Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors
Abstract
1. Introduction
2. Suicidal Behaviors Need Specific Therapeutics
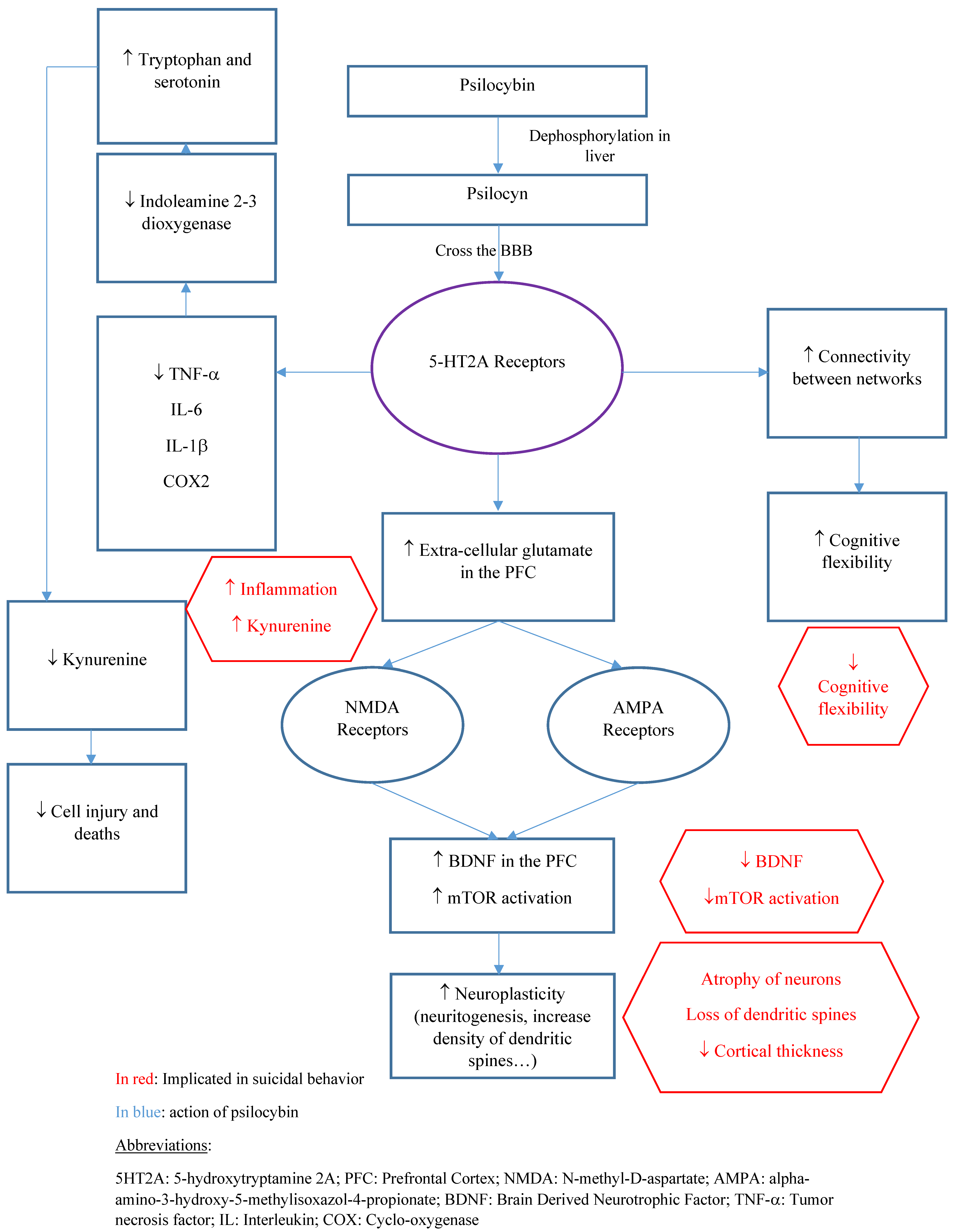
3. Psilocybin Pharmacological Properties
3.1. Pharmacokinetics
3.2. Pharmacodynamics
4. Neuroplastic Changes in Neurons and Synapses
5. Anti-Inflammatory Effects of Psilocybin
6. Antioxidant Effects of Psilocybin
7. Neuropsychological Aspects
8. Risks
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Nutt, D.; Erritzoe, D.; Carhart-Harris, R. Psychedelic Psychiatry’s Brave New World. Cell 2020, 181, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Foldi, C.J.; Liknaitzky, P.; Williams, M.; Oldfield, B.J. Rethinking Therapeutic Strategies for Anorexia Nervosa: Insights From Psychedelic Medicine and Animal Models. Front. Neurosci. 2020, 14, 43. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Nobile, B.; Olié, E.; Courtet, P. Commentary: Psychedelic Psychiatry’s Brave New World. Front. Psychiatry 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Courtet, P.; Nobile, B.; Olié, É. Soyons plus ambitieux pour traiter le suicide. Médecine/Sciences 2020, 36, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castroman, J.; Jaussent, I.; Gorwood, P.; Courtet, P. Suicidal depressed patients respond less well to antidepressants in the short term. Depress. Anxiety 2016, 33, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Courtet, P.; Jaussent, I.; Lopez-Castroman, J.; Gorwood, P. Poor response to antidepressants predicts new suicidal ideas and behavior in depressed outpatients. Eur. Neuropsychopharmacol. 2014, 24, 1650–1658. [Google Scholar] [CrossRef]
- Courtet, P.; Nobile, B.; Lopez-Castroman, J. Antidepressants and Suicide Risk: Harmful or Useful? In Handbook of Suicidal Behaviour; Kumar, U., Ed.; Springer: Singapore, 2017; pp. 329–347. ISBN 978-981-10-4815-9. [Google Scholar]
- Nobile, B.; Dubois, J.; Aouizerate, B.; Aubin, V.; Loftus, J.; Bellivier, F.; Belzeaux, R.; Dubertret, C.; Gard, S.; Haffen, E.; et al. Characterization of depressed bipolar patients with current suicidal ideation. Aust. N. Z. J. Psychiatry 2021, 55, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Nobile, B.; Olié, E.; Dubois, J.; Guillaume, S.; Gorwood, P.; Courtet, P. Characteristics and treatment outcome of suicidal depression: Two large naturalistic cohorts of depressed outpatients. Aust. N. Z. J. Psychiatry 2021, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rihmer, Z. Suicide risk in mood disorders. Curr. Opin. Psychiatry 2007, 20, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Chouinard, G. Acute and Persistent Withdrawal Syndromes Following Discontinuation of Psychotropic Medications. Psychother. Psychosom. 2020, 89, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Ducasse, D.; Jaussent, I.; Arpon-Brand, V.; Vienot, M.; Laglaoui, C.; Béziat, S.; Calati, R.; Carrière, I.; Guillaume, S.; Courtet, P.; et al. Acceptance and Commitment Therapy for the Management of Suicidal Patients: A Randomized Controlled Trial. Psychother. Psychosom. 2018, 87, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.V.; Quilty, L.C.; Ravitz, P.; Rosenbluth, M.; Pavlova, B.; Grigoriadis, S.; Velyvis, V.; Kennedy, S.H.; Lam, R.W.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can. J. Psychiatry 2016, 61, 524–539. [Google Scholar] [CrossRef]
- Zalsman, G.; Hawton, K.; Wasserman, D.; van Heeringen, K.; Arensman, E.; Sarchiapone, M.; Carli, V.; Höschl, C.; Barzilay, R.; Balazs, J.; et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry 2016, 3, 646–659. [Google Scholar] [CrossRef]
- Lengvenyte, A.; Olié, E.; Courtet, P. Suicide Has Many Faces, So Does Ketamine: A Narrative Review on Ketamine’s Antisuicidal Actions. Curr. Psychiatry Rep. 2019, 21, 132. [Google Scholar] [CrossRef]
- Bahji, A.; Vazquez, G.H.; Zarate, C.A. Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J. Affect. Disord. 2021, 278, 542–555. [Google Scholar] [CrossRef]
- Ross, S.; Agin-Liebes, G.; Lo, S.; Zeifman, R.J.; Ghazal, L.; Benville, J.; Franco Corso, S.; Bjerre Real, C.; Guss, J.; Bossis, A.; et al. Acute and Sustained Reductions in Loss of Meaning and Suicidal Ideation Following Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Life-Threatening Cancer. ACS Pharmacol. Transl. Sci. 2021, 4, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, P.S.; Thorne, C.B.; Clark, C.B.; Coombs, D.W.; Johnson, M.W. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol. 2015, 29, 280–288. [Google Scholar] [CrossRef]
- Zeifman, R.J.; Singhal, N.; Breslow, L.; Weissman, C.R. On the Relationship between Classic Psychedelics and Suicidality: A Systematic Review. ACS Pharmacol. Transl. Sci. 2021, 4, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Palhano-Fontes, F.; Hallak, J.; Arcoverde, E.; Maia-Oliveira, J.P.; Araujo, D.B. The Impact of Ayahuasca on Suicidality: Results From a Randomized Controlled Trial. Front. Pharmacol. 2019, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Singhal, N.; dos Santos, R.G.; Sanches, R.F.; de Lima Osório, F.; Hallak, J.E.C.; Weissman, C.R. Rapid and sustained decreases in suicidality following a single dose of ayahuasca among individuals with recurrent major depressive disorder: Results from an open-label trial. Psychopharmacology 2021, 238, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.T.; Danforth, A.; Daroff, P.R.; Stauffer, C.; Ekman, E.; Agin-Liebes, G.; Trope, A.; Boden, M.T.; Dilley, P.J.; Mitchell, J.; et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine 2020, 27, 100538. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder. JAMA Psychiatry 2021, 78, 481. [Google Scholar] [CrossRef]
- Courtet, P.; Nobile, B.; Guillaume, S.; Olié, E. An urgent need for rapid anti-suicidal drugs. French J. Psychiatry 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Courtet, P.; Nobile, B. Inclusion of Suicidal Individuals in Research Studies. J. Clin. Psychiatry 2020, 81, 2. [Google Scholar] [CrossRef]
- Iltis, A.S.; McCall, W.V.; Deria, R. Suicidality, Depression, and the FDA. J. Clin. Psychiatry 2020, 81, 3. [Google Scholar] [CrossRef]
- Obegi, J.H. Rethinking Suicidal Behavior Disorder. Crisis 2019, 40, 209–219. [Google Scholar] [CrossRef]
- Pompili, M. Critical appraisal of major depression with suicidal ideation. Ann. Gen. Psychiatry 2019, 18, 7. [Google Scholar] [CrossRef]
- Batterham, P.J.; Spijker, B.A.J.; Mackinnon, A.J.; Calear, A.L.; Wong, Q.; Christensen, H. Consistency of trajectories of suicidal ideation and depression symptoms: Evidence from a randomized controlled trial. Depress. Anxiety 2019, 36, 321–329. [Google Scholar] [CrossRef] [PubMed]
- van Ballegooijen, W.; Eikelenboom, M.; Fokkema, M.; Riper, H.; van Hemert, A.M.; Kerkhof, A.J.F.M.; Penninx, B.W.J.H.; Smit, J.H. Comparing factor structures of depressed patients with and without suicidal ideation, a measurement invariance analysis. J. Affect. Disord. 2019, 245, 180–187. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Morris, D.W.; Wisniewski, S.R.; Nierenberg, A.A.; Gaynes, B.N.; Kurian, B.T.; Warden, D.; Stegman, D.; Shores-Wilson, K.; Rush, A.J. Clinical and Sociodemographic Characteristics Associated with Suicidal Ideation in Depressed Outpatients. Can. J. Psychiatry 2013, 58, 113–122. [Google Scholar] [CrossRef]
- Bogers, I.C.H.M.; Zuidersma, M.; Boshuisen, M.L.; Comijs, H.C.; Oude Voshaar, R.C. The influence of thoughts of death and suicidal ideation on the course of depression in older depressed patients. Int. J. Geriatr. Psychiatry 2017, 32, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Oquendo, M.A.; Sullivan, G.M.; Sudol, K.; Baca-Garcia, E.; Stanley, B.H.; Sublette, M.E.; Mann, J.J. Toward a Biosignature for Suicide. Am. J. Psychiatry 2014, 171, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Hesselgrave, N.; Ogden, R.T.; Sullivan, G.M.; Oquendo, M.A.; Mann, J.J.; Parsey, R.V. Positron Emission Tomography Quantification of Serotonin Transporter in Suicide Attempters with Major Depressive Disorder. Biol. Psychiatry 2013, 74, 287–295. [Google Scholar] [CrossRef]
- Olié, E.; Jollant, F.; Deverdun, J.; de Champfleur, N.M.; Cyprien, F.; Le Bars, E.; Mura, T.; Bonafé, A.; Courtet, P. The experience of social exclusion in women with a history of suicidal acts: A neuroimaging study. Sci. Rep. 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Alacreu-Crespo, A.; Olié, E.; Le Bars, E.; Cyprien, F.; Deverdun, J.; Courtet, P. Prefrontal activation in suicide attempters during decision making with emotional feedback. Transl. Psychiatry 2020, 10, 313. [Google Scholar] [CrossRef]
- Lengvenyte, A.; Conejero, I.; Courtet, P.; Olié, E. Biological bases of suicidal behaviours: A narrative review. Eur. J. Neurosci. 2019, 53, ejn.14635. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Hofmann, A.; Frey, A.; Ott, H.; Petrzilka, T.; Troxler, F. Konstitutionsaufklärung und Synthese von Psilocybin. Experientia 1958, 14, 397–399. [Google Scholar] [CrossRef]
- Hofmann, A.; Heim, R.; Brack, A.; Kobel, H. Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen RauschpilzPsilocybe mexicana Heim. Experientia 1958, 14, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Laine, K.; Gynther, M.; Savolainen, J. Prodrug Approaches for CNS Delivery. AAPS J. 2008, 10, 92–102. [Google Scholar] [CrossRef]
- Geiger, H.A.; Wurst, M.G.; Daniels, R.N. DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem. Neurosci. 2018, 9, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef]
- Hasler, F.; Bourquin, D.; Brenneisen, R.; Bär, T.; Vollenweider, F.X. Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm. Acta Helv. 1997, 72, 175–184. [Google Scholar] [CrossRef]
- Nicholas, C.R.; Henriquez, K.M.; Gassman, M.C.; Cooper, K.M.; Muller, D.; Hetzel, S.; Brown, R.T.; Cozzi, N.V.; Thomas, C.; Hutson, P.R. High dose psilocybin is associated with positive subjective effects in healthy volunteers. J. Psychopharmacol. 2018, 32, 770–778. [Google Scholar] [CrossRef]
- Brown, R.T.; Nicholas, C.R.; Cozzi, N.V.; Gassman, M.C.; Cooper, K.M.; Muller, D.; Thomas, C.D.; Hetzel, S.J.; Henriquez, K.M.; Ribaudo, A.S.; et al. Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin. Pharmacokinet. 2017, 56, 1543–1554. [Google Scholar] [CrossRef]
- Shulgin, A.T. Psilocybin. J. Psychedelic Drugs 1980, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Williams, T.M.; Sessa, B.; Tyacke, R.J.; Rich, A.S.; Feilding, A.; Nutt, D.J. The administration of psilocybin to healthy, hallucinogen-experienced volunteers in a mock-functional magnetic resonance imaging environment: A preliminary investigation of tolerability. J. Psychopharmacol. 2011, 25, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocybin—Summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Hasler, F.; Bourquin, D.; Brenneisen, R.; Vollenweider, F.X. Renal excretion profiles of psilocin following oral administration of psilocybin: A controlled study in man. J. Pharm. Biomed. Anal. 2002, 30, 331–339. [Google Scholar] [CrossRef]
- Kalberer, F.; Kreis, W.; Rutschmann, J. The fate of psilocin in the rat. Biochem. Pharmacol. 1962, 11, 261–269. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Preller, K.H. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef]
- Willins, D.L.; Meltzer, H.Y. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J. Pharmacol. Exp. Ther. 1997, 282, 699–706. [Google Scholar]
- Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Galfalvy, H.; Dwork, A.J.; Mann, J.J.; Arango, V. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl. Psychiatry 2018, 8, 279. [Google Scholar] [CrossRef]
- Sudol, K.; Mann, J.J. Biomarkers of Suicide Attempt Behavior: Towards a Biological Model of Risk. Curr. Psychiatry Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.I.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef]
- Quednow, B.B.; Kometer, M.; Geyer, M.A.; Vollenweider, F.X. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology 2012, 37, 630–640. [Google Scholar] [CrossRef]
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Gouzoulis-Mayfrank, E. Neurometabolic Effects of Psilocybin, 3,4-Methylenedioxyethylamphetamine (MDE) and d-Methamphetamine in Healthy Volunteers A Double-Blind, Placebo-Controlled PET Study with [18F]FDG. Neuropsychopharmacology 1999, 20, 565–581. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Geyer, M.A. A systems model of altered consciousness: Integrating natural and drug-induced psychoses. Brain Res. Bull. 2001, 56, 495–507. [Google Scholar] [CrossRef]
- Vollenweider, F. Positron Emission Tomography and Fluorodeoxyglucose Studies of Metabolic Hyperfrontality and Psychopathology in the Psilocybin Model of Psychosis. Neuropsychopharmacology 1997, 16, 357–372. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.S. Psychedelics and the Human Receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Sakashita, Y.; Abe, K.; Katagiri, N.; Kambe, T.; Saitoh, T.; Utsunomiya, I.; Horiguchi, Y.; Taguchi, K. Effect of Psilocin on Extracellular Dopamine and Serotonin Levels in the Mesoaccumbens and Mesocortical Pathway in Awake Rats. Biol. Pharm. Bull. 2015, 38, 134–138. [Google Scholar] [CrossRef]
- Elman, I.; Borsook, D.; Volkow, N.D. Pain and suicidality: Insights from reward and addiction neuroscience. Prog. Neurobiol. 2013, 109, 1–27. [Google Scholar] [CrossRef]
- Dombrovski, A.Y.; Hallquist, M.N.; Brown, V.M.; Wilson, J.; Szanto, K. Value-Based Choice, Contingency Learning, and Suicidal Behavior in Mid- and Late-Life Depression. Biol. Psychiatry 2019, 85, 506–516. [Google Scholar] [CrossRef]
- Tsypes, A.; Owens, M.; Gibb, B.E. Reward Responsiveness in Suicide Attempters: An Electroencephalography/Event-Related Potential Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, L.; Mas-Herrero, E.; Zatorre, R.J.; Ripollés, P.; Gomez-Andres, A.; Alicart, H.; Olivé, G.; Marco-Pallarés, J.; Antonijoan, R.M.; Valle, M.; et al. Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. USA 2019, 116, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Michely, J.; Viswanathan, S.; Hauser, T.U.; Delker, L.; Dolan, R.J.; Grefkes, C. The role of dopamine in dynamic effort-reward integration. Neuropsychopharmacology 2020, 45, 1448–1453. [Google Scholar] [CrossRef]
- Corne, R.; Mongeau, R. Utilisation des psychédéliques en psychiatrie: Lien avec les neurotrophines. Biol. Aujourd’hui 2019, 213, 121–129. [Google Scholar] [CrossRef][Green Version]
- Vollenweider, F.X.; Kometer, M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010, 11, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, K.; Kuypers, K.P.C. Depression, Mindfulness, and Psilocybin: Possible Complementary Effects of Mindfulness Meditation and Psilocybin in the Treatment of Depression. A Review. Front. Psychiatry 2020, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [CrossRef] [PubMed]
- Jefsen, O.H.; Elfving, B.; Wegener, G.; Müller, H.K. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J. Psychopharmacol. 2021, 35, 483–493. [Google Scholar] [CrossRef]
- Shao, L.-X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- de Vos, C.M.H.; Mason, N.L.; Kuypers, K.P.C. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 1575. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Huang, T.-L. Brain-derived neurotrophic factor and mental disorders. Biomed. J. 2020, 43, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Aguglia, A.; Amerio, A.; Orsolini, L.; Salvi, V.; Serafini, G.; Volpe, U.; Amore, M.; Aguglia, E. Peripheral BDNF levels in psychiatric patients with and without a history of suicide attempt: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110342. [Google Scholar] [CrossRef] [PubMed]
- Misztak, P.; Pańczyszyn-Trzewik, P.; Nowak, G.; Sowa-Kućma, M. Epigenetic marks and their relationship with BDNF in the brain of suicide victims. PLoS ONE 2020, 15, e0239335. [Google Scholar]
- Le-Niculescu, H.; Levey, D.F.; Ayalew, M.; Palmer, L.; Gavrin, L.M.; Jain, N.; Winiger, E.; Bhosrekar, S.; Shankar, G.; Radel, M.; et al. Discovery and validation of blood biomarkers for suicidality. Mol. Psychiatry 2013, 18, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.D.; Donohue, D.; Muhie, S.; Yang, R.; Miller, S.A.; Hammamieh, R.; Ryberg, K.; Yehuda, R. Gene expression associated with suicide attempts in US veterans. Transl. Psychiatry 2017, 7, e1226. [Google Scholar] [CrossRef]
- Hercher, C.; Canetti, L.; Turecki, G.; Mechawar, N. Anterior cingulate pyramidal neurons display altered dendritic branching in depressed suicides. J. Psychiatr. Res. 2010, 44, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-G.; Cho, S.-E.; Na, K.-S.; Lee, J.S.; Joo, S.W.; Cho, S.-J.; Son, Y.-D.; Lee, Y.J. Differences in brain surface area and cortical volume between suicide attempters and non-attempters with major depressive disorder. Psychiatry Res. Neuroimaging 2020, 297, 111032. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.; Eloff, J.N. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts in vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J. Inflamm. Res. 2021, 14, 3729–3738. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Effects and safety of Psilocybe cubensis and Panaeolus cyanescens magic mushroom extracts on endothelin-1-induced hypertrophy and cell injury in cardiomyocytes. Sci. Rep. 2020, 10, 22314. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. Curr. Top. Behav. Neurosci. 2018, 36, 45–73. [Google Scholar] [CrossRef]
- Courtet, P.; Giner, L.; Seneque, M.; Guillaume, S.; Olie, E.; Ducasse, D. Neuroinflammation in suicide: Toward a comprehensive model. World J. Biol. Psychiatry 2016, 17, 564–586. [Google Scholar] [CrossRef]
- Arnone, D.; Saraykar, S.; Salem, H.; Teixeira, A.L.; Dantzer, R.; Selvaraj, S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018, 92, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Babcock, T.A.; Carlin, J.M. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor α in interferon-treated epithelial cells. Cytokine 2000, 12, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Hale, P.T.; Carlin, J.M. The Role of IFN-γ and TNF-α-Responsive Regulatory Elements in the Synergistic Induction of Indoleamine Dioxygenase. J. Interf. Cytokine Res. 2005, 25, 20–30. [Google Scholar] [CrossRef]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-Hydroxytryptamine 2A Receptor Activation Suppresses Tumor Necrosis Factor-α-Induced Inflammation with Extraordinary Potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- Kling, A.; Seddighzadeh, M.; Arlestig, L.; Alfredsson, L.; Rantapaa-Dahlqvist, S.; Padyukov, L. Genetic variations in the serotonin 5-HT2A receptor gene (HTR2A) are associated with rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 1111–1115. [Google Scholar] [CrossRef]
- Kraus, T.; Haack, M.; Schuld, A.; Hinze-Selch, D.; Koethe, D.; Pollmächer, T. Body Weight, the Tumor Necrosis Factor System, and Leptin Production during Treatment with Mirtazapine or Venlafaxine. Pharmacopsychiatry 2002, 35, 220–225. [Google Scholar] [CrossRef]
- Kast, R.E. Anti- and pro-inflammatory considerations in antidepressant use during medical illness: Bupropion lowers and mirtazapine increases circulating tumor necrosis factor-alpha levels. Gen. Hosp. Psychiatry 2003, 25, 495–496. [Google Scholar] [CrossRef]
- Kling, A.; Danell-Boman, M.; Stenlund, H.; Dahlqvist, R. Association between the use of serotonin receptor 2A-blocking antidepressants and joint disorders. Arthritis Rheum. 2009, 61, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Coupland, C.; Hill, T.; Morriss, R.; Arthur, A.; Moore, M.; Hippisley-Cox, J. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: Cohort study using a primary care database. BMJ 2015, 350, h517. [Google Scholar] [CrossRef]
- de Boer, T.; Ruigt, G.S.F. The Selective α2-Adrenoceptor Antagonist Mirtazapine (Org 3770) Enhances Noradrenergic and 5-HT1A-Mediated Serotonergic Neurotransmission. CNS Drugs 1995, 4, 29–38. [Google Scholar] [CrossRef]
- Conejero, I.; Jaussent, I.; Cazals, A.; Thouvenot, E.; Mura, T.; Le Bars, E.; Guillaume, S.; Squalli, S.; Courtet, P.; Olié, E. Association between baseline pro-inflammatory cytokines and brain activation during social exclusion in patients with vulnerability to suicide and depressive disorder. Psychoneuroendocrinology 2019, 99, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Van de Kar, L.D.; Javed, A.; Zhang, Y.; Serres, F.; Raap, D.K.; Gray, T.S. 5-HT 2A Receptors Stimulate ACTH, Corticosterone, Oxytocin, Renin, and Prolactin Release and Activate Hypothalamic CRF and Oxytocin-Expressing Cells. J. Neurosci. 2001, 21, 3572–3579. [Google Scholar] [CrossRef]
- Strajhar, P.; Schmid, Y.; Liakoni, E.; Dolder, P.C.; Rentsch, K.M.; Kratschmar, D.V.; Odermatt, A.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide on Circulating Steroid Levels in Healthy Subjects. J. Neuroendocrinol. 2016, 28, 3. [Google Scholar] [CrossRef]
- Hasler, F.; Grimberg, U.; Benz, M.A.; Huber, T.; Vollenweider, F.X. Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind, placebo-controlled dose? Effect study. Psychopharmacology 2004, 172, 145–156. [Google Scholar] [CrossRef]
- O’Connor, D.B.; Green, J.A.; Ferguson, E.; O’Carroll, R.E.; O’Connor, R.C. Cortisol reactivity and suicidal behavior: Investigating the role of hypothalamic-pituitary-adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology 2017, 75, 183–191. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatry 2018, 30, 363–375. [Google Scholar] [CrossRef]
- Romeo, B.; Hermand, M.; Pétillion, A.; Karila, L.; Benyamina, A. Clinical and biological predictors of psychedelic response in the treatment of psychiatric and addictive disorders: A systematic review. J. Psychiatr. Res. 2021, 137, 273–282. [Google Scholar] [CrossRef]
- Roseman, L.; Nutt, D.J.; Carhart-Harris, R.L. Quality of Acute Psychedelic Experience Predicts Therapeutic Efficacy of Psilocybin for Treatment-Resistant Depression. Front. Pharmacol. 2018, 8, 974. [Google Scholar] [CrossRef]
- Stefulj, J.; Jernej, B.; Cicin-Sain, L.; Rinner, I.; Schauenstein, K. mRNA Expression of Serotonin Receptors in Cells of the Immune Tissues of the Rat. Brain. Behav. Immun. 2000, 14, 219–224. [Google Scholar] [CrossRef]
- Kang, B.N.; Ha, S.G.; Bahaie, N.S.; Hosseinkhani, M.R.; Ge, X.N.; Blumenthal, M.N.; Rao, S.P.; Sriramarao, P. Regulation of Serotonin-Induced Trafficking and Migration of Eosinophils. PLoS ONE 2013, 8, e54840. [Google Scholar] [CrossRef] [PubMed]
- Cloez-Tayarani, I. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: Involvement of 5-hydroxytryptamine2A receptors. Int. Immunol. 2003, 15, 233–240. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.W.; Landry, A.N.; Hibicke, M.; Nichols, C.D. The 4 Position of 5-HT 2A Structural Agonists Imparts the Anti-Inflammatory Propensity Responsible for Reducing Airway Hyperrresponsiveness in Allergic Asthma. FASEB J. 2018, 32, 830–833. [Google Scholar] [CrossRef]
- Nichols, C.D. Serotonin 5- Receptor Function as a Contributing Factor to Both Neuropsychiatric and Cardiovascular Diseases. Cardiovasc. Psychiatry Neurol. 2009, 2009, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci. 2020, 11, 864–871. [Google Scholar] [CrossRef]
- Mendelson, S.D. The current status of the platelet 5-HT2A receptor in depression. J. Affect. Disord. 2000, 57, 13–24. [Google Scholar] [CrossRef]
- Pandey, G.N.; Dwivedi, Y.; Rizavi, H.S.; Ren, X.; Pandey, S.C.; Pesold, C.; Roberts, R.C.; Conley, R.R.; Tamminga, C.A. Higher Expression of Serotonin 5-HT 2A Receptors in the Postmortem Brains of Teenage Suicide Victims. Am. J. Psychiatry 2002, 159, 419–429. [Google Scholar] [CrossRef]
- Shelton, R.C.; Sanders-Bush, E.; Manier, D.H.; Lewis, D.A. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience 2009, 158, 1406–1415. [Google Scholar] [CrossRef]
- van Heeringen, C.; Audenaert, K.; Van Laere, K.; Dumont, F.; Slegers, G.; Mertens, J.; Dierckx, R.A. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. J. Affect. Disord. 2003, 74, 149–158. [Google Scholar] [CrossRef]
- Malone, K.M.; Ellis, S.P.; Currier, D.; John Mann, J. Platelet 5-HT2A receptor subresponsivity and lethality of attempted suicide in depressed in-patients. Int. J. Neuropsychopharmacol. 2007, 10, 335–343. [Google Scholar] [PubMed]
- Vaquero-Lorenzo, C.; Baca-Garcia, E.; Diaz-Hernandez, M.; Perez-Rodriguez, M.M.; Fernandez-Navarro, P.; Giner, L.; Carballo, J.J.; Saiz-Ruiz, J.; Fernandez-Piqueras, J.; Baldomero, E.B.; et al. Association study of two polymorphisms of the serotonin-2A receptor gene and suicide attempts. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 645–649. [Google Scholar] [CrossRef]
- Murnane, K.S. Serotonin 2A receptors are a stress response system: Implications for post-traumatic stress disorder. Behav. Pharmacol. 2019, 30, 151–162. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Nutt, D. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [PubMed]
- Muguruza, C.; Miranda-Azpiazu, P.; Díez-Alarcia, R.; Morentin, B.; González-Maeso, J.; Callado, L.F.; Meana, J.J. Evaluation of 5-HT2A and mGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: Effect of antidepressant treatment. Neuropharmacology 2014, 86, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef]
- Buchborn, T.; Schröder, H.; Höllt, V.; Grecksch, G. Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and hippocampal serotonin 5-HT 2 signalling. J. Psychopharmacol. 2014, 28, 545–552. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, antioxidant and cytoprotective activity evaluation of C-3 substituted indole derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef]
- Karbownik, M.; Gitto, E.; Lewiñski, A.; Reiter, R.J. Relative efficacies of indole antioxidants in reducing autoxidation and iron-induced lipid peroxidation in hamster testes. J. Cell. Biochem. 2001, 81, 693–699. [Google Scholar] [CrossRef]
- Estevão, M.S.; Carvalho, L.C.; Ribeiro, D.; Couto, D.; Freitas, M.; Gomes, A.; Ferreira, L.M.; Fernandes, E.; Marques, M.M.B. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010, 45, 4869–4878. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.L.; Eloff, J.N. Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom. Plants 2020, 9, 1127. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N,N-Dimethyltryptamine and 5-Methoxy-N,N-Dimethyltryptamine Modulate Innate and Adaptive Inflammatory Responses through the Sigma-1 Receptor of Human Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects against Hypoxia via Sigma-1 Receptor Activation in Human Primary iPSC-Derived Cortical Neurons and Microglia-Like Immune Cells. Front. Neurosci. 2016, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Uthaug, M.V.; Lancelotta, R.; Szabo, A.; Davis, A.K.; Riba, J.; Ramaekers, J.G. Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: Effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology 2020, 237, 773–785. [Google Scholar] [CrossRef]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Björkqvist, M.; Träskman-Bendz, L.; Brundin, L. Interleukin-6 Is Elevated in the Cerebrospinal Fluid of Suicide Attempters and Related to Symptom Severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef]
- Melhem, N.M.; Munroe, S.; Marsland, A.; Gray, K.; Brent, D.; Porta, G.; Douaihy, A.; Laudenslager, M.L.; DePietro, F.; Diler, R.; et al. Blunted HPA axis activity prior to suicide attempt and increased inflammation in attempters. Psychoneuroendocrinology 2017, 77, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Mariano, C.L.; Cruz, W.R. Serotonin 5ht2a receptor activation inhibits inducible nitric oxide synthase activity in c6 glioma cells. Life Sci. 1997, 61, 1819–1827. [Google Scholar] [CrossRef]
- Erdemir, F.; Atilgan, D.; Firat, F.; Markoc, F.; Parlaktas, B.S.; Sogut, E. The effect of Sertraline, Paroxetine, Fluoxetine and Escitalopram on testicular tissue and oxidative stress parameters in rats. Int. Braz. J. Urol. 2014, 40, 100–108. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Morsy, S.M.Y.; Sleem, A.A. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI J. 2011, 10, 290–302. [Google Scholar] [PubMed]
- Behr, G.A.; Moreira, J.C.F.; Frey, B.N. Preclinical and Clinical Evidence of Antioxidant Effects of Antidepressant Agents: Implications for the Pathophysiology of Major Depressive Disorder. Oxid. Med. Cell. Longev. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Morita, K.; Arimochi, H.; Her, S. Serotonergic 5-HT2A receptor stimulation induces steroid 5α-reductase gene expression in rat C6 glioma cells via transcription factor Egr-1. Mol. Brain Res. 2005, 139, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Odebrecht Vargas, H.; Vargas Nunes, S.O.; Pizzo de Castro, M.; Cristina Bortolasci, C.; Sabbatini Barbosa, D.; Kaminami Morimoto, H.; Venugopal, K.; Dodd, S.; Maes, M.; Berk, M. Oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts. J. Affect. Disord. 2013, 150, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Vasupanrajit, A.; Jirakran, K.; Tunvirachaisakul, C.; Maes, M. Suicide attempts are associated with activated immune-inflammatory, nitro-oxidative, and neurotoxic pathways: A systematic review and meta-analysis. J. Affect. Disord. 2021, 295, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Koweszko, T.; Gierus, J.; Zalewska, A.; Maciejczyk, M.; Waszkiewicz, N.; Szulc, A. The Relationship between Suicide and Oxidative Stress in a Group of Psychiatric Inpatients. J. Clin. Med. 2020, 9, 3462. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fernández, S.; Gurpegui, M.; Díaz-Atienza, F.; Pérez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Compared to Healthy Controls before and after Antidepressant Treatment. J. Clin. Psychiatry 2015, 76, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Evens, R.; Mertens, L.J.; Koslowski, M.; Betzler, F.; Gründer, G.; Jungaberle, H. Learning to Let Go: A Cognitive-Behavioral Model of How Psychedelic Therapy Promotes Acceptance. Front. Psychiatry 2020, 11, 5. [Google Scholar] [CrossRef]
- Nour, M.M.; Evans, L.; Nutt, D.; Carhart-Harris, R.L. Ego-Dissolution and Psychedelics: Validation of the Ego-Dissolution Inventory (EDI). Front. Hum. Neurosci. 2016, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Buchman-Schmitt, J.M.; Stanley, I.H.; Hom, M.A.; Tucker, R.P.; Hagan, C.R.; Rogers, M.L.; Podlogar, M.C.; Chiurliza, B.; Ringer, F.B.; et al. The interpersonal theory of suicide: A systematic review and meta-analysis of a decade of cross-national research. Psychol. Bull. 2017, 143, 1313–1345. [Google Scholar] [CrossRef]
- Stroud, J.B.; Freeman, T.P.; Leech, R.; Hindocha, C.; Lawn, W.; Nutt, D.J.; Curran, H.; Carhart-Harris, R.L. Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology 2018, 235, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Pokorny, T.; Hock, A.; Kraehenmann, R.; Stämpfli, P.; Seifritz, E.; Scheidegger, M.; Vollenweider, F.X. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. USA 2016, 113, 5119–5124. [Google Scholar] [CrossRef] [PubMed]
- Richard-Devantoy, S.; Berlim, M.T.; Jollant, F. Suicidal behaviour and memory: A systematic review and meta-analysis. World J. Biol. Psychiatry 2015, 16, 544–566. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Williams, T.M.; Erritzoe, D.; Abbasi, N.; Bargiotas, T.; Hobden, P.; Sharp, D.J.; Evans, J.; Feilding, A.; et al. Implications for psychedelic-assisted psychotherapy: Functional magnetic resonance imaging study with psilocybin. Br. J. Psychiatry 2012, 200, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.J. The acute effects of classic psychedelics on memory in humans. Psychopharmacology 2021, 238, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, M.J.; Kettner, H.; Carhart-Harris, R.L. Positive effects of psychedelics on depression and wellbeing scores in individuals reporting an eating disorder. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2021, 26, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Roseman, L.; Haijen, E.; Idialu-Ikato, K.; Kaelen, M.; Watts, R.; Carhart-Harris, R. Emotional breakthrough and psychedelics: Validation of the Emotional Breakthrough Inventory. J. Psychopharmacol. 2019, 33, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Hendricks, P.S.; Barrett, F.S.; Griffiths, R.R. Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 2019, 197, 83–102. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Richards, W.A.; Richards, B.D.; McCann, U.; Jesse, R. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology 2011, 218, 649–665. [Google Scholar] [CrossRef]
- Lawrence, R.E.; Oquendo, M.A.; Stanley, B. Religion and Suicide Risk: A Systematic Review. Arch. Suicide Res. 2016, 20, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Fisher, P.M.; Stenbæk, D.S.; Kristiansen, S.; Burmester, D.; Lehel, S.; Páleníček, T.; Kuchař, M.; Svarer, C.; Ozenne, B.; et al. A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur. Neuropsychopharmacol. 2020, 33, 71–80. [Google Scholar] [CrossRef]
- Tupper, K.W.; Wood, E.; Yensen, R.; Johnson, M.W. Psychedelic medicine: A re-emerging therapeutic paradigm. Can. Med. Assoc. J. 2015, 187, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Rubin-Kahana, D.S.; Hassan, A.N.; Le Foll, B. Posttraumatic Stress Disorder after a Psychedelic Experience, a Case Report. J. Addict. Med. 2021, 15, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.G.; Skladman, I.; Kodesh, A.; Sigal, M.; Shufman, E. LSD-induced Hallucinogen Persisting Perception Disorder treated with clonazepam: Two case reports. Isr. J. Psychiatry Relat. Sci. 2001, 38, 133–136. [Google Scholar] [PubMed]
- Skryabin, V.Y.; Vinnikova, M.; Nenastieva, A.; Alekseyuk, V. Hallucinogen persisting perception disorder: A literature review and three case reports. J. Addict. Dis. 2018, 37, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; Santacroce, R.; Pettorruso, M.; Montemitro, C.; Spano, M.; Lorusso, M.; di Giannantonio, M.; Lerner, A. Hallucinogen Persisting Perception Disorder: Etiology, Clinical Features, and Therapeutic Perspectives. Brain Sci. 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.R.F.; Hobus, J.; Faucett, J.; Mehra, V.; Giller, E.L.; Rudolph, R.L. Differential pattern of response in mood symptoms and suicide risk measures in severely ill depressed patients assigned to citalopram with placebo or citalopram combined with lithium: Role of lithium levels. J. Psychiatr. Res. 2011, 45, 1489–1496. [Google Scholar] [CrossRef]
- Tropini, C.; Moss, E.L.; Merrill, B.D.; Ng, K.M.; Higginbottom, S.K.; Casavant, E.P.; Gonzalez, C.G.; Fremin, B.; Bouley, D.M.; Elias, J.E.; et al. Transient Osmotic Perturbation Causes Long-Term Alteration to the Gut Microbiota. Cell 2018, 173, 1742–1754.e17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strumila, R.; Nobile, B.; Korsakova, L.; Lengvenyte, A.; Olie, E.; Lopez-Castroman, J.; Guillaume, S.; Courtet, P. Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors. Pharmaceuticals 2021, 14, 1213. https://doi.org/10.3390/ph14121213
Strumila R, Nobile B, Korsakova L, Lengvenyte A, Olie E, Lopez-Castroman J, Guillaume S, Courtet P. Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors. Pharmaceuticals. 2021; 14(12):1213. https://doi.org/10.3390/ph14121213
Chicago/Turabian StyleStrumila, Robertas, Bénédicte Nobile, Laura Korsakova, Aiste Lengvenyte, Emilie Olie, Jorge Lopez-Castroman, Sébastien Guillaume, and Philippe Courtet. 2021. "Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors" Pharmaceuticals 14, no. 12: 1213. https://doi.org/10.3390/ph14121213
APA StyleStrumila, R., Nobile, B., Korsakova, L., Lengvenyte, A., Olie, E., Lopez-Castroman, J., Guillaume, S., & Courtet, P. (2021). Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors. Pharmaceuticals, 14(12), 1213. https://doi.org/10.3390/ph14121213





