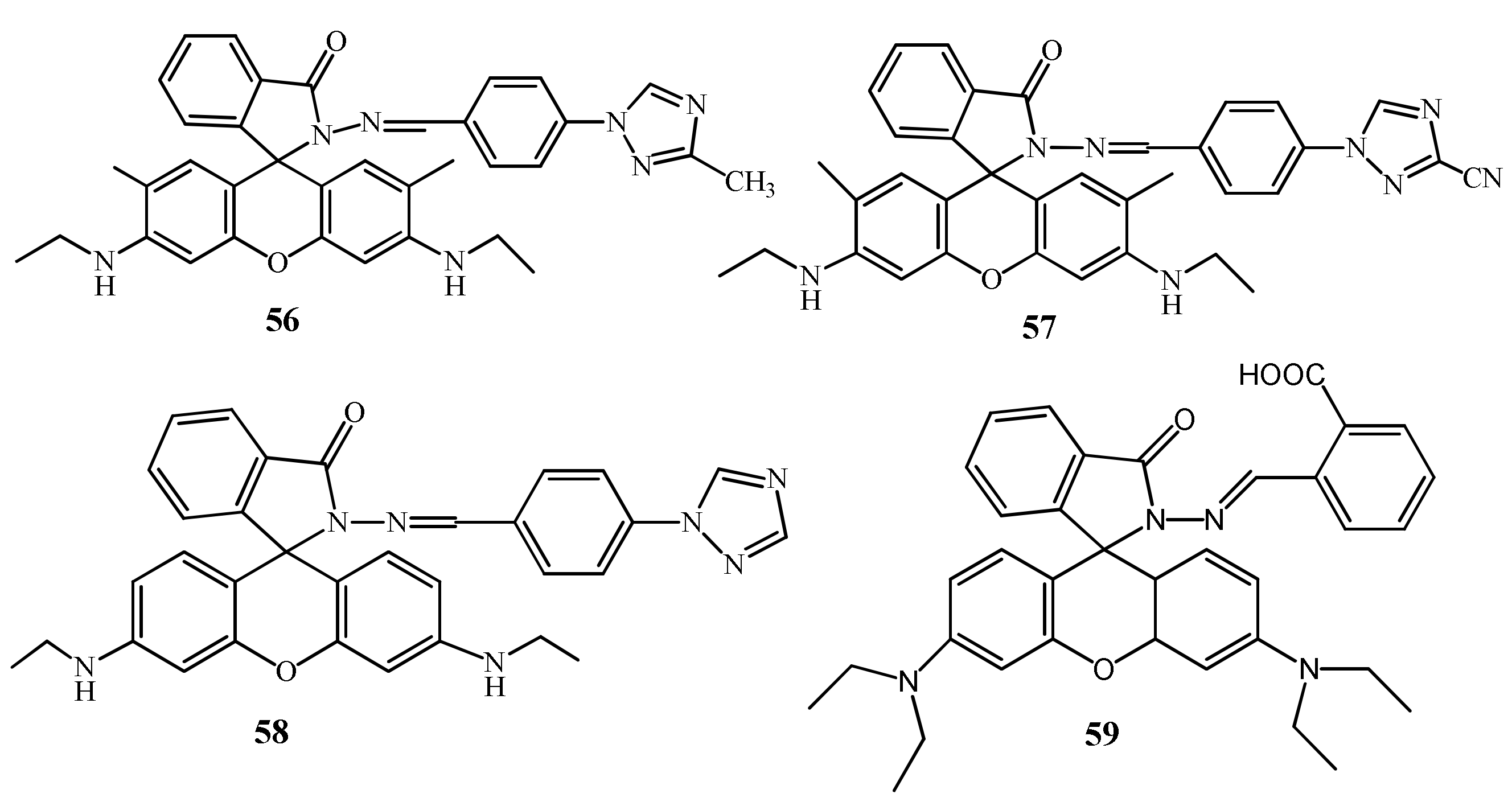

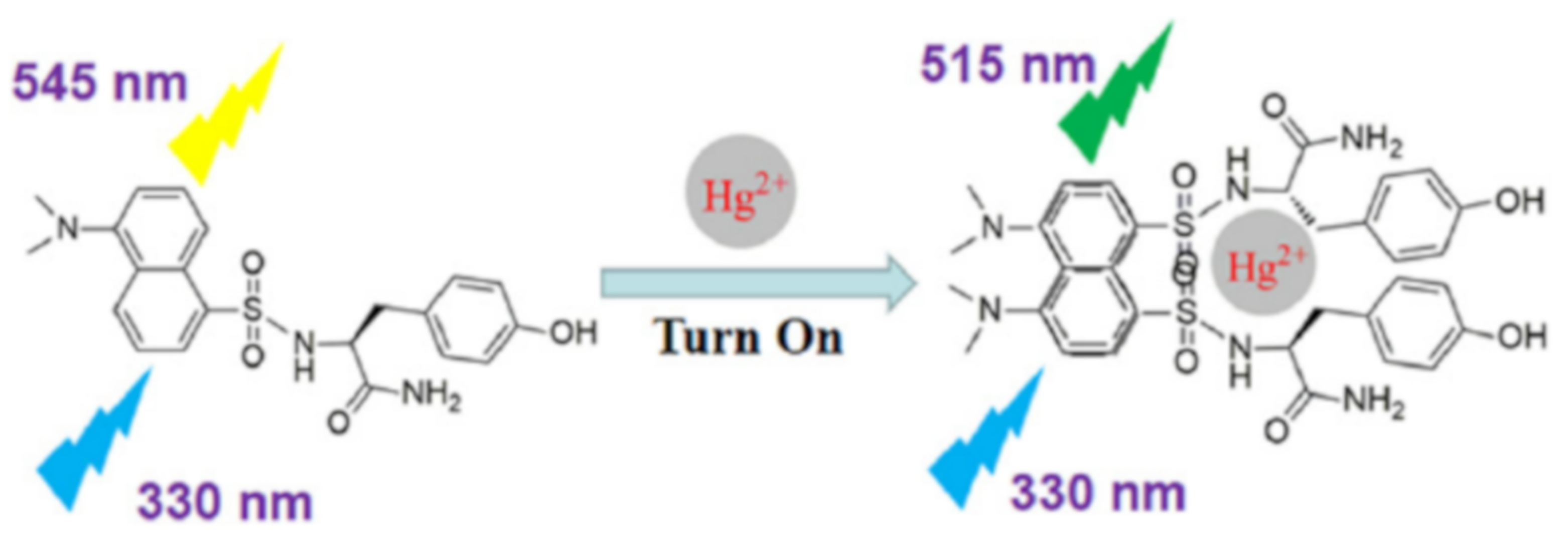
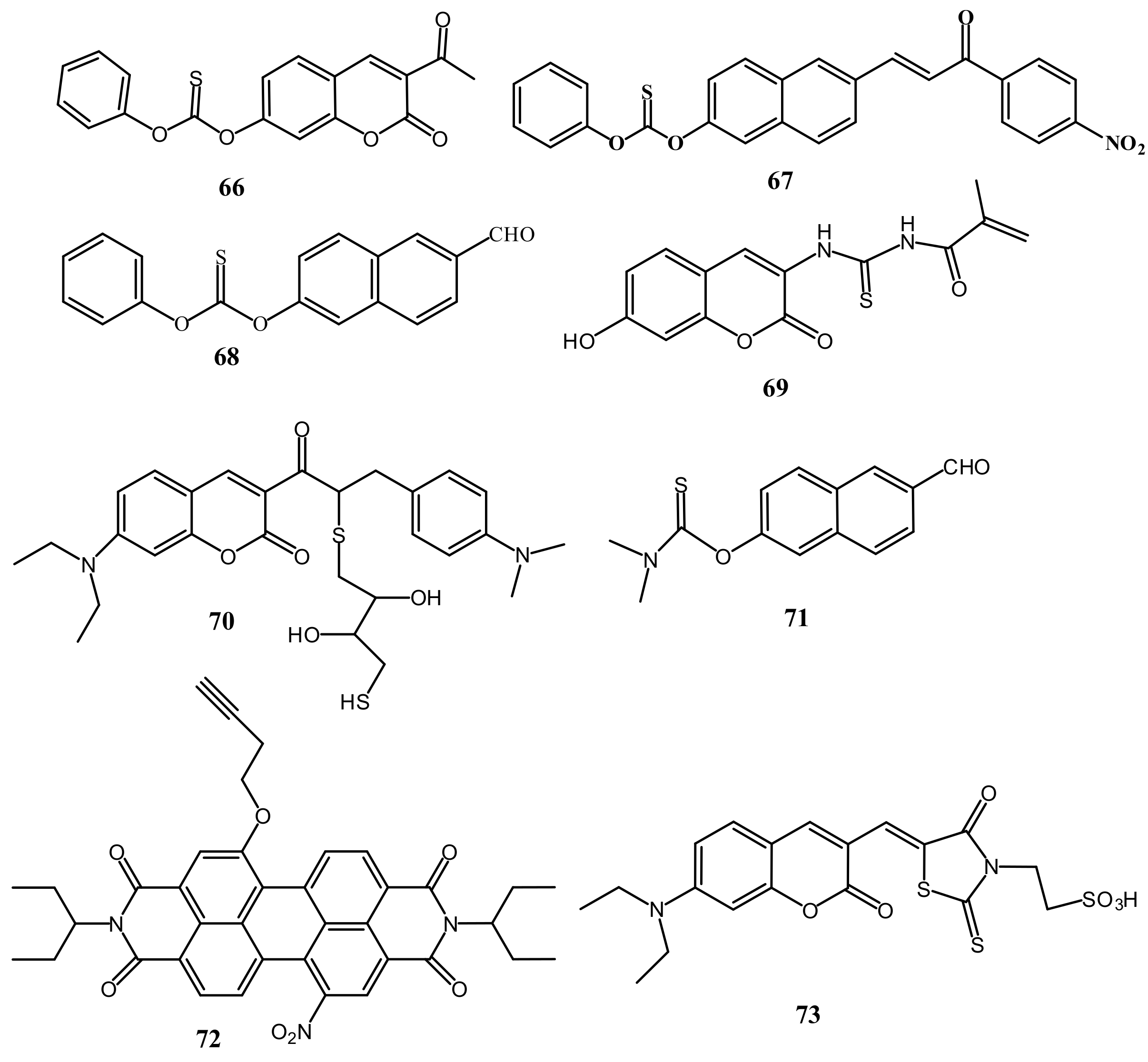
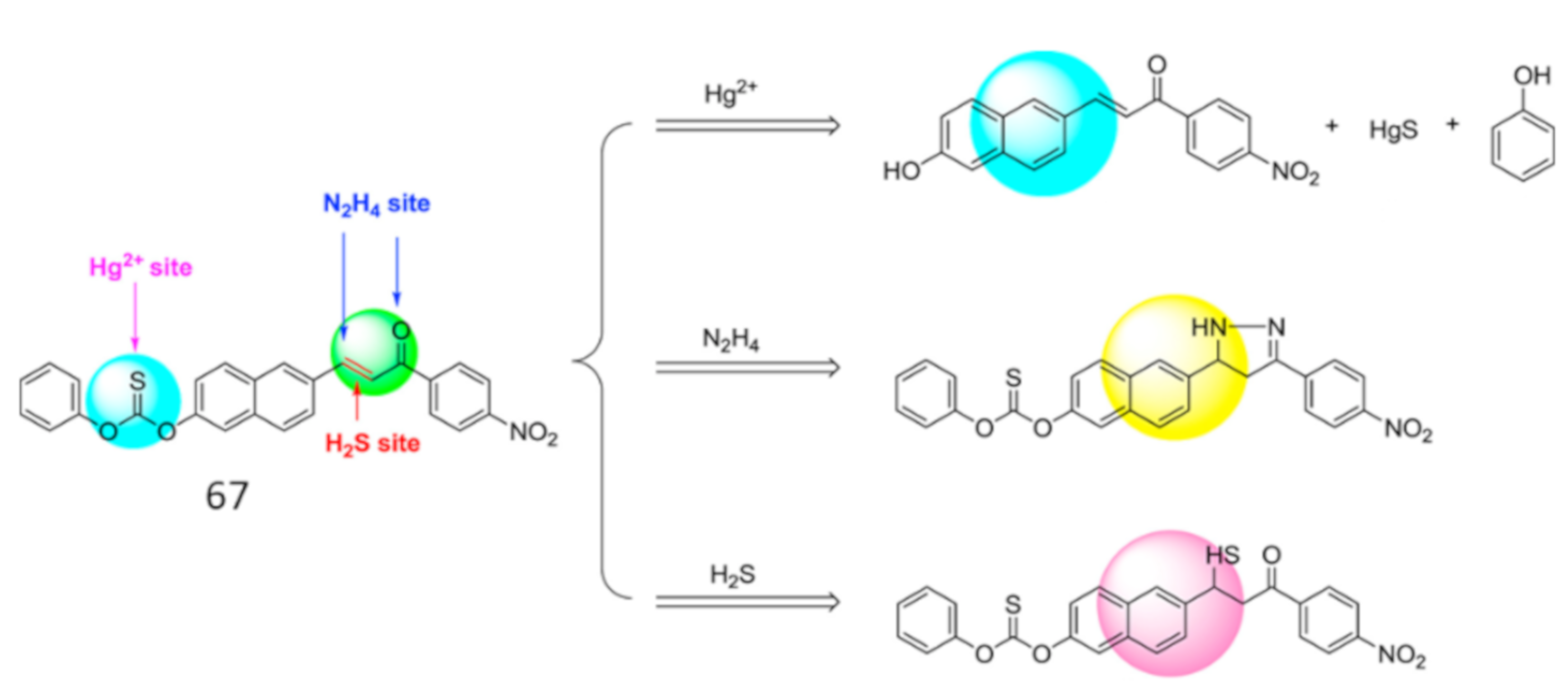
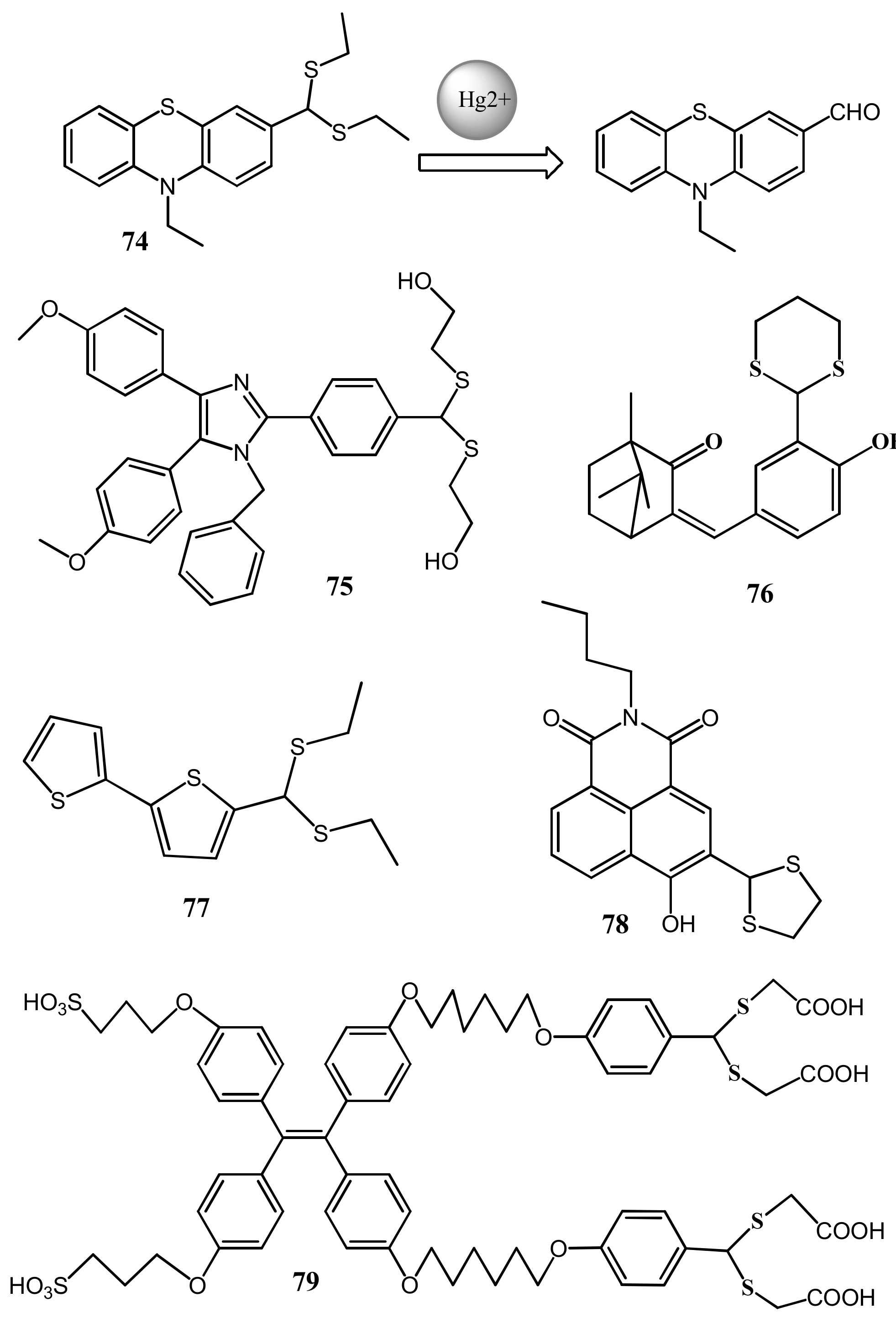
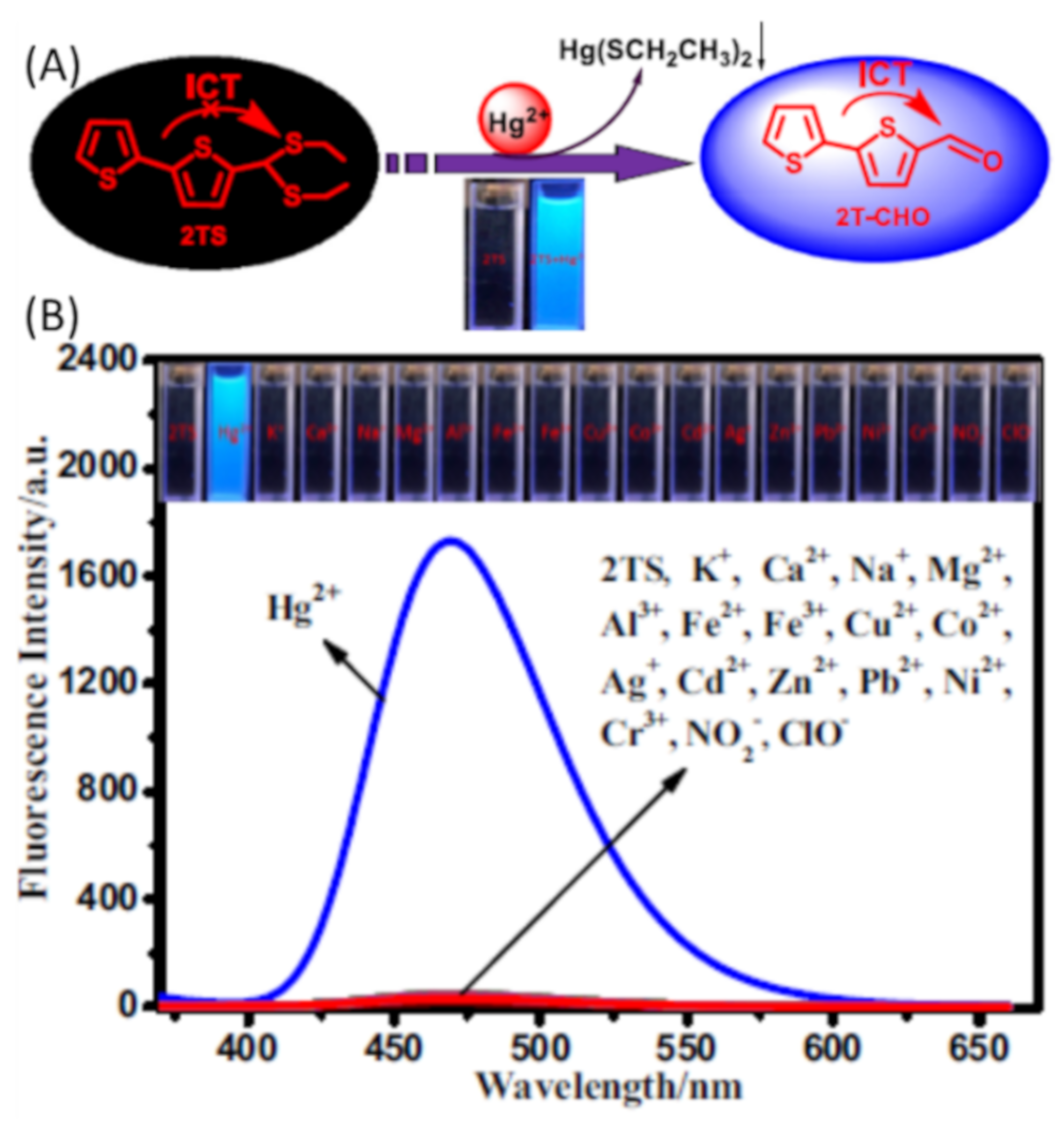

| Species | Occupational Exposure | Environmental Exposure | Routes of Exposure | Affected Organs |
|---|---|---|---|---|
| Elemental mercury, Hg | Chlor-alkali plants, gold extraction, incineration of wastes, coal burning, dental amalgam handling | Dental amalgam | Inhalation | Nervous system |
| Organic mercury, CH3Hg+ | - | Food (Fish and seafood) | Ingestion | Nervous system |
| Inorganic mercury, Hg2+ | - | Medicinal uses, dermatological creams | Ingestion, transdermal | Kidneys |
| Sensors | Medium | λexc/λem (nm) | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| 1 | H2O | 350/464 | 0.16 μM | Real water sample analysis | [32] |
| 2 | H2O | 500/632 | 39.2 nM | - | [33] |
| 3 | H2O | 316/416 | 0.1243 µM | Real water sample analysis | [34] |
| 4 | THF:H2O (1:49, v/v) | 380/500 | 22.8 ppb | - | [35] |
| 5 | CH3CN:H2O (1:1, v/v) | 270/380 | 0.68 μM | Real water analysis and pH sensing | [36] |
| 6 | EtOH | 410/485 | 172 nM | Living cell imaging | [37] |
| 7 | CH3CN:HEPES buffer (2:8, v/v) | 369/490 | 1 nM | Live-cell imaging | [38] |
| 8 | H2O | 490/574 | 312 nM | Real water sample analysis | [39] |
| 9 | Tris-HCL buffer | 358/445 | 4.40 μM | Real water sample analysis and live cell imaging | [40] |
| 10 | HEPES buffer | 330/550 | 7.59 nM | Real water samples and live cell imaging | [41] |
| 11 | CH3CN:H2O (6:4 v/v) | 375/485 | 1.26 nM | Test paper strip and real water analysis | [42] |
| 12 | CH3CN | 420/603 | 20 ppb | Removal from water | [43] |
| 13 | DMF | 325/463 | 17 nM | Air and real water samples | [44] |
| 14 | MeOH | 280/436 | 0.01 μM | - | [45] |
| 15 | H2O | 495/521 | 0.34 μM | Industrial effluents and paper strip | [46] |
| 16 | H2O | 430/512 | 5.02 mM | Real water analysis | [47] |
| 17 | MeOH:H2O (1/4, v/v) | 340/455 | 3.12 nM | Live cell imaging | [48] |
| 18 | CH3CN | 440/492 | 200 nM | Real water sample analysis | [49] |
| 19 | DMF:H2O (1:1, v/v) | 337/378 | 16 nM | - | [50] |
| 20 | H2O:DMSO (95:5, v/v) | 360/540 | 0.51 μM | - | [51] |
| 21 | H2O | 285/364,464 | 7.41 nM | Bioimaging | [52] |
| 22 | DMSO:HEPES buffer (9:1, v/v) | 355/455 | 8.12 nM | Live cell imaging | [53] |
| 23 | H2O | 359/495 | 22 nM | Real water analysis and bioimaging | [54] |
| 24 | MeOH:H2O (4:1, v/v) | 305/387 | 0.28 ppb | Real water analysis | [55] |
| 25 | DMF:HEPES buffer (1:1, v/v) | 315/495 | 0.645 µM | Real water analysis and live cell imaging | [56] |
| 26 | Acetonitrile:HEPES buffer (1:1, v/v) | 401/520 | 1.01 µM | Real water analysis | [57] |
| 27 | Acetonitrile:HEPES buffer (1:1, v/v) | 405/525 | 1.98 µM | - | [57] |
| 28 | THF:H2O (9:1, v/v) | 480/675 | 13.1 nM | Live cell imaging | [58] |
| 29 | H2O:DMSO (99.6: 0.4, v/v) | 495/543 | 19.2nM | Test color strips and bio-imaging | [59] |
| 30 | CH3CN/:H2O (4:6, v/v) | 520/587 | 3.9 ppb | Drinking water, live cells and plant tissues | [60] |
| 31 | CH3CN:HEPES (1:9, v/v) | 470/530 | 27 nM | Live cell imaging | [61] |
| 32 | MeOH:H2O (4:1, v/v) | 360/590 | 4.8 μM | Test paper strips, bioimaging | [62] |
| 33 | DMSO:H2O mixture | 360/453 | 406 nM | Adsorption of H2S | [63] |
| 34 | DMSO:H2O (2:8, v/v) | -/600 | 30 nM | Real food samples and live cell imaging | [64] |
| 35 | HEPES buffer (pH 7.4) | 480/518 | 3 nM | Real water and biological analysis | [65] |
| 36 | EtOH:H2O mixture | 366/491 | 750 nM | Real water sample analysis | [66] |
| 37 | CH3CN/H2O (0.2:99.8, v/v) | 366/463 | 2.1 nM | - | [67] |
| 38 | DMF:buffer (8:2, v/v) | 315/529 | 2.40 μM | Live cell imaging | [68] |
| 39 | DMF:buffer (8:2, v/v) | 470/621 | 2.86 μM | Live cell imaging | [68] |
| 40 | DMF:buffer (8:2, v/v) | 470/614 | 0.22 μM | Live cell imaging | [68] |
| 41 | H2O | 480/532 | 8.619 nM | Real water analysis and live cell imaging | [69] |
| 42 | PBS buffer | 460/515 | 0.4 nM | Real water analysis and live cell Imaging | [70] |
| 43 | CH3CN:HEPES buffer (1:9, v/v). | 545/580 | - | - | [71] |
| 44 | DMSO | 520/606 | 0.13 µM | INHIBIT logic gate | [72] |
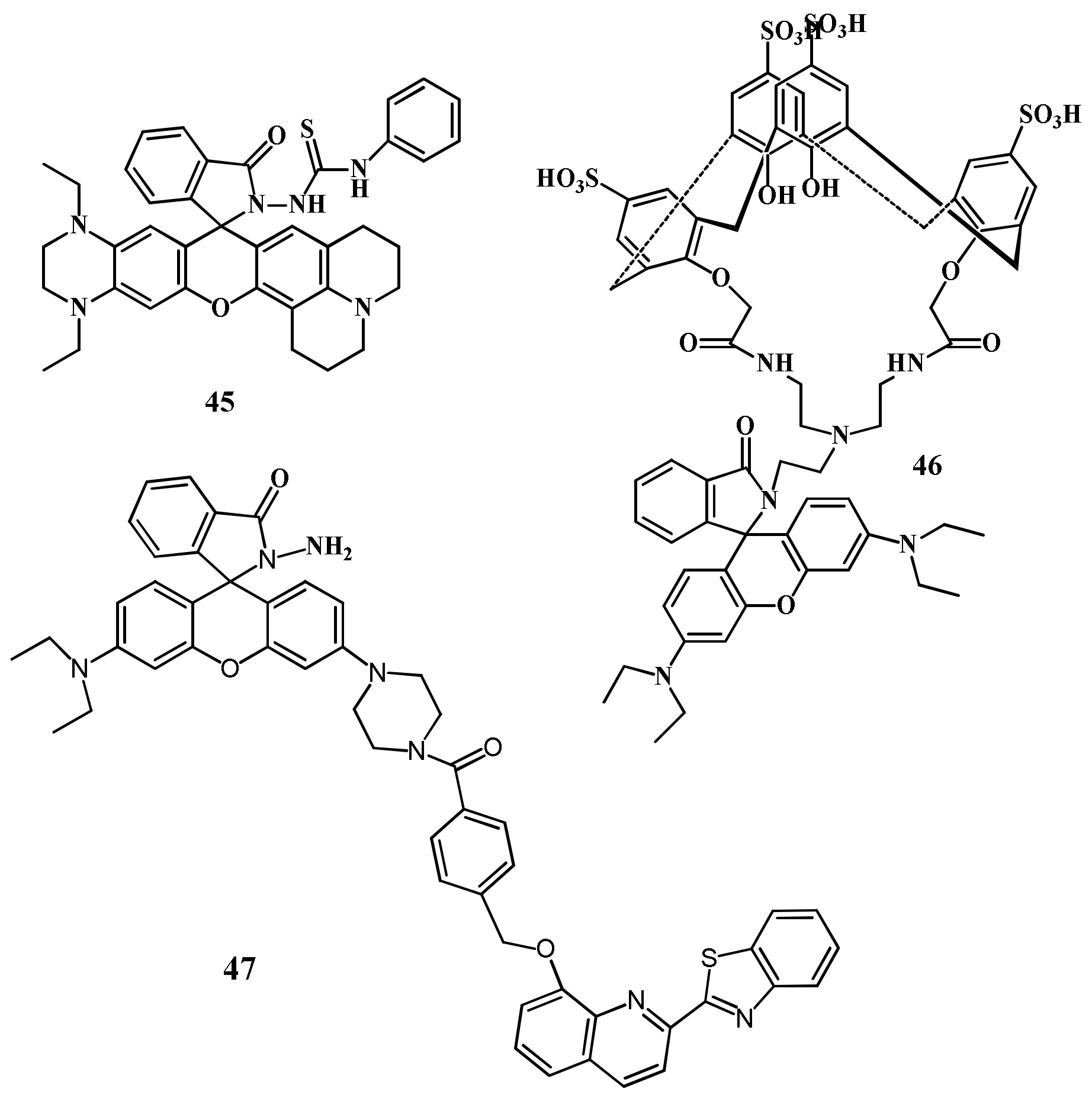
| 45 | CH3CN:HEPES buffer(2:8, v/v) | 580/691 | 1.5 nM | Living cells imaging | [73] |
| 45 | EtOH:HEPES buffer (1:1 v/v) | 590/664 | 1.87 ppb | Real water sample analysis | [74] |
| 46 | H2O | 335/574 | 3.55×10-13 mL−1 | - | [75] |
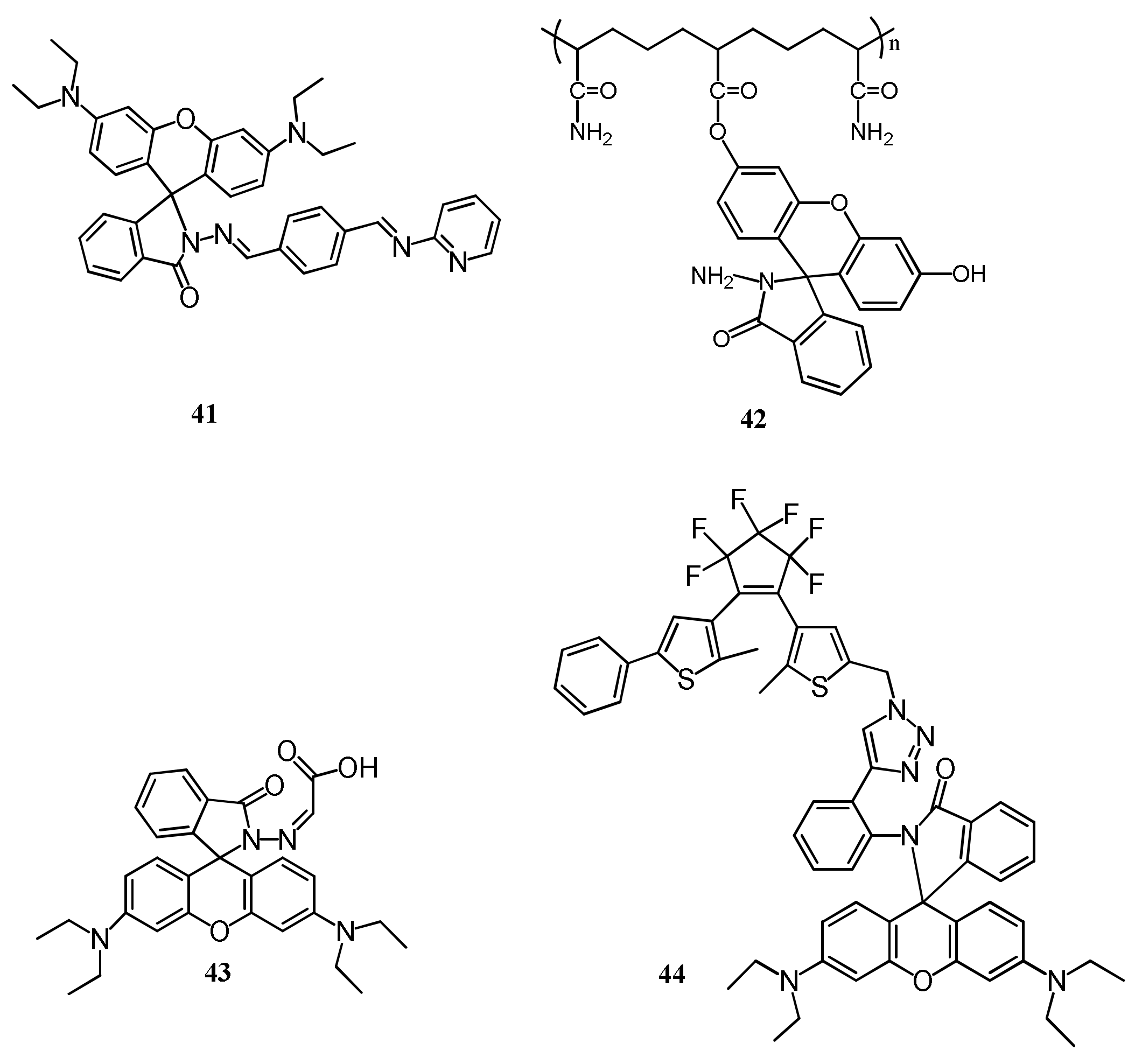
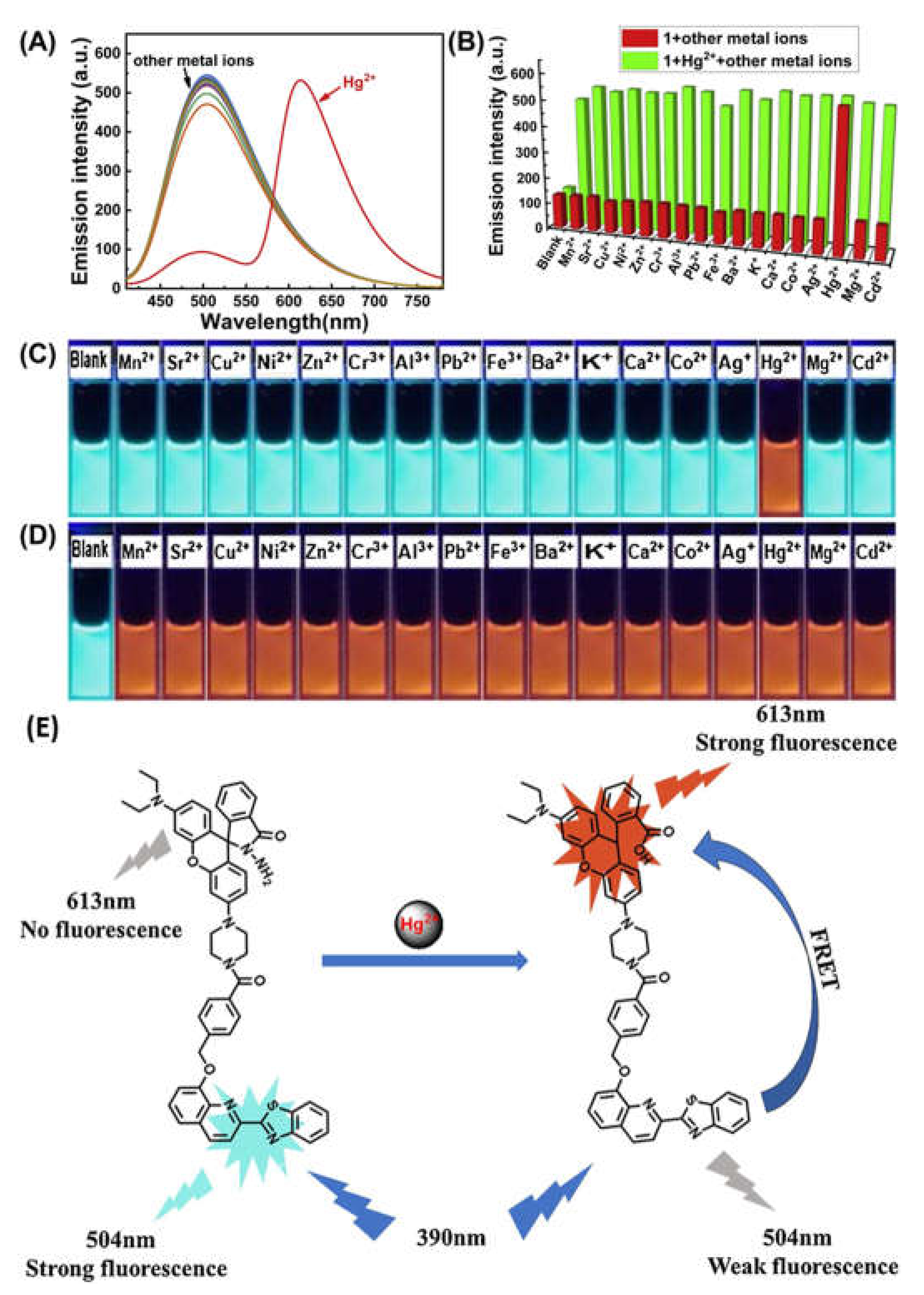
| 47 | DMF:H2O (7/3, v/v) | 390/504,613 | 0.2 µM | Living cells | [76] |
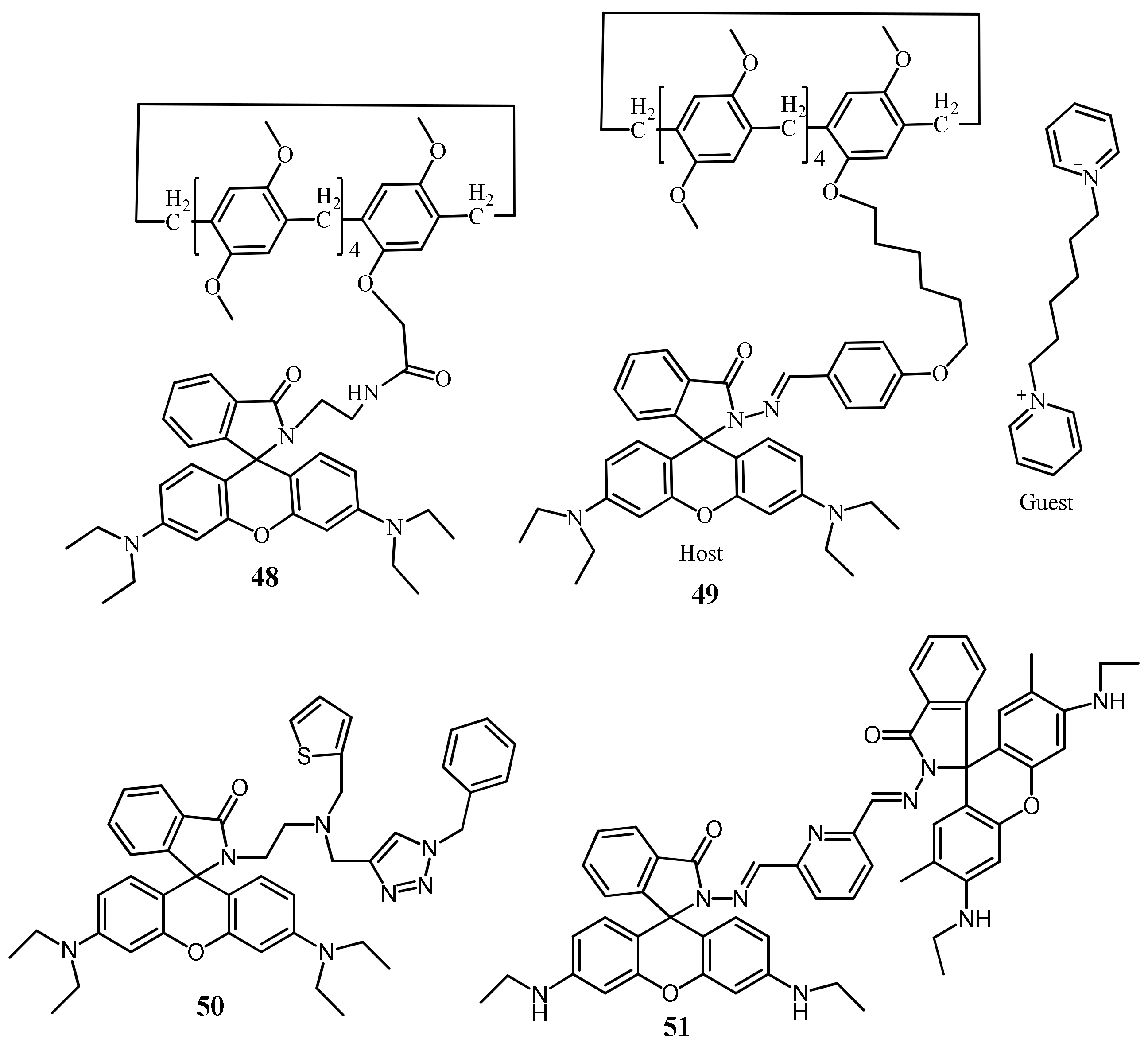
| 48 | CH3CN | 510/573 | 28.5 nM | - | [77] |
| 49 | DMSO:H2O (6:4, v/v) | 505/585 | 16.9 nM | - | [78] |
| 50 | CH3CN | 520/585 | 16 nM | Real water sample analysis | [79] |
| 51 | DMSO:H2O (1:1; v/v) | 500/562 | 26 nM | Real water sample analysis | [80] |
| 52 | DMSO:H2O (7/3, v/v) | 490/581 | 14.9 nM | - | [81] |
| 53 | CH3CN:H2O (7/3, v/v) | 520/604 | 0.38 μM | Test color strips and biosensing | [82] |
| 54 | DMF:Tris-HCl buffer (1:1, v/v) | 562/557 | 1.61 nM | Bio-sensing and live cell imaging | [83] |
| 55 | H2O:THF (4:1, v/v) | 565/590 | 16 nM | Live cell imaging | [84] |
| 56 | DMSO:H2O (7:3, v/v) | 480/582 | 13.4 nM | Test color strips and live cell imaging | [85] |
| 57 | DMSO:H2O (7:3, v/v) | 480/578 | 15.6 nM | Test color strips and live cell imaging | [85] |
| 58 | DMSO:H2O (7:3, v/v) | -/560 | 16.1 nM | Test paper strips and real water analysis | [86] |
| 59 | H2O | - | 120 nM | Real water sample analysis | [87] |
| 60 | HEPES buffer | 330/550 | 23.0 nM | Test color strips and sensing of biothiols | [88] |
| 61 | HEPES buffer | 310/430,548 | 1.3 nM | Environmental water samples | [89] |
| 62 | HEPES buffer | 330/545 | 22.65 nM | Biosensing | [90] |
| 63 | DMSO:HEPES medium (1:99, v/v) | 340/390 | 14.7 nM | Real water sample analysis | [91] |
| 64 | EtOH-H2O (9:1, v/v) | 500/385,447 | 0.22 μM | - | [92] |
| 65 | CH3CN:DMSO (99:1, v/v) | 340/395 | 8.11 nM | IMPLICATION logic gates | [93] |
| 66 | H2O | 390/455 | 7.9 | River water and live cell imaging | [94] |
| 67 | EtOH | 300 580 | 1.10 μM | Test color strips and real water analysis | [95] |
| 68 | DMSO:H2O (1:3, v/v) | 321/444,644 | 48.79 nM | Real water and beverages samples | [96] |
| 69 | EtOH:H2O (2:8, v/v) | 332/475 | 146 nM | Real waste water analysis | [97] |
| 70 | EtOH:HEPES buffer (1:9, v/v) | 450/495,600 | 1.6 nM | Real water analysis and live cell imaging | [98] |
| 71 | H2O | 300/443 | 39.28 nM | Test color strips and real water analysis | [99] |
| 72 | THF-H2O (1:9, v/v) | 490/667 | 33 nM | Biological sample and live cell imaging | [100] |
| 73 | HEPES-DMSO (99:1, v/v) | 325/630 | 15.1 μM | Bioimaging | [101] |
| 74 | PBS buffer (pH 7.4) | 390/445 | 21.2 nM | Real water analysis and live cell imaging | [102] |
| 75 | DMSO:PBS buffer (1:99, v/v) | 380/475 | 36 nM | Real water analysis and live cell imaging | [103] |
| 76 | DMSO:PBS buffer (1:99, v/v) | 365/518 | 19.3 nM | Real sample analysis, test color strips and cell imaging | [104] |
| 77 | H2O | 370/470 | 19 nM | Real water, seafood, human urine samples, test color strips and bio-imaging | [105] |
| 78 | PBS buffer | 414/510 | 40 nM | Living cell imaging | [106] |
| 79 | THF:H2O (1/99, v/v) | 353/477 | 20 nM | Test color strips and real water analysis | [107] |
| 80 | H2O | 390/461 | 1.11 μM | - | [108] |
| 81 | H2O | 390/464 | 1.89 μM | - | [108] |
| 82 | DMF:H2O (1:1, v/v) | 330/549 | 0.21 µM | - | [109] |
| 83 | DMF:H2O (1:1, v/v) | 331/550 | 0.63 µM | - | [109] |
| 84 | DMF:H2O (1:1, v/v) | 335/559 | 0.19 µM | - | [109] |
| Sensors | Medium | λabs (with/without Hg2+) | LOD | Applications | Ref. |
|---|---|---|---|---|---|
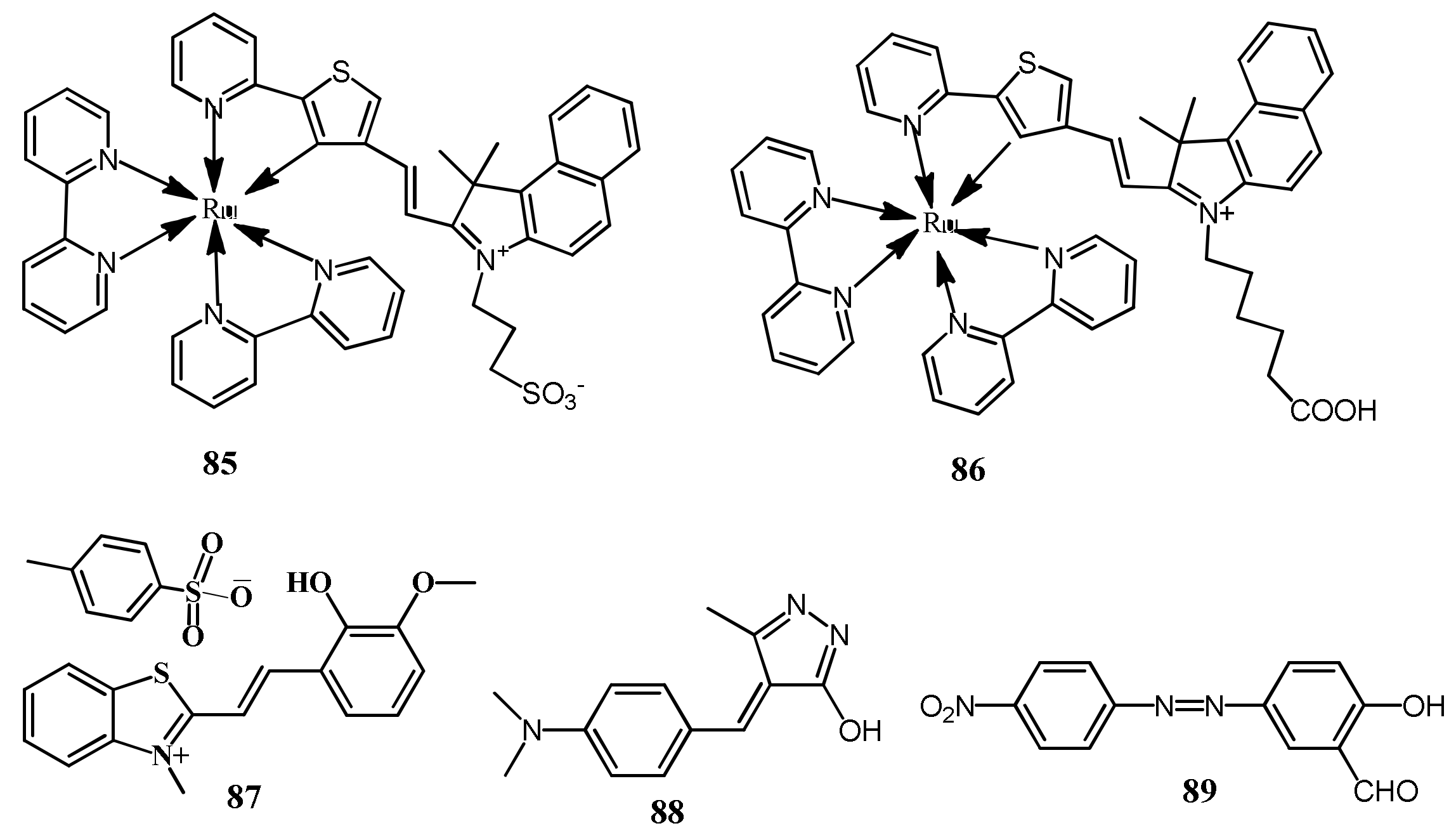
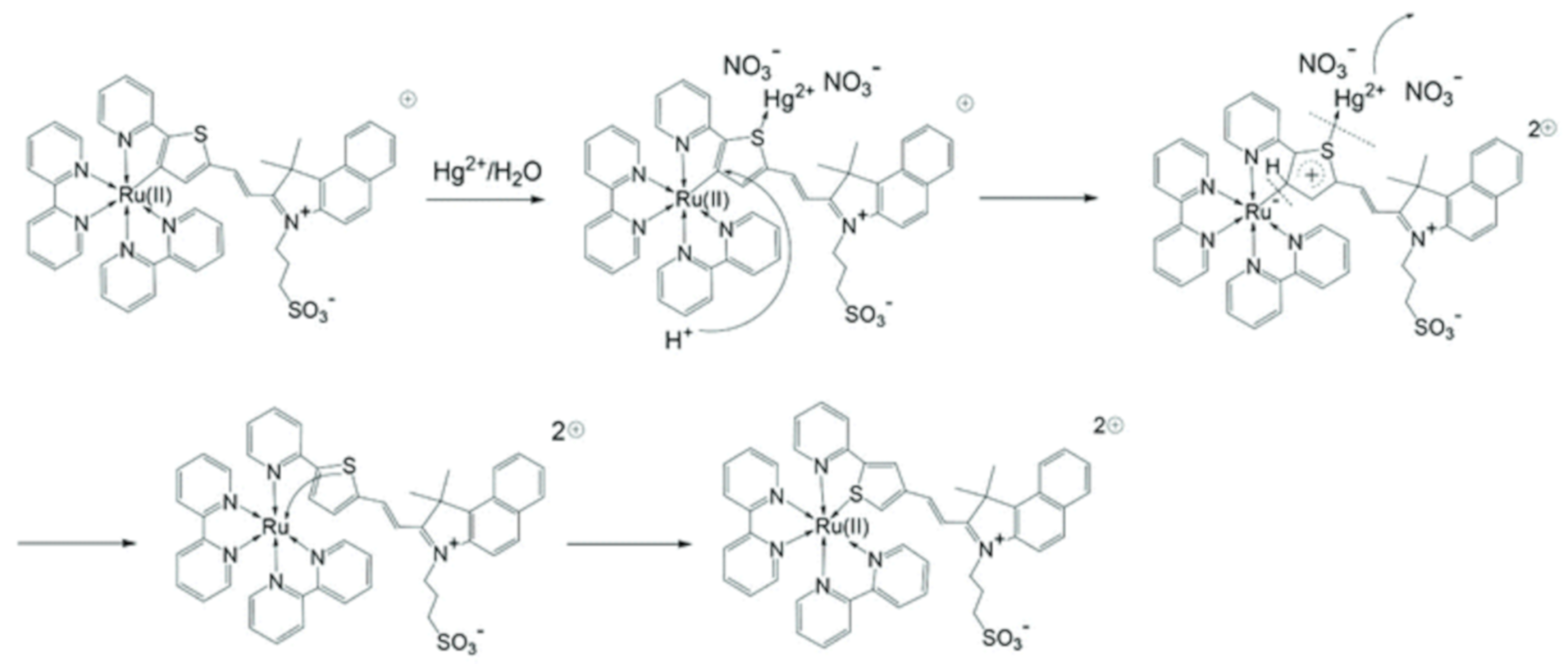
| 85 | Aqueous media | Quenching of bands at 506 and 730 nm | 21 nM | - | [110] |
| 86 | DMSO:HEPES (5:95, v/v) | Quenching at 503 with the new band formation at 610 nm | 53 nM | Polymer coated membrane | [111] |
| 87 | HEPES buffer | Quenching of bands at 390 and 530 nm | 0.27 μM | Test color strips | [112] |
| 88 | CH3CN:H2O (7:3, v/v) | Band at 447 nm shifted to 519 nm | 0.473 μM | Test color strips and real water analysis | [113] |
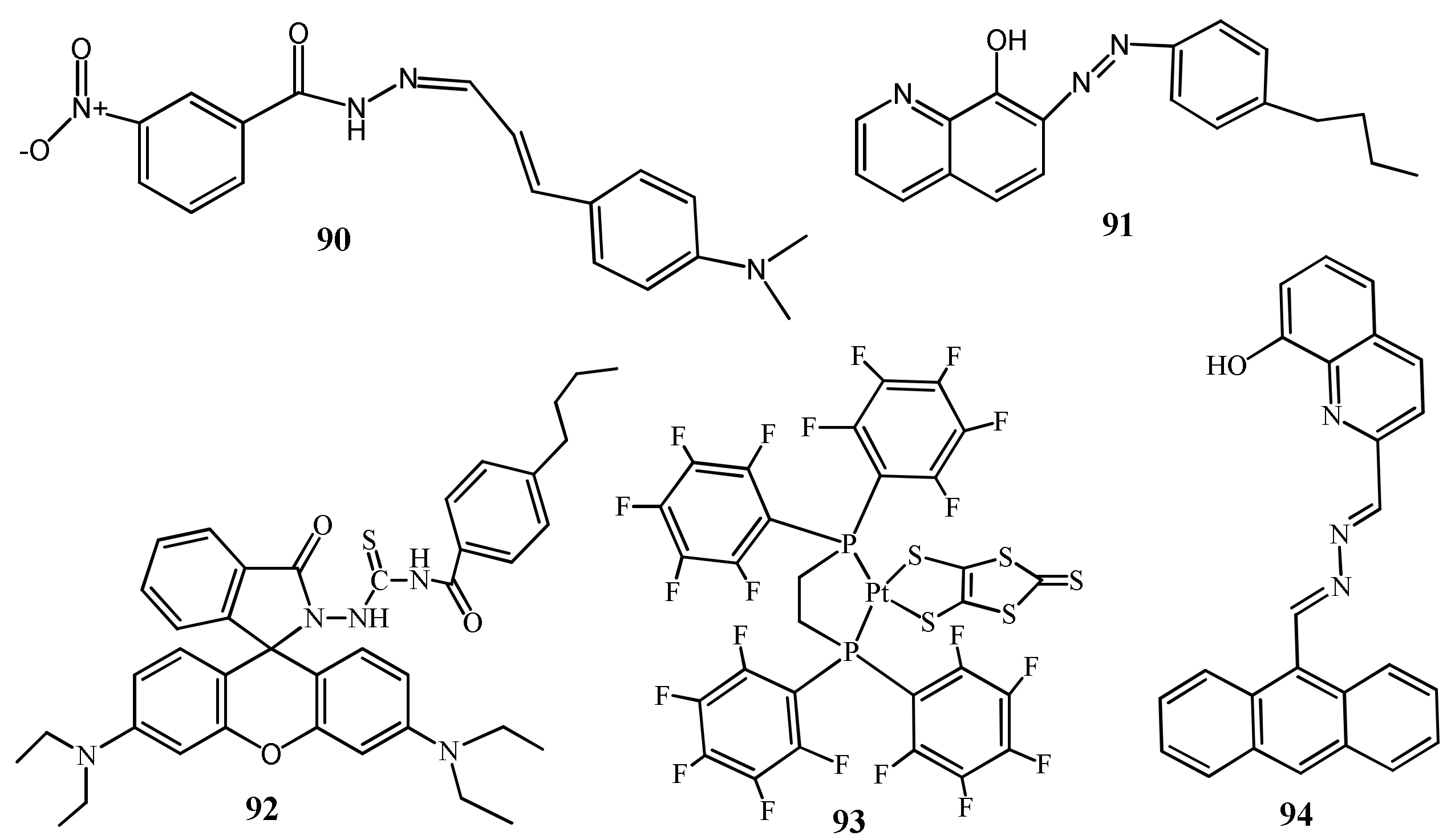
| 89 | DMSO:H2O (4:1 v/v) | Band at 502 nm shifted to 395 nm | 6.1 μM | Cellulose test strips, Logic Gate Operation | [114] |
| 90 | BufferDMF (98:2, v/v). | Quenching of bands at 350 and enhancement at 400 nm | 0.11 µM | Real water sample analysis | [115] |
| 91 | Aqueous medium | Enhancement of band at 540 nm | 0.100 and 0.180 μg/L | Real water sample analysis | [116] |
| 92 | Aqueous medium | Enhancement of band at 567 nm | 0.22 and 0.61µg/L | Real water sample analysis | [117] |
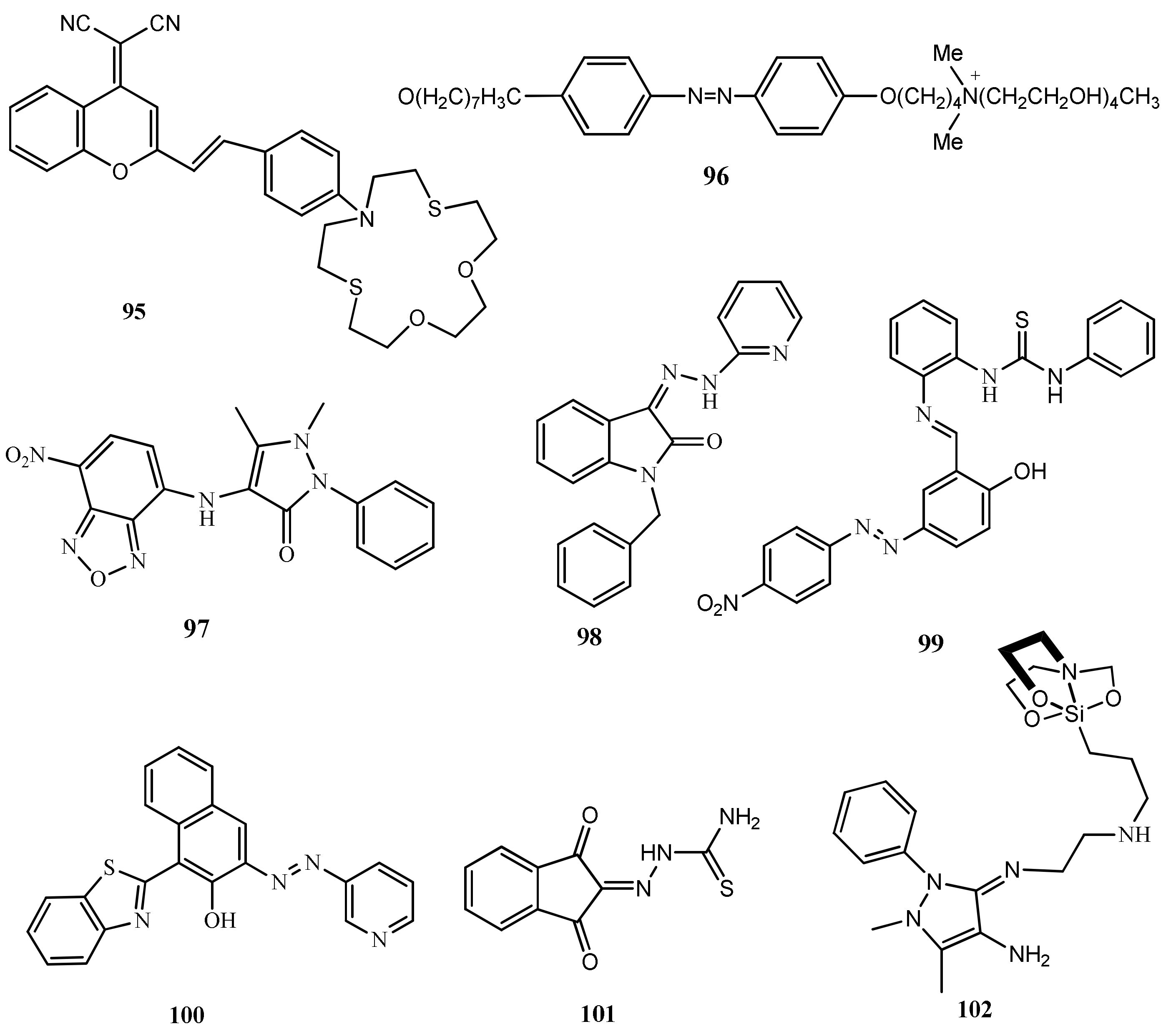
| 93 | CH3CN:H2O (1:1, v/v) | Band at 448 nm shifted to 523 nm | - | - | [118] |
| 94 | CH3OH:HEPES (7: 3, v/v) | Band at 414 nm shifted to 498 nm | 220 nM | Real water analysis, silica coating and test color strips | [119] |
| 95 | CH3CN:H2O (1:1, v/v). | Band at 517 nm shifted to 415 nm | - | Real water analysis | [120] |
| 96 | HEPES buffered | Band at 247 nm shifted to 234 nm | 40 nM | - | [121] |
| 97 | CH3OH:H2O (1: 1, v/v) | Band at 465 nm shifted to 485 nm | 25.7 nM | INHIBIT logic gate | [122] |
| 98 | MeCN:H2O (1:1, v/v) | Formation of new band at 470 and quenching at 380 nm | 0.95 nM | Real water sample analysis | [123] |
| 99 | DMSO:H2O (2:1, v/v) | Enhancement of band at 280 nm | 4.89 μM | Real water sample analysis | [124] |
| 100 | H2O:CH3CN (9:1, v/v) | Quenching of bands at 319 and 380 nm with the formation of new band at 610 nm | 8.5 μM | Test color strips and real water sample analysis | [125] |
| 101 | Aqueous medium | Band at 335 nm shifted to 305 nm | 1 μM | Real time application | [126] |
| 102 | DMSO:H2O (8:2, v/v) | Band at 290 nm shifted and two new bands formed at 255 and 292 nm | 0.10 mM | - | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, V.; Nurchi, V.M.; Sahoo, S.K. Mercury Toxicity and Detection Using Chromo-Fluorogenic Chemosensors. Pharmaceuticals 2021, 14, 123. https://doi.org/10.3390/ph14020123
Bhardwaj V, Nurchi VM, Sahoo SK. Mercury Toxicity and Detection Using Chromo-Fluorogenic Chemosensors. Pharmaceuticals. 2021; 14(2):123. https://doi.org/10.3390/ph14020123
Chicago/Turabian StyleBhardwaj, Vinita, Valeria M. Nurchi, and Suban K. Sahoo. 2021. "Mercury Toxicity and Detection Using Chromo-Fluorogenic Chemosensors" Pharmaceuticals 14, no. 2: 123. https://doi.org/10.3390/ph14020123
APA StyleBhardwaj, V., Nurchi, V. M., & Sahoo, S. K. (2021). Mercury Toxicity and Detection Using Chromo-Fluorogenic Chemosensors. Pharmaceuticals, 14(2), 123. https://doi.org/10.3390/ph14020123







