Effect of a Low Dose of Carvedilol on Cyclophosphamide-Induced Urinary Toxicity in Rats—A Comparison with Mesna
Abstract
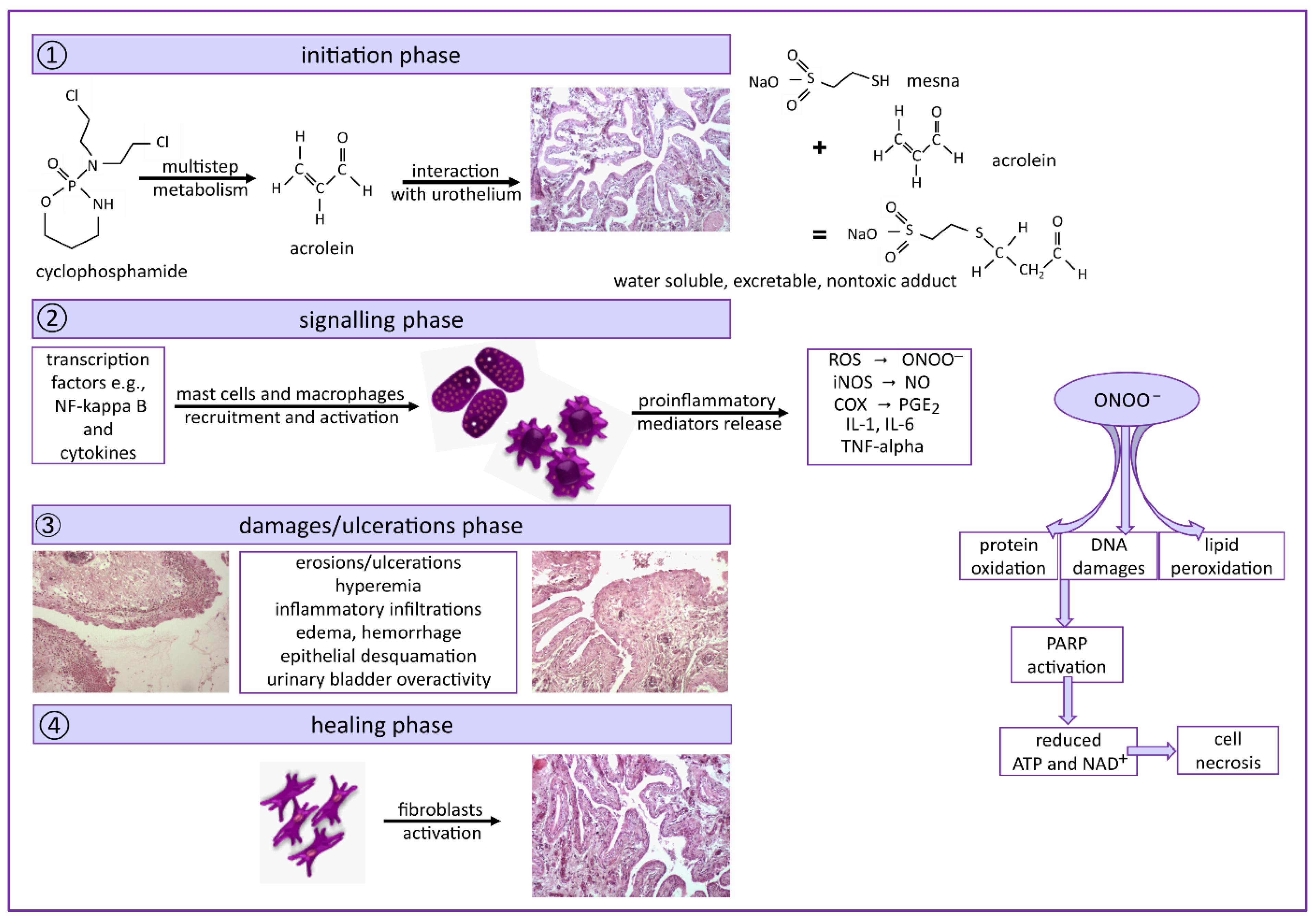
:1. Introduction
2. Results
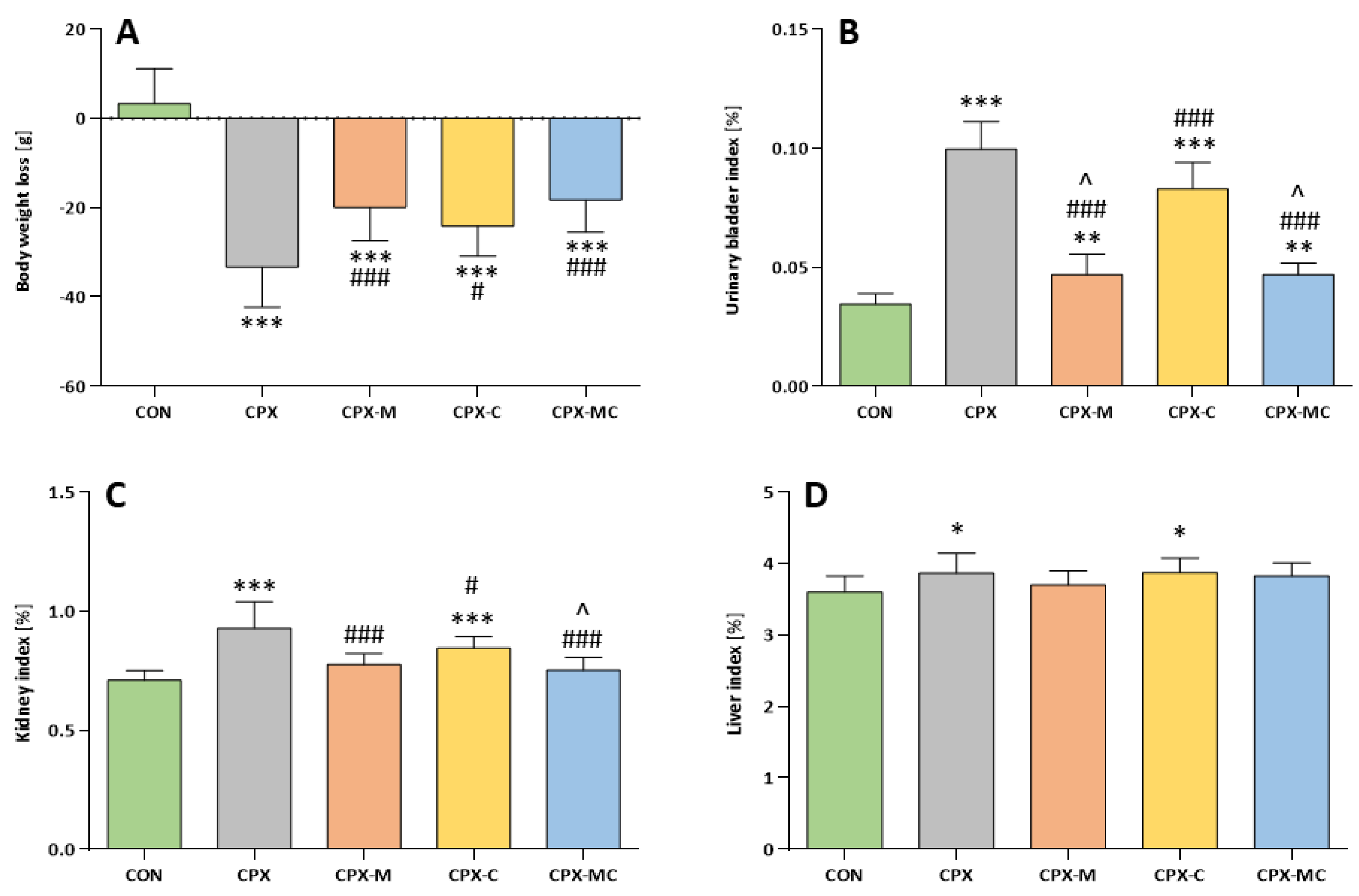
2.1. Body Weight Changes and Organ Weight
2.2. Serum Potassium and Creatinine Levels
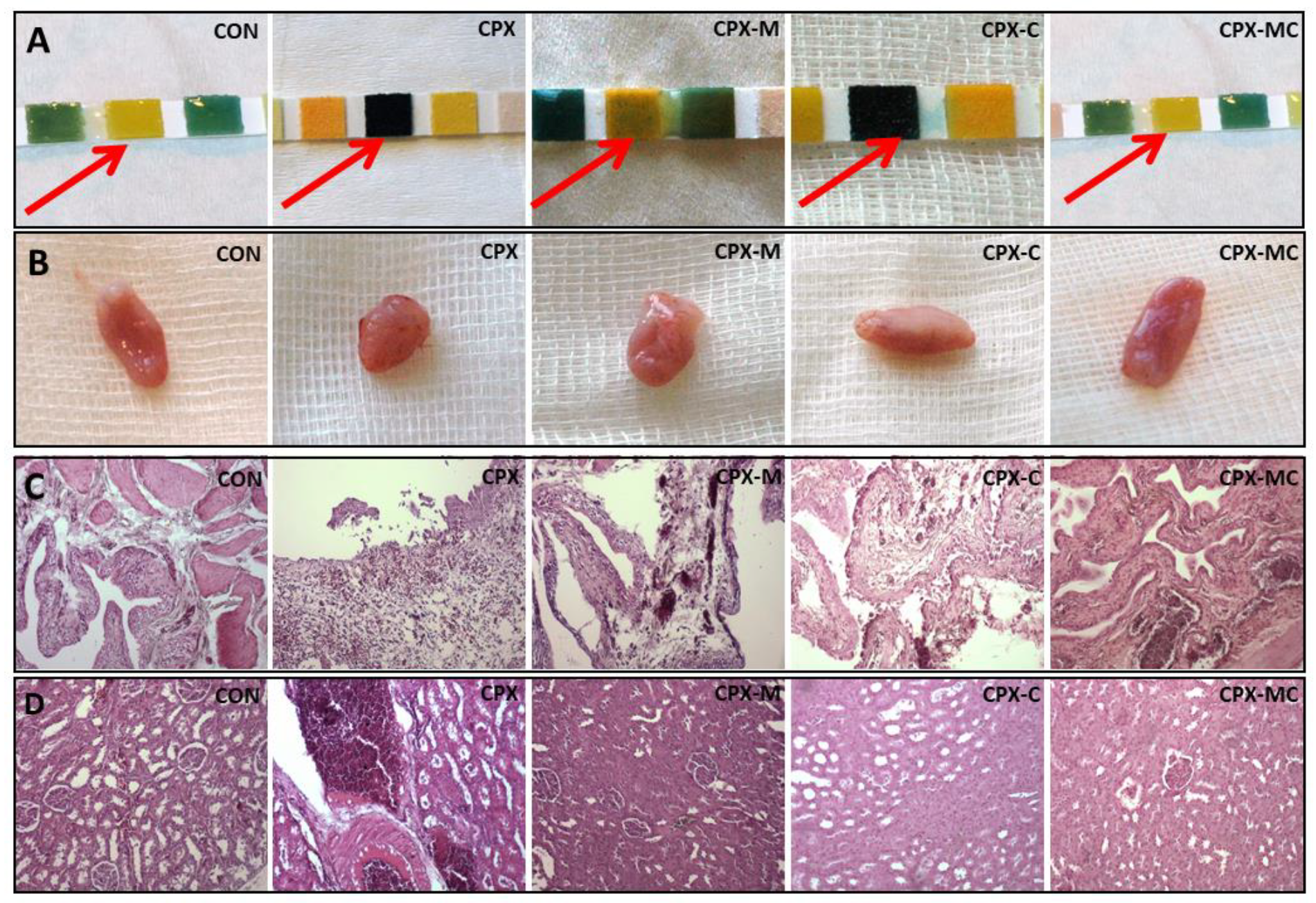
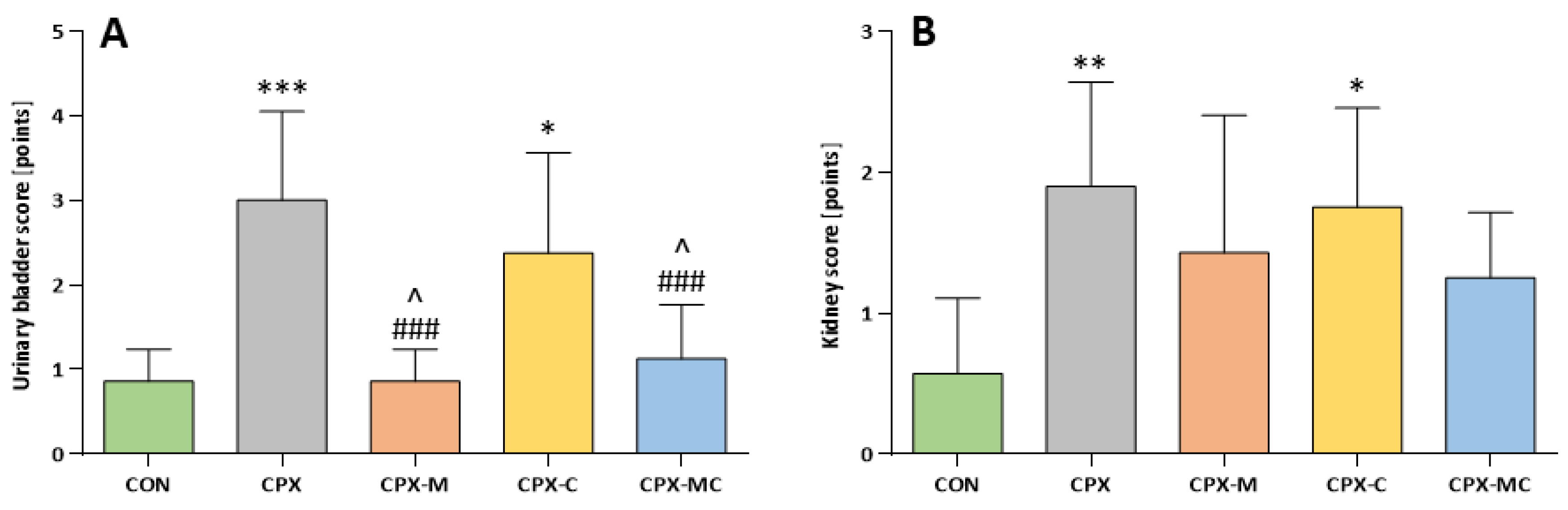
2.3. Hematuria, Urinary Bladder and Kidney Histology, Urinary Bladder and Kidney Scores
2.4. Oxidative Stress Parameters in Urinary Bladder, Kidney and Liver and Kidney IL-1β Level
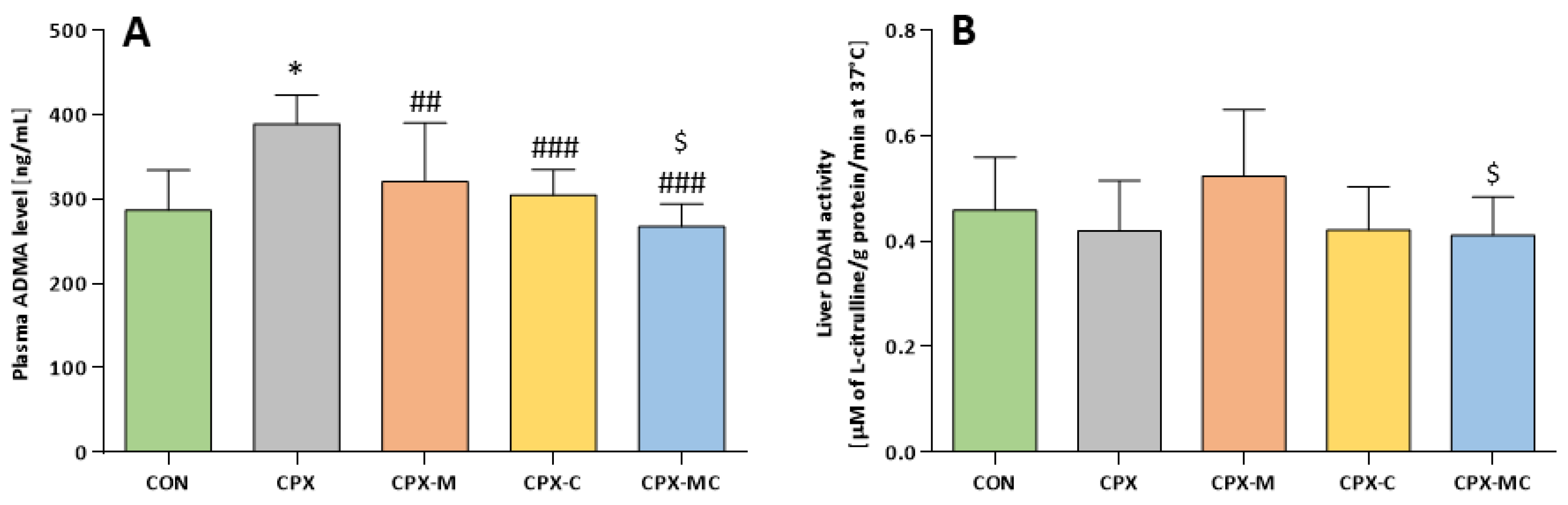
2.5. Asymmetric Dimethylarginine (ADMA) and Dimethylarginine Dimethylaminotransferase (DDAH) Assessment
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
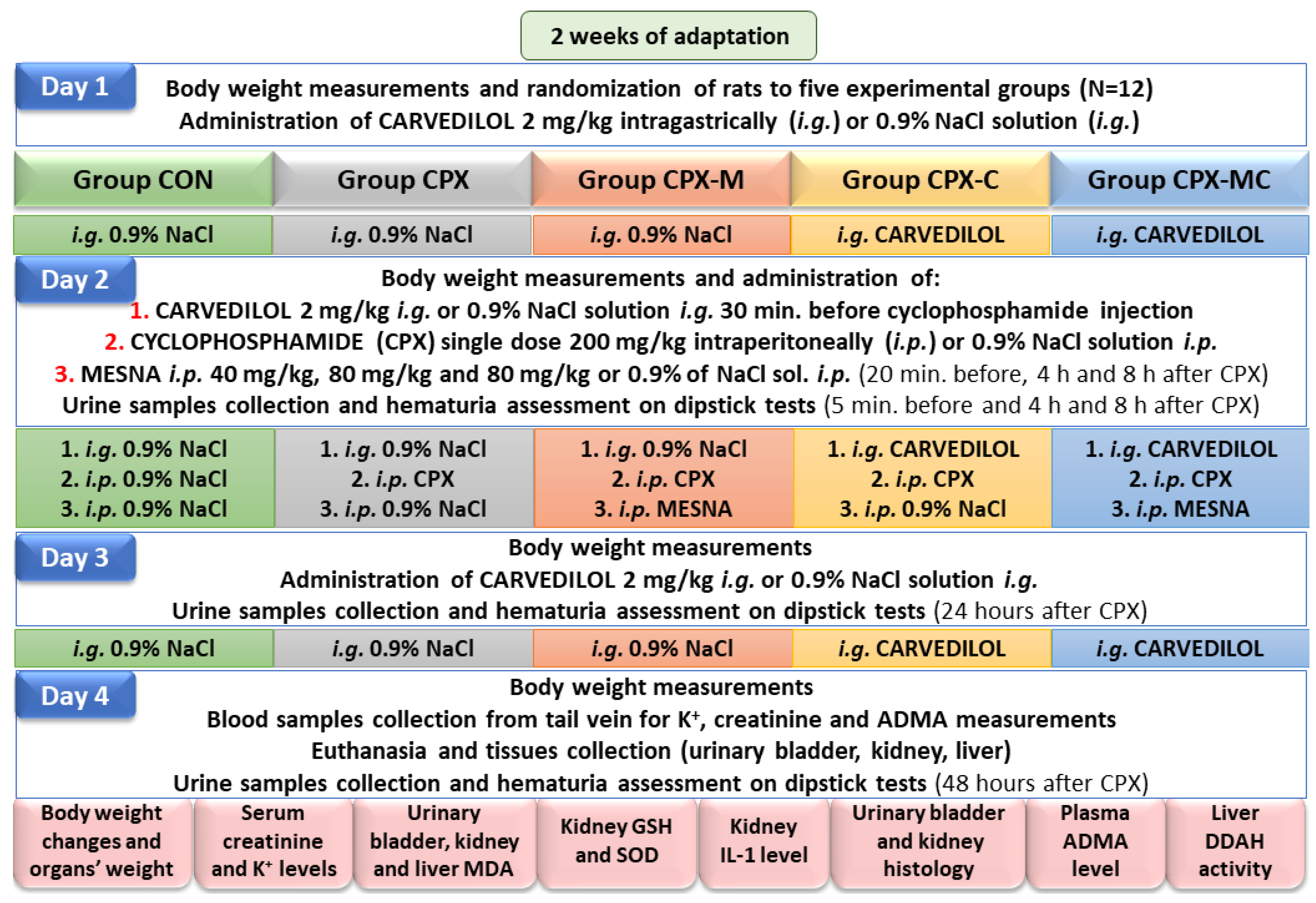
4.3. Experiment Design
4.4. Body Weight and Organ Weight
4.5. Serum Potassium and Creatinine Levels
4.6. Hematuria
4.7. Oxidative Stress Parameters in Urinary Bladder, Kidney and Liver and Kidney IL-1β Level
4.8. ADMA and DDAH Assessment
4.9. Histological Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| ATP | Adenosine triphosphate |
| CAT | Catalase |
| CCl4 | Carbon tetrachloride |
| CPX | Cyclophosphamide |
| COX | Cyclooxygenase |
| DDAH | Dimethylarginine dimethylaminotransferase |
| eNOS | Endothelial nitric oxide synthase |
| GSH | Glutathione |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| I/R | Ischemia reperfusion |
| MDA | Malondialdehyde |
| MDR | Multidrug resistance |
| MMP | Matrix metalloproteinases |
| NAD+ | Oxidized form of nicotinamide adenine dinucleotide |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NS | Not significant |
| ONOO– | Peroxynitrite |
| PAF | Platelet-activating factor |
| PARP | Poly (ADP-ribose) polymerase |
| PGE2 | Prostaglandin |
| PRMT | Protein arginine methyltransferase |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SOD | Superoxide dismutase |
| TGF-β1 | Transforming growth factor beta 1 |
| TNF-α | Tumor necrosis factor alpha |
References
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef]
- Cyclophosphamide 1000 mg Powder for Solution for Injection or Infusion–Summary of Product Characteristics (SmPC)–(emc). Available online: https://www.medicines.org.uk/emc/product/3525 (accessed on 27 October 2021).
- López-Beltrán, A.; Luque, R.J.; Mazzucchelli, R.; Scarpelli, M.; Montironi, R. Changes produced in the urothelium by traditional and newer therapeutic procedures for bladder cancer. J. Clin. Pathol. 2002, 55, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Demlova, R.; Valík, D.; Obermannova, R.; Zdrazilova-Dubska, L. The Safety of Therapeutic Monoclonal Antibodies: Implications for Cancer Therapy Including Immuno-Checkpoint Inhibitors. Physiol. Res. 2016, 65, S455–S462. [Google Scholar] [CrossRef]
- Linder, B.J.; Nelson, J.C.; Gounder, M.M. Chemotherapy and radiation-related hemorrhagic cystitis in cancer patients–UpToDate. Available online: https://www.uptodate.com/contents/chemotherapy-and-radiation-related-hemorrhagic-cystitis-in-cancer-patients?search=chemotherapy-and-radiation-related-hemorrhagic-cystitis-in-cancer-patientsrecommendationsforCPXHC&source=search_result&selectedTitle=1~ (accessed on 27 October 2021).
- Chopra, B.; Barrick, S.R.; Meyers, S.; Beckel, J.; Zeidel, M.L.; Ford, A.P.D.W.; De Groat, W.C.; Birder, L.A. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J. Physiol. 2005, 562, 859–871. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Lima-Junior, R.C.; Leite, C.A.V.; Mota, J.M.S.; Macedo, F.Y.; Lima, M.V.; Brito, G.A. Chemotherapy-induced hemorrhagic cystitis: Pathogenesis, pharmacological approaches and new insights. J. Exp. Integr. Med. 2012, 2, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Ko, I.G.; Moon, B.M.; Kim, S.E.; Jin, J.J.; Hwang, L.; Ji, E.S.; Kim, C.J.; Kim, T.H.; Choi, H.H.; Chung, K.J. Effects of Combination Treatment of Alpha 1-Adrenergic Receptor Antagonists on Voiding Dysfunction: Study on Target Organs in Overactive Bladder Rats. Int. Neurourol. J. 2016, 20, S150–S158. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, A.; Zapała, Ł.; Kluz, T.; Rogowski, A.; Misiek, M.; Juszczak, K.; Sieńko, J.; Gold, D.; Stangel-Wójcikiewicz, K.; Poleszak, E.; et al. The Potential of Asiatic Acid in the Reversion of Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats. Int. J. Mol. Sci. 2021, 22, 5853. [Google Scholar] [CrossRef]
- Dobrek, Ł.; Skowron, B.; Baranowska, A.; Płoszaj, K.; Badziul, D.; Thor, P. The influence of oxazaphosphorine agents on kidney function in rats. Medicina 2017, 53, 179–189. [Google Scholar] [CrossRef]
- Dobrek, L.; Nalik-Iwaniak, K.; Fic, K.; Arent, Z. The Effect of Acetylcysteine on Renal Function in Experimental Models of Cyclophosphamide-and Ifosfamide-Induced Cystitis. Curr. Urol. 2020, 14, 150–162. [Google Scholar] [CrossRef]
- Dorairajan, L.N.; Manikandan, R.; Kumar, S. Hemorrhagic cystitis: A challenge to the urologist. Indian J. Urol. 2010, 26, 159–166. [Google Scholar] [CrossRef]
- Matz, E.L.; Hsieh, M.H. Review of Advances in Uroprotective Agents for Cyclophosphamide- and Ifosfamide-induced Hemorrhagic Cystitis. Urology 2017, 100, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.; Schulz, G.; Langley, R.; Donze, K.; Winchester, K.; Rodgers, C. Evidence-Based Practice Recommendations for Hydration in Children and Adolescents With Cancer Receiving Intravenous Cyclophosphamide. J. Pediatr. Oncol. Nurs. 2014, 31, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, N.; Emmungil, H.; Gucenmez, S.; Ozen, G.; Yildiz, F.; Balkarli, A.; Kimyon, G.; Coskun, B.N.; Dogan, I.; Pamuk, O.N.; et al. Incidence of Cyclophosphamide-induced Urotoxicity and Protective Effect of Mesna in Rheumatic Diseases. J. Rheumatol. 2015, 42, 1661–1666. [Google Scholar] [CrossRef]
- Almalag, H.M.; Alasmari, S.S.; Alrayes, M.H.; A Binhameed, M.; A Alsudairi, R.; Alosaimi, M.M.; A Alnasser, G.; A Abuzaid, R.; Khalil, N.; Abouzaid, H.H.; et al. Incidence of hemorrhagic cystitis after cyclophosphamide therapy with or without mesna: A cohort study and comprehensive literature review. J. Oncol. Pharm. Pr. 2021, 27, 340–349. [Google Scholar] [CrossRef]
- Cleveland, K.H.; Yeung, S.; Huang, K.M.; Liang, S.; Andresen, B.T.; Huang, Y. Phosphoproteome profiling provides insight into the mechanism of action for carvedilol-mediated cancer prevention. Mol. Carcinog. 2018, 57, 997–1007. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Zhang, Q.; Yu, Z.; Gao, D. Carvedilol suppresses malignant proliferation of mammary epithelial cells through inhibition of the ROS-mediated PI3K/AKT signaling pathway. Oncol. Rep. 2018, 41, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Book, W.M. Carvedilol: A Nonselective β Blocking Agent With Antioxidant Properties. Congest. Heart Fail. 2002, 8, 173–190. [Google Scholar] [CrossRef]
- Jhorawat, R.; Kumari, S.; Varma, S.C.; Rohit, M.K.; Narula, N.; Suri, V.; Malhotra, P.; Jain, S.K. Preventive role of carvedilol in adriamycin-induced cardiomyopathy. Indian J. Med. Res. 2016, 144, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-Y.; Shen, H.-C.; Chen, C.-J.; Wu, S.-E.; Kao, H.-L.; Huang, J.-H.; Wang, D.L.; Chen, S.-C. The inhibition in tumor necrosis factor-α-induced attenuation in endothelial thrombomodulin expression by carvedilol is mediated by nuclear factor-κB and reactive oxygen species. J. Thromb. Thrombolysis 2009, 29, 52–59. [Google Scholar] [CrossRef]
- Júnior, R.F.D.A.; Souza, T.O.; De Medeiros, C.A.X.; De Souza, L.B.; Freitas, M.D.L.; De Lucena, H.F.; Alves, M.D.S.C.F.; De Araújo, A.A. Carvedilol Decrease IL-1β and TNF-α, Inhibits MMP-2, MMP-9, COX-2, and RANKL Expression, and Up-Regulates OPG in a Rat Model of Periodontitis. PLoS ONE 2013, 8, e66391. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; El-Desoky, K. Protective Effects of Carvedilol and Vitamin C against Azithromycin-Induced Cardiotoxicity in Rats via Decreasing ROS, IL1-β, and TNF-αProduction and Inhibiting NF-κB and Caspase-3 Expression. Oxidative Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Li, H.; Lan, Q.; Zhao, Z.; Cao, Y.; Zhou, P.; Wan, S.; Zhang, J.; Jiang, H.; Zhang, Q.; et al. Protective effects of S -carvedilol on doxorubicin-induced damages to human umbilical vein endothelial cells and rats. J. Appl. Toxicol. 2019, 39, 1233–1244. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Fadda, L.; Alhusaini, A.; Ahmad, R.; Hasan, I.H.; Mahmoud, A.M. Liposomal Resveratrol and/or Carvedilol Attenuate Doxorubicin-Induced Cardiotoxicity by Modulating Inflammation, Oxidative Stress and S100A1 in Rats. Antioxidants 2020, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.A.C.; Gobe, G.; Santos, N.A.G.; Santos, A.C. Carvedilol protects against apoptotic cell death induced by cisplatin in renal tubular epithelial cells. J. Toxicol. Environ. Health Part A 2012, 75, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Eid, A.H.; Abdelkader, N.F.; El-Raouf, O.M.A.; Fawzy, H.M.; El-Denshary, E.-E.-D.S. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch. Pharmacal Res. 2016, 39, 1693–1702. [Google Scholar] [CrossRef]
- Padi, S.S.; Chopra, K. Salvage of cyclosporine A-induced oxidative stress and renal dysfunction by carvedilol. Nephron 2002, 92, 685–692. [Google Scholar] [CrossRef]
- Ahmed, I.; Elkablawy, M.A.; El-Agamy, D.S.; A Bazarbay, A.; Ahmed, N. Carvedilol safeguards against aspirin-induced gastric damage in rats. Hum. Exp. Toxicol. 2020, 39, 1257–1267. [Google Scholar] [CrossRef]
- Takara, K.; Sakaeda, T.; Okumura, K. Carvedilol: A new candidate for reversal of MDR1/P-glycoprotein-mediated multidrug resistance. Anti-Cancer Drugs 2004, 15, 303–309. [Google Scholar] [CrossRef]
- Sloderbach, A.; Górska, A.; Sikorska, M.; Misiura, K.; Hładoń, B. Classical oxazaphosphorines – metabolism and therapeutic properties–New implications. Postępy Hig. Med. Dosw. 2013, 67, 1235–1253. [Google Scholar] [CrossRef]
- Dobrek, Ł.; Thor, P.J. Bladder urotoxicity pathophysiology induced by the oxazaphosphorine alkylating agents and its chemoprevention. Postępy Hig. Med. Dosw. 2012, 66, 592–602. [Google Scholar] [CrossRef]
- Sherif, I. Uroprotective mechanisms of natural products against cyclophosphamide-induced urinary bladder toxicity: A comprehensive review. Acta Sci. Pol. Technol. Aliment. 2015, 19, 333–346. [Google Scholar] [CrossRef]
- Tamamizu-Kato, S.; Wong, J.Y.; Jairam, V.; Uchida, K.; Raussens, V.; Kato, H.; Ruysschaert, J.-M.; Narayanaswami, V. Modification by Acrolein, a Component of Tobacco Smoke and Age-Related Oxidative Stress, Mediates Functional Impairment of Human Apolipoprotein E. Biochem. 2007, 46, 8392–8400. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.-P.; Yang, X.-M.; Luo, P.; Li, Y.-Q.; Tao, Y.-X.; Duan, Z.-H.; Xiao, W.; Zhang, D.-Y.; Liu, H.-Z. Inhibition of acrolein-induced autophagy and apoptosis by a glycosaminoglycan from Sepia esculenta ink in mouse Leydig cells. Carbohydr. Polym. 2017, 163, 270–279. [Google Scholar] [CrossRef]
- Yildizbayrak, N.; Orta-Yilmaz, B.; Aydin, Y.; Erkan, M. Acrolein exerts a genotoxic effect in the Leydig cells by stimulating DNA damage-induced apoptosis. Environ. Sci. Pollut. Res. 2020, 27, 15869–15877. [Google Scholar] [CrossRef]
- DeJarnett, N.; Conklin, D.J.; Riggs, D.W.; Myers, J.A.; O’Toole, T.E.; Hamzeh, I.; Wagner, S.; Chugh, A.; Ramos, K.S.; Srivastava, S.; et al. Acrolein Exposure Is Associated with Increased Cardiovascular Disease Risk. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, T.E.; Abplanalp, W.; Li, X.; Cooper, N.; Conklin, D.J.; Haberzettl, P.; Bhatnagar, A. Acrolein Decreases Endothelial Cell Migration and Insulin Sensitivity Through Induction of let-7a. Toxicol. Sci. 2014, 140, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Horinouchi, T.; Mazaki, Y.; Terada, K.; Miwa, S. Cigarette Smoke Extract and Its Cytotoxic Factor Acrolein Inhibit Nitric Oxide Production in Human Vascular Endothelial Cells. Biol. Pharm. Bull. 2020, 43, 1804–1809. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.-L.; Lin, T.; He, D.-W.; Wei, G.-H.; Liu, J.-H.; Li, L.-S. The cyclophosphamide metabolite, acrolein, induces cytoskeletal changes and oxidative stress in Sertoli cells. Mol. Biol. Rep. 2011, 39, 493–500. [Google Scholar] [CrossRef]
- Mesna Injection–Summary of Product Characteristics (SmPC)–(emc). Available online: https://www.medicines.org.uk/emc/product/1838/smpc (accessed on 27 October 2021).
- Kanat, O.; Kurt, E.; Yalcinkaya, U.; Evrensel, T.; Manavoglu, O. Comparison of uroprotective efficacy of mesna and amifostine in Cyclophosphamide- induced hemorrhagic cystitis in rats. Indian J. Cancer 2006, 43, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Mac, S.; Ngo, D.; Yang, D.; Chen, J.; Ali, H.; Arslan, S.; Dadwal, S.; Salhotra, A.; Cao, T.; Karras, N.; et al. Use of high-dose mesna and hyperhydration leads to lower incidence of hemorrhagic cystitis after posttransplant cyclophosphamide-based allogeneic transplantation. Bone Marrow Transplant. 2021, 56, 2464–2470. [Google Scholar] [CrossRef]
- Khaw, S.L.; Downie, P.A.; Waters, K.D.; Ashley, D.M.; Heath, J.A. Adverse hypersensitivity reactions to mesna as adjunctive therapy for cyclophosphamide. Pediatr. Blood Cancer 2007, 49, 341–343. [Google Scholar] [CrossRef] [PubMed]
- ETHYOL® ® (amifostine) for Injection RX only–Summary of Product Characteristics (SmPC). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/20221s12lbl.pdf (accessed on 27 October 2021).
- Acetylcysteine 200mg/ml Injection–Summary of Product Characteristics (SmPC)–(emc). Available online: https://www.medicines.org.uk/emc/product/3447/smpc (accessed on 27 October 2021).
- Dobrek, L.; Nalik-Iwaniak, K.; Kopanska, M.; Arent, Z.; Thor, P.J. Evaluation of selected protein biomarkers of renal function in rats with an experimental model of acute cyclophosphamide-induced cystitis treated with N-acetylcysteine. J. Physiol. Pharmacol. 2019, 70, 787–799. [Google Scholar]
- Mansour, H.H.; El Kiki, S.M.; Hasan, H. Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2015, 40, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer 2013, 108, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barut, E.N.; Engin, S.; Barut, B.; Kaya, C.; Kerimoglu, G.; Ozel, A.; Kadioglu, M. Uroprotective effect of ambroxol in cyclophosphamide-induced cystitis in mice. Int. Urol. Nephrol. 2019, 51, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Engin, S.; Barut, E.N.; Barut, B.; Duman, M.K.; Kaya, C.; Kerimoglu, G.; Ozel, A. Uroprotective effect of pantoprazole against cyclophosphamide-induced cystitis in mice. Support. Care Cancer 2019, 27, 4273–4281. [Google Scholar] [CrossRef]
- Ozguven, A.A.; Yılmaz, O.; Taneli, F.; Ulman, C.; Vatansever, S.; Onag, A. Protective effect of ketamine against hemorrhagic cystitis in rats receiving ifosfamide. Indian J. Pharmacol. 2014, 46, 147–151. [Google Scholar] [CrossRef]
- Eggertsen, R.; Andrén, L.; Sivertsson, R.; Hansson, L. Acute haemodynamic effects of carvedilol (BM 14190), a new combined beta-adrenoceptor blocker and precapillary vasodilating agent, in hypertensive patients. Eur. J. Clin. Pharmacol. 1984, 27, 19–22. [Google Scholar] [CrossRef]
- Carvedilol 25 mg Film-coated Tablets–Summary of Product Characteristics (SmPC)–(emc). Available online: https://www.medicines.org.uk/emc/product/2547/smpc (accessed on 27 October 2021).
- Dandona, P.; Ghanim, H.; Brooks, D.P. Antioxidant activity of carvedilol in cardiovascular disease. J. Hypertens. 2007, 25, 731–741. [Google Scholar] [CrossRef]
- Arozal, W.; Sari, F.R.; Watanabe, K.; Arumugam, S.; Veeraveedu, P.T.; Ma, M.; Thandavarayan, R.A.; Sukumaran, V.; Lakshmanan, A.P.; Kobayashi, Y.; et al. Carvedilol-Afforded Protection against Daunorubicin-Induced Cardiomyopathic Rats In Vivo: Effects on Cardiac Fibrosis and Hypertrophy. ISRN Pharmacol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-L.; Yang, J.-J.; Zhang, H.-S. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed. Pharmacother. 2019, 109, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Saitou, Y.; Nose, K.; Nishioka, T.; Ishii, T.; Uemura, H. Efficacy of Carvedilol for Ischemia/Reperfusion-Induced Oxidative Renal Injury in Rats. Transplant. Proc. 2008, 40, 2139–2141. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; De Velasco, M.A.; Saitou, Y.; Nose, K.; Nishioka, T.; Ishii, T.; Uemura, H. Carvedilol protects tubular epithelial cells from ischemia-reperfusion injury by inhibiting oxidative stress. Int. J. Urol. 2010, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yang, H.; Li, Q.; Bae, H.J.; Obianom, O.; Zeng, S.; Su, T.; Polli, J.E.; Shu, Y. Selective Inhibition on Organic Cation Transporters by Carvedilol Protects Mice from Cisplatin-Induced Nephrotoxicity. Pharm. Res. 2018, 35, 204. [Google Scholar] [CrossRef]
- Diogo, C.V.; Deus, C.M.; Lebiedzinska-Arciszewska, M.; Wojtala, A.; Wieckowski, M.R.; Oliveira, P.J. Carvedilol and antioxidant proteins in a type I diabetes animal model. Eur. J. Clin. Investig. 2017, 47, 19–29. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Zohouri, A. Carvedilol prevents pancreatic β-cell damage and the development of type 1 diabetes in mice by the inhibition of proinflammatory cytokines, NF-κB, COX-2, iNOS and oxidative stress. Cytokine 2021, 138, 155394. [Google Scholar] [CrossRef]
- Li, B.; Liao, Y.-H.; Cheng, X.; Ge, H.; Guo, H.; Wang, M. Effects of carvedilol on cardiac cytokines expression and remodeling in rat with acute myocardial infarction. Int. J. Cardiol. 2006, 111, 247–255. [Google Scholar] [CrossRef]
- Singh, D.; Chander, V.; Chopra, K. Carvedilol attenuates ischemia-reperfusion-induced oxidative renal injury in rats. Fundam. Clin. Pharmacol. 2004, 18, 627–634. [Google Scholar] [CrossRef]
- Watanabe, K.; Ohta, Y.; Nakazawa, M.; Higuchi, H.; Hasegawa, G.; Naito, M.; Fuse, K.; Ito, M.; Hirono, S.; Tanabe, N.; et al. Low dose carvedilol inhibits progression of heart failure in rats with dilated cardiomyopathy. Br. J. Pharmacol. 2000, 130, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Kawy, H.A. Low-dose carvedilol protects against acute septic renal injury in rats during the early and late phases. Can. J. Physiol. Pharmacol. 2015, 93, 443–450. [Google Scholar] [CrossRef]
- Moraes, J.P.; Pereira, D.S.; Matos, A.S.; Santana, D.G.; Santos, C.A.; Estevam, C.S.; Fakhouri, R.; Junior, W.D.L.; Camargo, E.A. The Ethanol Extract of the Inner Bark ofCaesalpinia pyramidalis(Tul.) Reduces Urinary Bladder Damage during Cyclophosphamide-Induced Cystitis in Rats. Sci. World J. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Özatik, F.Y.; Özatik, O.; Tekşen, Y.; Yiğitaslan, S.; Ari, N.S. Protective and therapeutic effect of Hydrogen sulfide on hemorrhagic cystitis and testis dysfunction induced with Cyclophosphamide. Turk. J. Med. Sci. 2021, 51, 1530–1542. [Google Scholar] [CrossRef]
- Elrashidy, R.A.; Hasan, R.A. Modulation of autophagy and transient receptor potential vanilloid 4 channels by montelukast in a rat model of hemorrhagic cystitis. Life Sci. 2021, 278, 119507. [Google Scholar] [CrossRef]
- Murali, V.P.; Kuttan, G. Curculigo orchioides Gaertn Effectively Ameliorates the Uro- and Nephrotoxicities Induced by Cyclophosphamide Administration in Experimental Animals. Integr. Cancer Ther. 2016, 15, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, N.; Eftekharzadeh, S.; Golmohammadi, M.; Khorramirouz, R.; Hashemi, J.; Kashani, Z.; Alijani, M.; Hamidieh, A.A.; Kajbafzadeh, A.-M. Alleviation of Cyclophosphamide-induced Hemorrhagic Cystitis by Dietary Pomegranate: A Comparative Experimental Study With Mesna. J. Pediatr. Hematol. 2018, 40, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Merwid-Ląd, A.; Ksiądzyna, D.; Hałoń, A.; Szkudlarek, D.; Trocha, M.; Szandruk-Bender, M.; Matuszewska, A.; Nowak, B.; Sozański, T.; Kuźniar, A.; et al. Morin-5′-Sulfonic Acid Sodium Salt (NaMSA) Attenuates Cyclophosphamide-Induced Histological Changes in Genitourinary Tract in Rats—Short Report. Pharmaceuticals 2021, 14, 192. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular Mechanisms of Acrolein Toxicity: Relevance to Human Disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, Z.; Huang, F.; Yang, Z.; Yu, F.; Tang, Y.; Ding, G. Protective Effect of Low Molecular Weight Peptides from Solenocera crassicornis Head against Cyclophosphamide-Induced Nephrotoxicity in Mice via the Keap1/Nrf2 Pathway. Antioxidants 2020, 9, 745. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, X.; Li, H.; Yuan, J.; Peng, Y.; Dong, L.; Dai, S. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. 2016, 84, 1899–1905. [Google Scholar] [CrossRef]
- Galal, S.M.; Mansour, H.H.; ElKhoely, A.A. Diallyl sulfide alleviates cyclophosphamide-induced nephropathic encephalopathy in rats. Toxicol. Mech. Methods 2019, 30, 208–218. [Google Scholar] [CrossRef]
- Hamzeh, M.; Amiri, F.T.; Beklar, S.Y.; Hosseinimehr, S.J. Nephroprotective effect of cerium oxide nanoparticles on cyclophosphamide-induced nephrotoxicity via anti-apoptotic and antioxidant properties in BALB/c mice. Marmara Pharm. J. 2018, 22, 180–189. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Kulurkar, P.; Singh, D.; Kumar, D.; Patial, V. Iridoid glycosides fraction from Picrorhiza kurroa attenuates cyclophosphamide-induced renal toxicity and peripheral neuropathy via PPAR-γ mediated inhibition of inflammation and apoptosis. Phytomedicine 2017, 36, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Sobeh, M.; El-Maadawy, W.H.; Mohammed, H.S.; Khalil, H.; Botros, S.; Wink, M. Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential Against Cyclophosphamide Induced Toxicity in Mice. Antioxidants 2019, 8, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagar, H.H.; Almubrik, S.A.; Attia, N.M.; AlJasser, S.N. Mesna Alleviates Cerulein-Induced Acute Pancreatitis by Inhibiting the Inflammatory Response and Oxidative Stress in Experimental Rats. Dig. Dis. Sci. 2020, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oh, C.-T.; Kwon, T.-R.; Bak, D.-H.; Kim, H.; Park, W.-S.; Kim, B.J. Inhibition of melanogenesis by sodium 2-mercaptoethanesulfonate. Korean J. Physiol. Pharmacol. 2020, 24, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, A.; Serefko, A.; Bańczerowska-Górska, M.; Szopa, A.; Dudka, J.; Poleszak, E. Intravesical administration of blebbistatin prevents cyclophosphamide-induced toxicity of the urinary bladder in female Wistar rats. Neurourol. Urodyn. 2019, 38, 1044–1052. [Google Scholar] [CrossRef]
- Wanas, H.; El-Shabrawy, M.; Mishriki, A.; Attia, H.; Emam, M.; Aboulhoda, B.E. Nebivolol protects against cyclophosphamide-induced nephrotoxicity through modulation of oxidative stress, inflammation, and apoptosis. Clin. Exp. Pharmacol. Physiol. 2021, 48, 811–819. [Google Scholar] [CrossRef]
- Refaie, M.M.M.; Shehata, S.; El-Hussieny, M.; Abdelraheem, W.M.; Bayoumi, A.M.A. Role of ATP-Sensitive Potassium Channel (KATP) and eNOS in Mediating the Protective Effect of Nicorandil in Cyclophosphamide-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2019, 20, 71–81. [Google Scholar] [CrossRef]
- Savitz, S.I.; Erhardt, J.A.; Anthony, J.V.; Gupta, G.; Li, X.; Barone, F.C.; Rosenbaum, D.M. The Novel β-Blocker, Carvedilol, Provides Neuroprotection in Transient Focal Stroke. Br. J. Pharmacol. 2000, 20, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- Amiri, F.T.; Hamzeh, M.; Hosseinimehr, S.J.; Khalatbary, A.R.; Mohammadi, H.R.; Dashti, A. Atorvastatin mitigates cyclophosphamide-induced hepatotoxicity via suppression of oxidative stress and apoptosis in rat model. Res. Pharm. Sci. 2018, 13, 440–449. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; El-Nashar, H.A.S.; Al-Mohammadi, A.G.A.; Eldahshan, O.A. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 2021, 12, 9443–9455. [Google Scholar] [CrossRef] [PubMed]
- Khordad, E.; Alipour, F.; Pourabbas, M.; Mansouri, S.; Salimnejad, R. Hepatoprotective Impact of Ghrelin against Cyclophosphamide-Induced Toxicity in the Male Mice. Drug Res. 2021, 71, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Shafie, B.; Pourahmad, J.; Rezaei, M. N-acetylcysteine is more effective than ellagic acid in preventing acrolein induced dysfunction in mitochondria isolated from rat liver. J. Food Biochem. 2021, 45, e13775. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawad, F.H.; Al-Attar, Z.; Abbood, M.S. The Protective Effect of Nitroglycerin, N-Acetyl Cysteine and Metoprolol in CCL4 Induced Animal Model of Acute Liver Injury. Open Access Maced. J. Med. Sci. 2019, 7, 1739–1743. [Google Scholar] [CrossRef] [Green Version]
- El-Wakeel, S.A.; Rahmo, R.M.; El-Abhar, H.S. Anti-fibrotic impact of Carvedilol in a CCl-4 model of liver fibrosis via serum microRNA-200a/SMAD7 enhancement to bridle TGF-β1/EMT track. Sci. Rep. 2018, 8, 14327. [Google Scholar] [CrossRef]
- El Sayed, N.F.; Abdallah, D.M.; Awad, A.S.; Ahmed, K.A.; El-Abhar, H.S. Novel peripheral role of Nurr-1/GDNF/AKT trajectory in carvedilol and/or morin hydrate hepatoprotective effect in a model of hepatic ischemia/reperfusion. Life Sci. 2021, 273, 119235. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Urinary Dimethylamine (DMA) and Its Precursor Asymmetric Dimethylarginine (ADMA) in Clinical Medicine, in the Context of Nitric Oxide (NO) and Beyond. J. Clin. Med. 2020, 9, 1843. [Google Scholar] [CrossRef]
- Hulin, J.-A.; Gubareva, E.A.; Jarzebska, N.; Rodionov, R.N.; Mangoni, A.A.; Tommasi, S. Inhibition of Dimethylarginine Dimethylaminohydrolase (DDAH) Enzymes as an Emerging Therapeutic Strategy to Target Angiogenesis and Vasculogenic Mimicry in Cancer. Front. Oncol. 2020, 9, 1455. [Google Scholar] [CrossRef]
- Dowsett, L.; Higgins, E.; Alanazi, S.; Alshuwayer, N.A.; Leiper, F.C.; Leiper, J. ADMA: A Key Player in the Relationship between Vascular Dysfunction and Inflammation in Atherosclerosis. J. Clin. Med. 2020, 9, 3026. [Google Scholar] [CrossRef]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [Green Version]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (DDAH): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3227–H3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Merwid-Ląd, A.; Trocha, M.; Chlebda-Sieragowska, E.; Sozański, T.; Magdalan, J.; Ksiądzyna, D.; Szuba, A.; Kopacz, M.; Kuźniar, A.; Nowak, D.; et al. Effect of cyclophosphamide and morin-5’-sulfonic acid sodium salt, alone or in combination, on ADMA/DDAH pathway in rats. Pharmacol. Rep. 2013, 65, 201–207. [Google Scholar] [CrossRef]
- Jayachandran, I.; Sundararajan, S.; Paramasivam, P.; Venkatesan, B.; Subramanian, S.C.; Balasubramanyam, M.; Mohan, V.; Manickam, N. Association of circulatory asymmetric dimethylarginine (ADMA) with diabetic nephropathy in Asian Indians and its causative role in renal cell injury. Clin. Biochem. 2017, 50, 835–842. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Ghiadoni, L.; Tripepi, G.; Bruno, R.M.; Mancia, G.; Zoccali, C. Sympathetic Nerve Traffic and Asymmetric Dimethylarginine in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2620–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.-M.; Hsu, C.-P.; Chang, C.-F.; Lin, C.-C.; Lee, T.-S.; Lin, S.-J.; Chan, W.-L. Asymmetric dimethylarginine predicts the risk of contrast-induced acute kidney injury in patients undergoing cardiac catheterization. Atheroscler. 2016, 254, 161–166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, K.; Dong, W.; Li, R.; Huang, R.; Zhang, H.; Shi, W.; Liu, S.; Li, Z.; Chen, Y.; et al. Raised Plasma Levels of Asymmetric Dimethylarginine Are Associated with Pathological Type and Predict the Therapeutic Effect in Lupus Nephritis Patients Treated with Cyclophosphamide. Kidney Dis. 2020, 6, 355–363. [Google Scholar] [CrossRef]
- Betz, B.; Möller-Ehrlich, K.; Kress, T.; Kniepert, J.; Schwedhelm, E.; Böger, R.H.; Wanner, C.; Sauvant, C.; Schneider, R. Increased symmetrical dimethylarginine in ischemic acute kidney injury as a causative factor of renal L-arginine deficiency. Transl. Res. 2013, 162, 67–76. [Google Scholar] [CrossRef]
- Wang, Y.; An, W.; Zhang, F.; Niu, M.; Liu, Y.; Shi, R. Nebivolol ameliorated kidney damage in Zucker diabetic fatty rats by regulation of oxidative stress/NO pathway: Comparison with captopril. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1135–1148. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, M.; Yin, S.; Zhang, F.; Shi, R. Nephroprotective effects of nebivolol in 2K1C rats through regulation of the kidney ROS-ADMA-NO pathway. Pharmacol. Rep. 2018, 70, 917–929. [Google Scholar] [CrossRef]
- Alfieri, A.B.; Briceno, L.; Fragasso, G.; Spoladore, R.; Palloshi, A.; Bassanelli, G.; Montano, C.; Arioli, F.; Cuko, A.; Ruotolo, G.; et al. Differential Long-term Effects of Carvedilol on Proinflammatory and Antiinflammatory Cytokines, Asymmetric Dimethylarginine, and Left Ventricular Function in Patients With Heart Failure. J. Cardiovasc. Pharmacol. 2008, 52, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Inrig, J.K.; Van Buren, P.; Kim, C.; Vongpatanasin, W.; Povsic, T.J.; Toto, R. Probing the Mechanisms of Intradialytic Hypertension: A Pilot Study Targeting Endothelial Cell Dysfunction. Clin. J. Am. Soc. Nephrol. 2012, 7, 1300–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewedy, W.A.; Mostafa, D.K. Nebivolol suppresses asymmetric dimethylarginine and attenuates cyclosporine-induced nephrotoxicity and endothelial dysfunction in rats. Pharmacol. Rep. 2016, 68, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Szajerski, P.; Zielonka, J.; Sikora, A.; Adamus, J.; Marcinek, A.; Gebicki, J.; Kozlovski, V.I.; Drelicharz, Ł.; Chłopicki, S. Radical scavenging and NO-releasing properties of selected ?-adrenoreceptor antagonists. Free. Radic. Res. 2006, 40, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Ueda, S.; Yamagishi, S.-I.; Obara, N.; Taguchi, K.; Ando, R.; Kaida, Y.; Iwatani, R.; Kaifu, K.; Yokoro, M.; et al. Asymmetric dimethylarginine accumulates in the kidney during ischemia/reperfusion injury. Kidney Int. 2014, 85, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Giannikouris, I. The effect of N-acetylcysteine on oxidative serum biomarkers of hemodialysis patients. Hippokratia 2016, 19, 131–135. [Google Scholar]
- Tain, Y.-L.; Baylis, C. Determination of dimethylarginine dimethylaminohydrolase activity in the kidney. Kidney Int. 2007, 72, 886–889. [Google Scholar] [CrossRef] [Green Version]

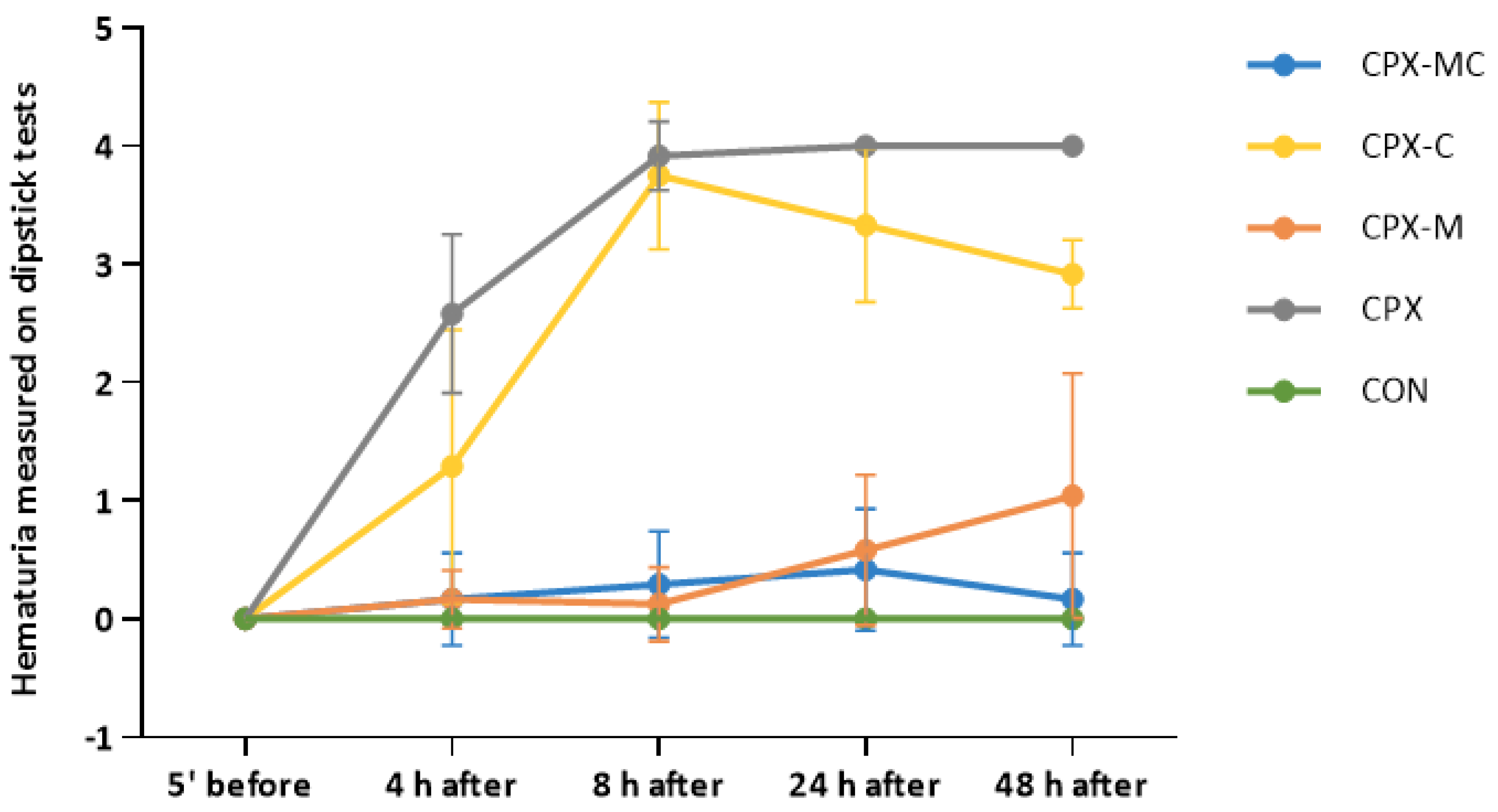

| 5 min before i.p. CPX or Normal Saline Injection | 4 h after i.p. CPX or Normal Saline Injection | 8 h after i.p. CPX or Normal Saline Injection | 24 h after i.p. CPX or Normal Saline Injection | 48 h after i.p. CPX or Normal Saline Injection | |
|---|---|---|---|---|---|
| CON | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| CPX | 0.0 ± 0.0 | 2.58 ± 0.67 *** | 3.92 ± 0.29 *** | 4.00 ± 0.0 *** | 4.00 ± 0.0 *** |
| CPX-M | 0.0 ± 0.0 | 0.17 ± 0.25 ### | 0.13 ± 0.31 ###,^^^ | 0.58 ± 0.63 ###,^ | 1.04 ± 1.03 ### |
| CPX-C | 0.0 ± 0.0 | 1.29 ± 1.16 * | 3.75 ± 0.62 *** | 3.33 ± 0.65 *** | 2.92 ± 0.29 *** |
| CPX-MC | 0.0 ± 0.0 | 0.17 ± 0.39 ### | 0.29 ± 0.45 ##,^^ | 0.42 ± 0.51 ###,^^ | 0.17 ± 0.39 ###,^^ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merwid-Ląd, A.; Ziółkowski, P.; Szandruk-Bender, M.; Matuszewska, A.; Szeląg, A.; Trocha, M. Effect of a Low Dose of Carvedilol on Cyclophosphamide-Induced Urinary Toxicity in Rats—A Comparison with Mesna. Pharmaceuticals 2021, 14, 1237. https://doi.org/10.3390/ph14121237
Merwid-Ląd A, Ziółkowski P, Szandruk-Bender M, Matuszewska A, Szeląg A, Trocha M. Effect of a Low Dose of Carvedilol on Cyclophosphamide-Induced Urinary Toxicity in Rats—A Comparison with Mesna. Pharmaceuticals. 2021; 14(12):1237. https://doi.org/10.3390/ph14121237
Chicago/Turabian StyleMerwid-Ląd, Anna, Piotr Ziółkowski, Marta Szandruk-Bender, Agnieszka Matuszewska, Adam Szeląg, and Małgorzata Trocha. 2021. "Effect of a Low Dose of Carvedilol on Cyclophosphamide-Induced Urinary Toxicity in Rats—A Comparison with Mesna" Pharmaceuticals 14, no. 12: 1237. https://doi.org/10.3390/ph14121237
APA StyleMerwid-Ląd, A., Ziółkowski, P., Szandruk-Bender, M., Matuszewska, A., Szeląg, A., & Trocha, M. (2021). Effect of a Low Dose of Carvedilol on Cyclophosphamide-Induced Urinary Toxicity in Rats—A Comparison with Mesna. Pharmaceuticals, 14(12), 1237. https://doi.org/10.3390/ph14121237









