Abstract
Presently, notwithstanding the progress regarding wound-healing management, the treatment of the majority of skin lesions still represents a serious challenge for biomedical and pharmaceutical industries. Thus, the attention of the researchers has turned to the development of novel materials based on cellulose derivatives. Cellulose derivatives are semi-synthetic biopolymers, which exhibit high solubility in water and represent an advantageous alternative to water-insoluble cellulose. These biopolymers possess excellent properties, such as biocompatibility, biodegradability, sustainability, non-toxicity, non-immunogenicity, thermo-gelling behavior, mechanical strength, abundance, low costs, antibacterial effect, and high hydrophilicity. They have an efficient ability to absorb and retain a large quantity of wound exudates in the interstitial sites of their networks and can maintain optimal local moisture. Cellulose derivatives also represent a proper scaffold to incorporate various bioactive agents with beneficial therapeutic effects on skin tissue restoration. Due to these suitable and versatile characteristics, cellulose derivatives are attractive and captivating materials for wound-healing applications. This review presents an extensive overview of recent research regarding promising cellulose derivatives-based materials for the development of multiple biomedical and pharmaceutical applications, such as wound dressings, drug delivery devices, and tissue engineering.
1. Introduction
Skin is the largest and the main organ that forms the body covering, with a complex structure of tissues, and creates an exterior defense barrier, which protects the internal organs from mechanical impairments, radiation, chemicals, and foreign invaders (bacteria and viruses) [1]. More than being a passive barrier, the skin defends the body against contamination, infection, and surrounding environment influence [2]. Skin is also a sensory organ and contains a large category of sensory neuron subtypes (thermoreceptors, nociceptors, pruriceptors, and low-threshold mechanoreceptors), that take over and transfer to the brain information about the environment [3]. Moreover, the skin has an important role in homeostasis, elimination of toxins, sustaining regular hydration levels, prevention of electrolytes loss [4], and in control of body temperature and blood pressure. The skin is made up of three particular layers: epidermis, dermis, and hypodermis or subcutaneous layer [5].
The epidermis is a physical protective barrier against the external factors, which does not contain blood vessels. It is comprised of two main categories of cells: dendritic cells and keratinocytes (keratin synthesis), along with Langerhans cells (engaged in the immune reaction), Merkel cells (sensory corpuscles), and melanocytes (melanin synthesis) [6,7]. The dermis is situated between the epidermis and hypodermis, and it is responsible for skin thickness. The dermis structure is principally fibrous because it contains collagen and elastic fibers [8]. Moreover, this layer also includes hair follicles, sebaceous glands, sweat glands, nerves, and blood vessels. The fundamental component of the dermis is collagen and the most abundant are type I and type III (approximately 95%) [9,10]. The dermis plays an essential role in thermoregulation, skin protection, preservation of skin support, and perception of sensation [11]. The hypodermis (subcutaneous tissue), the widest and the thickest part of the skin, is located between the dermis and muscles or bones beneath it and it is made up of elastin and loose connective tissue [12]. The principal roles of subcutaneous tissue are thermal insulation, energy resource, nutritional reserve, and mechanical conservation [13,14].
There are many factors and systemic diseases that can alter skin functions, for example, pathogens, pollution, radiation exposure, smoking, malnutrition, obesity, diabetes mellitus, peripheral vascular diseases, pressure ulcer, inflammatory, bleeding, or metabolic conditions and immunosuppression [15,16]. In many cases, all these factors can delay wound healing, with harmful risks for patients, such as oxidative stress, chronic inflammation, infection, increased tissue loss, and necrosis [17]. Thus, skin tissue disorders are a public health problem worldwide, with a higher incidence from year to year. For example, in 2005 there were estimated ~5 million skin and subcutaneous conditions, in 2015 ~6.1 million [18], and in 2018 there were ~8.2 million people, who suffered from wounds. Total costs of treatments for wound healing vary between $28.1 billion and $96.8 billion [19]. With a prevalence of 1–2% in the global population, chronic wounds have the largest frequency from all types of skin tissue injuries, especially surgical wounds, and leg/foot ulcers (pressure ulcer and diabetic foot ulcer) [20]. An injury involves physical impact, such as pain, inflammation, mobility limitation, disturbance of sleep, alterations of skin appearance, and restriction of daily activities; consequently, these effects have a negative impact on the patient quality of life, affecting emotional, social, and physical states [21]. To restore the impaired tissue and to rebalance the quality of life for patients with wounds, optimal and multidisciplinary wound management has an essential role. Its main purpose is to obtain a proper functional, structural, and cosmetic result [22]. Frequently, to alleviate the pain and inflammation, which accompany the wounds, analgesic and anti-inflammatory drugs are prescribed. Because of their side effects on the gastrointestinal system when they are administered orally [23], a more advantageous and simple treatment is to apply on the lesion site a wound dressing [24]. The main purpose of wound management is to reduce the period of wound healing through the prevention of infection, alleviation of inflammation and pain, and diminishing the scars [25].
In this review, we mainly present studies from the last 10 years regarding the cellulose derivatives-based wound dressings with various bioactive agents to accelerate the regeneration of skin tissue injuries. Cellulose derivatives have proper and optimal applicability to develop novel wound dressings that can enhance wound healing, obtained by substituting the hydroxyl groups from cellulose molecule with different alkyl groups [26]. Thus, they exhibit high solubility in water and represent a feasible alternative to water-insoluble cellulose. Moreover, these semi-synthetic biopolymers possess other multiple characteristics, such as biocompatibility, biodegradability, proper physicochemical properties, low toxicity and costs, poor immunogenicity, adequate absorption of wound exudates, thermo-gelling power, and antibacterial effect [27,28]. Besides wound-dressings development, cellulose derivatives are promising biomaterials for tissue engineering, drug delivery, hemodialysis, osseointegration, and biosensors [29].
2. Wound Classification
A wound represents a lesion, break, tear, or damage of skin structure and function, produced by physical, mechanical (surgery), thermal, chemical, and electrical (burns) factors; an injury can also be the result of an underlying medical or physiological disorder (diabetes and malignancies) [30,31,32]. The Wound Healing Society define a wound as a consequence of ‘disruption of normal anatomic structure and function’ [33]. The National Institutes of Health from the United States assesses that approximately 3% of people over the age of 65 can suffer at any one time from a cutaneous lesion [34].
Wounds are classified according to many parameters:
- etiology: surgical, traumatic, radiation and malignant wound, chemical or thermal injury, pressure ulcer, diabetic foot ulcer, vascular ulcer, or atypical injury;
- healing time (duration) and nature of the injury restoration process: acute or chronic wounds;
- depth of injury or number of skin layers affected: superficial, partial thickness (deep dermal) or full-thickness wounds;
- complexity: simple, complex, or complicated wounds;
- contamination and postoperative infection risk: clean wound (class I), clean/contaminated wound (class II), contaminated wound (class III) or dirty wound (class IV);
- mode of lesion: abrasion, ulceration, incision, laceration or degloving;
- tissue loss: without tissue loss (surgical wounds) and with tissue loss (burns, traumatic wounds, diabetic foot ulcers, and iatrogenic wounds);
- appearance: necrotic, sloughy, infected, malodorous, granulating, and epithelializing wound;
- injured tissue coloration: black, green, yellow, white, brown, purple, beefy red, or pale pink wounds [35,36,37].
From all of the classification criteria, the most significant and decisive criterion for selection of an adequate dressing and for optimal wound-healing management is the healing time (duration) and the nature of the injury restoration process. Hence, an acute injury heals totally, without external support, with minimum scarring, and usually demands a period for healing from 8 to 12 weeks [38]. An acute lesion can be simple or complex, but it depends on the affected anatomical parts and on the dimension and depth of this lesion. In this category are found mechanical injuries, burns, and chemical wounds [33,39]. In contrast to the acute wound, a chronic injury heals slowly, requires a long time for healing, more than 12 weeks, usually reoccurs, and leaves severe scars; mostly, a chronic wound does not have any time limitation for the repair process [40]. The main chronic wounds are venous ulcer, ischemic injuries (especially of atherosclerotic origin), diabetic foot ulcer, pressure ulcer, and malignant wounds [41,42].
3. Wound-Healing Process
Wound healing is a sophisticated and well-coordinated process [43], which involves a variety of cellular and biochemical reactions that need a complex and dynamic cascade of biological processes [44,45] for the reestablishment of skin layers, growth and tissue regeneration, anatomical continuity, and skin functions [46]. Damaged skin tissue has the capacity to repair itself to form a new epithelium that closes the wound and repairs the barrier function, through an intricate process [47].
3.1. Wound-Healing Stages
The wound repair process consists of four different, overlapping, and exactly programmed stages: hemostasis, inflammation, proliferation, and remodeling (maturation) phases [48], illustrated in Figure 1.
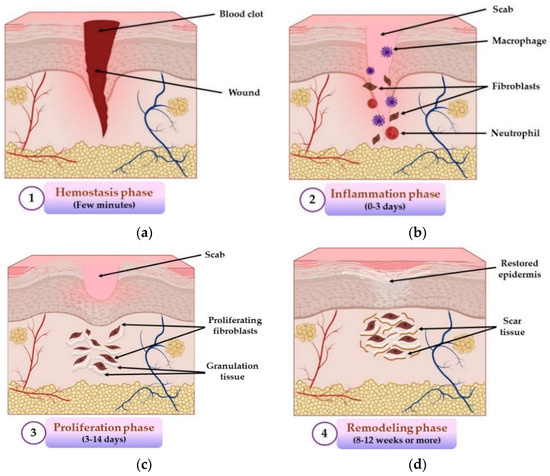
Figure 1.
The four stages of wound healing process: (a) hemostasis phase; (b) inflammation phase; (c) proliferation phase; (d) remodeling phase. All illustrations have been created with BioRender.com, Inkscape, and PowerPoint.
The first and the shortest stage (5–10 min) of the wound repair process is hemostasis, an instantaneous reaction towards lesion [49], whose main purpose is to stop the bleeding through vasoconstriction, primary hemostasis (thrombocytes aggregation with thrombocyte plug formation) and secondary hemostasis (fibrin clot formation) [50]. The inflammation stage happens approximately at the same time as the hemostatic stage and consists of enrollment of neutrophils and macrophages, cytokines secretion, destruction and elimination of bacteria and formation of a wound bed [51]. Inflammation induces accumulation of leukocytes at the lesion’s level, activating different mediators and chemotactic factors in 1–2 days after injury and lasts for about 3 days [47]. The proliferation stage begins on day 3 and can have a duration up to 14 days after tissue damage [52]. This phase represents a complex process that includes the next events: neoangiogenesis, production of granulation tissue through fibroblasts proliferation and collagen deposition, synthesis of extracellular matrix, re-epithelialization, and injury retirement, all these happening simultaneously [53]. The final phase of the wound repair process is the remodeling (maturation) stage, and its major purpose is the production of cellular connective tissue and hardening of the new epithelium that establishes the final scar nature [54]. It is the longest stage of all five and can last from weeks to 1–2 years or more. The main event of this phase is the remodeling of granulation tissue, where collagen type I will take the place of collagen type III because type I is more stable [55].
3.2. Factors Affecting Wound-Healing Process
Many factors can interfere with the wound-healing phases, the consequence being an improper or damaged wound repair process. In general, these factors can be categorized as local and systemic. Local factors affect features of the lesion itself, and systemic factors represent the general health or condition states of one person, which influence the capacity to heal [56]. The main factors that affect the wound-healing process are presented in Figure 2.
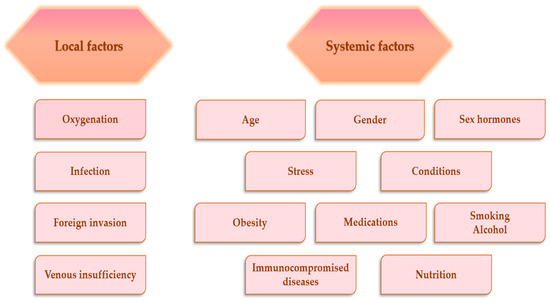
Figure 2.
Factors affecting wound-healing process [57,58].
3.2.1. Local Factors That Affect Wound-Healing Process
In injured tissues, vascular disruption generates depletion of oxygen, causing hypoxia, and, consequently, impaired wound healing. Temporary hypoxia stimulates the lesion repair process, but persistent hypoxia prolongs this process [59]. Hypoxia is characterized by high levels of reactive oxygen species (ROS) in cells and the impact on tissue healing is deleterious [31,57]. Oxygen has many roles in the injured tissue: avoids infection, activates the angiogenesis, enhances keratinocytes differentiation, movement, and re-epithelialization, increases fibroblast proliferation and biosynthesis of collagen, and stimulates lesion contraction [60]. When skin tissues are injured, the physical protective barrier against foreign invaders is damaged, these germs easily invade the lesion and contaminate or colonize it, causing local infection, and in severe cases, when the injury is not treated properly, they cause systemic infection [61]. Moreover, bacteria and endotoxins may induce the extended elevation of matrix metalloproteinases and pro-inflammatory cytokines (IL-1 and TNF-α), prolonging the inflammatory phase [56].
3.2.2. Systemic Factors That Affect Wound-Healing Process
A main risk factor for the damaged lesion repair process is increased age due to multiple comorbidities [62]. Acute injuries have a prolonged healing time for elderly males compared to elderly females. This fact can be explained through sex hormones, which have an essential role in the wound repair process [63]. Alongside them, stress causes the decrease of pro-inflammatory cytokine levels and the reduction of chemo-attractants expression, which are involved in the inflammation stage of wound healing [64]. Regarding the conditions, the major disease, which strongly and negatively influences the wound-healing process, is diabetes mellitus, because of the diabetic foot ulcer, which causes hypoxia, inhibition of the expansion of macrophages and neutrophils and reduction of fibroblasts proliferation [65]. Obesity represents another major factor that affects the normal repair process, because it is characterized by an augmented workload of the heart to provide oxygenated blood to skin tissues, it cannot perfuse them, the outcome being the onset of ischemia and a higher risk to develop infections [66]. Among medications, steroids and chemotherapeutic drugs can lead to delayed healing. Mechanisms through steroids that affect the wound healing are the inhibition of lesion contraction, and fibroblasts proliferation, the decrease of tensile strength, and collagen production [67]. Chemotherapeutic drugs disturb the proliferative stage through slowing cells’ movement to the lesion, angiogenesis inhibition, reduction of biosynthesis of collagen and decrease of fibrin deposition [68]. The quality and rate of the normal repair process can be also altered by smoking and alcohol because they lead to lesion infection and dehiscence, reduction of neutrophils, lessening of angiogenesis, inhibition of epithelial reconstruction and lesion contraction, and in severe cases, to necrosis of tissues [69]. Poor nutrition slows the lesion repair process through inflammation extension, inhibition of fibroblasts functions, decline of angiogenesis, and reduction of collagen biosynthesis and deposition [70].
4. Wound Dressings: Properties and Classification
In past years, due to the technology’s noteworthy progress, various wound dressings were formulated worldwide to cure all types of tissue lesion. Dressings play a fundamental role in wound-healing management because these protect tissue lesions from external invasion (wound dressings are permeable for oxygen and moisture and function as physical barriers) [71], preventing the infection on the wound site [72]. Moreover, dressings contribute to the regeneration and restoration of epidermis and dermis layers [73,74].
4.1. Wound Dressing Properties
For the development of dressings, which allow rapid healing, with minimal scars on the body surface, it is necessary to develop new biopolymeric materials that accomplish some properties to create the ideal wound dressing that are reviewed in Figure 3.
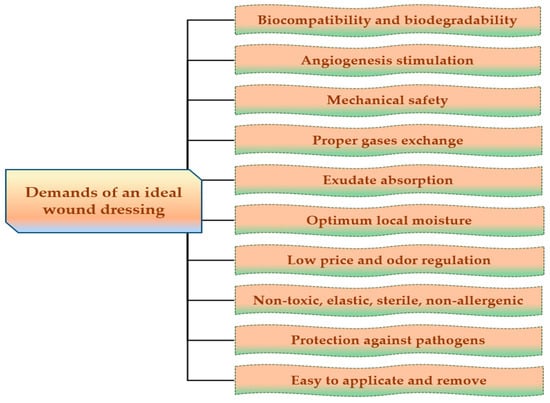
Figure 3.
Major demands of an ideal wound dressing.
The ideal wound dressing preferably presents the following features: biocompatibility, biodegradability, non-toxicity, chemical inertness [75], to be applied effortlessly, to have the capacity to keep local moisture, to ensure a suitable exchange of gases (O2 and CO2), to absorb exudates that form on the lesion site [76], to stimulate the angiogenesis, to protect against extraneous pathogens, to clear the injured tissue, to eliminate nonviable tissues, to reduce the exposed area [77], to be able to be removed and replaced without difficulty [78], to adjust the odor, to sustain an adequate temperature to the lesion bed, to promote the blood circulation, and to stimulate cell expansion, to ensure mechanical safety [79]. Also, wound dressings materials must be elastic, sterile, non-adherent, non-allergenic [80], to have an acceptable price and to provide thermal insulation [81].
4.2. Wound-Dressing Classification
A potential classification of wound dressings comprises passive dressings and active dressings, depending on the presence or absence of one or more pharmacologically active substances or natural substances [82], which can act to the site of the lesion, with local or systemic action, conditioned by the depth of the wound. Moreover, the progress of manufacturing led to the evolution of wound dressings from traditional dressings to modern (advanced) dressings [83].
Passive dressings can be considered dry traditional dressings, which are fundamental for a faster wound-healing process. There is a wide simple range of passive dressings for several types of skin lesion: cotton wool, lint, gauze, natural, and synthetic bandages–they work as primary dressing or secondary dressing [79,84]. Active dressings contain a large variety of pharmacologically active substances (antibiotics or other antimicrobials, non-steroidal anti-inflammatory, analgesic, antifungal, and local anesthetics drugs) or natural substances (plant extracts) with anti-inflammatory, astringent, emollient, epithelializing, antioxidant, demulcent, and antimicrobial properties [73].
Modern or advanced dressings were designed to cover tissue lesions and in this category are included the hydrogels, hydrocolloids, semi-permeable films, semi-permeable foams, and alginate dressings [52,85]. The biggest difference between traditional and modern dressings is local moisture maintenance. Thus, traditional dressings have a lower capacity to maintain the local moisture on the wound site [83], and modern dressings sustain excellent local moisture to enhance wound healing [86]. The classification of wound dressings is illustrated in Figure 4.
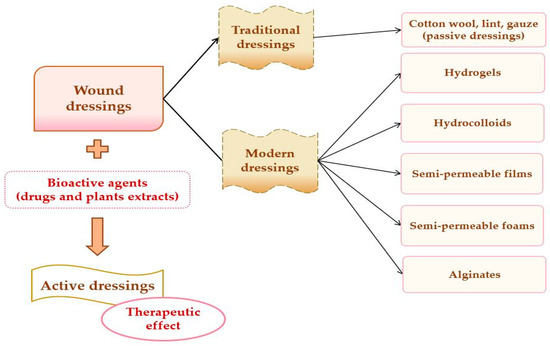
Figure 4.
Wound dressings classification.
The main materials underlying the modern wound dressings are polymers, which can be natural (collagen, gelatin, cellulose, hemicellulose, chitin, chitosan, pectins, gums, chondroitin sulfate, alginic acid, alginates, agar, dextran, carrageenan, elastin, hyaluronic acid, silk fibroin, fibrinogen, and fibrin) [87,88], semi-synthetic (cellulose derivatives) [89] or synthetic (poly(α-ester)s, polyanhydrides, polycarbonates, poly(amide), poly(esteramide)s, polyphosphazenes, polyurethanes, pseudo poly(amino acids), polyacetals) [90,91,92].
The first class of modern wound dressings includes hydrogels, also called hungry or smart networks, which can be defined as three-dimensional networks, consisting of cross-linked polymeric materials [93], with a significant capacity to absorb inside their structure a massive volume of water or body fluids, without dissolution in these liquids [94]. Hydrogels are transparent dressings, and this fact allows the tissue lesion to be observed and controlled without the dressing being eliminated [95]. Hydrogels dressings have a remarkable application in many domains due to their high water content (up to 96%) [96], such as biomedical and pharmaceutical sciences (wound dressings, drug delivery systems, diagnostics, tissue engineering, contact lenses, regenerative medicines) [97], agriculture, food industry, biotechnology, separation technology (cells and biomolecules), electroconductive hydrogels and biosensors, oil recovery, the cosmetic industry, and hygienic products [98,99,100]. Hydrogels, which stimulate autolytic debridement, are used as wound dressings in pressure ulcers, thermal injuries, and lesions caused by surgery [101].
Hydrocolloids are another class of modern wound dressings, which are based on a combination between elastomers, alginates, and colloidal materials. They present the ability to take in a small or medium quantity of exudates, have good biocompatibility, biodegradability, and adhere to the skin [102]. These dressings are occlusive, so they do not allow microorganisms to penetrate tissue lesions, do not afford gases exchanges, and are water-resistant [103].
The third class of modern wound dressing includes semi-permeable films. They are flexible and elastic sheets, made from transparent polyurethane. For a good attachment to the skin, polyurethane films present an acrylic adhesive on one part [104,105]. Films are impermeable to pathogens and water but allow the exchange of oxygen and water vapor. Films are used in surgical injuries or wounds with a reduced volume of exudates [106]. They cannot be applied on tissue lesions with necrosis or infection, on sensitive skin (newborns and elder people), and also on wounds, which have a substantial amount of exudates because films offer a poor capacity to absorb wound fluids [41,96].
The following category of modern dressing includes semi-permeable foams, which showed a vast improvement and favorable biocompatibility [107]. They have a considerable capacity to swallow a great quantity of liquids formed at the injury site, due to their content of hydrophilic polyurethane and silicone, so they can be recommended for tissue lesions with a medium to high status of wound fluids [108]. The disadvantages of foams consist in the limitation of use for dry wounds (foams have a dehydration effect) and in the impossibility to follow the evolution of wound healing because foams are totally opaque [109].
The last class of modern wound dressings is represented by alginates, a category of polysaccharides extracted from brown algae and kelp, with remarkable absorption properties [109]. Alginates are the result of the alginic acid reaction with calcium and sodium; therefore, alginates are called salts [50,110]. The formation of alginate gel is based on the exchange of calcium ions, which are inside the dressing, with sodium ions, which are in lesion exudates. Thus, the alginate gel presents an exceptional power to absorb a large volume of wound fluids, especially in the case of foot ulcers [111]. Another advantage of alginates dressings is that they have a hemostatic effect due to calcium ions (known as clotting factor IV, which plays an important role in blood coagulation); accordingly, they can be used if an injury bleeds [112].
5. Cellulose Derivatives as Wound Dressings
During the last decades, cellulose derivatives, also known as cellulosics, have become extensively used in many fields, from food, cosmetics, biomedical, and pharmaceutical industry [113] to biofuels and oilfield industry (petrochemicals) [114]. These semi-synthetic biopolymers present many advantageous characteristics, such as biocompatibility, biodegradability, non-toxicity, sustainability, abundance, and a suitable price; therefore, cellulose derivatives represent the first option for wound dressings development [115,116].
5.1. Cellulose Derivatives Classification
Cellulose, discovered by Anselme Payen in the 19th century, is a natural polymer, an organic polysaccharide from plant origin, non-toxic, with a structural role, being the most plentiful and renewable biopolymer on Earth [117]. Structurally, cellulose is a linear macromolecule composed of many molecules of D-glucose (the number of the glucose units can reach more than ten thousand), which are bound through 1-4-β-glycosidic linkages and its chemical formula is (C6H10O5)n [118]. The chemical structure of cellulose shows the presence of free hydroxyl groups at C2, C3, and C6 of each molecule of glucose, which have a good capacity to form powerful inter- and intramolecular hydrogen bonds [119]. As a result of this property, cellulose has a crystalline and stiff structure and, consequently, it is insoluble in water and the majority of the organic solvents; moreover, this natural biopolymer cannot be digested by the human digestive system [120,121]. Cellulose has good stability to pH fluctuations and temperature [122].
To improve the solubility problems of cellulose and to extend its applications, the chemical structure of this polymer can suffer several changes to obtain the cellulose derivatives, which have suitable physicochemical properties to be used in many fields, especially in the pharmaceutical and biomedical industry [123]. The modifications in the cellulose molecule can be chemical, physical, or biological [114], but the most used and significant of the three is the chemical modification. Targeted by this method are the hydroxyl groups, which suffer an esterification or an etherification reaction [124]. Therefore, the cellulose derivatives can be classified in two major classes: cellulose esters derivatives and cellulose ethers derivatives, which have particular mechanical and physicochemical characteristics [125]. The chemical structures of cellulose and cellulose derivatives are presented in Figure 5.
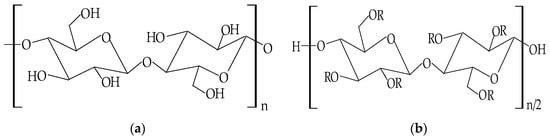
Figure 5.
Chemical structures of: (a) cellulose; (b) cellulose derivatives.
Cellulose ethers derivatives are characterized by high molecular weight and the greatest applicability in the pharmaceutical domain of all these derivatives are: sodium carboxymethylcellulose (NaCMC), hydroxypropylmethylcellulose (HPMC), methylcellulose (MC), hydroxyethylcellulose (HEC), ethylcellulose (EC), hydroxypropylcellulose (HPC), hydroxyethylmethylcellulose (HEMC) and benzylcellulose (BC) [126,127]. The cellulose ethers are illustrated in Table 1.

Table 1.
Main cellulose ether derivatives according to R groups [128].
Cellulose esters derivatives are extensively used in the pharmaceutical industry as enteric coated drug delivery devices, and they also have excellent properties to form films. There are two categories of cellulose esters: organic and inorganic, but the most common in the pharmaceutical practice are organic esters [129]. Among them are cellulose acetate (CA), cellulose acetate butyrate (CAB), cellulose acetate phthalate (CAP), cellulose acetate trimelitate (CAT), hydroxypropylmethylcellulose phthalate (HPMCP), and hydroxypropylmethylcellulose acetate succinate (HPMCAS). With fewer applications in the pharmaceutical industry are inorganic esters, such as cellulose nitrate (CN) and cellulose sulphate (CS) [130]. The cellulose esters are illustrated in Table 2.

Table 2.
Main cellulose ester derivatives according to R groups [128].
Another classification of cellulose derivatives depends on the water solubility of these polymers; thus, there are described in water-soluble cellulose derivatives and water-insoluble cellulose derivatives. In the first category are included the majority of cellulose ethers (methylcellulose (MC), sodium carboxymethylcellulose (NaCMC), hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), hydroxyethylmethylcellulose (HEMC) and hydroxypropylmethylcellulose (HPMC)) [131], while the other cellulose ethers (ethylcellulose and benzylcellulose) and cellulose esters are included in the category of water-insoluble cellulose derivatives. Between the two categories, water-soluble cellulose derivatives are the most used biopolymers in the pharmaceutical and biomedical industry [115,132], because they present several favorable features, such as solubility, surface activity, viscosity in solution, similar properties to thermoplastic film, and proper stability to oxidative and hydrolytic reactions, heat and biodegeneration [130,133].
Due to the general properties of wound dressings presented in Section 4.1, but also the particular properties, such as hydrophilicity, mechanical toughness, pH stability, and rheological characteristics, cellulose and cellulose derivatives have multiple applications in many fields [134]. Areas of the applicability of all these biopolymers involve: biomedical and pharmaceutical industries, where they can act as drug-delivery devices, wound dressings, muco- and bioadhesive drugs, excipients for drug formulations, and support for tissue engineering [29]; also, they can be used for cosmetic and hygienic products, in the textile area, in the food industry and agriculture [128,135]. The representation of cellulose derivatives-based wound dressing on an open wound is illustrated in Figure 6.
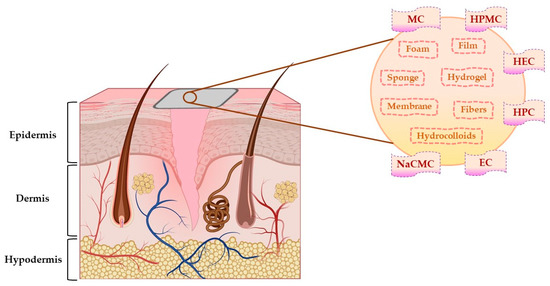
Figure 6.
The representation of cellulose derivatives-based wound dressing on an open wound. This illustration has been created with BioRender.com, Inkscape, and PowerPoint.
Cellulose ethers derivatives (Table 1) are the most used biopolymers for tailoring of new wound dressings, compared to cellulose esters derivatives. Therefore, we will further describe them, and we will present their main different types of wound dressings for an optimal wound management, from gels to foams, as we summarized in Section 4.2.
5.2. Sodium Carboxymethylcellulose-Based Wound Dressings
Carboxymethylcellulose (CMC), also known as carmellose, is a semi-synthetic and hydrophilic polymer, a water-soluble cellulose ether derivative, and one of the polymers with the lowest price [136]. Sodium carboxymethylcellulose (NaCMC) is the sodium salt of CMC, an anionic polymer, with a great solubility in water [137]. NaCMC was the first compound from the group of cellulose derivatives; therefore, all the researchers’ attention was focused on it because, compared to other cellulose derivatives, NaCMC can be synthesized through simple methods with low-cost materials [138]. It results from the etherification reaction of the cellulose with sodium monochloroacetate in an alkaline solution (NaOH) [139]. In the cellulose molecule, three hydroxyl groups (from 2, 3, and 6 positions) are substituted by carboxymethyl groups [140], resulting in different values of substitution degree from 0.4 to 1.5 and different molecular weights of NaCMC, varying from 90,000 to 2,000,000 g/mol [51]. The optimal substitution degree to be used in the pharmaceutical industry is from 0.60 to 1.00 [139]. The chemical structure of NaCMC is shown in Figure 7.
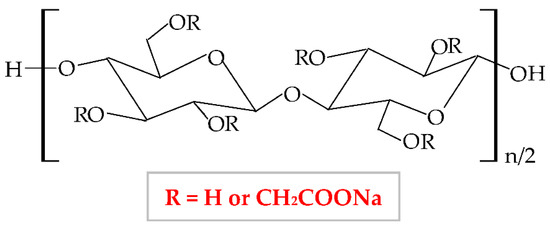
Figure 7.
Chemical structure of sodium carboxymethylcellulose (NaCMC).
The NaCMC network illustrates a thixotropic behavior to generate 3D structures through intermolecular attraction. Its thixotropy is influenced by concentration and degree of substitution [141]. NaCMC presents excellent physicochemical and mechanical properties [142], optimal biocompatibility and biodegradability, proper capacity to absorb the water and to swell, high gelation behavior, non-toxicity, and low-immunogenicity [143]. It is the most used cellulose derivative in the pharmaceutical industry, mainly for the development of new wound dressings because it has the capacity to absorb heavy exudates [144,145], to ensure excellent moisture at the lesion site, and to avoid skin tissues water loss and tissues necrosis. Moreover, an optimal local humidity can impede dehydration, facilitate the synergy between target cells and growth factors, promote angiogenesis advancement, the mitigation of the ache, and the disruption of the fibrin network [146]. NaCMC is also used as a drug-delivery device and excipient for drug formulations (used as an emulsifier, thickener, stabilizer, and film-maker) [147]. Besides its applicability in the pharmaceutical area, this biopolymer possesses different usefulness in the food (E466 food additive) industry [148], in paper, textile and cosmetics domains [51,149], for tissue culture and dental medicine field [150].
NaCMC can be combined with other polymers to enhance its properties and to develop its applicability. Thus, it is more advantageous to blend two or more polymers for the development of a new material comparative to the chemical industrial development of that material. Moreover, the new material obtained by mixing other well-known polymers presents all the properties or is more favorable than the component polymers [75]. Furthermore, the blend of polymers can be realized to compensate for their drawbacks. Hence, Liu et al., combined NaCMC with HEC by electrostatic complexing and obtained a sponge and a membrane with a porous network, enhanced viscoelastic properties, and high swelling behavior [151]. Hu et al., mixed NaCMC with PVA and quaternized chitosan and designed a new composite with enhanced flexibility, water absorption rate, mechanical strength, swelling ratio, and humidity permeability [152]. A novel NaCMC/PVA-based composite was formulated, with higher properties than two polymers: improved swelling capacity, elasticity, water solubility, porosity, water vapor transmission rate, bioavailability, and biodegradability for the tissue repair process; this formulation also presented an extension of its applicability, such as agriculture, biomedical field as drug delivery systems and food packaging [153,154]. NaCMC was blended with PEG through a photo-click reaction based on thiol-norbornene. It formed a pH-sensitive hydrogel with an augmented swelling ratio [150]. Zhang et al., designed a novel hydrogel based on NaCMC and sodium alginate. In a ratio of 1:4, the hydrogel exhibited high biocompatibility, mechanical characteristics, degradation rate, and local humidity [155]. Shin et al., blended NaCMC with PVA and PEG 400 through cyclic freezing/thawing method and obtained a hydrogel with improved properties: the swelling rate, the compressive strength, and cytocompatibility [156]. NaCMC can also be blended with diverse biopolymers to develop a new potential wound dressing with better properties to accelerate the wound healing process. All these combinations are illustrated in Table 3.

Table 3.
Recent studies on the use of sodium carboxymethylcellulose as a wound dressing.
5.3. Hydroxypropylmethylcellulose-Based Wound Dressings
Hydroxypropylmethylcellulose (HPMC), hypromellose [187], is a semi-synthetic hydrophilic polymer, a nonionic cellulose ether derivative [188], with higher stability at a lower pH. In terms of physical properties, HPMC is a white, fibrous, or granular powder, whose particles are not cohesive, and it does not have a taste and odor [189]. This biopolymer results from hydroxyl groups substitution from cellulose molecule with methyl and hydroxypropyl groups. The chemical structure of HPMC is illustrated in Figure 8.
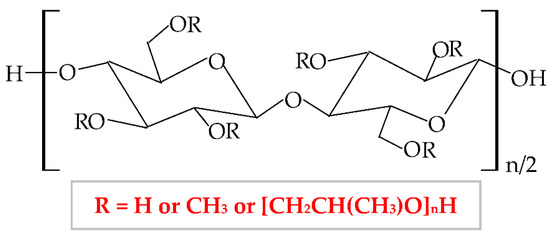
Figure 8.
Chemical structure of hydroxypropylmethylcellulose (HPMC).
Therefore, HPMC presents many degrees of substitution, that give to this biopolymer different molecular weight and physicochemical features (rheological properties and crystalline nature) [190,191]. The hydrophilic or hydrophobic nature is related to the values of the degree of substitution (DS) and the molar substitution (MS). Thus, the HPMC molecule with decreased values of DS and MS is more hydrophilic and the HPMC molecule with increased values of DS and MS is more hydrophobic [192]. Following this chemical substitution, HPMC gets both polar (hydroxypropyl) and non-polar (methyl) character; consequently, it can form hydrophobic, intermolecular, and intramolecular linkages with many other materials [190]. The non-ionic character leads to a limited adhesive capacity [193]. At high temperature, the biopolymer can suffer a thermoreversible phase transition from sol to gel, with a temperature of gelation over 60 °C, superior to the temperature of the body (37 °C) [194]. HPMC-based hydrogels are temperature-responsive [195].
According to United States Pharmacopeia (USP), there are four distinct forms of HPMC, which are categorized by the content of methoxy, respectively hydroxypropoxy groups in: HPMC 1828, HPMC 2208, HPMC 2906, and HPMC 2910 [196]. This biopolymer has been approved as a food additive, E464 [197], by the American Institute, Food and Drug Administration (FDA), by the European Institution, European Parliament, and Council Directive, and by the Joint Expert Committee on Food Additives [198].
HPMC has a proper solubility in water, and it is one of the most used cellulose derivatives in many industries. It is widely used in the biotechnological field (construction, food, cosmetics, biomedical, and pharmaceutical industry), due to its excellent characteristics, such as biocompatibility, biodegradability, superior stability, large availability, excellent swelling, high surface activity, and mechanical properties [199], remarkable ability to form films and poor toxicity [200]. Regarding the applicability of HPMC in biomedical and pharmaceutical domains, it is used as a drug-delivery device, with a large practice for wound dressings development and it can also have remarkable applicability in tissue engineering [201]. HPMC can also be used as an excipient because it possesses proper abilities of emulsification, stabilization, suspension, and thickening [202,203].
HPMC can be combined with other polymers to enhance its properties and to develop its applicability [204]. To improve the physicochemical properties of a new composite, HPMC has been blended with several natural, semi-synthetic, or synthetic polymers [205]. In this way, to improve the thermal stability, HPMC has been blended with collagen [206,207], gelatin [204], chitosan [208], chitosan, and xanthan gum [209]; to improve the mechanical properties (tensile strength and ultimate elongation), HPMC has been mixed with chitosan [210], collagen [207], poloxamer 407 [211], silk fibroin [212], PVA and PVP [213], chitosan and xanthan gum [209]; to increase the swelling rate, HPMC has been combined with methylcellulose [214], κ-carrageenan [215], chitosan and hyaluronic acid [216], chitosan and xanthan gum [209]. HPMC can also be blended with diverse biopolymers and multiple bioactive agents (plants extracts, organic or inorganic substances, and chemical drugs) to develop new potential wound dressing to accelerate the wound-healing process. All these studies are summarized in Table 4.

Table 4.
Recent studies on the use of hydroxypropylmethylcellulose as a wound dressing.
5.4. Methylcellulose-Based Wound Dressings
Methylcellulose (MC) is a semi-synthetic and non-ionic polymer, a cellulose ether derivative with high solubility in water, which is influenced by temperature [247]. It forms through the etherification of cellulose molecule with methyl chloride or dimethyl sulfate in basic solution [27] when the hydroxyl groups from the mother molecule are substituted with methyl groups, which leads to a diminishing of crystallinity [248]. The chemical structure of MC is presented in Figure 9.
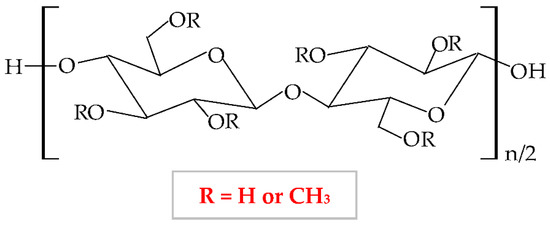
Figure 9.
Chemical structure of methylcellulose (MC).
At a variation of temperature, MC has a thermo-sensitive behavior with a reversible sol-gel transition in an aqueous solution [249]. At a lower temperature than lower critical solution temperature, it realizes the hydration of the MC network in solution, with the formation of hydrogen bonds. At a higher temperature than lower critical solution temperature, the MC aqueous solution takes in the heat, with the disintegration of hydrogen bonds [195]. Thus, MC presents increased viscosity at higher temperatures, and at lower temperatures it exhibits a reduced viscosity [250].
The degree of substitution for commercial MC varies from 1.7 to 2.2 when it results in a semiflexible biopolymer because the inter-and intra- hydrogen bonds from cellulose molecule break off [251]. There are many substances, which influence the gelation behavior of MC, such as inorganics salts, ethanol, propylene glycol, polyethylene glycol 400, sucrose, glycerin, sorbitol, and different surfactants (sodium dodecyl sulfate and cetyltrimethylammonium bromide) [252]. MC is extensively used in biomedical, pharmaceutical, cosmetic, and food industries as a thickening, binding, and film-forming agent because it possesses excellent biocompatibility, biodegradability, and reduced toxicity [253,254,255].
To improve the characteristics of MC, it can be blended with other polymers in different ratios to enhance the physicochemical, morphological, and structural properties of both polymers and of the resulting composite [255]. Abu et al., illustrated that a higher concentration of MC led to increased hydrophilicity and porosity of the MC-chitosan scaffold due to the hydroxyl groups from the MC molecule, which can attract water molecules. The higher wettability has been described by the suitable results of the water uptake capacity [256]. Another combination of MC and chitosan was studied by Tan et al., They illustrated that an augmented concentration of MC led to improved tensile strength, moisture content, whitish index, and elongation at break [257]. El-Naggar et al., mixed MC with PVA and doxycycline hyclate (drug model) to develop a new drug delivery device, which showed a proper swelling capacity and a high drug release at basic medium [253]. The combination between MC and poly(acrylic acid) presented optimal mechanical properties and thermal stability [258]. The novel composite resulting by blending MC and tragacanth gum exhibited a higher capacity to form a gel and adequate mechanical and rheological properties [259]. MC can also be blended with diverse biopolymers and multiple bioactive agents (plants extracts, organic or inorganic substances, and chemical drugs) to develop a new potential wound dressing to accelerate the wound-healing process. All these mixtures are presented in Table 5.

Table 5.
Recent studies on the use of methylcellulose as a wound dressing.
5.5. Hydroxyethylcellulose-Based Wound Dressings
Hydroxyethylcellulose (HEC) is a semi-synthetic, nonionic, and inert polymer, a water-soluble cellulose ether derivative [289]. It forms through etherification of alkaline cellulose with chlorohydrin or ethylene oxide, when hydroxyl groups from cellulose molecule are substituted with hydroxyethyl groups [290]. The chemical structure of HEC is illustrated in Figure 10.
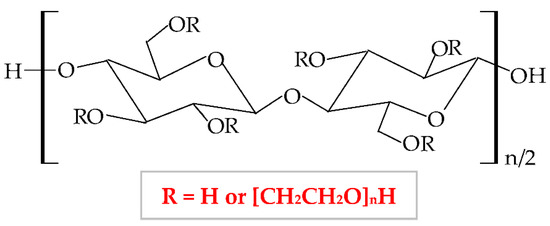
Figure 10.
Chemical structure of hydroxyethylcellulose (HEC).
It has a low price, without taste and smell, with no color to light yellowish [291]; presents optimal stability at pH values between 2 and 12 [292]. HEC exhibits a proper capacity to scavenge free radicals and to form hydrogen and electrostatic bonds [293]. HEC is regarded as a hydrogel-like material, with two important characteristics: liquid-like and solid-like. Due to its polysaccharide structure, this hydrophilic biopolymer exhibits a high capacity to absorb and hold a large quantity of water or wound exudates. The elastic strength of its structure leads to an expansion of the molecule dimensions, without the modification of the structural stability and the gel form [294]. HEC possesses excellent physicochemical properties: rheological, hydrodynamic, and thermodynamic [295]. HEC also presents adequate biocompatibility, biodegradability, insignificant toxicity, immunogenicity, and cementing properties [296]. Due to its nonionic behavior, HEC exhibits the ability to coexist with a large field of other polymers, which have an appropriate solubility in water, salts, or surfactants. Therefore, HEC presents optimal toughness in a dielectric solution with a large concentration [297]. This biopolymer presents the largest commercial availability from all cellulose derivatives [298]; therefore, HEC is a noticeable biopolymer, which can be used successfully as an emulsifier, film-coating, stabilizer, suspender, and thickener agent in biomedical, pharmaceutical (wound dressing development) [299], cosmetic, food, adhesive, and textile industries [291,300,301]. The most predictive method for hydrogels synthesis is the crosslinking of free radicals generated by irradiation (electron beam and gamma-radiation) [302].
To enhance its properties, HEC can be blended with other polymers. For example, Zia et al., mixed HEC with poly(lactic acid) and polyurethane. They obtained a new composite with higher thermal stability and mechanical (tensile strength and elongation) properties compared to other polymers [303,304]. Moreover, HEC has been blended with polyvinyl alcohol (PVA), resulting in suitable electrical conductibility, viscoelasticity, stretchability, and thermosensitivity [305]. Guo et al., combined HEC with poly(caprolactone) by trimethylsilyl group technology and the result was the formation of a new copolymer with enhanced thermal properties [306]. HEC was also blended with chitosan to obtain a copolymer with improved physicochemical and mechanical characteristics [307], with gelatin to obtain a superparamagnetic composite [308], with sodium alginate to form a copolymer with enhanced swelling efficacy and drug delivery profile. HEC can also be combined with diverse biopolymers to formulate novel wound dressing, which can stimulate the wound healing process and restore the damaged skin. The main combinations are summarized in Table 6.

Table 6.
Recent studies on the use of hydroxyethylcellulose as a wound dressing.
5.6. Ethylcellulose-Based Wound Dressings
Ethylcellulose (EC) is a nonionic semi-synthetic polymer, a cellulose ether derivative insoluble in water [318]. It forms through the etherification of alkali cellulose with ethyl chloride when the hydroxyl groups from cellulose molecule are substituted with ethyl groups [27]. The chemical structure of MC is presented in Figure 11.
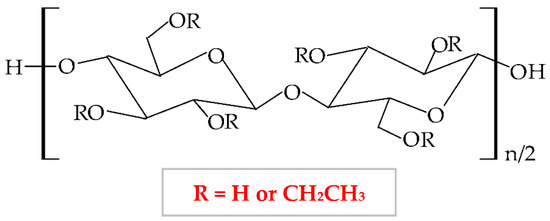
Figure 11.
Chemical structure of ethylcellulose (EC).
This biopolymer presents numerous advantageous characteristics, such as mechanical properties, biodegradability, flexibility, low toxicity, hydrophobicity, gelling capacity [319], light, moisture, oxygen resistance, thermoplasticity [320], and low price, which make EC an excellent material for use in different industries (pharmaceutical, cosmetic and food) [321]. Moreover, this biopolymer has several particular features in addition to the other cellulose derivatives: high film-forming capacity, suitable chemical strength, and optimal mechanical properties [322]. EC represents the most extensively analyzed biopolymer due to its capacity to form film for coating solid pharmaceutical forms (tablets, microcapsules, and microspheres) and formulation of new topical forms [323]. EC is a promising material to be used for encapsulation due to its optimal optical transparency, processing temperature, and electronic insulation [324]. It also presents a good capacity to bind, preserve and dissolve [325], and possesses a proper control of drug delivery [326]. Films based on EC are brittle because of the stiffness of hydrogen linkages from its molecule. This biopolymer has high stability to chemical substances and can be associated with different plasticizers to design heavy and impermeable films [327].
EC can be mixed with various polymers to enhance the physicochemical and mechanical properties and thus, its applicability. To develop a novel drug-delivery device, Li et al., blended EC by electrospinning method with poly(di(ethylene glycol) methyl ether methacrylate), a thermosensitive polymer. The new formulation showed normal morphology, a large porosity, and an increased wettability at a higher temperature, which led to more hydrophobic behavior, causing an extended release of the drug [328]. EC was mixed with poly (ethylene-co-vinyl acetate) and resulted in a new composite with higher mechanical properties [329]. Chen et al., mixed EC and poly(β-hydroxybutyrate) when EC acted as a thickening agent because it increased the viscosity of the new composite. In a concentration of 1%, EC augmented the tensile strength [330]. Li et al., blended EC with konjac glucomannan to formulate a novel composite with higher mechanical properties, moisture resistance, permeability of oxygen, and stability at a high temperature [331]. EC was also associated with another cellulose derivative, HPC, and obtained a scaffold with enhanced mechanical properties and 3D printing capacity [332].
EC can also be combined with other polymers to develop new wound dressings, with enhanced physicochemical and mechanical properties that can accelerate the wound-healing process. Principal blends are presented in Table 7.

Table 7.
Recent studies on the use of ethylcellulose as a wound dressing.
5.7. Hydroxypropylcellulose-Based Wound Dressings
Hydroxypropyl cellulose (HPC) is a semi-synthetic hydrophilic polymer, a cellulose ether derivative, with proper solubility in water and organic solvents [339]. Its solubility depends on the degree of substitution. At values smaller than 12%, HPC is water-soluble and at values higher than 12%, HPC is ethanol-soluble [340]. This biopolymer results from the etherification reaction of alkali cellulose with 1,2-propylene oxide. Thus, the 2,3,6-hydroxyl groups from the cellulose molecule are replaced with hydroxypropyl groups [27]. The chemical structure of HPC is presented in Figure 12.
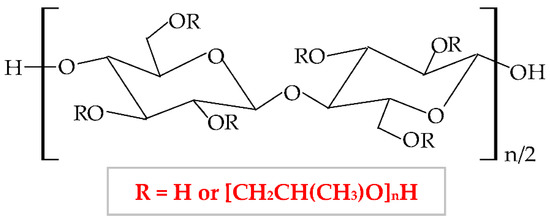
Figure 12.
Chemical structure of hydroxypropylcellulose (HPC).
It has numerous advantageous properties, such as amphiphilicity, low price, electrical neutrality, biocompatibility, biodegradability, non-toxicity, high power of swelling the wounds exudate [341,342], adequate chemical strength, and film-forming efficiency [236]. At a high temperature and in a concentrated aqueous solution, HPC generates a cholesteric liquid crystalline network, depending on its concentration [343]. HPC exhibits a thermoplastic behavior and develops temperature-responsive hydrogels [195,344]. Regarding the HPC-based films, these are defined by high flexibility, good impermeability for oil and fat, and a low value of Tg (glass transition temperature) at excessive humidity. The LCST (lower critical solution temperature) water value is about 41 °C. At a slightly higher temperature than LCST, HPC presents a phase change because the water solution of this biopolymer generates metastable nanosphere aggregates [345]. Moreover, the solubility of HPC is influenced by LCST values. At a lower temperature than LCST, HPC dissolves easily in water and at a higher temperature than LCST, HPC does not dissolve [346]. Thus, this cellulose derivative is an optimal material to be used in biomedical and pharmaceutical fields as a binding, disintegrating, emulsifying, thickening, filler, and coating agent [347,348] and in the construction domain [349]. It can also be used in the food industry because the United States Food and Drug Administration (FDA) authorized HPC as a safe food additive [350].
HPC can be blended with other polymers to improve the physicochemical and mechanical properties and thus, to extend its applicability. For instance, Veerapur et al., combined HPC and chitosan, and the new formulated composite presented higher hydrophilicity, swelling capacity, and permeation rate [351]. By mixing HPC with cellulose acetate phthalate resulted a composite with higher properties than compounds: increased pseudoplasticity and viscoelastic behavior [352]. Gan et al., prepared a high-performance hydrogel with enhanced tensile strength, toughness, biocompatibility, wear resistance, and low friction coefficient from HPC, sodium alginate, and poly(vinyl alcohol); these excellent characteristics extend the area of use to biosensors and nerve replacement [353]. Lu et al., blended HPC with poly(vinyl alcohol) to obtain a new scaffold with augmented toughness, elasticity, conductivity, and mechanical strength that is a promising material for the development of biosensors and interaction between humans and machines [354]. HPC can also be mixed with other polymers to develop novel wound dressing, with higher physicochemical traits that can restore the impaired skin tissue. The main combinations are summarized in Table 8.

Table 8.
Recent studies on the use of hydroxypropylcellulose as a wound dressing.
5.8. Combinations of Cellulose Derivatives-Based Wound Dressings
One or more cellulose derivatives may combine with other cellulose derivatives to formulate novel wound dressings, with enhanced properties that can accelerate the wound-healing process and alleviate the pain, inflammation, and stress caused by damaged skin tissue. Moreover, they can be combined to counteract their drawbacks [75]. The main combinations are summarized in Table 9.

Table 9.
Recent studies on the use of combinations of cellulose derivatives as wound dressing.
6. Conclusions and Future Perspectives
This review has focused on different types of wound dressing (gel, hydrogel, sponge, hydrocolloid, film, membrane, foam, and nanofibers) based on cellulose derivatives as biopolymeric scaffolds, and various bioactive agents, from plant extracts to chemical drugs. We have considered the cellulose ethers derivatives (NaCMC, HPMC, MC, HEC, EC, and HPC). It has been illustrated that cellulose derivatives can manifest a therapeutic effect on wound healing, alone or in combination with other natural, semi-synthetic, and synthetic polymers. The major advantage of mixing two or more biopolymers is, besides the beneficial action on damaged tissue, the improvement of physicochemical properties of the novel dressing. Cellulose derivatives have a particular chemical structure, obtained by etherification of hydroxyl groups from cellulose molecule with different alkyl groups, the consequence being the improvement of water solubility. Therefore, these biopolymers can be successfully used as a base for diverse formulations, due to their high gelation properties. Cellulose derivatives exhibit an efficient capacity to absorb the exudates on the site of the lesion, retain them, and swell. Consequently, the newly formulated wound dressings show an excellent ability to maintain relevant moisture on the wound bed and allow gas exchanges with the environment. Due to their high biocompatibility, biodegradability, physicochemical properties, eco-friendliness, and low cost, cellulose derivatives are promising materials for biomedical and pharmaceutical domains (electrochemical biosensors for medical diagnosis, bone tissue engineering, hemodialysis, drug delivery and 3D printing), for oilfields, carbon capture and the food industry.
Author Contributions
The authors had equal contribution. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was financially supported by the Carol Davila University of Medicine and Pharmacy Bucharest through Contract No. CNFIS-FDI-2021-0300 and RDI Capability consolidation at the Institutional level of the multidisciplinary research teams involved in the sustainability of UMFCD priority research directions.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, e1801019. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.; Bai, L.; Ginty, D.D. The gentle touch receptors of mammalian skin. Science 2014, 346, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Kimiz, I.; Sendemir, A.; Hasirci, V.; Hasirci, N. A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1764–1784. [Google Scholar] [CrossRef]
- Kolarsick, P.A.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- McLafferty, E.; Hendry, C.; Alistair, F. The integumentary system: Anatomy, physiology and function of skin. Nurs. Stand. 2012, 27, 35–42. [Google Scholar] [CrossRef]
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hashmi, S.; Marinkovich, M.P. Molecular organization of the basement membrane zone. Clin. Dermatol. 2011, 29, 398–411. [Google Scholar] [CrossRef]
- Zimoch, J.; Zielinska, D.; Michalak-Micka, K.; Rütsche, D.; Böni, R.; Biedermann, T.; Klar, A.S. Bioengineering a prevascularized human tri-layered skin substitute containing a hypodermis. Acta Biomater. 2021, 134, 215–227. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Sellami, M.; Salem, I.; Conic, R.; Kimak, M.; Pigatto, P.D.M.; Damiani, G. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients 2019, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; de Assis, A.M.; Moura, D.J.; Halmenschlager, G.; Saffi, J.; Xavier, L.L.; Fernandes Mda, C.; Wink, M.R. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS ONE 2014, 9, e96241. [Google Scholar] [CrossRef]
- Okoye, E.; Okolie, T. Development and in vitro characterization of ciprofloxacin loaded polymeric films for wound dressing. Int. J. Health Allied Sci. 2015, 4, 234. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Minaiyan, M.; Rastegarnasab, F.; Baradaran, A. Development and in vitro/in vivo evaluation of HPMC/chitosan gel containing simvastatin loaded self-assembled nanomicelles as a potent wound healing agent. Drug Dev. Ind. Pharm. 2018, 44, 276–288. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Jarbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Walker, J.; Cullen, M.; Chambers, H.; Mitchell, E.; Steers, N.; Khalil, H. Identifying wound prevalence using the Mobile Wound Care program. Int. Wound J. 2014, 11, 319–325. [Google Scholar] [CrossRef]
- Kapp, S.; Miller, C.; Santamaria, N. The quality of life of people who have chronic wounds and who self-treat. J. Clin. Nurs. 2018, 27, 182–192. [Google Scholar] [CrossRef]
- Moreira, M.E.; Markovchick, V.J. Wound Management. Crit. Care Nurs. Clin. N. Am. 2012, 24, 215–237. [Google Scholar] [CrossRef]
- Ghica, M.V.; Kaya, M.G.A.; Dinu-Pirvu, C.E.; Lupuleasa, D.; Udeanu, D.I. Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef] [PubMed]
- Clark, M. Alginates in Dressings and Wound Management. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11, pp. 213–222. [Google Scholar]
- Aramwit, P. Introduction to biomaterials for wound healing. In Wound Healing Biomaterials, Vol. 2: Functional Biomaterials; Agren, M.S., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publ. Ltd.: Cambridge, UK, 2016; Volume 115, pp. 3–38. [Google Scholar]
- Lee, D.; Yoo, B. Cellulose derivatives agglomerated in a fluidized bed: Physical, rheological, and structural properties. Int. J. Biol. Macromol. 2021, 181, 232–240. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.F.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Jia, B.; Zhou, J.; Zhang, L. Electrospun nano-fiber mats containing cationic cellulose derivatives and poly (vinyl alcohol) with antibacterial activity. Carbohydr. Res. 2011, 346, 1337–1341. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Soni, S.; Mittal, G.; Bhatnagar, A. Role of polymeric biomaterials as wound healing agents. Int. J. Low Extrem. Wounds 2014, 13, 180–190. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog Biomater 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Serena, T.E. A Global Perspective on Wound Care. Adv. Wound Care 2014, 3, 548–552. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Kordestani, S.S. Chapter 6—Wound Classification. In Atlas of Wound Healing; Kordestani, S.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–50. [Google Scholar]
- Kumar, K.S.; Reddy, B.E.J.S.; Processing, I. Wound image analysis classifier for efficient tracking of wound healing status. Signal Image Process. 2014, 5, 15–27. [Google Scholar]
- Wang, M.; Huang, X.; Zheng, H.; Tang, Y.; Zeng, K.; Shao, L.; Li, L. Nanomaterials applied in wound healing: Mechanisms, limitations and perspectives. J. Control. Release 2021, 337, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Low, J.S.; Mak, K.-K.; Zhang, S.; Pichika, M.R.; Marappan, P.; Mohandas, K.; Balijepalli, M.K. In vitro methods used for discovering plant derived products as wound healing agents—An update on the cell types and rationale. Fitoterapia 2021, 154, 105026. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Khodaei, M.; Alizadeh, Z.; Banitalebi-Dehkordi, M. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int. J. Biol. Macromol. 2021, 192, 298–322. [Google Scholar] [CrossRef] [PubMed]
- Skorkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Surgical Dressings and Wound Management; Dr Stephen Thomas: Joplin, MI, USA, 2010. [Google Scholar]
- Hachem, R.; Parikh, U.M.; Reitzel, R.; Rosenblatt, J.; Kaul, A.; Vargas-Cruz, N.; Hill, L.; Moore, L.; Meyer, J.; Chaftari, A.M.; et al. Novel antimicrobial ointment for infected wound healing in an in vitro and in vivo porcine model. Wound Repair Regen. 2021, 29, 830–842. [Google Scholar] [CrossRef]
- Albu, M.G.; Ghica, M.V.; Stefanescu, G.A.; Hodorogea, M.; Marin, M.M.; Marin, S.; Danila, E.; Voicu, S.; Simonca, A.G.; Popa, L. Design and Characterization of Collagen-Sodium Carboxymethylcellulose-Lidocaine 3D Composites for Wound Management. Key Eng. Mater. 2016, 695, 309–316. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; D’Autilia, F.; Rossi, S.; Ferrari, F.; Grisoli, P.; Sorrenti, M.; Catenacci, L.; Del Fante, C.; Perotti, C.; et al. Wound dressings based on silver sulfadiazine solid lipid nanoparticles for tissue repairing. Eur. J. Pharm. Biopharm. 2013, 84, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Verma, Y.K.; Verma, R.; Sing, A.K.; Gangenahalli, G. LiCl Containing Thermosensitive Formulation Improves Hemostasis, Wound Healing, and Hair Regrowth. Regen. Eng. Transl. Med. 2020, 17, 362–378. [Google Scholar] [CrossRef]
- Kenet, G.; Barg, A.A.; Nowak-Gottl, U. Hemostasis in the Very Young. Semin. Thromb. Hemost. 2018, 44, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Dragan, M.; Ionescu, O.M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupascu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloid Surf. B Biointerfaces 2013, 102, 102–110. [Google Scholar] [CrossRef]
- Harper, D.; Young, A.; McNaught, C.-E.J.S. The physiology of wound healing. Adv. Ski. Wound Care 2014, 32, 445–450. [Google Scholar] [CrossRef]
- Yang, L.; Liang, F.; Zhang, X.; Jiang, Y.; Duan, F.; Li, L.; Ren, F. Remodeling microenvironment based on MOFs-Hydrogel hybrid system for improving diabetic wound healing. Chem. Eng. J. 2022, 427, 131506. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P.J. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Elements affecting wound healing time: An evidence based analysis. Wound Repair Regen. 2015, 23, 550–556. [Google Scholar] [CrossRef]
- Desmet, C.M.; Preat, V.; Gallez, B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 262–284. [Google Scholar] [CrossRef]
- Gueldner, J.; Zhang, F.; Zechmann, B.; Bruce, E.D. Evaluating a novel oxygenating therapeutic for its potential use in the advancement of wound healing. Toxicol. In Vitro 2017, 43, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Paunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, S.; Albu, M.G.; Constantin, V.D.; Dinu-Pirvu, C.; Vuluga, Z.; Corobea, M.C.; Ghica, M.V. New Collagen-Dextran-Zinc Oxide Composites for Wound Dressing. J. Nanomater. 2016, 2016, 5805034. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Emmerson, E.; Hardman, M.J. The role of estrogen deficiency in skin ageing and wound healing. Biogerontology 2012, 13, 3–20. [Google Scholar] [CrossRef]
- Gouin, J.P.; Kiecolt-Glaser, J.K. The Impact of Psychological Stress on Wound Healing: Methods and Mechanisms. Crit. Care Nurs. Clin. N. Am. 2012, 24, 201–213. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Goodarzi, P.; Alavi-Moghadam, S.; Sarvari, M.; Beik, A.T.; Falahzadeh, K.; Aghayan, H.; Payab, M.; Larijani, B.; Gilany, K.; Rahim, F.; et al. Adipose Tissue-Derived Stromal Cells for Wound Healing. In Cell Biology and Translational Medicine, Vol. 4: Stem Cells and Cell Based Strategies in Regeneration; Turksen, K., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing AG: Cham, Switzerland, 2018; Volume 1119, pp. 133–149. [Google Scholar]
- Serra, M.B.; Barroso, W.A.; Silva, N.N.; Silva, S.d.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M.O.d.R. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflamm. 2017, 2017, 3406215. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The Effect of Comorbidities on Wound Healing. Surg. Clin. N. Am. 2020, 100, 695–705. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.C.; Browning, K.K. Smoking, chronic wound healing, and implications for evidence-based practice. J. Wound Ostomy Cont. Nurs. 2014, 41, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Kus, K.J.B.; Ruiz, E.S. Wound Dressings—A Practical Review. Curr. Dermatol. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J. Biol. Macromol. 2016, 85, 327–334. [Google Scholar] [CrossRef]
- Trevisol, T.C.; Fritz, A.R.M.; de Souza, S.M.A.G.U.; Bierhalz, A.C.K.; Valle, J.A.B. Alginate and carboxymethyl cellulose in monolayer and bilayer films as wound dressings: Effect of the polymer ratio. J. Appl. Polym. Sci. 2019, 136, 46941. [Google Scholar] [CrossRef]
- Mishra, S.K.; Mary, D.S.; Kannan, S. Copper incorporated microporous chitosan-polyethylene glycol hydrogels loaded with naproxen for effective drug release and anti-infection wound dressing. Int. J. Biol. Macromol. 2017, 95, 928–937. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pirvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Akrami-Hasan-Kohal, M.; Tayebi, L.; Ghorbani, M. Curcumin-loaded naturally-based nanofibers as active wound dressing mats: Morphology, drug release, cell proliferation, and cell adhesion studies. New J. Chem. 2020, 44, 10343–10351. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Sharma, G.; Lee, S.W.; Atanacio, O.; Parvizi, J.; Kim, T.K. In search of the optimal wound dressing material following total hip and knee arthroplasty: A systematic review and meta-analysis. Int. Orthop. 2017, 41, 1295–1305. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Q.; You, J.; Cheng, Y.; Hong, C.; Chen, Z.; Jiang, T.; Hao, T. Adhesion loss mechanism based on carboxymethyl cellulose-filled hydrocolloid dressings in physiological wounds environment. Carbohydr. Polym. 2020, 235, 115953. [Google Scholar] [CrossRef]
- Yadav, V.; Mittal, A.; Bansal, P.; Singh, S.K. Regulatory approval process for advanced dressings in India: An overview of rules. J. Wound Care 2019, 28, S32–S42. [Google Scholar] [CrossRef]
- Olatunji, O. Classification of Natural Polymers. In Natural Polymers; Olatunji, O., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar]
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S. Natural polymers–A comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Alves, T.F.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P.J.C. Applications of natural, semi-synthetic, and synthetic polymers in cosmetic formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Bhatia, S. Natural Polymers vs. Synthetic Polymer. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 95–118. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Sklenar, Z.; Vitkova, Z.; Herdova, P.; Horackova, K.; Simunkova, V. Formulation and release of alaptide from cellulose-based hydrogels. Acta Vet. Brno 2012, 81, 301–306. [Google Scholar] [CrossRef]
- Keogh, S.J.; Nelson, A.; Webster, J.; Jolly, J.; Ullman, A.J.; Chaboyer, W.P. Hydrocolloid dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2018, 2018, CD010364. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Meuleneire, F. A vapour-permeable film dressing used on superficial wounds. Br. J. Nurs. 2014, 23, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Chamsai, B.; Settharaksa, S.; Opanasopit, P. Synergistic antibacterial activity of alpha mangostin and resveratrol loaded polymer-based films against bacteria infected wound. J. Drug Deliv. Sci. Technol. 2020, 57, 101629. [Google Scholar] [CrossRef]
- Shi, C.Y.; Wang, C.Y.; Liu, H.; Li, Q.J.; Li, R.H.; Zhang, Y.; Liu, Y.Z.; Shao, Y.; Wang, J.C. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Lundin, J.G.; McGann, C.L.; Daniels, G.C.; Streifel, B.C.; Wynne, J.H. Hemostatic kaolin-polyurethane foam composites for multifunctional wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 702–709. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Powers, J.G.; Morton, L.M.; Phillips, T.J. Dressings for chronic wounds. Dermatol. Ther. 2013, 26, 197–206. [Google Scholar] [CrossRef]
- Dumville, J.C.; Keogh, S.J.; Liu, Z.; Stubbs, N.; Walker, R.M.; Fortnam, M. Alginate dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 5, CD011277. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Crabbe-Mann, M.; Tsaoulidis, D.; Parhizkar, M.; Edirisinghe, M. Ethyl cellulose, cellulose acetate and carboxymethyl cellulose microstructures prepared using electrohydrodynamics and green solvents. Cellulose 2018, 25, 1687–1703. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al. Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef] [PubMed]
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016; Volume 2, p. 64. [Google Scholar]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, A.H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 271, pp. 1–52. [Google Scholar]
- Miao, J.J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, X.J.; Xie, Y.J.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- Dmour, I.; Taha, M.O. Chapter 2—Natural and semisynthetic polymers in pharmaceutical nanotechnology. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 35–100. [Google Scholar]
- Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef]
- Raucci, M.; Alvarez-Perez, M.; Demitri, C.; Giugliano, D.; De Benedictis, V.; Sannino, A.; Ambrosio, L.J. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 2045–2056. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.P.; Wang, J.F.; Chu, F.X. Synthesis and Characterization of Ethyl Cellulose Based Acrylate. In Proceedings of the 4th International Conference on Manufacturing Science and Engineering (ICMSE 2013), Dalian, China, 30–31 March 2013; pp. 124–130. [Google Scholar]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L.J.C. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157–4165. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S.F. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Abdelhak, M.J. A Review: Application of Biopolymers in the Pharmaceutical Formulation. J. Adv. Bio-Pharm. Pharmacovigil. 2019, 1, 15–25. [Google Scholar] [CrossRef]
- Goncalves, C.; Favre, C.; Feuardant, P.; Klein, S.; Vaca-Garcia, C.; Cecutti, C.; Thiebaud-Roux, S.; Vedrenne, E. Synthesis of new cellulose ethers using Suzuki-Miyaura reactions. Carbohydr. Polym. 2015, 116, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Gao, C.Z.; Liu, S.; Edgar, K.J. Regioselective chlorination of cellulose esters by methanesulfonyl chloride. Carbohydr. Polym. 2018, 193, 108–118. [Google Scholar] [CrossRef]
- Shokri, J.; Adibki, K. Application of Cellulose and Cellulose Derivatives in Pharmaceutical Industries. In Cellulose—Medical, Pharmaceutical and Electronic Applications; van de Ven, T., Godbout, L., Eds.; IntechOpen: London, UK, 2013. [Google Scholar]
- Karlsson, K.; Schuster, E.; Stading, M.; Rigdahl, M. Foaming behavior of water-soluble cellulose derivatives: Hydroxypropyl methylcellulose and ethyl hydroxyethyl cellulose. Cellulose 2015, 22, 2651–2664. [Google Scholar] [CrossRef]
- Ambrosio, L.; Demitri, C.; Sannino, A. Superabsorbent cellulose-based hydrogels for biomedical applications. In Biomedical Hydrogels; Rimmer, S., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 25–50. [Google Scholar]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Mao, J.; Li, S.; Huang, J.; Meng, K.; Chen, G.; Lai, Y. Recent Advances of Multifunctional Cellulose-Based Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M., Ed.; Springer: Cham, Switzerland, 2018; pp. 37–64. [Google Scholar]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef]
- Burduşel, A.-C.; Stancu, I.; Marin, I.B.M.Ş.; Chelaru, C.; Serafim, A.; Drăguşin, D.; Kaya, M.G.A.; Coară, G. Development and characterization of collagen–carboxymethylcellulose materials for lenses. In Proceedings of the International Conference on Advanced Materials and Systems (ICAMS), Qingdao, China, 26–27 March 2016; pp. 215–220. [Google Scholar]
- Vinklarkova, L.; Masteikova, R.; Vetchy, D.; Dolezel, P.; Bernatoniene, J. Formulation of Novel Layered Sodium Carboxymethylcellulose Film Wound Dressings with Ibuprofen for Alleviating Wound Pain. Biomed. Res. Int. 2015, 2015, 892671. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.; Wong, T.W. Sodium carboxymethylcellulose scaffolds and their physicochemical effects on partial thickness wound healing. Int. J. Pharm. 2011, 403, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Peng, M.; Zhou, X.Y.; Wu, H.; Hu, J.; Xie, W.G.; Liu, S.H. Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohydr. Polym. 2014, 112, 32–38. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef]
- Udeanu, D.I.; Kaya, M.G.A.; Ghica, M.V.; Marin, S.; Marin, M.M.; Kaya, D.A.; Popa, L.; Dinu-Pirvu, C. Anti-inflammatory drug-loaded biopolymeric spongious matrices with therapeutic perspectives in burns treatment. Farmacia 2018, 66, 783–790. [Google Scholar] [CrossRef]
- Miraftab, M.; Qiao, Q.; Kennedy, J.F.; Knill, C.J.; Groocock, M.R. Advanced Wound-care Materials: Ultra High Absorbing Fibres made from Alginates Containing Branan Ferulate and Carboxymethyl Cellulose. J. Text. Inst. 2010, 95, 341–348. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Antimicrobial and Wound Treatment Aspects of Micro- and Nanoformulations of Carboxymethyl, Dialdehyde, and TEMPO-Oxidized Derivatives of Cellulose: Recent Advances. Macromol. Biosci. 2020, 20, 1900362. [Google Scholar] [CrossRef]
- Wong, T.W.; Ramli, N.A. Carboxymethylcellulose film for bacterial wound infection control and healing. Carbohydr. Polym. 2014, 112, 367–375. [Google Scholar] [CrossRef]
- Ali, M.; Khan, N.R.; Basit, H.M.; Mahmood, S. Physico-chemical based mechanistic insight into surfactant modulated sodium Carboxymethylcellulose film for skin tissue regeneration applications. J. Polym. Res. 2019, 27, 20. [Google Scholar] [CrossRef]
- Krizova, H.; Wiener, J. Development of carboxymethyl cellulose/polyphenols gels for textile applications. Autex Res. J. 2013, 13, 33–36. [Google Scholar] [CrossRef][Green Version]
- Fukuyama, Y.; Maruo, T.; Nishiyama, Y.; Nemoto, Y.; Murayama, K.; Kayanuma, H.; Kawarai, S. Application of a novel carboxymethyl cellulose-based Mohs sol-gel on malignant wounds in three dogs. J. Vet. Med. Sci. 2021, 83, 385–389. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.H.; Ki, C.S. Fabrication of PEG-carboxymethylcellulose hydrogel by thiol-norbornene photo-click chemistry. Int. J. Biol. Macromol. 2016, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhao, Y.; Tong, Z.R.; Chen, S.S. Superabsorbent Sponge and Membrane Prepared by Polyelectrolyte Complexation of Carboxymethyl Cellulose/Hydroxyethyl Cellulose-Al3+. BioResources 2015, 10, 6479–6495. [Google Scholar] [CrossRef]
- Hu, D.; Qiang, T.; Wang, L. Quaternized chitosan/polyvinyl alcohol/sodium carboxymethylcellulose blend film for potential wound dressing application. Wound Med. 2017, 16, 15–21. [Google Scholar] [CrossRef]
- Nargesi Khoramabadi, H.; Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, J.H.; Piao, M.G.; Lee, M.K.; Oh, D.H.; Hwang, D.H.; Quan, Q.Z.; Yong, C.S.; Choi, H.G. Effect of sodium carboxymethylcellulose and fucidic acid on the gel characterization of polyvinylalcohol-based wound dressing. Arch. Pharm. Res. 2010, 33, 1073–1081. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.N.; Wei, Q.H.; Li, X.P.; Guo, Y.; Zhang, S. Design and Fabrication of Sodium Alginate/Carboxymethyl Cellulose Sodium Blend Hydrogel for Artificial Skin. Gels 2021, 7, 115. [Google Scholar] [CrossRef]
- Shin, J.Y.; Lee, D.Y.; Kim, B.Y.; Yoon, J.I. Effect of polyethylene glycol molecular weight on cell growth behavior of polyvinyl alcohol/carboxymethyl cellulose/polyethylene glycol hydrogel. J. Appl. Polym. Sci. 2020, 137, 49568. [Google Scholar] [CrossRef]
- Fan, L.H.; Zhou, X.Y.; Wu, P.H.; Xie, W.G.; Zheng, H.; Tan, W.; Liu, S.H.; Li, Q.Y. Preparation of carboxymethyl cellulose sulfates and its application as anticoagulant and wound dressing. Int. J. Biol. Macromol. 2014, 66, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Im, J.N.; Kim, T.H.; Chung, D.J.; Doh, S.J. Structure and Liquid Handling Properties of Water-insoluble Carboxymethyl Cellulose Foam. Fiber. Polym. 2015, 16, 726–734. [Google Scholar] [CrossRef]
- Marchianti, A.C.N.; Sakinah, E.N.; Elfiah, U.; Putri, N.K.S.; Wahyuliswari, D.I.; Maulana, M.; Ulfa, E.U. Gel formulations of Merremia mammosa (Lour.) accelerated wound healing of the wound in diabetic rats. J. Tradit. Complement. Med. 2021, 11, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; An, S.J.; Jeong, S.I.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Amin, M.; Ng, S.F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J. Biomater. Sci.-Polym. Ed. 2019, 30, 629–645. [Google Scholar] [CrossRef]
- Ng, S.F.; Jumaat, N. Carboxymethyl cellulose wafers containing antimicrobials: A modern drug delivery system for wound infections. Eur. J. Pharm. Sci. 2014, 51, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Nabi, M.; Shadi, M.; Amini, M.M.; Shaabani, A. Carboxymethyl cellulose/tetracycline@UiO-66 nanocomposite hydrogel films as a potential antibacterial wound dressing. Int. J. Biol. Macromol. 2021, 188, 811–819. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Vinklarkova, L.; Masteikova, R.; Foltynova, G.; Muselik, J.; Pavlokova, S.; Bernatoniene, J.; Vetchy, D. Film wound dressing with local anesthetic based on insoluble carboxymethycellulose matrix. J. Appl. Biomed. 2017, 15, 313–320. [Google Scholar] [CrossRef]
- Shi, D.D.; Wang, F.J.; Lan, T.; Zhang, Y.H.; Shao, Z.Q. Convenient fabrication of carboxymethyl cellulose electrospun nanofibers functionalized with silver nanoparticles. Cellulose 2016, 23, 1899–1909. [Google Scholar] [CrossRef]
- Tu, C.; Zhang, R.D.; Yan, C.; Guo, Y.; Cui, L. A pH indicating carboxymethyl cellulose/chitosan sponge for visual monitoring of wound healing. Cellulose 2019, 26, 4541–4552. [Google Scholar] [CrossRef]
- Bang, S.; Ko, Y.G.; Kim, W.I.; Cho, D.; Park, W.H.; Kwon, O.H. Preventing postoperative tissue adhesion using injectable carboxymethyl cellulose-pullulan hydrogels. Int. J. Biol. Macromol. 2017, 105, 886–893. [Google Scholar] [CrossRef]
- Siritientong, T.; Aramwit, P. Characteristics of carboxymethyl cellulose/sericin hydrogels and the influence of molecular weight of carboxymethyl cellulose. Macromol. Res. 2015, 23, 861–866. [Google Scholar] [CrossRef]
- Wang, P.; He, H.W.; Cai, R.; Tao, G.; Yang, M.R.; Zuo, H.; Umar, A.; Wang, Y.J. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.K.; Kalarikkal, N.; Elain, A.; Wong, T.W.; Thomas, S.; Grohens, Y. Wound healing analysis of pectin/carboxymethyl cellulose/microfibrillated cellulose based composite scaffolds. Mater. Lett. 2014, 132, 34–37. [Google Scholar] [CrossRef]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and Characterization of Low Methoxyl Pectin/Gelatin/Carboxymethyl Cellulose Absorbent Hydrogel Film for Wound Dressing Applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Z.M.; Wang, F.P.; Liu, L.; Wei, R.N.; Zhang, M. Preparation of self-regulating/anti-adhesive hydrogels and their ability to promote healing in burn wounds. J. Biomed. Mater. Res. Part B 2019, 107, 1471–1482. [Google Scholar] [CrossRef]
- Li, D.F.; Ye, Y.X.; Li, D.R.; Li, X.Y.; Mu, C.D. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef]
- Doh, S.J.; Lee, J.Y.; Lim, D.Y.; Im, J.N. Manufacturing and analyses of wet-laid nonwoven consisting of carboxymethyl cellulose fibers. Fibers Polym. 2013, 14, 2176–2184. [Google Scholar] [CrossRef]
- Nitipir, C.; Marin, S.; Marin, M.M.; Kaya, M.A.; Ghica, M.V.; Mederle, N. Hybrid Collagen-NaCMC Matrices Loaded with Mefenamic Acid for Wound Healing. Rev. Chim. 2017, 68, 2605–2609. [Google Scholar] [CrossRef]
- Leau, S.A.; Marin, S.; Coara, G.; Albu, L.; Constantinescu, R.R.; Kaya, M.A.; Neacsu, I.A. Study of wound-dressing materials based on collagen, sodium carboxymethylcellulose and silver nanoparticles used for their antibacterial activity in burn injuries. In Proceedings of the 7th International Conference on Advanced Materials and Systems, Bucharest, Romania, 18–20 October 2018; pp. 123–128. [Google Scholar]
- Maver, T.; Kurecic, M.; Pivec, T.; Maver, U.; Gradisnik, L.; Gasparic, P.; Kaker, B.; Bratusa, A.; Hribernik, S.; Kleinschek, K.S. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 2020, 27, 4487–4508. [Google Scholar] [CrossRef]
- Maver, T.; Kurecic, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J. Sol-Gel Sci. Technol. 2016, 79, 475–486. [Google Scholar] [CrossRef]
- Wang, F.J.; Shi, D.D.; Lan, T.; Zhang, Y.H.; Shao, Z.Q. One-step preparation of carboxymethyl cellulose/polyoxyethylene nanofiber mats containing silver nanoparticles. Integr. Ferroelectr. 2016, 169, 50–57. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Moreira, A.P.D.; Thire, R.; Quilty, B.; Passos, T.M.; Simon, P.; Mancini, M.C.; McGuinness, G.B. Absorbent Polyvinyl Alcohol-Sodium Carboxymethyl Cellulose Hydrogels for Propolis Delivery in Wound Healing Applications. Polym. Eng. Sci. 2017, 57, 1224–1233. [Google Scholar] [CrossRef]
- Joorabloo, A.; Khorasani, M.T.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Fabrication of heparinized nano ZnO/poly(vinylalcohol)/carboxymethyl cellulose bionanocomposite hydrogels using artificial neural network for wound dressing application. J. Ind. Eng. Chem. 2019, 70, 253–263. [Google Scholar] [CrossRef]
- Pagano, C.; Calarco, P.; Di Michele, A.; Ceccarini, M.R.; Beccari, T.; Primavilla, S.; Scuota, S.; Marmottini, F.; Ramella, D.; Ricci, M.; et al. Development of sodium carboxymethyl cellulose based polymeric microparticles for in situ hydrogel wound dressing formation. Int. J. Pharm. 2021, 602, 120606. [Google Scholar] [CrossRef]
- Maver, T.; Hribernik, S.; Mohan, T.; Smrke, D.M.; Maver, U.; Stana-Kleinschek, K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015, 5, 77873–77884. [Google Scholar] [CrossRef]
- Trevisol, T.C.; Scartazzini, L.; Valerio, A.; de Souza, S.; Bierhalz, A.C.K.; Valle, J.A.B. Diclofenac release from alginate/carboxymethyl cellulose mono and bilayer films for wound dressing applications. Cellulose 2020, 27, 6629–6642. [Google Scholar] [CrossRef]
- Birsan, M.; Bibire, N.; Vieriu, M.; Panainte, A.D.; Cojocaru, I. Influence of Hydroxypropyl Methylcellulose on Flowing and Swelling Parameters in Biomucoadhesive Tablets with Miconazole Nitrate. Rev. Chim. 2017, 68, 2346–2349. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.Q.; Qian, J.Y.; Yue, L.N.; Li, Q.; Xiao, L.X.; Ding, X.L.; Guan, C.R. Microstructures, physical and sustained antioxidant properties of hydroxypropyl methylcellulose based microporous photophobic films. Int. J. Biol. Macromol. 2020, 152, 1002–1009. [Google Scholar] [CrossRef]
- Rao, B.L.; Shivananda, C.S.; Shetty, G.R.; Harish, K.V.; Madhukumar, R.; Sangappa, Y. Influence of UV Irradiation on Hydroxypropyl Methylcellulose Polymer Films. In Proceedings of the 2nd International Conference on Condensed Matter and Applied Physics (ICC), Bikaner, India, 24–25 November 2017. [Google Scholar]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sanchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef]
- Akinosho, H.; Hawkins, S.; Wicker, L. Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276–281. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Mostaco, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Kamel, R.; El-batanony, R.; Salama, A. Pioglitazone-loaded three-dimensional composite polymeric scaffolds: A proof of concept study in wounded diabetic rats. Int. J. Pharm. 2019, 570, 118667. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.M.; Shen, T.T.; Wu, D.Y. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Fare, S. Thermo-Responsive Methylcellulose Hydrogels: From Design to Applications as Smart Biomaterials. Tissue Eng. Part B Rev. 2020, 27, 486–513. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Kumari, S.; Harjai, K.; Chhibber, S. Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. J. Infect. Dev. Ctries. 2010, 4, 367–377. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Asl, A.K. Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose—A review. Food Sci. Nutr. 2019, 7, 3363–3377. [Google Scholar] [CrossRef]
- Liu, H.W.; Chaw, J.R.; Shih, Y.C.; Huang, C.C. Designed hydrocolloid interpenetrating polymeric networks for clinical applications of novel drug-carrying matrix systems using Tris (6-isocyanatohexyl) isocyanurate and hydroxypropylmethylcellulose. Bio-Med. Mater. Eng. 2014, 24, 2065–2072. [Google Scholar] [CrossRef]
- Song, J.; Feng, H.; Wu, M.; Chen, L.; Xia, W.; Zhang, W. Preparation and characterization of arginine-modified chitosan/hydroxypropyl methylcellose antibacterial film. Int. J. Biol. Macromol. 2020, 145, 750–758. [Google Scholar] [CrossRef]
- Yin, J.; Fang, Y.; Xu, L.; Ahmed, A. High-throughput fabrication of silk fibroin/hydroxypropyl methylcellulose (SF/HPMC) nanofibrous scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1210–1221. [Google Scholar] [CrossRef]
- Uslu, I.; Aytimur, A.; Serincay, H. Preparation of PVA/PAA/PEG/PVP Nanofibers with HPMC and Aloe Vera. Curr. Nanosci. 2013, 9, 489–493. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, L.N.; Qian, J.Y.; Ding, X.L. Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose. Foods 2021, 10, 34. [Google Scholar] [CrossRef]
- Liu, X.X.; Ji, Z.L.; Peng, W.W.; Chen, M.; Yu, L.; Zhu, F. Chemical mapping analysis of compatibility in gelatin and hydroxypropyl methylcellulose blend films. Food Hydrocoll. 2020, 104, 105734. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yu, L.; Xie, F.W.; Li, S.; Sun, Q.J.; Liu, H.S.; Chen, L. On the investigation of thermal/cooling-gel biphasic systems based on hydroxypropyl methylcellulose and hydroxypropyl starch. Ind. Crop. Prod. 2018, 124, 418–428. [Google Scholar] [CrossRef]
- Ding, C.C.; Zhang, M.; Li, G.Y. Rheological Properties of Collagen/Hydroxypropyl Methylcellulose (COL/HPMC) Blended Solutions. J. Appl. Polym. Sci. 2014, 131, 40042. [Google Scholar] [CrossRef]
- Shao, X.R.; Sun, H.T.; Zhou, R.; Zhao, B.B.; Shi, J.F.; Jiang, R.P.; Dong, Y. Effect of bovine bone collagen and nano-TiO2 on the properties of hydroxypropyl methylcellulose films. Int. J. Biol. Macromol. 2020, 158, 937–944. [Google Scholar] [CrossRef]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Costa, C.M.; Lanceros-Mendez, S.; Maciavello, M.N.T.; Ribelles, J.L.G.; Sentanin, F.; Pawlicka, A.; Silva, M.M. Thermal-mechanical behaviour of chitosan-cellulose derivative thermoreversible hydrogel films. Cellulose 2015, 22, 1911–1929. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Zandraa, O.; Patwa, R.; Saha, N.; Capakova, Z.; Saha, P. Self-crosslinked chitosan/dialdehyde xanthan gum blended hypromellose hydrogel for the controlled delivery of ampicillin, minocycline and rifampicin. Int. J. Biol. Macromol. 2021, 167, 1468–1478. [Google Scholar] [CrossRef]
- da Silva, M.N.; Fonseca, J.D.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Campos, C.E.M.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- Dong, T.; Mi, R.X.; Wu, M.; Zhong, N.P.; Zhao, X.; Chen, X.; Shao, Z.Z. The regenerated silk fibroin hydrogel with designed architecture bioprinted by its microhydrogel. J. Mat. Chem. B 2019, 7, 4328–4337. [Google Scholar] [CrossRef]
- Tekko, I.A.; Chen, G.Y.; Dominguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larraneta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Y.H. Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2019, 655, 958–967. [Google Scholar] [CrossRef]
- Sun, G.H.; Liang, T.Q.; Tan, W.Y.; Wang, L.J. Rheological behaviors and physical properties of plasticized hydrogel films developed from kappa-carrageenan incorporating hydroxypropyl methylcellulose. Food Hydrocoll. 2018, 85, 61–68. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.L.; Xu, J.H. Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Drug Deliv. 2021, 28, 607–619. [Google Scholar] [CrossRef]
- Bagheri, M.; Shokoohinia, Y.; Pourmanouchehri, Z.; Jalilian, F.; Khaledian, S.; Mirzaie, S.; Behbood, L. Formulation and evaluation of the novel herbal antibacterial gel to the treatment of cutaneous burn infections. J. Rep. Pharm. Sci. 2021, 10, 93–100. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, G.X.; Huang, H.H.; Kuo, S.M. Liposome-encapsulated farnesol accelerated tissue repair in third-degree burns on a rat model. Burns 2019, 45, 1139–1151. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Morales, S.; Okamoto, Y.; Chan, H.-K. Topical application of bacteriophages for treatment of wound infections. Transl. Res. 2020, 220, 153–166. [Google Scholar] [CrossRef]
- Saddik, M.S.; Alsharif, F.M.; El-Mokhtar, M.A.; Al-Hakkani, M.F.; El-Mahdy, M.M.; Farghaly, H.S.; Abou-Taleb, H.A. Biosynthesis, Characterization, and Wound-Healing Activity of Phenytoin-Loaded Copper Nanoparticles. AAPS PharmSciTech 2020, 21, 175. [Google Scholar] [CrossRef]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly Developed Topical Cefotaxime Sodium Hydrogels: Antibacterial Activity and In Vivo Evaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef]
- Febriyenti; Noor, A.M.; Baie, S.B. Mechanical properties and water vapour permeability of film from haruan (Channa striatus) and fusidic acid spray for wound dressing and wound healing. Pak. J. Pharm. Sci. 2010, 23, 155–159. [Google Scholar]
- Huang, T.W.; Lu, H.T.; Ho, Y.C.; Lu, K.Y.; Wang, P.; Mi, F.L. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111396. [Google Scholar] [CrossRef]
- Claudio-Rizo, J.A.; Hernandez-Hernandez, N.G.; Cano-Salazar, L.F.; Flores-Guia, T.E.; de la Cruz-Duran, F.N.; Cabrera-Munguia, D.A.; Becerra-Rodriguez, J.J. Novel semi-interpenetrated networks based on collagen-polyurethane-polysaccharides in hydrogel state for biomedical applications. J. Appl. Polym. Sci. 2021, 138, 49739. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, W.; Wang, J.; Tang, R.P.; Zhang, D.; Wang, X. Hypromellose succinate-crosslinked chitosan hydrogel films for potential wound dressing. Int. J. Biol. Macromol. 2016, 91, 85–91. [Google Scholar] [CrossRef]
- Chen, C.P.; Hsieh, C.M.; Tsai, T.; Yang, J.C.; Chen, C.T. Optimization and Evaluation of a Chitosan/Hydroxypropyl Methylcellulose Hydrogel Containing Toluidine Blue O for Antimicrobial Photodynamic Inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef]
- Anuroop, U.P.; Nair, S.S.; Chacko, A.J. Development and evaluation of medicated biodegradable film for wounds and burns. Int. J. Pharm.l Sci. Res. 2019, 10, 5664–5672. [Google Scholar] [CrossRef]
- Pathan, I.B.; Munde, S.J.; Shelke, S.; Ambekar, W.; Setty, C.M. Curcumin loaded fish scale collagen-HPMC nanogel for wound healing application: Ex-vivo and In-vivo evaluation. Int. J. Polym. Mater. Polym. Biomat. 2019, 68, 165–174. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Sun, X.X.; Lin, X.L.; Yi, W.Y.; Jiang, J.Y. An injectable metal nanoparticle containing cellulose derivative-based hydrogels: Evaluation of antibacterial and in vitro-vivo wound healing activity in children with burn injuries. Int. Wound J. 2021, 13, 13664. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.E.; Machado, B.E.K.; da Silva, M.G.C.; da Silva, M.M.A.; Dal Bosco, L.; Marques, M.S.; Horn, A.P.; Persich, L.; Geller, F.C.; Argenta, D.; et al. Coumestrol/hydroxypropyl-beta-cyclodextrin association incorporated in hydroxypropyl methylcellulose hydrogel exhibits wound healing effect: In vitro and in vivo study. Eur. J. Pharm. Sci. 2018, 119, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Henriques, M.; Soares, G. Cyclodextrin/cellulose hydrogel with gallic acid to prevent wound infection. Cellulose 2014, 21, 4519–4530. [Google Scholar] [CrossRef]
- Uslu, I.; Aytimur, A. Production and characterization of poly(vinyl alcohol)/poly(vinylpyrrolidone) iodine/poly(ethylene glycol) electrospun fibers with (hydroxypropyl)methyl cellulose and aloe vera as promising material for wound dressing. J. Appl. Polym. Sci. 2012, 124, 3520–3524. [Google Scholar] [CrossRef]
- Lakshmi, P.K.; Thangellapalli, N.; Chennuri, A.; Prasanthi, D.; Veeresh, B. Wound healing activity of topical lawsone gel on rat model. Int. J. Pharm. Sci. Res. 2017, 8, 3162–3169. [Google Scholar] [CrossRef]
- Abraham, S.; Harsha, G.G.S.; Desai, K.; Furtado, S.; Srinivasan, B. Nano Calcium Oxide Incorporated Hydrocolloid Dressings for Wound Care. J. Pharm. Innov. 2020, 12. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Damato, T.C.; Carmona-Ribeiro, A.M.; Sierakowski, M.R.; Petri, D.F.S. Sustainable hydroxypropyl methylcellulose/xyloglucan/gentamicin films with antimicrobial properties. Carbohydr. Polym. 2017, 165, 285–293. [Google Scholar] [CrossRef]
- Prabu, D.; Majdalawieh, A.F.; Abu-Yousef, I.A.; Inbasekaran, K.; Balasubramaniam, T.; Nallaperumal, N.; Gunasekar, C.J. Preparation and characterization of gatifloxacin-loaded sodium alginate hydrogel membranes supplemented with hydroxypropyl methylcellulose and hydroxypropyl cellulose polymers for wound dressing. Int. J. Pharm. Investig. 2016, 6, 86–95. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, Y.L. Combination of the Silver-Ethylene Interaction and 3D Printing to Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef]
- Grip, J.; Engstad, R.E.; Skjaeveland, I.; Skalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsaeter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Sainz, C.; Chiou, B.-S.; Valenzuela-Medina, D.; Du, W.-X.; Gregorski, K.S.; Williams, T.G.; Wood, D.F.; Glenn, G.M.; Orts, W.J. Solution blow spun poly(lactic acid)/hydroxypropyl methylcellulose nanofibers with antimicrobial properties. Eur. Polym. J. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Namuiriyachote, N.; Lipipun, V.; Althhatuattananglzul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; De, A.; Das, B.; Mukherjee, B.; Samanta, A. Development of gum odina-gelatin based antimicrobial loaded biodegradable spongy scaffold: A promising wound care tool. J. Appl. Polym. Sci. 2021, 138, 50057. [Google Scholar] [CrossRef]
- Wikene, K.O.; Hegge, A.B.; Bruzell, E.; Tonnesen, H.H. Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): Studies on curcumin and curcuminoids LII. Drug Dev. Ind. Pharm. 2015, 41, 969–977. [Google Scholar] [CrossRef]
- Pinho, E.; Machado, S.; Soares, G. Smart Hydrogel for the pH-Selective Drug Delivery of Antimicrobial Compounds. In Proceedings of the 24th Polymer-Networks-Group Conference/82nd Prague Meeting on Macromolecules, Prague, Czech Republic, 17–21 June 2018. [Google Scholar]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef]
- Agubata, C.O.; Okereke, C.; Nzekwe, I.T.; Onoja, R.I.; Obitte, N.C. Development and evaluation of wound healing hydrogels based on a quinolone, hydroxypropyl methylcellulose and biodegradable microfibres. Eur. J. Pharm. Sci. 2016, 89, 1–10. [Google Scholar] [CrossRef]
- Pišlová, M.; Kolářová, K.; Vokatá, B.; Brož, A.; Ulbrich, P.; Bačáková, L.; Kolská, Z.; Švorčík, V. A new way to prepare gold nanoparticles by sputtering—Sterilization, stability and other properties. Mater. Sci. Eng. C 2020, 115, 111087. [Google Scholar] [CrossRef]
- Schuhladen, K.; Mukoo, P.; Liverani, L.; Nescakova, Z.; Boccaccini, A.R. Manuka honey and bioactive glass impart methylcellulose foams with antibacterial effects for wound-healing applications. Biomed. Mater. 2020, 15, 065002. [Google Scholar] [CrossRef]
- Fattahpour, S.; Shamanian, M.; Tavakoli, N.; Fathi, M.; Sadeghi-Aliabadi, H.; Sheykhi, S.R.; Fesharaki, M.; Fattahpour, S. An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int. J. Biol. Macromol. 2020, 151, 220–229. [Google Scholar] [CrossRef]
- Kar, A.K.; Shil, A.; Kar, B.; Dey, S. Formulation development and statistical optimization of zingiberol incorporated sodium alginate-methyl cellulose blend microspheres. Int. J. Biol. Macromol. 2020, 162, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P. Methyl cellulose solutions and gels: Fibril formation and gelation properties. Prog. Polym. Sci. 2021, 112, 101324. [Google Scholar] [CrossRef]
- Sangfai, T.; Tantishaiyakul, V.; Hirun, N.; Li, L. Microphase Separation and Gelation of Methylcellulose in the Presence of Gallic Acid and NaCl as an In Situ Gel-Forming Drug Delivery System. AAPS PharmSciTech 2017, 18, 605–616. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.W.M.; Senna, M.M.; Mostafa, T.A.; Helal, R.H. Radiation synthesis and drug delivery properties of interpenetrating networks (IPNs) based on poly(vinyl alcohol)/methylcellulose blend hydrogels. Int. J. Biol. Macromol. 2017, 102, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Bullock, A.; Ralston, D.; MacNeil, S. Development of a one-step approach for the reconstruction of full thickness skin defects using minced split thickness skin grafts and biodegradable synthetic scaffolds as a dermal substitute. Burns 2014, 40, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Grafova, M.; Slepicka, P.; Gedeon, O.; Synytsya, A. Modification of chitosan-methylcellulose composite films with meso-tetrakis(4-sulfonatophenyl)porphyrin. Biomacromolecules 2012, 13, 489–498. [Google Scholar] [CrossRef]
- Abu, N.; Kasim, S.H.; Hisham, S.F.; Shamsudin, S.; Noorsal, K.; Mastor, A. Effect of Methylcellulose on the Hydrophilicity of Chitosan 3D-Porous Scaffold. Adv. Mater. Res. 2016, 1133, 55–59. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z. Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2020, 146, 53–61. [Google Scholar] [CrossRef]
- Negim, E.S.M.; Nurpeissova, Z.A.; Mangazbayeva, R.A.; Khatib, J.M.; Williams, C.; Mun, G.A. Effect of pH on the physico-mechanical properties and miscibility of methyl cellulose/poly(acrylic acid) blends. Carbohydr. Polym. 2014, 101, 415–422. [Google Scholar] [CrossRef]
- Varshosaz, J.; Sajadi-Javan, Z.S.; Kouhi, M.; Mirian, M. Effect of bassorin (derived from gum tragacanth) and halloysite nanotubes on physicochemical properties and the osteoconductivity of methylcellulose-based injectable hydrogels. Int. J. Biol. Macromol. 2021, 192, 869–882. [Google Scholar] [CrossRef]
- Blinova, A.A.; Blinov, A.V.; Baklanova, O.A.; Yasnaya, M.A.; Baklanov, I.S.; Siddiqui, A.A.; Golik, A.B.; Okolelova, A.I.; Gvozdenko, A.A.; Simonov, A.N.; et al. Study of Wound-Healing Ointment Composition based on Highly Dispersed Zinc Oxide Modified with Nanoscale Silver. Int. J. Pharm. Phytopharm. Res. 2021, 11, 134–142. [Google Scholar] [CrossRef]
- Ghodrati Azadi, H.; Fathi, B.; Kazemi Mehrjerdi, H.; Maleki, M.; Shaterzadeh, H.; Abyazi, M.J. Macroscopic evaluation of wound healing activity of the Persian shallot, Allium hirtifolium in rat. Iran. J. Vet. Sci. Technol. 2011, 3, 31–38. [Google Scholar]
- Volkova, N.; Yukhta, M.; Pavlovich, O.; Goltsev, A. Application of Cryopreserved Fibroblast Culture with Au Nanoparticles to Treat Burns. Nanoscale Res. Lett. 2016, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, H.; Shin, J.Y.; Park, W.H. Effect of vitamin derivatives on gelation rate and gel strength of methylcellulose. Carbohydr. Polym. 2018, 196, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Sriwiroch, W.; Chungsamarnyart, N.; Chantakru, S.; Pongket, P.; Saengprapaitip, K.; Pongchairerk, U.J.A.; Resources, N. The effect of Pedilanthus tithymaloides (L.) Poit crude extract on wound healing stimulation in mice. Agric. Nat. Resour. 2010, 44, 1121–1127. [Google Scholar]
- Pislova, M.; Jackova, T.; Fajstavr, D.; Kolarova, K.; Svorcik, V. Release of Silver Nanoparticles from Polysaccharide Films Based on Methylcellulose; Tanger Ltd.: Slezska, Czech Republic, 2019; pp. 517–522. [Google Scholar]
- Jung, H.S.; Kim, H.C.; Park, W.H. Robust methylcellulose hydrogels reinforced with chitin nanocrystals. Carbohydr. Polym. 2019, 213, 311–319. [Google Scholar] [CrossRef]
- Denisova, E.V.; Suprunchuk, V.E.; Dronova, A.A. Biopolymeric materials containing brown algae polysaccharides. Entomol. Appl. Sci. Lett. 2017, 4, 19–23. [Google Scholar] [CrossRef]
- Das, B.; Basu, A.; Maji, S.; Dutta, K.; Dewan, M.; Adhikary, A.; Maiti, T.K.; Chattopadhyay, D. Nanotailored hyaluronic acid modified methylcellulose as an injectable scaffold with enhanced physico-rheological and biological aspects. Carbohydr. Polym. 2020, 237, 116146. [Google Scholar] [CrossRef]
- Nowald, C.; Penk, A.; Chiu, H.Y.; Bein, T.; Huster, D.; Lieleg, O. A Selective Mucin/Methylcellulose Hybrid Gel with Tailored Mechanical Properties. Macromol. Biosci. 2016, 16, 567–579. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, W.; Zhang, J.; Peng, X.; Li, G.; Zhang, L.-M.; Yang, L. Thermosensitive hydrogels based on methylcellulose derivatives for prevention of postoperative adhesion. Cellulose 2019, 27, 1555–1571. [Google Scholar] [CrossRef]
- Laffleur, F.; Strasdat, B. Gelatin-based formulations for dermal application. Eur. Polym. J. 2019, 118, 542–550. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Park, W.H. Dual-crosslinked, self-healing and thermo-responsive methylcellulose/chitosan oligomer copolymer hydrogels. Carbohydr. Polym. 2021, 258, 117705. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Liang, C.Y.; Wang, R.; Yao, X.L.; Guo, P.; Yuan, W.Z.; Liu, Y.; Song, Y.; Li, Z.H.; Xie, X.Y. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef]
- Mayol, L.; De Stefano, D.; De Falco, F.; Carnuccio, R.; Maiuri, M.C.; De Rosa, G. Effect of hyaluronic acid on the thermogelation and biocompatibility of its blends with methyl cellulose. Carbohydr. Polym. 2014, 112, 480–485. [Google Scholar] [CrossRef]
- Jiang, J.; Han, X.; Xu, H. Injectable Methylcellulose and Hyaluronic Acid Hydrogel Containing Silver Nanoparticles for Their Effective Anti-Microbial and Wound Healing Activity in Nursing Care for Burn Injuries in Children. J. Polym. Environ. 2021. under review. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.S.; Kim, J.S.; Choi, Y.C.; Cho, Y.W. Injectable and Thermosensitive Soluble Extracellular Matrix and Methylcellulose Hydrogels for Stem Cell Delivery in Skin Wounds. Biomacromolecules 2016, 17, 4–11. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Deguchi, S.; Ueno, A.; Kawasaki, N.; Ito, Y.J.B.; Bulletin, P. Combination ointment containing solid tranilast nanoparticles and dissolved sericin is efficacious for treating skin wound-healing deficits and redness in diabetic rats. Biol. Pharm. Bull. 2017, 40, 444–450. [Google Scholar] [CrossRef]
- Park, C.H.; Jeong, L.; Cho, D.; Kwon, O.H.; Park, W.H. Effect of methylcellulose on the formation and drug release behavior of silk fibroin hydrogel. Carbohydr. Polym. 2013, 98, 1179–1185. [Google Scholar] [CrossRef]
- Karavasili, C.; Tsongas, K.; Andreadis, I.I.; Andriotis, E.G.; Papachristou, E.T.; Papi, R.M.; Tzetzis, D.; Fatouros, D.G. Physico-mechanical and finite element analysis evaluation of 3D printable alginate-methylcellulose inks for wound healing applications. Carbohydr. Polym. 2020, 247, 116666. [Google Scholar] [CrossRef]
- Rastin, H.; Ramezanpour, M.; Hassan, K.; Mazinani, A.; Tung, T.T.; Vreugde, S.; Losic, D. 3D bioprinting of a cell-laden antibacterial polysaccharide hydrogel composite. Carbohydr. Polym. 2021, 264, 117989. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Lim, S.M.; Ismail, M.F.; Majeed, A.B. Antimicrobial and in vitro wound healing properties of novel clay based bionanocomposite films. J. Mater. Sci. Mater. Med. 2014, 25, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Niziol, M.; Paleczny, J.; Junka, A.; Shavandi, A.; Dawiec-Lisniewska, A.; Podstawczyk, D. 3D Printing of Thermoresponsive Hydrogel Laden with an Antimicrobial Agent towards Wound Healing Applications. Bioengineering 2021, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.Y.; Zhang, L.M.; Yang, L.Q.; Wu, J.F.; Li, N.; Pan, C.L.; Wang, X.Y.; Zeng, L.X.; Yan, L.; Yang, C.; et al. Sustained delivery of MMP-9 siRNA via thermosensitive hydrogel accelerates diabetic wound healing. J. Nanobiotechnol. 2021, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Pereira, A.D.; Castro, L.D.; Santa Brigida, A.; Lamarao, M.L.N.; Barbosa, W.L.R.; Silva, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide-Metilcellulose Hydrogels Containing Aloe barbadensis Extract as Dressing for Treatment of Chronic Cutaneous Skin Lesions. Polymers 2020, 12, 690. [Google Scholar] [CrossRef]
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Nescakova, Z.; Boccaccini, A.R. Production of a novel poly(e-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J. Biomed. Mater. Res. Part B 2021, 109, 180–192. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xie, Y.W.; Tang, Y.; Ni, S.H.; Wang, B.; Chen, Z.; Liu, X.J. Development and characterization of thermo-sensitive films containing asiaticoside based on polyvinyl alcohol and Methylcellulose. J. Drug Deliv. Sci. Technol. 2015, 30, 133–145. [Google Scholar] [CrossRef]
- Yadav, B.K.N.; Patel, G.C. Fabrication and characterization of coblended methyl cellulose with polyvinyl alcohol electrospun nanofibers as a carrier for drug delivery system. Polym. Bull. 2021, 20, 1–29. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef]
- Lin, P.J.; Liu, L.L.; He, G.H.; Zhang, T.; Yang, M.; Cai, J.Z.; Fan, L.H.; Tao, S.X. Preparation and properties of carboxymethyl chitosan/oxidized hydroxyethyl cellulose hydrogel. Int. J. Biol. Macromol. 2020, 162, 1692–1698. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef]
- Yamane, L.T.; de Paula, E.; Jorge, M.P.; de Freitas-Blanco, V.S.; Montanari, I.; Figueira, G.M.; Anholeto, L.A.; de Oliveira, P.R.; Rodrigues, R.A. Acmella oleracea and Achyrocline satureioides as Sources of Natural Products in Topical Wound Care. Evid.-Based Complement. Altern. Med. 2016, 2016, 3606820. [Google Scholar] [CrossRef] [PubMed]
- Rosic, R.; Kocbek, P.; Baumgartner, S.; Kristl, J. Electro-spun hydroxyethyl cellulose nanofibers: The relationship between structure and process. J. Drug Deliv. Sci. Technol. 2011, 21, 229–236. [Google Scholar] [CrossRef]
- Raafat, A.I.; Ali, A.E.H. A novel Lawsonia inermis (Henna)/(hydroxyethylcellulose/polyvinylpyrrolidone) wound dressing hydrogel: Radiation synthesis, characterization and biological evaluation. Polym. Bull. 2019, 76, 4069–4086. [Google Scholar] [CrossRef]
- Luo, P.F.; Liu, L.L.; Xu, W.Y.; Fan, L.H.; Nie, M. Preparation and characterization of aminated hyaluronic acid/oxidized hydroxyethyl cellulose hydrogel. Carbohydr. Polym. 2018, 199, 170–177. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zhou, G.; Ding, J.F.; Li, S.L.; Wang, G. Preparation and characterization of an agglomeration-cementing agent for dust suppression in open pit coal mining. Cellulose 2018, 25, 4011–4029. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, J.; Kang, M.X.; Ma, C.P.; Li, H.; Qiu, Z.M. Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2021, 138, 49880. [Google Scholar] [CrossRef]
- Kang, M.; Oderinde, O.; Han, X.; Fu, G.; Zhang, Z. Development of oxidized hydroxyethyl cellulose-based hydrogel enabling unique mechanical, transparent and photochromic properties for contact lenses. Int. J. Biol. Macromol. 2021, 183, 1162–1173. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Shahitha, F.; Yusuff, M.M.; Hamidon, N.N.; Chahal, S. Cross-Linking Effect on Electrospun Hydroxyethyl Cellulose/Poly(Vinyl Alcohol) Nanofibrous Scaffolds. Procedia Eng. 2013, 53, 689–695. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef]
- Taheri, H.; Hietala, M.; Oksman, K. One-step twin-screw extrusion process of cellulose fibers and hydroxyethyl cellulose to produce fibrillated cellulose biocomposite. Cellulose 2020, 27, 8105–8119. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zia, F.; Zia, K.M.; Aftab, W.; Tabasum, S.; Nazli, Z.-i.-H.; Mohammadi, M.; Zuber, M. Synthesis and characterization of graphene nanoplatelets-hydroxyethyl cellulose copolymer-based polyurethane bionanocomposite system. Int. J. Biol. Macromol. 2020, 165, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Zia, F.; Zia, K.M.; Nazli, Z.-i.-H.; Tabasum, S.; Khosa, M.K.; Zuber, M. Preparation of hydroxyethyl cellulose/halloysite nanotubes graft polylactic acid-based polyurethane bionanocomposites. Int. J. Biol. Macromol. 2020, 153, 591–599. [Google Scholar] [CrossRef]
- Huang, S.; Shuyi, S.; Gan, H.; Linjun, W.; Lin, C.; Danyuan, X.; Zhou, H.; Lin, X.; Qin, Y. Facile fabrication and characterization of highly stretchable lignin-based hydroxyethyl cellulose self-healing hydrogel. Carbohydr. Polym. 2019, 223, 115080. [Google Scholar] [CrossRef]
- Guo, M.; Yin, X.X.; Wang, P. C-13 NMR Characteristics and Thermal Properties of Hydroxyethyl Cellulose Grafting Poly(caprolactone) Copolymer. Asian J. Chem. 2013, 25, 4501–4505. [Google Scholar] [CrossRef]
- Demina, T.S.; Birdibekova, A.V.; Svidchenko, E.A.; Ivanov, P.L.; Kuryanova, A.S.; Kurkin, T.S.; Khaibullin, Z.I.; Goncharuk, G.P.; Zharikova, T.M.; Bhuniya, S.; et al. Solid-State Synthesis of Water-Soluble Chitosan-g-Hydroxyethyl Cellulose Copolymers. Polymers 2020, 12, 611. [Google Scholar] [CrossRef]
- Balaita, L.; Peptu, C.A.; Postolache, P.; Lisa, G.; Popa, M. Gelatin-hydroxyethyl cellulose magnetic microparticles as drug carriers: Preparation and characterization. J. Optoelectron. Adv. Mater. 2012, 14, 1023–1033. [Google Scholar]
- Jamlang, J.; Marañon, M.T.; Rigor, G.J.; Tumolva, T. Developing a physically cross-linked hydroxyethyl cellulose hydrogel for wound dressing applications. In Materials Science Forum; Trans Tech Publications Ltd.: Kapellweg, Switzerland, 2019; pp. 3–12. [Google Scholar]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Zahran, M.K.; Hakeim, O.A. Greener therapeutic pH-sensing wound dressing based on Curcuma Longa and cellulose hydrogel. Eur. Polym. J. 2021, 159, 110744. [Google Scholar] [CrossRef]
- Wang, C.W.; Niu, H.Y.; Ma, X.Y.; Hong, H.; Yuan, Y.; Liu, C.S. Bioinspired, Injectable, Quaternized Hydroxyethyl Cellulose Composite Hydrogel Coordinated by Mesocellular Silica Foam for Rapid, Noncompressible Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 34595–34608. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Yang, R.D. Novel Nanocomposites Based on Hydroxyethyl Cellulose and Graphene Oxide. Fiber Polym. 2017, 18, 334–341. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Zeyohannes, S.S.; Rasad, M.S.B.A.; Yusuff, M.M. A facile synthesis method of hydroxyethyl cellulose-silver nanoparticle scaffolds for skin tissue engineering applications. Mater. Sci. Eng. C 2017, 79, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Anumary, A.; Thanikaivelan, P.; Ashokkumar, M.; Kumar, R.; Sehgal, P.K.; Chandrasekaran, B. Synthesis and Characterization of Hybrid Biodegradable Films From Bovine Hide Collagen and Cellulose Derivatives for Biomedical Applications. Soft Mater. 2013, 11, 181–194. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Jahir Hussain, F.S.; Abdull Rasad, M.S.B.; Mohd Yusoff, M. In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym. Degrad. Stab. 2014, 110, 473–481. [Google Scholar] [CrossRef]
- Tohamy, K.M.; Soliman, I.E.; Mabrouk, M.; ElShebiney, S.; Beherei, H.H.; Aboelnasr, M.A.; Das, D.B. Novel polysaccharide hybrid scaffold loaded with hydroxyapatite: Fabrication, bioactivity, and in vivo study. Mater. Sci. Eng. C 2018, 93, 1–11. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose-hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, Z.W.; Godakanda, V.U.; Chiu, Y.J.; Angkawinitwong, U.; Patel, K.; Stapleton, P.G.; de Silva, R.M.; de Silva, K.M.N.; Zhu, L.M.; et al. The effect of collection substrate on electrospun ciprofloxacin-loaded poly (vinylpyrrolidone) and ethyl cellulose nanofibers as potential wound dressing materials. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 109917. [Google Scholar] [CrossRef]
- Zhang, Y.; Sadgrove, M.P.; Mumper, R.J.; Jay, M. Transdermal Prodrug Delivery for Radionuclide Decorporation: Nonaqueous Gel Formulation Development and In Vitro and In Vivo Assessment. Drug Dev. Res. 2013, 74, 322–331. [Google Scholar] [CrossRef]
- Lin, Y.M.; Li, M.; Xia, J.L.; Ding, H.Y.; Xu, L.N.; Yang, X.H.; Li, S.H. Synthesis of plant oil derived polyols and their effects on the properties of prepared ethyl cellulose composite films. Cellulose 2021, 28, 4211–4222. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ramezani, S.; Rashidi, M.R. Fabrication of honey-loaded ethylcellulose/gum tragacanth nanofibers as an effective antibacterial wound dressing. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 621, 126615. [Google Scholar] [CrossRef]
- Xu, J.Y.; Jia, H.G.; Yang, N.; Wang, Q.J.; Yang, G.X.; Zhang, M.Y.; Xu, S.P.; Zang, Y.; Ma, L.Q.; Jiang, P.F.; et al. High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids. Polymers 2019, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Mahnaj, T.; Ahmed, S.U.; Plakogiannis, F.M. Characterization of ethyl cellulose polymer. Pharm. Dev. Technol. 2013, 18, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Yan, G.H.; Sun, Y.; Tang, X.; Zeng, X.H.; Lin, L.; Lei, T.Z. Preparation of Ethyl Cellulose Composite Film with Down Conversion Luminescence Properties by Doping Perovskite Quantum Dots. ChemistrySelect 2019, 4, 6516–6523. [Google Scholar] [CrossRef]
- Djerafi, R.; Masmoudi, Y.; Crampon, C.; Meniai, A.; Badens, E. Supercritical anti-solvent precipitation of ethyl cellulose. J. Supercrit. Fluids 2015, 105, 92–98. [Google Scholar] [CrossRef]
- Suksaeree, J.; Thuengernthong, A.; Pongpichayasiri, K.; Maneewattanapinyo, P.; Settharaksa, S.; Pichayakorn, W. Formulation and Evaluation of Matrix Type Transdermal Patch Containing Silver Nanoparticles. J. Polym. Environ. 2018, 26, 4369–4375. [Google Scholar] [CrossRef]
- Su, X.C.; Yang, Z.; Tan, K.B.; Chen, J.F.; Huang, J.L.; Li, Q.B. Preparation and characterization of ethyl cellulose film modified with capsaicin. Carbohydr. Polym. 2020, 241, 116259. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, K.L.; Sang, Q.Q.; Williams, G.R.; Wu, J.Z.; Wang, H.J.; Wu, J.R.; Zhu, L.M. A thermosensitive drug delivery system prepared by blend electrospinning. Colloid Surf. B Biointerfaces 2017, 159, 277–283. [Google Scholar] [CrossRef]
- Girija, B.G.; Sailaja, R.R.N.; Biswas, S.; Deepthi, M.V. Mechanical and Thermal Properties of Eva Blended with Biodegradable Ethyl Cellulose. J. Appl. Polym. Sci. 2010, 116, 1044–1056. [Google Scholar] [CrossRef]
- Chen, J.X.; Wu, D.F.; Pan, K.R. Effects of ethyl cellulose on the crystallization and mechanical properties of poly(beta-hydroxybutyrate). Int. J. Biol. Macromol. 2016, 88, 120–129. [Google Scholar] [CrossRef]
- Li, X.; Jiang, F.T.; Ni, X.W.; Yan, W.L.; Fang, Y.P.; Corke, H.; Xiao, M. Preparation and characterization of konjac glucomannan and ethyl cellulose blend films. Food Hydrocoll. 2015, 44, 229–236. [Google Scholar] [CrossRef]
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589. [Google Scholar] [CrossRef]
- Godakanda, V.U.; Li, H.Y.; Alquezar, L.; Zhao, L.X.; Zhu, L.M.; de Silva, R.; de Silva, K.M.N.; Williams, G.R. Tunable drug release from blend poly(vinyl pyrrolidone)-ethyl cellulose nanofibers. Int. J. Pharm. 2019, 562, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Wang, K.; Yu, D.G.; Yang, Y.Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.M.N.; Kyazze, G.; Locke, I.C.; Tron, T.; Keshavarz, T. Poly(3-hydroxybutyrate)-ethyl cellulose based bio-composites with novel characteristics for infection free wound healing application. Int. J. Biol. Macromol. 2015, 81, 552–559. [Google Scholar] [CrossRef]
- Ahmadian, S.; Ghorbani, M.; Mahmoodzadeh, F. Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing. Int. J. Biol. Macromol. 2020, 162, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Q.Q.; Chen, Y.J.; Xia, S.F.; Huang, W.; Wei, Q.F. A Novel Multilayer Composite Membrane for Wound Healing in Mice Skin Defect Model. Polymers 2020, 12, 573. [Google Scholar] [CrossRef]
- Kapileshwari, G.R.; Barve, A.R.; Kumar, L.; Bhide, P.J.; Joshi, M.; Shirodkar, R.K. Novel drug delivery system of luliconazole—Formulation and characterisation. J. Drug Deliv. Sci. Technol. 2020, 55, 101302. [Google Scholar] [CrossRef]
- Ogawa, A.; Nakayama, S.; Uehara, M.; Mori, Y.; Takahashi, M.; Aiba, T.; Kurosaki, Y. Pharmaceutical properties of a low-substituted hydroxypropyl cellulose (L-HPC) hydrogel as a novel external dressing. Int. J. Pharm. 2014, 477, 546–552. [Google Scholar] [CrossRef]
- Bhatt, N.; Gupta, P.M.; Naithani, S. Hydroxypropyl cellulose from alpha-cellulose isolated from Lantana camara with respect to DS and rheological behavior. Carbohydr. Polym. 2011, 86, 1519–1524. [Google Scholar] [CrossRef]
- Lin, C.M.; Chang, Y.C.; Cheng, L.C.; Liu, C.H.; Chang, S.C.; Hsien, T.Y.; Wang, D.M.; Hsieh, H.J. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Miao, L.; Hu, J.W.; Lu, M.G.; Tu, Y.Y.; Chen, X.; Li, Y.W.; Lin, S.D.; Li, F.; Hu, S.Y. Alkynyl-functionalization of hydroxypropyl cellulose and thermoresponsive hydrogel thereof prepared with P(NIPAAm-co-HEMAPCL). Carbohydr. Polym. 2016, 137, 433–440. [Google Scholar] [CrossRef]
- Szabo, P.; Kallai-Szabo, B.; Kallai-Szabo, N.; Sebe, I.; Zelko, R. Preparation of hydroxypropyl cellulose microfibers by high-speed rotary spinning and prediction of the fiber-forming properties of hydroxypropyl cellulose gels by texture analysis. Cellulose 2014, 21, 4419–4427. [Google Scholar] [CrossRef]
- Ishii, D.; Ueda, K.; Stroeve, P.; Nakaoki, T.; Hayashi, H. Transport properties of chemically crosslinked hydroxypropyl cellulose in solvated state. Cell Chem. Technol. 2016, 50, 755–760. [Google Scholar]
- Ma, L.; Wang, L.L.; Wu, L.X.; Zhuo, D.X.; Weng, Z.X.; Ren, R.R. Cellulosic nanocomposite membranes from hydroxypropyl cellulose reinforced by cellulose nanocrystals. Cellulose 2014, 21, 4443–4454. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Green Electrospining of Hydroxypropyl Cellulose Nanofibres for Drug Delivery Applications. J. Nanosci. Nanotechnol. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Saraiva, D.V.; Chagas, R.; de Abreu, B.M.; Gouveia, C.N.; Silva, P.E.S.; Godinho, M.H.; Fernandes, S.N. Flexible and Structural Coloured Composite Films from Cellulose Nanocrystals/Hydroxypropyl Cellulose Lyotropic Suspensions. Crystals 2020, 10, 122. [Google Scholar] [CrossRef]
- Bielska, D.; Karewicz, A.; Kaminski, K.; Kielkowicz, I.; Lachowicz, T.; Szczubialka, K.; Nowakowska, M. Self-organized thermo-responsive hydroxypropyl cellulose nanoparticles for curcumin delivery. Eur. Polym. J. 2013, 49, 2485–2494. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Kassem, N.F.; Hassan, M.L. Hydroxypropyl cellulose/rice straw oxidized cellulose nanocrystals nanocomposites and their use in paper coating. Ind. Crop. Prod. 2016, 93, 186–192. [Google Scholar] [CrossRef]
- Sudo, S. Dielectric Properties of the Free Water in Hydroxypropyl Cellulose. J. Phys. Chem. B 2011, 115, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Veerapur, R.S.; Gudasi, K.B.; Aminabhavi, T.M. Pervaporation dehydration of isopropanol using blend membranes of chitosan and hydroxypropyl cellulose. J. Membr. Sci. 2007, 304, 102–111. [Google Scholar] [CrossRef]
- Onofrei, M.D.; Dobos, A.M.; Stoica, I.; Olaru, N.; Olaru, L.; Ioan, S. Lyotropic Liquid Crystal Phases in Cellulose Acetate Phthalate/Hydroxypropyl Cellulose Blends. J. Polym. Environ. 2014, 22, 99–111. [Google Scholar] [CrossRef]
- Gan, S.C.; Bai, S.H.; Chen, C.; Zou, Y.L.; Sun, Y.J.; Zhao, J.H.; Rong, J.H. Hydroxypropyl cellulose enhanced ionic conductive double-network hydrogels. Int. J. Biol. Macromol. 2021, 181, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Wang, C.P.; Zhang, D.H.; Wang, J.F.; Yong, Q.; Chu, F.X. Ultra-strong hydroxypropyl cellulose/polyvinyl alcohol composite hydrogel by combination of triple-network and mechanical training. Int. J. Biol. Macromol. 2021, 184, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Bjorn, C.; Noppa, L.; Salomonsson, E.N.; Johansson, A.L.; Nilsson, E.; Mahlapuu, M.; Hakansson, J. Efficacy and safety profile of the novel antimicrobial peptide PXL150 in a mouse model of infected burn wounds. Int. J. Antimicrob. Agents 2015, 45, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Shi, L.J.; Wu, H.P.; Li, Q.Y.; Hu, W.; Zhang, Z.B.; Huang, L.H.; Zhang, J.X.; Chen, D.J.; Deng, S.P.; et al. Graphene Oxide-IPDI-Ag/ZnO@Hydroxypropyl Cellulose Nanocomposite Films for Biological Wound-Dressing Applications. ACS Omega 2019, 4, 15373–15381. [Google Scholar] [CrossRef]
- Wang, J.J.; Wei, J. Interpenetrating network hydrogels with high strength and transparency for potential use as external dressings. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 460–467. [Google Scholar] [CrossRef]
- Rojewska, A.; Karewicz, A.; Karnas, K.; Wolski, K.; Zajac, M.; Kaminski, K.; Szczubialka, K.; Zapotoczny, S.; Nowakowska, M. Pioglitazone-Loaded Nanostructured Hybrid Material for Skin Ulcer Treatment. Materials 2020, 13, 2050. [Google Scholar] [CrossRef]
- Tolouei, S.; Hasheminia, S.J.; Narimani, M.; Khamesipour, A.; Shatalebi, M.A.; Hejazi, S.H. Leishmanicidal Activity of Films Containing Paromomycin and Gentamicin Sulfate both In Vitro and In Vivo. Iran. J. Parasitol. 2011, 6, 60–65. [Google Scholar]
- Mohebian, Z.; Tajmohammadi, I.; Maroufi, L.Y.; Ramezani, S.; Ghorbani, M. A Novel Aloe Vera-Loaded Ethylcellulose/Hydroxypropyl Methylcellulose Nanofibrous Mat Designed for Wound Healing Application. J. Polym. Environ. 2021, 11. [Google Scholar] [CrossRef]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose-grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Bordoloi, D.; Kunnumakkara, A.B.; Anandalakshmi, R. Preparation and characterization of cellulose-based nanocomposite hydrogel films containing CuO/Cu2O/Cu with antibacterial activity. J. Appl. Polym. Sci. 2020, 137, 49216. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Anandalakshmi, R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int. J. Biol. Macromol. 2019, 134, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K.; Bordoloi, D.; Kunnumakkara, A.B.; Anandalakshmi, R. Formation and characterization of zinc oxide complexes in composite hydrogel films for potential wound healing applications. Polym. Compos. 2020, 41, 2274–2287. [Google Scholar] [CrossRef]
- Amanat, S.; Taymouri, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A. Carboxymethyl cellulose-based wafer enriched with resveratrol-loaded nanoparticles for enhanced wound healing. Drug Deliv. Transl. Res. 2020, 10, 1241–1254. [Google Scholar] [CrossRef]
- Rezvanian, M.; Tan, C.K.; Ng, S.F. Simvastatin-loaded lyophilized wafers as a potential dressing for chronic wounds. Drug Dev. Ind. Pharm. 2016, 42, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).