An Update on the Anticancer Activity of Xanthone Derivatives: A Review
Abstract
1. Introduction
2. Xanthone Derivatives as Anticancer Agents
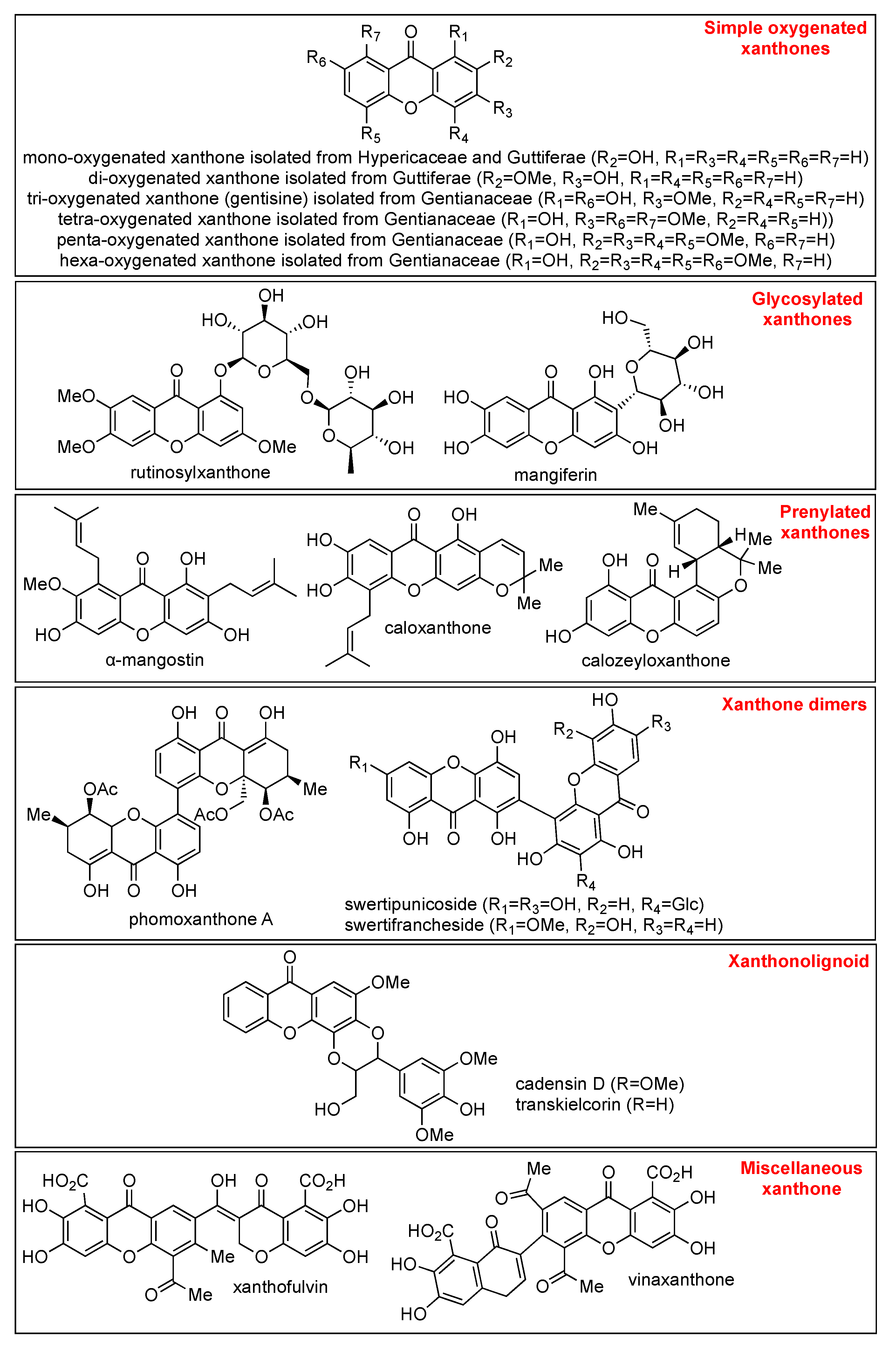
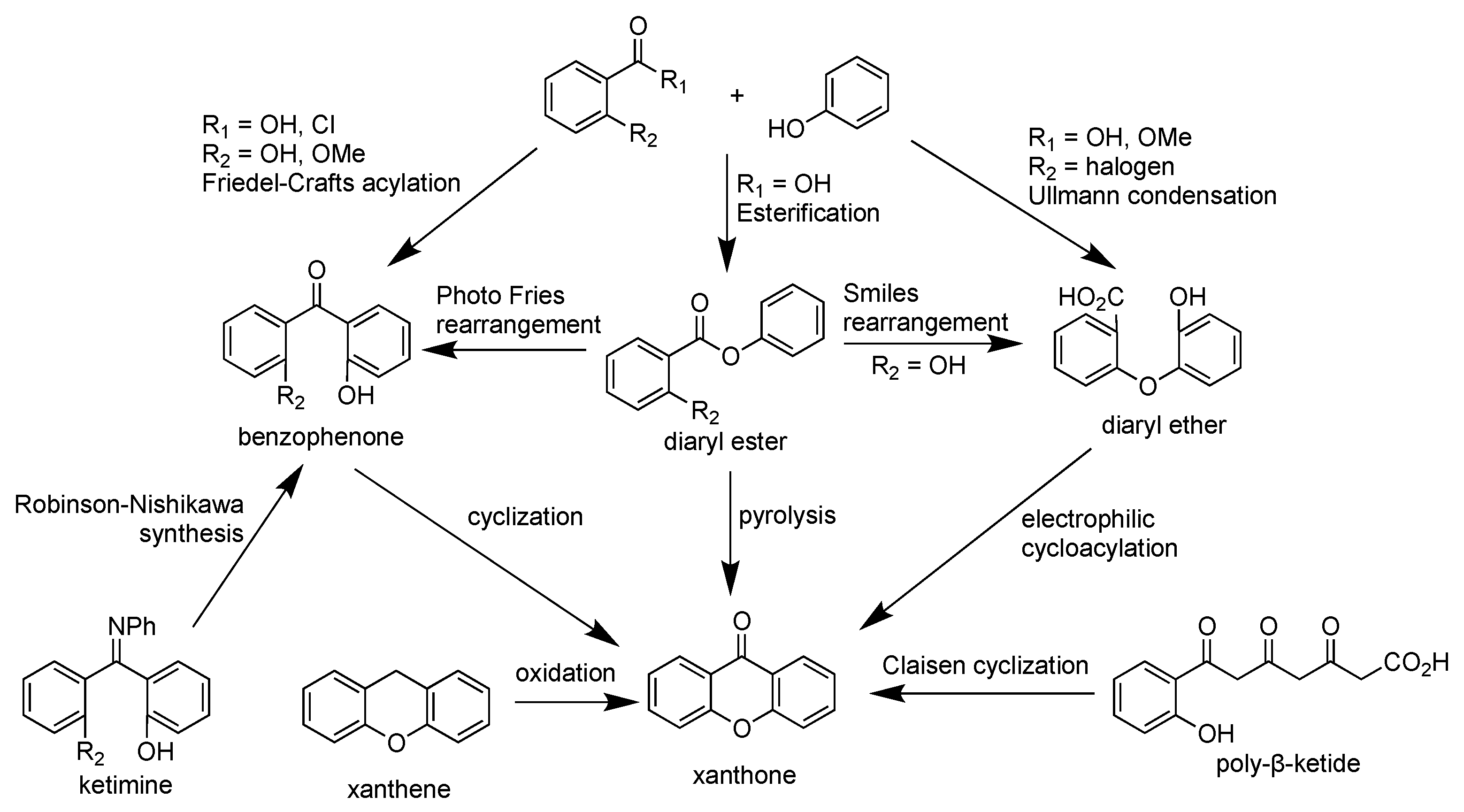
2.1. Xanthone Derivatives
2.2. Characteristics on Chemical Identification of Xanthone Derivatives
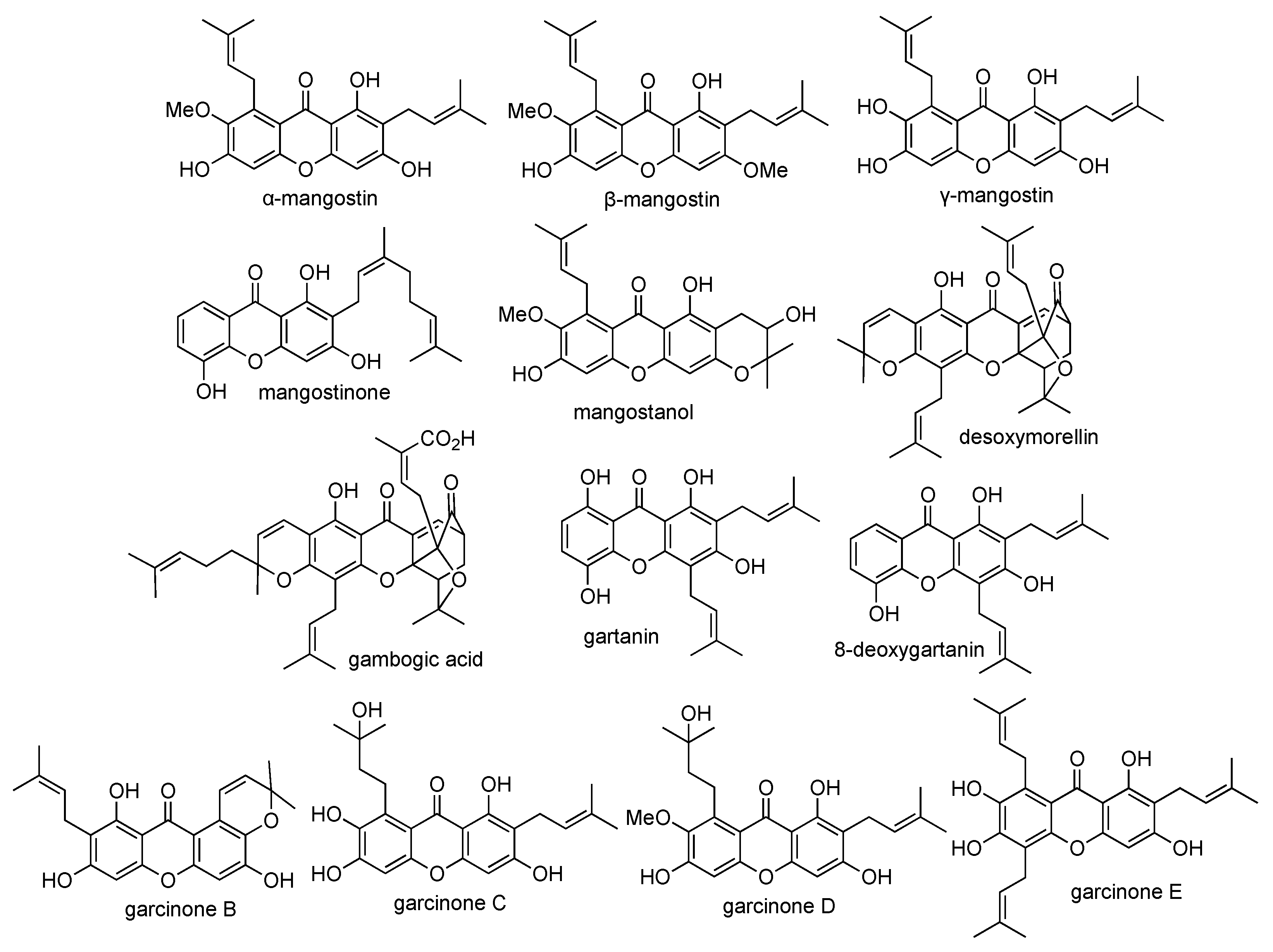
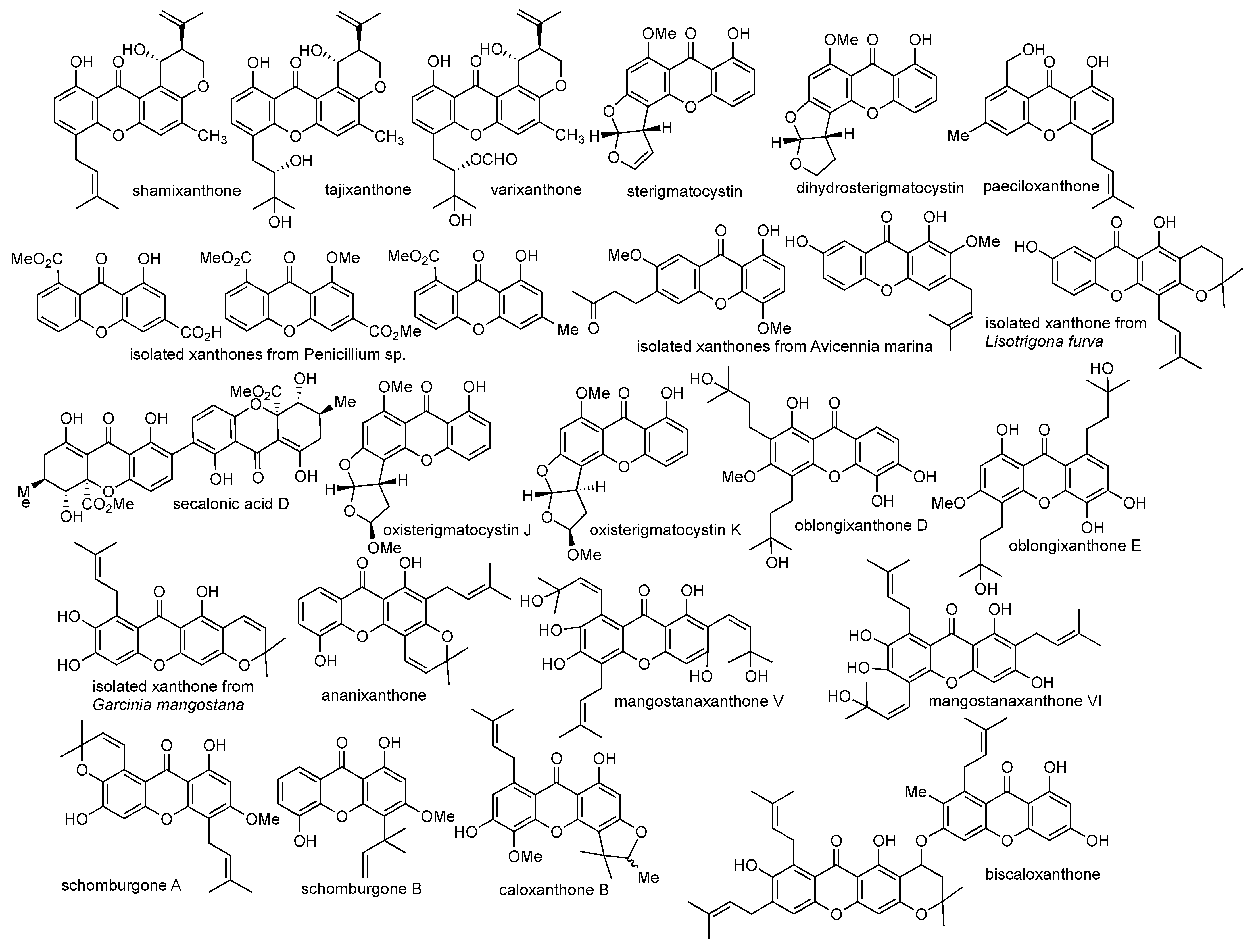
2.3. Isolation of Xanthone Derivatives
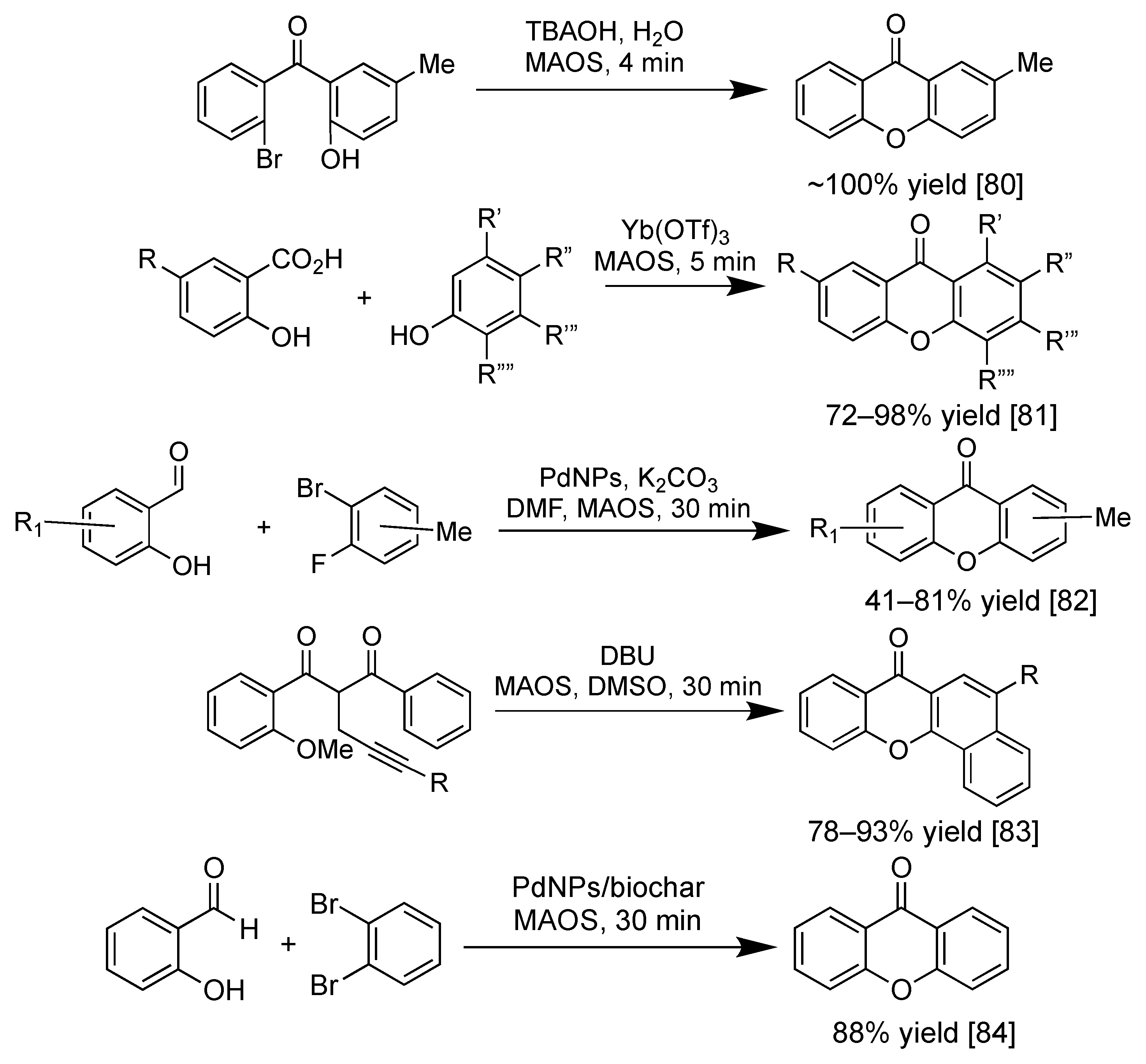
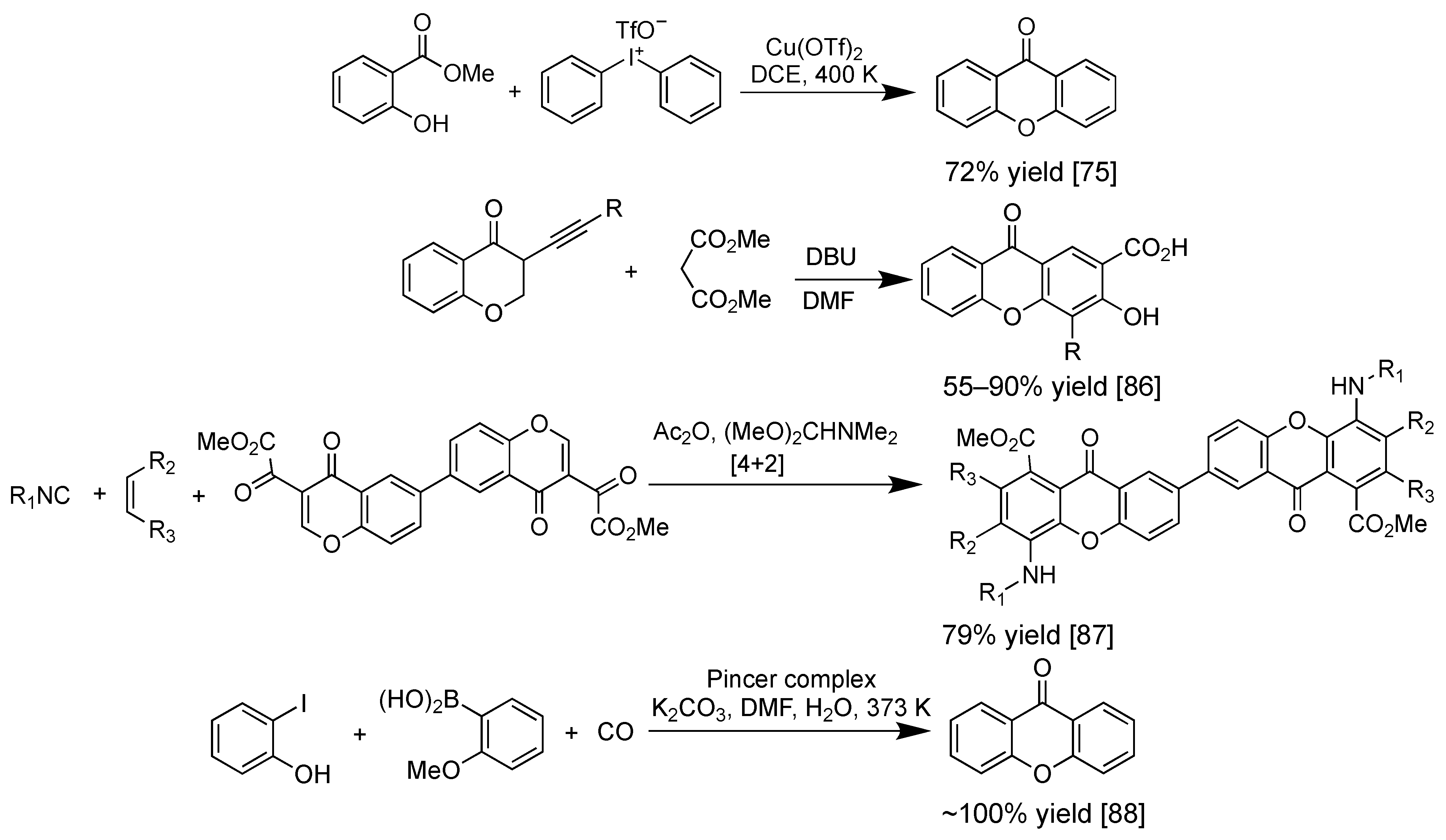
2.4. Synthesis of Xanthone Derivatives
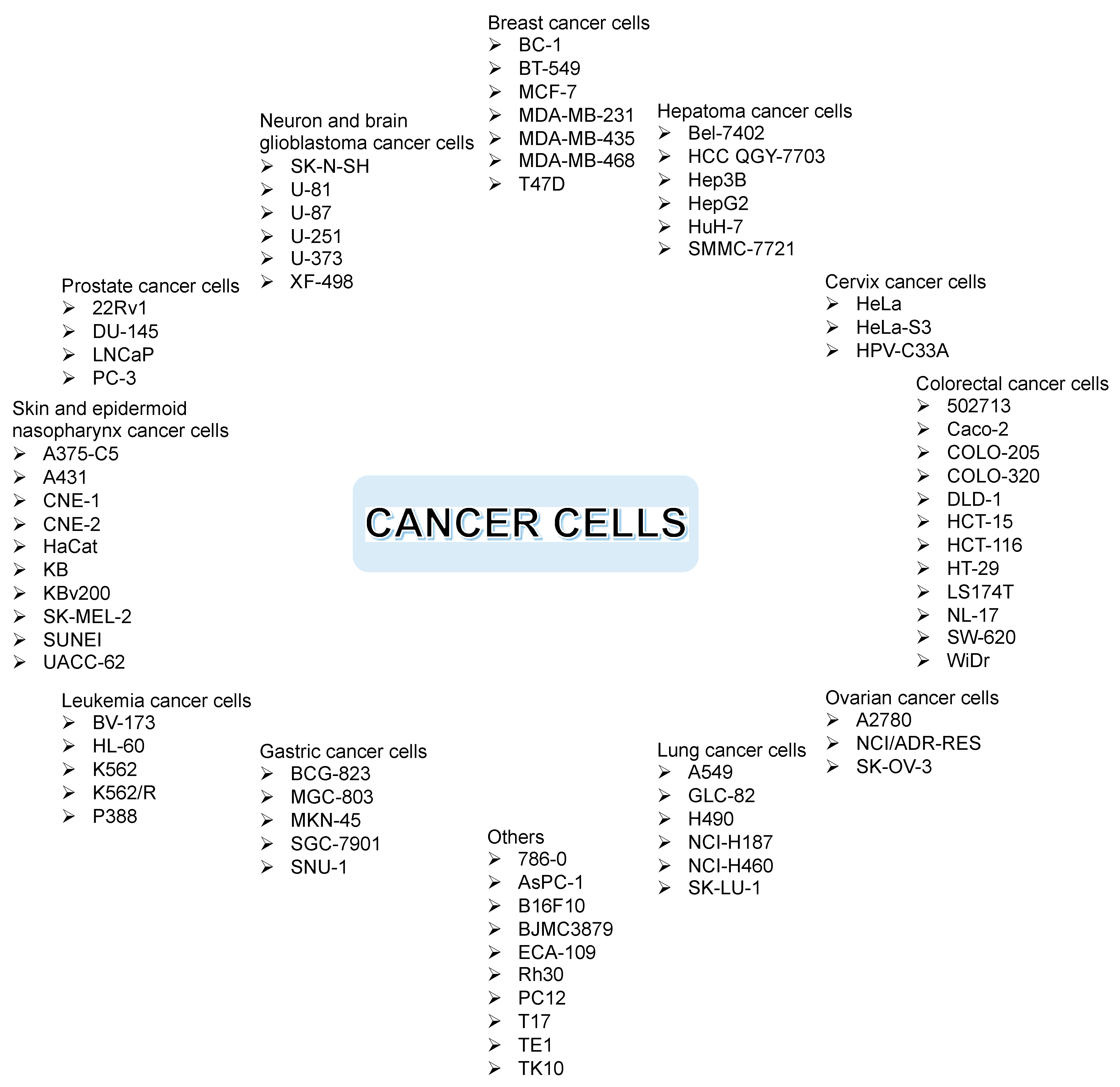
2.5. In Vitro Anticancer Assay of Xanthone Derivatives
2.6. In Vivo and Clinical Anticancer Assays of Xanthone Derivatives
3. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Carugo, A.; Draetta, G.F. Academic discovery of anticancer drugs: Historic and future perspectives. Annu. Rev. Cancer Biol. 2019, 3, 385–408. [Google Scholar] [CrossRef]
- Olgen, S. Overview on anticancer drug design and development. Curr. Med. Chem. 2018, 25, 1704–1719. [Google Scholar] [CrossRef]
- Buyel, J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotech. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millenium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Pereira-Terra, P.; Moreira, J.; Freitas-Silva, J.; Lemos, A.; Gales, L.; Pinto, E.; De Sousa, M.E.; Da Costa, P.M.; Pinto, M.M.M. Synthesis of a small library of nature-inspired xanthones and study of their antimicrobial activity. Molecules 2020, 25, 2405. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; de Blanco, E.J.C.; Fuchs, J.R.; Soejarto, D.D.; Burdette, J.E.; Swanson, S.M.; Kinghorn, A.D. Potential anticancer agents characterized from selected tropical plants. J. Nat. Prod. 2019, 82, 657–679. [Google Scholar] [CrossRef]
- Alves, A.; Correia-da-Silva, M.; Nunes, C.; Campos, J.; Sousa, E.; Silva, P.M.A.; Bousbaa, H.; Rodrigues, F.; Ferreira, D.; Costa, P.C.; et al. Discovery of a new xanthone against glioma: Synthesis and development of (pro)liposome formulations. Molecules 2019, 24, 409. [Google Scholar] [CrossRef] [PubMed]
- Chukaew, A.; Saithong, S.; Chusri, S.; Limsuwan, S.; Watanapokasin, R.; Voravuthikunchai, S.P.; Chakthong, S. Cytotoxic xanthones from the roots of Mesua ferrea L. Phytochemistry 2019, 157, 64–70. [Google Scholar] [CrossRef]
- Lemos, A.; Gomes, A.S.; Loureiro, J.B.; Brandao, P.; Palmeira, A.; Pinto, M.M.M.; Saraiva, L.; Sousa, M.E. Synthesis, biological evaluation, and in silico studies of novel aminated xanthones as potential p53-activating agents. Molecules 2019, 24, 1975. [Google Scholar] [CrossRef]
- Raksat, A.; Maneerat, W.; Andersen, R.J.; Pyne, S.G.; Laphookhieo, S. A tocotrienol quinone dimer and xanthones from the leaf extract of Garcinia nigrolineata. Fitoterapia 2019, 136, 104175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.J.; Fu, W.-W.; Wu, R.; Yang, J.-L.; Yao, C.-Y.; Yan, B.-X.; Tan, H.-S.; Zheng, C.-W.; Song, Z.-J.; Xu, H.-X. Bioactive scalemic caged xanthones from the leaves of Garcinia bracteata. Bioorg. Chem. 2019, 82, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, D.; Ding, Z.J.; Lao, Y.Z.; Tan, H.S.; Xu, H.X. UPLC-PDA-QTOFMS-guided isolation of prenylated xanthones and benzoylphloroglucinols from the leaves of Garcinia oblongifolia and their migration-inhibitory activity. Sci. Rep. 2016, 6, 35789. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, H.; Wang, H.; Luo, Q.; Zuo, J.; Liu, Z.; Qiu, S.; Sun, X.; Liu, X. Synthesis of xanthone derivatives and anti-hepatocellular carcinoma potency evaluation: Induced apoptosis. RSC Adv. 2019, 9, 40781–40791. [Google Scholar] [CrossRef]
- Na, Y. Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 2009, 61, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C. Naturally occurring xanthones. Chem. Rev. 1961, 61, 591–605. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; da Silva, M.C.; Cravo, S. From natural products to new synthetic small molecules: A journey through the world of xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef] [PubMed]
- Shagufta, S.; Ahmad, I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.; Fernandes, C.; Pinto, M.; Tiritan, M.E. Chiral derivatives of xanthones with antimicrobial activity. Molecules 2019, 24, 314. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Freitas, M.; Fernandes, E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitor. Eur. J. Med. Chem. 2018, 157, 1460–1479. [Google Scholar] [CrossRef]
- Salman, Z.; Yu-Qing, J.; Bin, L.; Cai-Yun, P.; Iqbal, C.M.; Atta-ur, R.; Wei, W. Antioxidant nature adds further therapeutic value: An updated review on natural xanthones and their glycosides. Digit. Chin. Med. 2019, 2, 166–192. [Google Scholar] [CrossRef]
- Cruz, M.I.; Cidade, H.; Pinto, M. Dual/multitargeted xanthone derivatives for Alzheimer’s disease: Where do we stand? Future Med. Chem. 2017, 9, 1611–1630. [Google Scholar] [CrossRef]
- Gunter, N.V.; Teh, S.S.; Lim, Y.M.; Mah, S.H. Natural xanthones and skin inflammatory disease: Multitargeting mechanisms of action and potential application. Front. Pharmacol. 2020, 11, 594202. [Google Scholar] [CrossRef]
- Feng, Z.; Lu, X.; Gan, L.; Zhang, Q.; Lin, L. Xanthones, a promising anti-inflammatory scaffold: Structure, activity, and drug likeness analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef]
- Rosa, G.P.; Palmeira, A.; Resende, D.I.S.P.; Almeida, I.F.; Kane-Pagès, A.; Barreto, M.C.; Sousa, E.; Pinto, M.M.M. Xanthones for melanogenesis inhibition: Molecular docking and QSAR studies to understand their anti-tyrosinase activity. Bioorg. Med. Chem. 2021, 29, 115873. [Google Scholar] [CrossRef]
- Yu, F.-C.; Lin, X.-R.; Liu, Z.-C.; Zhang, J.-H.; Liu, F.-F.; Wu, W.; Ma, Y.-L.; Qu, W.-W.; Yan, S.-J.; Lin, J. Beyond the antagonism: Self-labeled xanthone inhibitors as modeled “two-in-one” drugs in cancer therapy. ACS Omega 2017, 2, 873–889. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone glucosides: Isolation, bioactivity and synthesis. Molecules 2021, 26, 5575. [Google Scholar] [CrossRef]
- Zhang, B.J.; Fu, W.-W.; Wu, R.; Yang, J.-L.; Yao, C.-Y.; Yan, B.-X.; Tan, H.-S.; Zheng, C.-W.; Song, Z.-J.; Xu, H.-X. Cytotoxic prenylated xanthones from the leaves of Garcinia bracteata. Planta Med. 2019, 85, 444–452. [Google Scholar] [CrossRef]
- Markowicz, J.; Uram, L.; Sobich, J.; Mangiardi, L.; Maj, P.; Rode, W. Antitumor and anti-nematode activities of α-mangostin. Eur. J. Pharmacol. 2019, 863, 172678. [Google Scholar] [CrossRef] [PubMed]
- Castanheiro, R.A.P.; Silva, A.M.S.; Campos, N.A.N.; Nascimento, M.S.J.; Pinto, M.M.M. Antitumor activity of some prenylated xanthones. Pharmaceuticals 2009, 2, 33–43. [Google Scholar] [CrossRef]
- Bedi, P.; Gupta, R.; Pramanik, T. Synthesis and biological properties of pharmaceutically important xanthones and benzoxanthone analogs: A brief review. Asian J. Pharm. Clin. Res. 2018, 11, 12–20. [Google Scholar] [CrossRef]
- Klein-Junior, L.C.; Campos, A.; Niero, R.; Correa, R.; Heyden, Y.V.; Filho, V.C. Xanthones and cancer: From natural sources to mechanisms of action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Castanheiro, R.A.P.; Kijjoa, A. Xanthones from marine-derived microorganism: Isolation, structure elucidation, and biological activities. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 12–17. [Google Scholar] [CrossRef]
- Arends, P.; Helboe, P. Xanthone studies. IV. Hydroxyl proton chemical shifts in the structural investigation of xanthones. Acta Chem. Scand. 1972, 26, 4180–4182. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. the absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Fernandes, C.; Carraro, M.L.; Ribeiro, J.; Araujo, J.; Tiritan, M.E.; Pinto, M.M.M. Synthetic chiral derivatives of xanthones: Biological activities and enantioselectivity studies. Molecules 2019, 24, 791. [Google Scholar] [CrossRef]
- Sukandar, E.R.; Kaennakam, S.; Rassamee, K.; Ersam, T.; Siripong, P.; Tip-pyang, S. Tetrandraxanthones A−I, prenylated and geranylated xanthones from the stem bark of Garcinia tetrandra. J. Nat. Prod. 2019, 82, 1312–1318. [Google Scholar] [CrossRef]
- Sukandar, E.R.; Kaennakam, S.; Rassamee, K.; Siripong, P.; Fatmawati, S.; Ersam, T.; Tip-pyang, S. Xanthones and biphenyls from the stems of Garcinia cylindrocarpa and their cytotoxicity. Fitoterapia 2018, 130, 112–117. [Google Scholar] [CrossRef]
- Yang, H.X.; Li, W.; Li, Q.; Ai, H.-L.; Li, Z.-H.; Huang, R.; Feng, T.; Liu, J.-K. Piperidine alkaloids and xanthone from the roots of Caulophyllum robustum Maxim. Fitoterapia 2019, 132, 22–25. [Google Scholar] [CrossRef]
- Meechai, I.I.; Phupong, W.; Chunglok, W.; Meepowpan, P. Dihydroosajaxanthone: A new natural xanthone from the branches of Garcinia schomburgkiana Pierre. Iran J. Pharm. Res. 2018, 17, 1347–1352. [Google Scholar]
- He, K.; Fan, L.-L.; Wu, T.-T.; Du, J. A new xanthone glycoside from Pyrrosia sheareri. Nat. Prod. Res. 2019, 33, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, M.; Bibi, Y.; Qayyum, A.; Iriti, M. Isolation and structural confirmation of xanthone isomers from Dryopteris ramose (Hope) C. Chr. and their in vitro antioxidant mechanism. Arab. J. Sci. Eng. 2021, 46, 5327–5337. [Google Scholar] [CrossRef]
- Tanjung, M.; Tjahjandarie, T.S.; Saputri, R.D.; Kurnia, B.D.; Rachman, M.F.; Syah, Y.M. Calotetrapterins A-C, three new pyranoxanthones and their cytotoxicity from the stem bark of Calophyllum tetrapterum Miq. Nat. Prod. Res. 2021, 35, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kashiwada, Y. Characteristic metabolites of Hypericum plants: Their chemical structures and biological activities. J. Nat. Med. 2021, 75, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Umoh, Y.F.; Thomas, P.S.; Essien, E.E.; Okokon, J.E.; Leo, M.D.; Ajibesin, K.K.; Flamini, G.; Eseyin, O.A. Isolation and characterization of bioactive xanthones from Hippocratea africana (Willd.)Loes.ex Engl. (Celastraceae). J. Ethnopharmacol. 2021, 280, 114031. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kim, S.B.; Ahn, J.H.; Turk, A.; Kown, E.-B.; Kim, M.-O.; Hwang, B.Y.; Lee, M.K. Xanthones from the stems of Cudrania tricuspidata and their inhibitory effects on pancreatic lipase and fat accumulation. Bioorg. Chem. 2019, 92, 103234. [Google Scholar] [CrossRef]
- Tang, Y.X.; Fu, W.-W.; Xi, Z.-C.; Yang, J.-L.; Zheng, C.-W.; Lu, Y.; Shen, Z.-W.; Xu, H.-X. Xanthone derivatives from the leaves of Garcinia oligantha. Eur. J. Med. Chem. 2019, 181, 111536. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Fahmi, M.R.G.; Yuliati, L. Isolation and optical properties of natural pigments from purple mangosteen peels. IOP Conf. Ser. 2020, 833, 012018. [Google Scholar] [CrossRef]
- Birch, A.J.; Baldas, J.; Hlubucek, J.R.; Simpson, T.J.; Westerman, P.W. Biosynthesis of the fungal xanthone ravenelin. J. Chem. Soc. Perkin Trans. I 1976, 8, 898–904. [Google Scholar] [CrossRef]
- Fujita, M.; Inoue, T. New hypocholesterolemic abietamide derivatives. structure-activity relationship. Chem. Pharm. Bull. 1980, 28, 453–458. [Google Scholar] [CrossRef][Green Version]
- Anantachoke, N.; Tuchinda, P.; Kuhakarn, C.; Pohmakotr, M.; Reutrakul, V. Prenylated caged xanthones: Chemistry and biology. Pharm. Biol. 2012, 50, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Kumla, D.; Dethoup, T.; Gales, L.; Pereira, J.A.; Freitas-Silva, J.; Costa, P.M.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A. Erubescensoic Acid, a new polyketide and a xanthonopyrone SPF-3059-26 from the culture of the marine sponge-associated fungus penicillium erubescens KUFA 0220 and Antibacterial activity evaluation of some of its constituents. Molecules 2019, 24, 208. [Google Scholar] [CrossRef]
- Loureiro, D.R.P.; Soares, J.X.; Costa, J.C.; Magalhães, Á.F.; Azevedo, C.M.G.; Pinto, M.M.M.; Afonso, C.M.M. Structures, activities and drug-likeness of anti-infective xanthone derivatives isolated from the marine environment: A review. Molecules 2019, 24, 243. [Google Scholar] [CrossRef]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, J.; Christophersen, C.; Barrero, A.F.; Oltra, J.E.; Justicia, J.; Rosales, A. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. J. Nat. Prod. 2002, 65, 364–367. [Google Scholar] [CrossRef]
- Shao, C.; Wang, C.; Wei, M.; Gu, Y.; She, Z.; Lin, Y. Structure elucidation of two new xanthone derivatives from the marine fungus Penicillium sp. (ZZF 32#) from South China Sea. Magn. Reson. Chem. 2008, 46, 1066–1069. [Google Scholar] [CrossRef]
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.-K.; Jung, J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, R.; Guo, Z.; She, Z.; Lin, Y. A new xanthone derivative from mangrove endophytic fungus No. ZSU-H16. Chem. Nat. Compd. 2010, 46, 348–351. [Google Scholar] [CrossRef]
- Xu, J. Biomolecules produced by mangrove-associated microbes. Curr. Med. Chem. 2011, 18, 5224–5266. [Google Scholar] [CrossRef]
- Tang, Y.X.; Fu, W.W.; Wu, R.; Tan, H.S.; Shen, Z.W.; Xu, H.X. Bioassay-guided isolation of prenylated xanthone derivatives from the leaves of Garcinia oligantha. J. Nat. Prod. 2016, 79, 1752–1761. [Google Scholar] [CrossRef]
- Yang, R.; Li, P.; Li, N.; Zhang, Q.; Bai, X.; Wang, L.; Xiao, Y.; Sun, L.; Yang, Q.; Yan, J. Xanthones from the pericarp of Garcinia mangostana. Molecules 2017, 22, 683. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.K.; Zamakshshari, N.H.; Ee, G.C.L.; Mah, S.H.; Nor, S.M.M. Isolation and structural modifications of ananixanthone from Calophyllum teysmannii and their cytotoxic activities. Nat. Prod. Res. 2018, 32, 2147–2151. [Google Scholar] [CrossRef]
- Kaennakam, S.; Mudsing, K.; Rassamee, K.; Siripong, P.; Tip-pyang, S. Two new xanthones and cytotoxicity from the bark of Garcinia schomburgkiana. J. Nat. Med. 2019, 73, 257–261. [Google Scholar] [CrossRef]
- Zamakshshari, N.H.; Ee, G.C.L.; Ismail, I.S.; Ibrahim, Z.; Mah, S.H. Cytotoxic xanthones isolated from Calophyllum depressinervosum and Calophyllum buxifolium with antioxidant and cytotoxic activities. Food Chem. Toxicol. 2019, 133, 110800. [Google Scholar] [CrossRef] [PubMed]
- Oanh, V.T.K.; Thoa, H.T.; Hang, N.T.M.; Phuong, D.T.L.; Lien, N.T.P.; Popova, M.; Trusheva, B.; Bankova, V.; Le, T.N. New dihydrochromene and xanthone derivatives from Lisotrigona furva propolis. Fitoterapia 2021, 149, 104821. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ma, N.; Liu, C.-Y.; Feng, Y.-X.; Zhang, F.-X.; Li, C.; Pei, Y.-H. Xanthones and anthraquinones from the soil fungus Penicillium sp. DWS10-P-6. RSC Adv. 2021, 11, 3162–3167. [Google Scholar] [CrossRef]
- Kostanecki, S.V.; Nessler, B. Synthesen von oxyxanthonen. Ber. Dtsch. Chem. Ges. 1891, 24, 1894–1897. [Google Scholar] [CrossRef]
- Grover, P.K.; Shah, G.D.; Shah, R.C. Xanthones. Part IV. A new synthesis of hydroxyxanthones and hydroxybenzophenones. J. Chem. Soc. 1955, 3982–3985. [Google Scholar] [CrossRef]
- Sousa, M.E.; Pinto, M.M.M. Synthesis of xanthones: An overview. Curr. Med. Chem. 2005, 12, 2447–2479. [Google Scholar] [CrossRef]
- Liu, G.; Wu, C.; Chen, B.; He, R.; Chen, C. Concise synthesis of xanthones by the tandem etherification: Acylation of diaryliodonium salts with salicylates. Chin. Chem. Lett. 2018, 29, 985–988. [Google Scholar] [CrossRef]
- Atwell, G.J.; Yang, S.; Denny, W.A. An improved synthesis of 5,6-dimethylxanthenone-4-acetic acid (DMXAA). Eur. J. Med. Chem. 2002, 37, 825–828. [Google Scholar] [CrossRef]
- Otrubova, K.; Fitzgerald, A.E.; Mani, N.S. A novel entry to xanthones by an intramolecular Diels-Alder reaction involving 2-(1,2-dichlorovinyloxy)aryldienones. Tetrahedron 2018, 74, 5715–5724. [Google Scholar] [CrossRef]
- Nagarajan, S.; Arjun, P.; Raaman, N.; Das, T.M. Regioselective facile one-pot Friedländer synthesis of sugar-based heterocyclic biomolecules. Carbohydr. Res. 2010, 345, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Krisbiantoro, P.A.; Imawan, A.C. Green chemistry influences in organic synthesis: A Review. J. Multidiscipl. Appl. Nat. Sci. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, L.; Wu, Y.J.; Du, J.Y.; Mao, Y.L.; Wang, X.; Luan, S.J.; Lei, Y.H.; Li, X.; Sun, H.P.; et al. Microwave-assisted transition-metal-free intramolecular Ullmann-type O-arylation in water for the synthesis of xanthones and azaxanthones. Tetrahedron Lett. 2014, 55, 4883–4887. [Google Scholar] [CrossRef]
- Genovese, S.; Fiorito, S.; Specchiulli, M.C.; Taddeo, V.A.; Epifano, F. Microwave-assisted synthesis of xanthones promoted by ytterbium triflate. Tetrahedron Lett. 2015, 56, 847–850. [Google Scholar] [CrossRef]
- Shen, C.R.; Wu, X.F. Selective preparation of xanthones from 2-bromofluorobenzenes and salicylaldehydes via palladium-catalyzed acylation-SNAr approach. Synlett 2016, 27, 1269–1273. [Google Scholar] [CrossRef]
- Liang, Y.E.; Barve, B.D.; Kuo, Y.-H.; Fang, H.-W.; Kuo, T.-S.; Li, W.-T. Metal-free, DBU-mediated, microwave assisted synthesis of benzo[c]xanthones by tandem reactions of alkynyl-1,3-diketones. Adv. Synth. Catal. 2021, 363, 505–511. [Google Scholar] [CrossRef]
- Steingruber, H.S.; Mendioroz, P.; Diez, A.S.; Gerbino, D.C. A green nanopalladium-supported catalyst for the microwave-assisted direct synthesis of xanthones. Synthesis 2020, 52, 619–628. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, B.; Chen, H.; Huang, H.; Hu, Y. Promiscuous enzyme-catalyzed cascade reaction: Synthesis of xanthone derivatives. Bioorg. Chem. 2018, 80, 555–559. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Xie, F.C.; Cheng, G. A base-promoted tandem reaction of 3-(1-alkynyl)chromones with 1,3-dicarbonyl compounds: An efficient approach to functional xanthones. Ang. Chem. Int. 2009, 48, 6520–6523. [Google Scholar] [CrossRef] [PubMed]
- Bornadiego, A.; Diaz, J.; Marcos, C.F. Expedious multicomponent synthesis of xanthone dimers. Proceedings 2019, 9, 13. [Google Scholar] [CrossRef]
- Loureiro, D.R.P.; Soares, J.X.; Maia, A.; Silva, A.M.N.; Rangel, M.; Azevedo, C.M.G.; Hansen, S.V.; Ulven, T.; Pinto, M.M.M.; Reis, S.; et al. One-pot synthesis of xanthone by carbonylative Suzuki coupling reaction. ChemistrySelect 2021, 6, 4511–4514. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Duraes, F.; Maia, M.; Sousa, E.; Pinto, M.M.M. Recent advances in the synthesis of xanthones and azaxanthones. Org. Chem. Front. 2020, 7, 3027–3066. [Google Scholar] [CrossRef]
- Loureiro, D.R.P.; Magalhães, Á.F.; Soares, J.X.; Pinto, J.; Azevedo, C.M.G.; Vieira, S.; Henriques, A.; Ferreira, H.; Neves, N.; Bousbaa, H.; et al. Yicathins B and C and analogues: Total synthesis, lipophilicity and biological activities. ChemMedChem 2020, 15, 749–755. [Google Scholar] [CrossRef]
- Xu, D.; Nie, Y.; Liang, X.; Ji, L.; Hu, S.; You, Q.; Wang, F.; Ye, H.; Wang, J. A concise and efficient total synthesis of α-mangostin and β-mangostin from Garcinia mangostana. Nat. Prod. Commun. 2013, 8, 1101–1103. [Google Scholar] [CrossRef]
- Wei, X.; Liang, D.; Wang, Q.; Meng, X.; Li, Z. Total synthesis of mangiferin, homomangiferin, and neomangiferin. Org. Biomol. Chem. 2016, 14, 8821–8831. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kitamura, T.; Arulmozhiraja, S.; Manabe, K.; Tokiwa, H.; Suzuki, Y. Total synthesis of Termicalcicolanone A via organocatalysis and regioselective Claisen rearrangement. Org. Lett. 2019, 21, 2777–2781. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and cancer: Insight into tumor progression and immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.R.; Miao, F.-P.; Zhang, J.; Wang, G.; Yin, X.-L.; Ji, N.-Y. Three new xanthone derivatives from an algicolous isolate of Aspergillus wentii. Magn. Reson. Chem. 2013, 51, 65–68. [Google Scholar] [CrossRef]
- Park, A.; Lee, Y.; Kim, M.S.; Kang, Y.J.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. Prostaglandin E2 secreted by thyroid cancer cells contributes to immune escape through the suppression of natural killer (NK) cell cytotoxicity and NK cell differentiation. Front. Immunol. 2018, 9, 1859. [Google Scholar] [CrossRef]
- Su, X.; Li, Q. Prostaglandin EP2 receptor: Novel therapeutic target for human cancers. Int. J. Mol. Med. 2018, 42, 1203–1214. [Google Scholar] [CrossRef]
- Forterre, P.; Gribaldo, S.; Gadelle, D.; Serre, M.C. Origin and evolution of DNA topoisomerases. Biochimie 2007, 89, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Gil-Martins, E.; Silva, B.; Rocha-Pereira, C.; Sousa, M.E.; Remião, F.; Silva, R. Xanthones as P-glycoprotein modulators and their impact on drug bioavailability. Expert Opin. Drug Metab. Toxicol. 2021, 17, 441–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chen, J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef]
- Koulgi, S.; Jani, V.; Uppuladinne, V.N.; Sonavane, U.; Joshi, R. Natural plant products as potential inhibitors of RNA dependent RNA polymerase of severe acute respiratory syndrome coronavirus-2. PLoS ONE 2021, 16, e0251801. [Google Scholar] [CrossRef]
- Woo, S.; Jung, J.; Lee, C.; Kwon, Y.; Na, Y. Synthesis of new xanthone analogues and their biological activity test—cytotoxicity, topoisomerase II inhibition, and DNA cross-linking study. Bioorg. Med. Chem. Lett. 2007, 17, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Kang, D.; Nam, J.M.; Lee, C.S.; Ha, E.-M.; Lee, E.-S.; Kwon, Y.; Na, Y. Synthesis and pharmacological evaluation of new methyloxiranylmethoxyxanthone analogues. Eur. J. Med. Chem. 2010, 45, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.Y.; Lee, E.-Y.; Jung, M.-J.; Lee, O.-H.; Lee, E.-S.; Choo, H.-Y.P.; Na, Y.; Kwon, Y. Synthesis, biological evaluation, and molecular docking study of 3-(3’-heteroatom substituted-2’-hydroxy-1’-propyloxy)xanthone analogues as novel topoisomerase IIa catalytic inhibitor. Eur. J. Med. Chem. 2011, 46, 1964–1971. [Google Scholar] [CrossRef]
- Varache-Lembège, M.; Moreau, S.; Larrouture, S.; Montaudon, D.; Robert, J.; Nuhrich, A. Synthesis and antiproliferative activity of aryl- and heteroaryl-hydrazones derived from xanthone carbaldehydes. Eur. J. Med. Chem. 2008, 43, 1336–1343. [Google Scholar] [CrossRef]
- Luo, L.; Qin, J.K.; Dai, Z.-K.; Gao, S.-H. Synthesis and biological evaluation of novel benzo[b]xanthone derivatives as potential antitumor agents. J. Serb Chem. Soc. 2013, 78, 1301–1308. [Google Scholar] [CrossRef]
- Sypniewski, D.; Szkaradek, N.; Loch, T.; Waszkielewicz, A.M.; Gunia-Krzyzak, A.; Matczynska, D.; Soltysik, D.; Marona, H.; Bednarek, I. Contribution of reactive oxygen species to the anticancer activity of aminoalkanol derivatives of xanthone. Investig. New Drugs 2018, 36, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Huang, J.; Qin, J.-K.; Dai, Z.-K.; Lan, W.-L.; Su, G.-F.; Tang, H.; Yang, F. Design, synthesis and biological evaluation of novel 1-hydroxyl-3-aminoalkoxy xanthone derivatives as potent anticancer agents. Eur. J. Med. Chem. 2014, 85, 487–497. [Google Scholar] [CrossRef]
- Shen, R.; Wang, W.; Yang, G. DNA binding property and antitumor evaluation of xanthone with dimethylamine side chain. J. Fluoresc. 2014, 24, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Masawang, K.; Tiritan, M.E.; Sousa, E.; de Lima, V.; Afonso, C.; Bousbaa, H.; Sudprasert, W.; Petro, M.; Pinto, M.M. New chiral derivatives of xanthones: Synthesis and investigation of enantioselectivity as inhibitors of growth of human tumor cell lines. Bioorg. Med. Chem. 2014, 22, 1049–1062. [Google Scholar] [CrossRef]
- Carraro, M.L.; Marques, S.; Silva, A.S.; Freitas, B.; Silva, P.M.A.; Pedrosa, J.; De Marco, P.; Bousbaa, H.; Fernandes, C.; Tiritan, M.E.; et al. Synthesis of new chiral derivatives of xanthones with enantioselective effect on tumor cell growth and DNA crosslinking. ChemistrySelect 2020, 5, 10285–10291. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Zhang, Z.; Zhang, S.B.; Yang, S.; Zhang, A.; Yin, L.; Swarts, S.; Vidyasagar, S.; Zhang, L.; et al. Synthesis and anticancer potential of novel xanthone derivatives with 3,6-substituted chains. Bioorg. Med. Chem. 2016, 24, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Yuan, X.; Kang, J.; Zhu, Z.-J.; Yue, R.-C.; Hu, Y.; Chen, B.-Y.; Zhang, W.-D.; Liu, R.-H.; Sun, Q.-Y. Synthesis and biological evaluation of phenyl substituted polyoxygenated xanthone derivatives as anti-hepatoma agents. Eur. J. Med. Chem. 2013, 69, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Minniti, E.; Byl, J.A.W.; Riccardi, L.; Sissi, C.; Rosini, M.; Vivo, M.D.; Minarini, A.; Osheroff, N. Novel xanthone-polyamine conjugates as catalytic inhibitors of human topoisomerase IIα. Bioorg. Med. Chem. Lett. 2017, 27, 4687–4693. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Wang, H.; Liu, Z.; Zhang, C.; Jiang, Z.; Chen, H. Synthesis of xanthone derivatives and studies on the inhibition against cancer cells growth and synergistic combinations of them. Eur. J. Med. Chem. 2017, 133, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-D.; Zeng, L.-L.; Tong, Y.-G.; Fang, J.-Y.; Ruan, Z.-P.; Zeng, X.-Y.; Fang, Y.-Y.; Xu, G.-F.; Hu, D.-B. Synthesis and antitumor, antityrosinase, and antioxidant activities of xanthone. J. Asian Nat. Prod. Res. 2018, 20, 467–476. [Google Scholar] [CrossRef]
- Pedro, M.; Cerqueira, F.; Sousa, M.E.; Nascimento, M.S.J.; Pinto, M. Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorg. Med. Chem. 2002, 10, 3725–3730. [Google Scholar] [CrossRef]
- Amanatie, A.; Jumina, J.; Mustofa, M.; Hanafi, M.; Armunanto, R. QSAR study of xanthone derivatives as antiplasmodial agents. Indones. J. Chem. 2010, 10, 357–362. [Google Scholar] [CrossRef]
- Amanatie, A.; Jumina, J.; Mustofa, M.; Hanafi, M.; Kadidae, L.O.; Sahidin, I. Synthesis of 2-hydroxyxanthone from xanthone as a basic material for new antimalarial drugs. Asian J. Pharm. Clin. Res. 2017, 10, 242–246. [Google Scholar] [CrossRef]
- Fitriastuti, D.; Jumina, J.; Priatmoko, P. Heme polymerization inhibition activity (HPIA) assay of synthesized xanthone derivative as antimalarial compound. AIP Conf. Proc. 2017, 1823, 020120. [Google Scholar] [CrossRef]
- Fitriastuti, D.; Jumina, J.; Priatmoko, P. Synthesis and characterization of 2,3,4-trihydroxy-5-methyl xanthone as antimalarial compound. EKSAKTA J. Sci. Data Anal. 2016, 16, 94–102. [Google Scholar] [CrossRef]
- Miladiyah, I.; Tahir, I.; Jumina, J.; Mubarika, S.; Mustofa, M. Quantitative structure-activity relationship analysis of xanthone derivatives as cytotoxic agents in liver cancer cell line HepG2. Molekul 2016, 11, 143–157. [Google Scholar] [CrossRef][Green Version]
- Yuanita, E.; Pranowo, H.D.; Jumina, J.; Mustofa, M. Design of hydroxy xanthones derivatives as anticancer using quantitative structure-activity relationship. Asian J. Pharm. Clin. Res. 2016, 9, 180–185. [Google Scholar]
- Yuanita, E.; Pranowo, H.D.; Mustofa, M.; Swasono, R.T.; Syahri, J.; Jumina, J. Synthesis, characterization and molecular docking of chloro-substituted hydroxyxanthone derivatives. Chem. J. Mold. 2019, 14, 68–76. [Google Scholar] [CrossRef]
- Yuanita, E.; Pranowo, H.D.; Siswanta, D.; Swasono, R.T.; Mustofa, M.; Zulkarnain, A.K.; Syahri, J.; Jumina, J. One-pot synthesis, antioxidant activity and toxicity evaluation of some hydroxyxanthones. Chem. Chem. Technol. 2018, 12, 290–295. [Google Scholar] [CrossRef]
- Zakiah, M.; Syarif, R.A.; Mustofa, M.; Jumina, J.; Fatmasari, N.; Sholikhah, E.N. In vitro antiplasmodial, heme polymerization, and cytotoxicity of hydroxyxanthone derivatives. J. Trop. Med. 2021, 2021, 8866681. [Google Scholar] [CrossRef]
- Miladiyah, I.; Jumina, J.; Haryana, S.M.; Mustofa, M. Biological activity, quantitative structure–activity relationship analysis, and molecular docking of xanthone derivatives as anticancer drugs. Drug Des. Dev. Ther. 2018, 12, 149–158. [Google Scholar] [CrossRef]
- Miladiyah, I.; Yuanita, E.; Nuryadi, S.; Jumina, J.; Haryana, S.M.; Mustofa, M. Synergistic effect of 1,3,6-trihydroxy-4,5,7-trichloroxanthone in combination with doxorubicin on b-cell lymphoma cells and its mechanism of action through molecular docking. Curr. Ther. Res. 2020, 92, 100576. [Google Scholar] [CrossRef]
- Yuanita, E.; Ulfa, M.; Sudirman, S.; Sumarlan, I.; Sudarma, I.M.; Dharmayani, N.K.T.; Syahri, J.; Jumina, J. Synthesis, cytotoxic evaluation and molecular docking of bromo-substituted 1,3,6-trihydroxyxanthone as protein tyrosine kinase inhibitor. Malays. J. Chem. 2021, 23, 24–32. [Google Scholar]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Selles, D.J.N.; Daglia, M.; Rastrelli, L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors 2016, 42, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, S.-M.; Si, H.-Z.; Li, Y.-B.; Li, Y.-S.; Fan, J.-H.; Liang, Q.-Q.; He, H.-B.; Ye, H.-M.; Cui, Z.-N. Synthesis and bioactivity of novel xanthone and thioxanthone l-rhamnopyranosides. RSC Adv. 2015, 5, 36092–36103. [Google Scholar] [CrossRef]
- Sordat-Diserens, I.; Hamburger, M.; Rogers, C.; Hostettmannt, K. Dimeric xanthones from Garcinia livingstonei. Phytochemistry 1992, 31, 3589–3593. [Google Scholar] [CrossRef]
- Heald, R.A.; Dexheimer, T.S.; Vankayalapati, H.; Siddiqui-Jain, A.; Szabo, L.Z.; Gleason-Guzman, M.C.; Hurley, L.H. Conformationally restricted analogues of psorospermin: Design, synthesis, and bioactivity of natural-product-related bisfuranoxanthones. J. Med. Chem. 2005, 48, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yang, L.; Liu, Y.; Wang, Y.; Qiao, C.; Song, J.; Xu, L.; Yang, D.; Chen, S.; Xu, H. Gambogic acid and epigambogic acid, C-2 epimers with novel anticancer effects from Garcinia hanburyi. Planta Med. 2006, 72, 281–284. [Google Scholar] [CrossRef]
- Asano, J.; Chiba, K.; Tada, M.; Yoshii, T. Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry 1996, 41, 815–820. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Syers, J.K.; Kiattansakul, R.; Chantrapromma, K. Cytotoxic and antimalarial prenylated xanthones from Cratoxylum cochinchinense. Chem. Pharm. Bull. 2006, 54, 745–747. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 54, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Li, I.V.; Wang, Q.; Wang, X.; Li, G.; Shen, S.; Wei, X. Gambogic acid exhibits anti-metastatic activity on malignant melanoma mainly through inhibition of PI3K/Akt and ERK signaling pathways. Eur. J. Pharmacol. 2019, 864, 172719. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, Q.; Wang, S.; Gao, Y.; Chen, X.; Zhao, Y.; Qian, X. Gambogic acid impairs tumor angiogenesis by targeting YAP/STAT3 signaling axis. Phytother. Res. 2019, 33, 1579–1591. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Q.L.; You, Q.D.; Wu, Z.Q.; Gu, H.Y. Gambogic acid induces apoptosis and regulates expressions of bax and bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol. Pharm. Bull. 2004, 27, 998–1003. [Google Scholar] [CrossRef]
- Tao, S.J.; Guan, S.H.; Wang, W.; Lu, Z.Q.; Chen, G.T.; Sha, N.; Yue, Q.X.; Liu, X.; Guo, D.A. Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. J. Nat. Prod. 2009, 72, 117–124. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Zhao, Q.; Qi, Q.; Lu, N.; Yang, Y.; Nei, F.F.; Rong, J.J.; You, Q.D.; Guo, Q.L. Synergistic effect of 5-fluorouracil with gambogic acid on BGC-823 human gastric carcinoma. Toxicology 2009, 256, 135–140. [Google Scholar] [CrossRef]
- Yen, C.T.; Goto, K.N.; Hwang, T.-L.; Natschke, S.L.M.; Bastow, K.F.; Wu, Y.-C.; Lee, K.-H. Design and synthesis of gambogic acid analogs as potent cytotoxic and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2012, 22, 4018–4022. [Google Scholar] [CrossRef]
- Khaing, E.I.; Saenpunya, T.; Kerdklai, P.; Pangpongma, S.; Vongvijit, S.; Phaechamud, T.; Intaraphairot, T. Combination effects of gambogic acid on imatinib mesylate cytotoxicity in colon cancer cells. Key Eng. Mater. 2020, 859, 27–33. [Google Scholar] [CrossRef]
- Hahnvajanawong, C.; Boonyanugomol, W.; Nasomyon, T.; Loilome, W.; Namwat, N.; Anantachoke, N.; Tassaneeyakul, W.; Sripa, B.; Namwat, W.; Reutrakul, V. Apoptotic activity of caged xanthones from Garcinia hanburyi in cholangiocarcinoma cell lines. World J. Gastroenterol. 2010, 16, 2235–2243. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Jessen, K.A.; Maliartchouk, S.; Wang, J.Y.; English, N.M.; Drewe, J.; Qiu, L.; Archer, S.P.; Ponce, A.E.; Sirisoma, N.; et al. A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc. Natl. Acad. Sci. USA 2005, 102, 12095–12100. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Sung, B.; Ahn, K.S.; Kunnumakkara, A.B.; Chaturvedi, M.M.; Aggarwal, B.B. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood 2007, 110, 3517–3525. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Li, C.P.; Chen, J.H.; Ouyang, J. Apoptosis-inducing effect of gambogic acid on K562 cells and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009, 17, 1443–1447. [Google Scholar] [PubMed]
- Nie, F.; Zhang, X.; Qi, Q.; Yang, L.; Yang, Y.; Liu, W.; Lu, N.; Wu, Z.; You, Q.; Guo, Q. Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology 2019, 260, 60–67. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, M.; Zhou, H.; Chen, D.; Liu, L. Gambogic acid inhibits the growth of ovarian cancer tumors by regulating p65 activity. Oncol. Lett. 2017, 13, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Okada, M.; Sayeed, I.; Xiao, G.; Stein, D.; Jin, P.; Ye, K. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc. Natl. Acad. Sci. USA 2007, 104, 16329–16334. [Google Scholar] [CrossRef]
- Cao, S.; Brodie, P.J.; Miller, J.S.; Randrianaivo, R.; Ratovoson, F.; Birkinshaw, C.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Antiproliferative xanthones of Terminalia calcicole from the Madagascar rain forest. J. Nat. Prod. 2007, 70, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 7, 36977. [Google Scholar] [CrossRef]
- França, F.; Silva, P.M.A.; Soares, J.X.; Henriques, A.C.; Loureiro, D.R.P.; Azevedo, C.M.G.; Afonso, C.M.M.; Bousbaa, H. A pyranoxanthone as a potent antimitotic and sensitizer of cancer cells to low doses of paclitaxel. Molecules 2020, 25, 5845. [Google Scholar] [CrossRef]
- Palmeira, A.; Vasconcelos, M.H.; Paiva, A.; Fernandes, M.X.; Pinto, M.; Sousa, E. Dual inhibitors of P-glycoprotein and tumor cell growth: (re)discovering thioxanthones. Biochem. Pharmacol. 2012, 83, 57–68. [Google Scholar] [CrossRef]
- Johnson, J.J.; Petiwala, S.M.; Syed, D.N.; Rasmussen, J.T.; Adhami, V.M.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2011, 33, 413–419. [Google Scholar] [CrossRef]
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int. J. Oncol. 2016, 48, 2155–2165. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2004, 90, 161–166. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Yi, H.; Ohguchi, K.; Ito, T.; Tanaka, T.; Kobayashi, E.; Iinuma, M.; Nozawa, Y. Preferential target is mitochondria in α-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorg. Med. Chem. 2004, 12, 5799–5806. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg. Med. Chem. 2005, 13, 6064–6069. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Kamalidehghan, B.; Ghaderian, M.; Dehghan, F.; Ali, L.Z.; Karimian, H.; et al. Involvement of Nf-kB and HSP-70 signaling pathways in the apoptosis of MDA-MB-231 cells induced by a prenylated xanthone compound, α-mangostin from Cratoxylum arborescens. Drug Des. Dev. Ther. 2014, 8, 2193–2211. [Google Scholar] [CrossRef][Green Version]
- Quang, T.H.; Ngan, N.T.T.; Yoon, C.-S.; Cho, K.-H.; Kang, D.G.; Lee, H.S.; Kim, Y.-C.; Oh, H. Protein tyrosine phosphatase 1B inhibitors from the root of Cudrania tricuspidata. Molecules 2015, 20, 11173–11183. [Google Scholar] [CrossRef]
- Zhang, K.J.; Gu, Q.L.; Yang, K.; Ming, X.J.; Wang, J.X. Anticarcinogenic effects of α-mangostin: A review. Planta Med. 2017, 83, 188–202. [Google Scholar] [CrossRef]
- Khan, P.; Queen, A.; Smita, T.M.; Khan, N.S.; Hafeez, Z.B.; Hassan, M.I.; Ali, S. Identification of α-mangostin as a potential inhibitor of microtubule affinity regulating kinase 4. J. Nat. Prod. 2019, 82, 2252–2261. [Google Scholar] [CrossRef]
- Pérez-Rojas, J.M.; González-Macías, R.; González-Cortes, J.; Jurado, R.; Pedraza-Chaverri, J.; García-López, P. Synergic effect of α-mangostin on the cytotoxicity of cisplatin in a cervical cancer model. Oxid. Med. Cell. Longev. 2016, 2016, 7981397. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.M.G.; Afonso, C.M.M.; Sousa, D.; Lima, R.T.; Helena Vasconcelos, M.; Pedro, M.; Barbosa, J.; Corrêa, A.G.; Reis, S.; Pinto, M.M.M. Multidimensional optimization of promising antitumor xanthone derivatives. Bioorg. Med. Chem. 2013, 21, 2941–2959. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Jo, M.; Lee, B.; Han, S.-B.; Lee, K.; Jung, J.-K.; Seo, S.-Y.; Kwak, Y.-S. Synthesis of xanthone derivatives based on α-mangostin and their biological evaluation for anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 2062–2065. [Google Scholar] [CrossRef]
- Chang, H.F.; Yang, L.-L. Gamma-mangostin, a micronutrient of mangosteen fruit, induces apoptosis in human colon cancer cells. Molecules 2012, 17, 8010–8021. [Google Scholar] [CrossRef]
- Cheng, P.; Zhu, L.; Guo, W.; Liu, W.; Yao, J.; Dong, G.; Zhang, Y.; Zhuang, C.; Sheng, C.; Miao, Z.; et al. Synthesis of novel benzoxanthone analogues as non-camptothecin topoisomerase I inhibitors. J. Enzym. Inhib. Med. Chem. 2011, 27, 437–442. [Google Scholar] [CrossRef]
- Niu, S.L.; Li, Z.-L.; Ji, F.; Liu, G.-Y.; Zhao, N.; Liu, X.-Q.; Jing, Y.-K.; Hua, H.-M. Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry 2012, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Tho, L.-Y.; Lim, Y.M.; Syed, A.A.S.; Weber, J.F.F. Synthesis of 1,3,6-trioxygenated prenylated xanthone derivatives as potential antitumor agents. Lett. Org. Chem. 2012, 9, 549–555. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Sun, H.; Jiang, Z.; Tao, L.; Gao, Y.; Guo, Q.; You, Q. Synthesis and evaluation of novel aza-caged Garcinia xanthones. Org. Biomol. Chem. 2012, 10, 3288. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Sun, H.; Wang, X.; Zhao, L.; Gao, Y.; Liu, X.; Zhang, S.; Wang, Y.; Yang, Y.; et al. Garcinia xanthones as orally active antitumor agents. J. Med. Chem. 2013, 56, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, L.; Chen, X.-H.; Zhu, X.-F.; Qian, X.-J.; Feng, G.-K.; Lan, W.-J.; Li, H.-J. Cytotoxic prenylated xanthones from the pericarps of Garcinia mangostana. Molecules 2014, 19, 1820–1827. [Google Scholar] [CrossRef]
- Mariano, L.N.B.; Vendramini-Costa, D.B.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Corrêa, R.; Filho, V.C.; Monache, F.D.; Niero, R. In vitro antiproliferative activity of uncommon xanthones from branches of Garcinia achachairu. Pharm. Biol. 2015, 54, 1697–1704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, D.H.; Li, C.-X.; Jia, C.-C.; Sun, Y.-T.; Xue, C.-M.; Bai, J.; Hua, H.-M.; Liu, X.-Q.; Li, Z.-L. Xanthones from Garcinia paucinervis with in vitro anti-proliferative activity against HL-60 cells. Arch. Pharm. Res. 2015, 39, 172–177. [Google Scholar] [CrossRef]
- Jia, C.; Xue, J.; Gong, C.; Li, X.; Li, D.; Li, Z.; Hua, H. Chiral resolution and anticancer effect of xanthones from Garcinia paucinervis. Fitoterapia 2018, 127, 220–225. [Google Scholar] [CrossRef]
- Paiva, A.M.; Pinto, M.M.; Sousa, E. A century of thioxanthones: Through synthesis and biological applications. Curr. Med. Chem. 2013, 20, 2438–2457. [Google Scholar] [CrossRef]
- Almeida, J.R.; Palmeira, A.; Campos, A.; Cunha, I.; Freitas, M.; Felpeto, A.B.; Turkina, M.V.; Vasconcelos, V.; Pinto, M.; Correia-Dasilva, M.; et al. Structure-antifouling activity relationship and molecular targets of bio-inspired (thio)xanthones. Biomolecules 2020, 10, 1126. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, T.-C.; Lee, C.-C.; Shih, L.-C.; Lin, C.-Y.; Hsieh, Y.-Y.; Ali, A.A.A.; Huang, H.-S. Synthesis and evaluation of new 3-substituted-4-chloro-thioxanthone derivatives as potent anti-breast cancer agents. Arab. J. Chem. 2015, 12, 3503–3516. [Google Scholar] [CrossRef]
- Barbosa, J.; Lima, R.; Sousa, D.; Gomes, A.; Palmeira, A.; Seca, H.; Choosang, K.; Pakkong, P.; Bousbaa, H.; Pinto, M.M.; et al. Screening a small library of xanthones for antitumor activity and identification of a hit compound which induces apoptosis. Molecules 2016, 21, 81. [Google Scholar] [CrossRef]
- Ataci, N.; Kazancioglu, E.O.; Kalındemirtas, F.D.; Kuruca, S.E.; Arsu, N. The interaction of light-activatable 2-thioxanthone thioacetic acid with ct-DNA and its cytotoxic activity: Novel theranostic agent. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118491. [Google Scholar] [CrossRef]
- Hermawan, F.; Jumina, J.; Pranowo, H.D. Design of thioxanthone derivatives as potential tyrosine kinase inhibitor: A molecular docking study. Rasayan J. Chem. 2020, 13, 2626–2632. [Google Scholar] [CrossRef]
- Iresha, M.R.; Jumina, J.; Pranowo, H.D. Molecular docking study of xanthyl chalcone derivatives as potential inhibitor agents against KIT tyrosine kinase and KIT kinase domain mutant D816H. J. Appl. Pharm. Sci. 2020, 10, 18–26. [Google Scholar] [CrossRef]
- Azevedo, C.M.G.; Afonso, C.M.M.; Soares, J.X.; Reis, S.; Sousa, D.; Lima, R.T.; Vasconcelos, M.H.; Pedro, M.; Barbosa, J.; Gales, L.; et al. Pyranoxanthones: Synthesis, growth inhibitory activity on human tumor cell lines and determination of their lipophilicity in two membrane models. Eur. J. Med. Chem. 2013, 69, 798–816. [Google Scholar] [CrossRef]
- Chantarasriwong, C.; Milcarek, A.T.; Morales, T.H.; Settle, A.L.; Rezende, C.O., Jr.; Althufairi, B.D.; Theodoraki, M.A.; Alpaugh, M.L.; Theodorakis, E.A. Synthesis, structure-activity relationship and in vitro pharmacodynamics of A-ring modified caged xanthones in a preclinical model of inflammatory breast cancer. Eur. J. Med. Chem. 2019, 168, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Sugara, T.H.; Jumina, J.; Sholikhah, E.N.; Pranowo, H.D. QSAR and molecular docking approaches for development of haloxanthones as the anticancer agent against MCF-7 and HepG2. Rasayan J. Chem. 2021, 14, 1927–1937. [Google Scholar] [CrossRef]
- Alam, S.; Khan, F. QSAR and docking studies on xanthone derivatives for anticancer activity targeting DNA topoisomerase IIα. Drug Des. Dev. Ther. 2014, 8, 183–195. [Google Scholar] [CrossRef]
- Alam, S.; Khan, F. Virtual screening, docking, ADMET and system pharmacology studies on Garcinia caged xanthone derivatives for anticancer activity. Sci. Rep. 2018, 8, 5524. [Google Scholar] [CrossRef]
- Kosem, N.; Ichikawa, K.; Utsumi, H.; Moongkarndi, P. In vivo toxicity and antitumor activity of mangosteen extract. J. Nat. Med. 2013, 67, 255–263. [Google Scholar] [CrossRef]
- Nabandith, V.; Suzui, M.; Morioka, T.; Kaneshiro, T.; Kinjo, T.; Matsumoto, K.; Akao, Y.; Iinuma, M.; Yoshimi, N. Inhibitory effects of crude α-mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in the rat. Asian Pac. J. Cancer Prev. 2004, 5, 433–438. [Google Scholar] [PubMed]
- Chao, A.C.; Hsu, Y.L.; Liu, C.K.; Kuo, P.L. α-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J. Agric. Food Chem. 2011, 59, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Shibata, M.A.; Shibata, E.; Morimoto, J.; Akao, Y.; Iinuma, M.; Tanigawa, N.; Otsuki, Y. Panaxanthone isolated from pericarp of Garcinia mangostana L. suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res. 2009, 29, 2485–2495. [Google Scholar] [PubMed]
- Shibata, M.; Iinuma, M.; Morimoto, J.; Kurose, H.; Akamatsu, K.; Okuno, Y.; Akao, Y.; Otsuki, Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn.) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011, 9, 69. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement. Altern. Med. 2012, 12, 104. [Google Scholar] [CrossRef]
- Kondo, M.; Zhang, L.; Ji, H.; Kou, Y.; Ou, B. Bioavailability and antioxidant effects of a xanthone-rich mangosteen (Garcinia mangostana) product in humans. J. Agric. Food Chem. 2009, 57, 8788–8792. [Google Scholar] [CrossRef]
- Xie, G.; Sintara, M.; Chang, T.; Ou, B. Functional beverage of Garcinia mangostana (mangosteen) enhances plasma antioxidant capacity in healthy adults. Food Sci. Nutr. 2015, 3, 32–38. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, D.A.; Failla, M.L. Bioavailability of xanthones from mangosteen juice in healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef]
- Han, S.Y.; You, B.H.; Kim, Y.C.; Chin, Y.-W.; Choi, Y.H. Dose-independent ADME properties and tentative identification of metabolites of α-mangostin from Garcinia mangostana in mice by automated microsampling and UPLC-MS/MS methods. PLoS ONE 2015, 10, e0131587. [Google Scholar] [CrossRef]
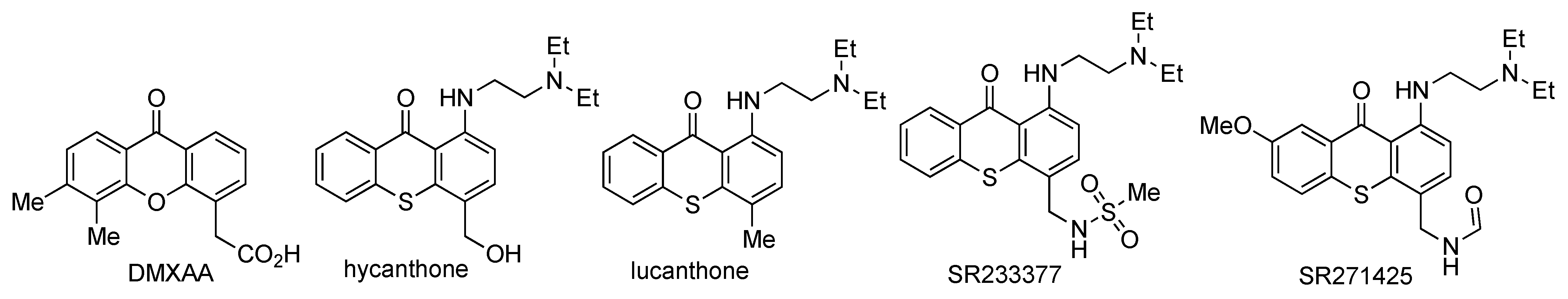
- Jameson, M.B.; Thompson, P.I.; Baguley, B.C.; Evans, B.D.; Harvey, V.J.; Porter, D.J.; McCrystal, M.R.; Small, M.; Bellenger, K.; Gumbrell, L.; et al. Clinical aspects of a phase I trial of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent. Br. J. Cancer 2003, 88, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, S.; Belluti, F.; Rampa, A.; Bisi, A. Flavonoid-inspired vascular disrupting agents: Exploring flavone-8-acetic acid and derivatives in the new century. Molecules 2021, 26, 4228. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.T.; Sousa, D.; Gomes, A.S.; Mendes, N.; Matthiesen, R.; Pedro, M.; Marques, F.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. The antitumor activity of a lead thioxanthone is associated with alterations in cholesterol localization. Molecules 2018, 23, 3301. [Google Scholar] [CrossRef]
- Lopes, A.; Martins, E.; Silva, R.; Pinto, M.M.M.; Remião, F.; Sousa, E.; Fernandes, C. Chiral thioxanthones as modulators of P-glycoprotein: Synthesis and enantioselectivity studies. Molecules 2018, 23, 626. [Google Scholar] [CrossRef]
- Naidu, M.D.; Agarwal, R.; Pena, L.A.; Cunha, L.; Mezei, M.; Shen, M.; Wilson, D.M., III; Liu, Y.; Sanchez, Z.; Chaudhary, P.; et al. Lucanthone and its derivative hycanthone inhibit apurinic endonuclease-1 (APE1) by direct protein binding. PLoS ONE 2011, 6, e23679. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Dieras, V.; Girre, V.; Yovine, A.; Mialaret, K.; Armand, J.-P. QTc Monitoring during a phase I study: Experience with SR271425. Am. J. Clin. Oncol. 2007, 30, 106–112. [Google Scholar] [CrossRef]
- Goncalves, P.H.; High, F.; Juniewicz, P.; Shackleton, G.; Li, J.; Boerner, S.; LoRusso, P.M. Phase I dose-escalation study of the thioxanthone SR271425 administered intravenously once every 3 weeks in patients with advanced malignancies. Investig. New Drugs 2008, 26, 347–354. [Google Scholar] [CrossRef]
- LoRusso, P.M.; Foster, B.J.; Wozniak, A.; Heilbrun, L.K.; McCormick, J.I.; Ruble, P.E.; Graham, M.A.; Purvis, J.; Rake, J.; Drozd, M.; et al. Phase I pharmacokinetic study of the novel antitumor agent SR233377. Clin. Cancer Res. 2000, 6, 3088–3094. [Google Scholar]
- Santos, A.; Soares, J.X.; Cravo, S.; Tiritan, M.E.; Reis, S.; Afonso, C.; Fernandes, C.; Pinto, M.M.M. Lipophilicity assessment in drug discovery: Experimental and theoretical methods applied to xanthone derivatives. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Pedro, M.; Nascimento, M.S.J.; Pinto, M.M.M.; Barbosa, C.M. Development and characterization of PLGA nanoparticles containing 1,3-dihydroxy-2-methylxanthone with improved antitumor activity on a human breast cancer cell line. Pharm. Dev. Technol. 2019, 24, 1104–1114. [Google Scholar] [CrossRef]
- Liu, F.; Huang, X.; Han, L.; Sang, M.; Hu, L.; Liu, B.; Duan, B.; Jiang, P.; Wang, X.; Qiao, Z.; et al. Improved drugability of gambogic acid using core–shell nanoparticles. Biomater. Sci. 2019, 7, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Guler, E.; Barlas, F.B.; Timur, S.; Yagci, Y. Polymeric thioxanthones as potential anticancer and radiotherapy agents. Macromol. Rapid Commun. 2016, 37, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
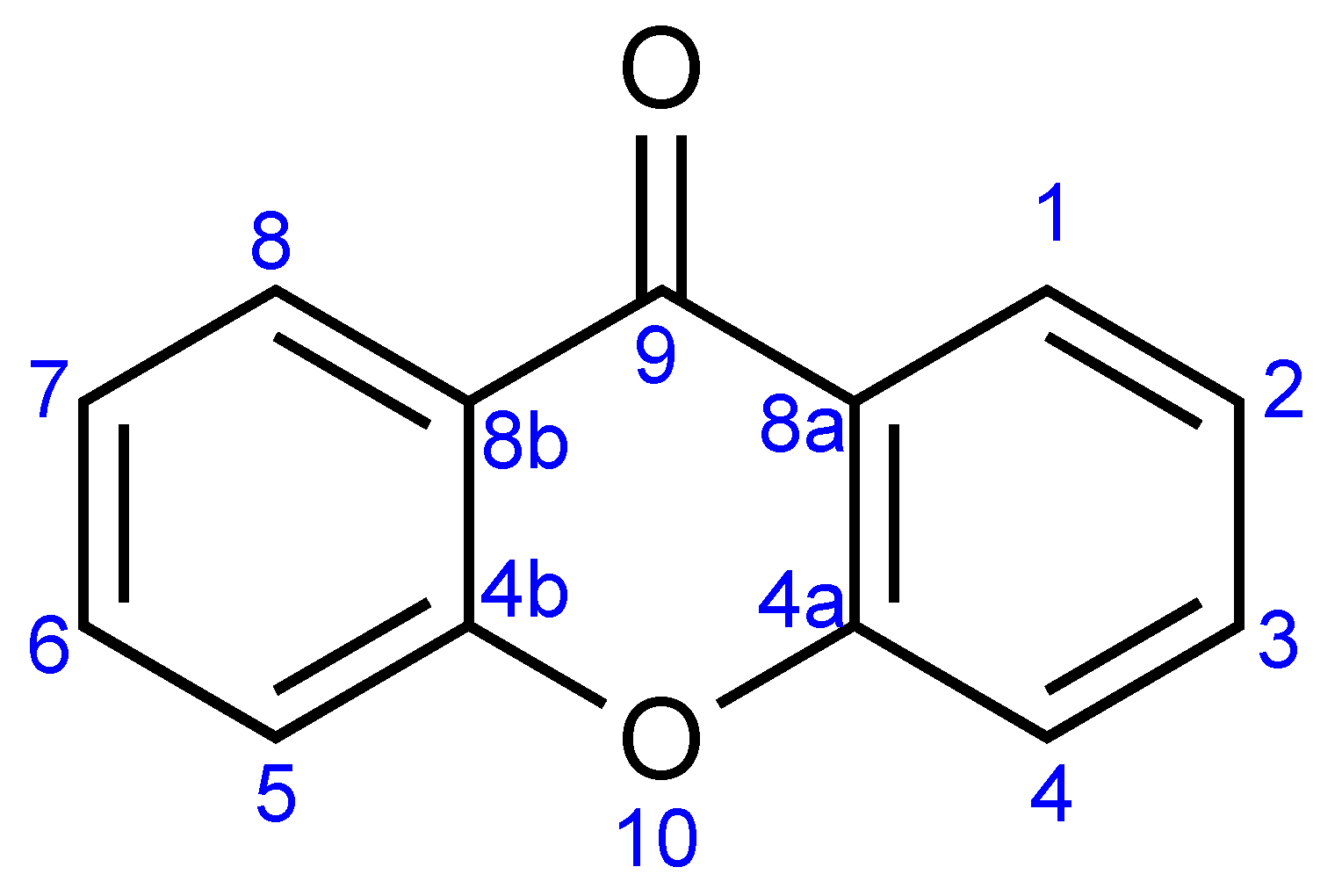


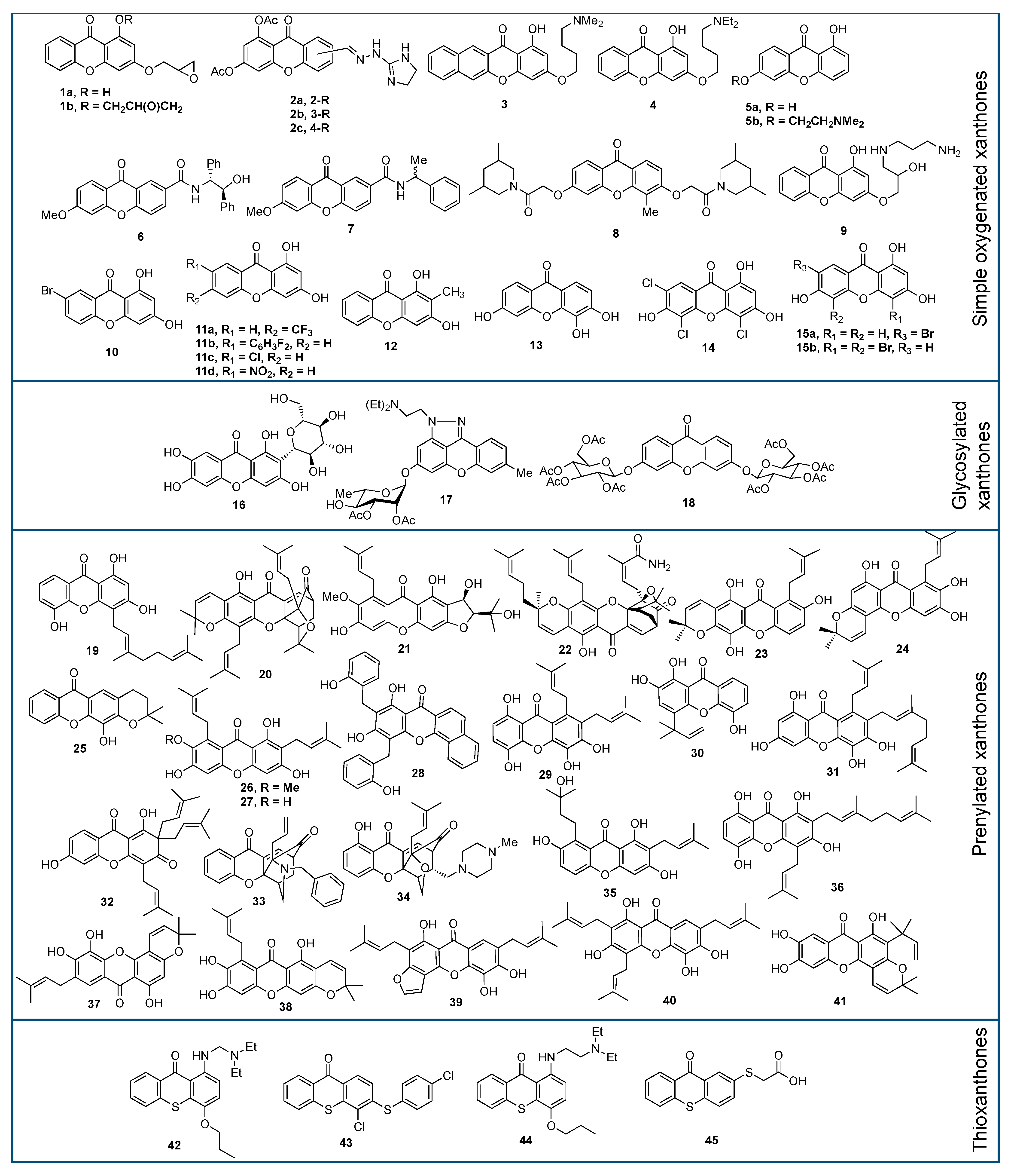
| Xanthone Structure | IR (cm−1) | UV (nm) | 1H-NMR (ppm) | 13C-NMR (ppm) |
|---|---|---|---|---|
| Csp2-H | 3000–3100 | - | 6–9 | - |
| C=O | 1650–1720 | 200–400 | - | 176 |
| C=C aromatic | 1450–1600 | - | 126 (C-1) | |
| 124 (C-2) | ||||
| 135 (C-3) | ||||
| 118 (C-4) | ||||
| 156 (C-4a) | ||||
| 121 (C-8a) | ||||
| C-O-C ether | 1000–1200 | - | - | - |
| O-H | 3300–3500 | - | 9–10 (C-2 or C-7) | - |
| 9–11 (C-4 or C-5) | ||||
| 10–11 (C-3 or C-6) | ||||
| 12–14 (C-1 or C-8) |
| Xanthone Derivative | Source | IC50 (Cancer Cells) | Ref. |
|---|---|---|---|
| Crude extract of xanthones | Fungus Penicillium sp. strain ZZF 32#. | 1.50 µg/mL (KB) | [61] |
| 2.50 µg/mL (KBv200) | |||
| Sterigmatocystin | Fungus Aspergillus versicolor | 3.76 µM (SK-MEL-2) | [62] |
| 1,7-Dihydroxy-2-methoxy-3-(3-methylbut-2-enyl)-9H-xanthen-9-one | Fungus Avicennia marina | 20 µM (KB) | [63] |
| 30 µM (KBv200) | |||
| 1-Hydroxy-4,7-dimethoxy-6-(3-oxobutyl)-9H-xanthen-9-one | 35 µM (KB) | ||
| 41 µM (KBv200) | |||
| Secalonic acid D | Fungus Penicillum oxalicum | 0.43 µM (K562) | [64] |
| 0.38 µM (HL60) | |||
| Dihydroxanthone | Leaf of Garcinia oligantha | 3.90 µM (A549) | [65] |
| 3.20 µM (PC-3) | |||
| Tetrahydroxanthone | 5.50 µM (A549) | ||
| 4.60 µM (PC-3) | |||
| Prenylated xanthone | Pericarp of Garcinia mangostana | 3.35 µM (CNE-1) | [66] |
| 4.01 µM (CNE-2) | |||
| 5-methoxyananixanthone | Stem bark of Calophyllum teysmanni | 14.7 µM (K562) | [67] |
| schomburgones A | Bark of Garcinia schomburgkiana | 45.05 µM (HepG2) | [68] |
| 52.21 µM (HeLa S-3) | |||
| Ananixanthone | Stem bark of Calophyllum species | 7.21 µM (K562) | [69] |
| Caloxanthone B | 3.00 µM (K562) | ||
| Isolated xanthone | Propolis of the stingless bee Lisotrigona furva | 12.63 µg/mL (HepG2) | [70] |
| 14.36 µg/mL (SK-LU-1) | |||
| Oxisterigmatocystins J | Fungus Penicillium sp. strain DWS10-P-6 | 15.14 µM (HL-60) | [71] |
| Oxisterigmatocystins K | 21.62 µM (MDA-MB-231) | ||
| 12.06 µM (HL-60) |
| Xanthone Derivative | Source | IC50 (µM) (Cancer Cells) | Main Mechanism | Ref. |
|---|---|---|---|---|
| 1a | Synthesis | 68.4 (MCF-7) | Topoisomerase inhibition, DNA crosslinking | [102] |
| 1b | 3.28 (MCF-7) | |||
| 2a | Synthesis | 1.3 (MCF-7) | - | [105] |
| 2b | 0.8 (MCF-7) | |||
| 2c | 1.05 (KB) | |||
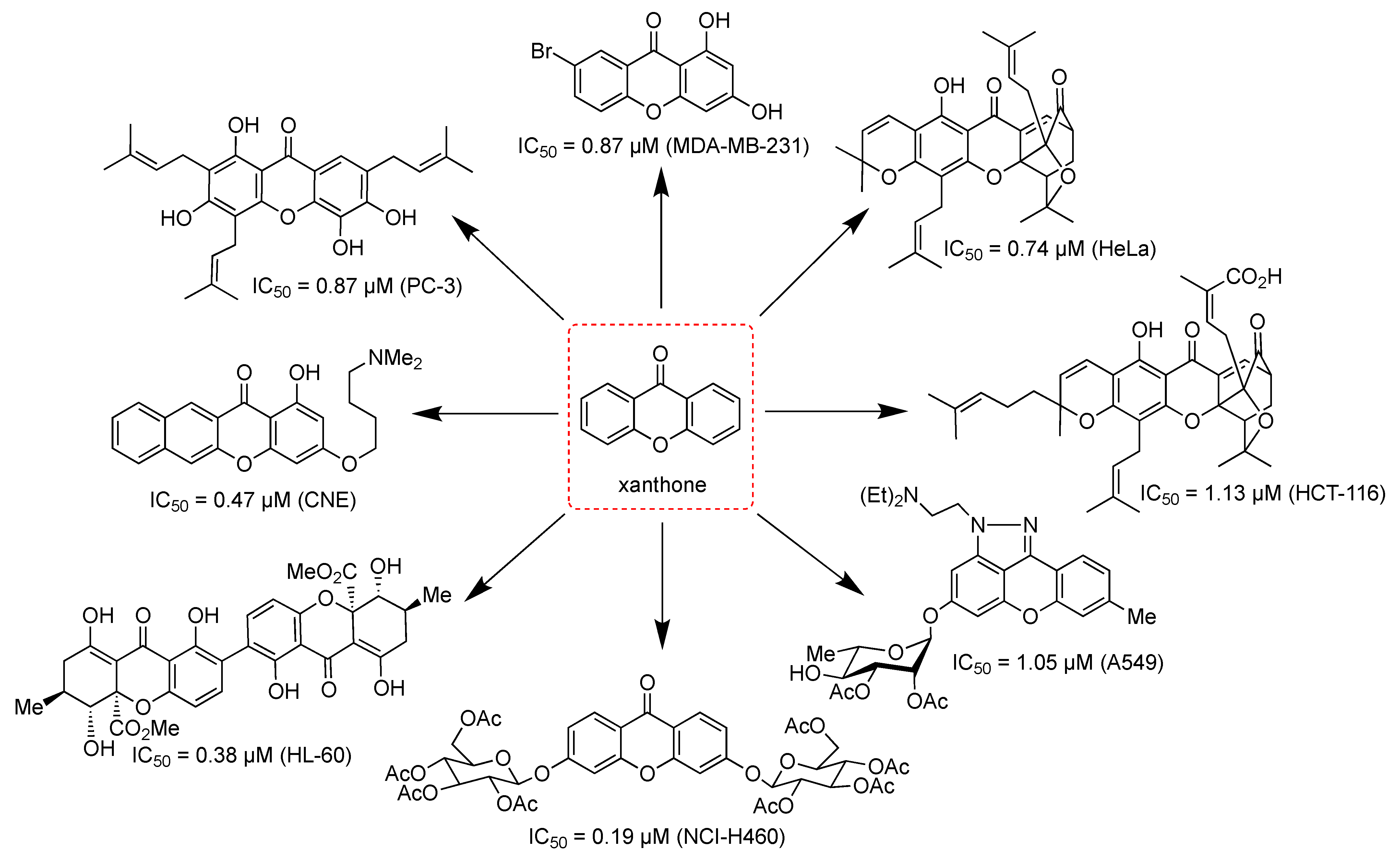
| 3 | Synthesis | 0.47 (CNE) | Mitochondrial dysfunction | [106] |
| 4 | Synthesis | 3.57 (MGC-803) | Mitochondrial dysfunction | [108] |
| 5a | Synthesis | 25.7 (ECA109) | DNA binding | [109] |
| 5b | 9.56 (ECA109) | |||
| 6 | Synthesis | 22.6 (MCF-7) | DNA crosslinking | [110] |
| (R)-7 | Synthesis | 24.0 (MCF-7) | DNA crosslinking | [111] |
| (S)-7 | 112 (MCF-7) | |||
| 8 | Synthesis | 4.59 (A549) | Promoting cell cycle arrest | [112] |
| 9 | Synthesis | 1.00 (-) | Topoisomerase IIα inhibition | [114] |
| 10 | Synthesis | 0.46 (MDA-MB-231) | Apoptosis induction | [115] |
| 11a | Synthesis | 27.16 (SMMC-7721) | - | [116] |
| 11b | 24.9 (A549) | |||
| 11c | 6.14 (SMMC-7721) | |||
| 11d | 14.02 (SMMC-7721) | |||
| 12 | Synthesis | 20.0 (UACC-62) | - | [117] |
| 13 | Synthesis | 37.8 (WiDr) | Suppressing mRNA COX-2 expression | [127] |
| 14 | Synthesis | 5.21 (P388) | Raf-1 and c-JNK inhibition | [124] |
| 15a | Synthesis | 6.34 (P388) | c-KIT inhibition | [129] |
| 15b | 10.7 (P388) | |||
| 16 | Isolation | 274 (BT-549) | bcr/abl gene expression inhibition | [130] |
| 17 | Synthesis | 0.19 (NCl-H460) | Promoting cell cycle arrest | [133] |
| 18 | Synthesis | 0.19 (NCI-H460) | - | [12] |
| 19 | Isolation | 1.58 (WiDr) | - | [134] |
| 20 | Isolation | 0.74 (HeLa) | Apoptosis induction | [136] |
| 21 | Isolation | 6.11 (KB) | - | [140] |
| 22 | Isolation | 0.005 (T17) | Apoptosis induction | [153] |
| 23 | Isolation | 40.6 (A2780) | - | [154] |
| 24 | 8.10 (A2780) | |||
| 25 | Synthesis | 7.00 (HL-60) | Apoptosis induction | [157] |
| 26 | Isolation | 5.90 (LNCaP) | Apoptosis induction | [158] |
| 27 | Isolation | 68.5 (HT-29) | Apoptosis induction | [170] |
| 28 | Synthesis | 5.17 (HTC116) | Topoisomerase inhibition | [171] |
| 29 | Isolation | 2.80 (HL-60) | Caspase activation and PG-E2 inhibition | [172] |
| 30 | 3.40 (HL-60) | |||
| 31 | 3.10 (HL-60) | |||
| 32 | Synthesis | 4.50 (MDA-MB-231) | - | [173] |
| 33 | Synthesis | 2.10 (A549) | Protein kinase inhibition | [174] |
| 34 | Synthesis | 3.25 (HepG2) | - | [175] |
| 35 | Isolation | 0.73 (CNE-2) | Antiproliferative induction | [176] |
| 36 | 8.61 (NCI/ADR-RES) | |||
| 37 | Isolation | 1.30 (HL-60) | Antiproliferative induction | [178] |
| 38 | Isolation | 3.35 (CNE-1) | Apoptosis induction | [67] |
| 39 | Isolation | 0.87 (HL-60) | - | [179] |
| 40 | 4.66 (PC-3) | |||
| 41 | Synthesis | 18.6 (HepG2) | Caspase activation | [18] |
| 42 | Synthesis | 1.90 (K562) | - | [157] |
| 43 | Synthesis | 3.90 (MDA-MB-468) | - | [182] |
| 44 | Synthesis | 3.60 (A375-C5) | Apoptosis induction | [183] |
| 45 | Synthesis | >165 (HT-29) | - | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An Update on the Anticancer Activity of Xanthone Derivatives: A Review. Pharmaceuticals 2021, 14, 1144. https://doi.org/10.3390/ph14111144
Kurniawan YS, Priyangga KTA, Jumina, Pranowo HD, Sholikhah EN, Zulkarnain AK, Fatimi HA, Julianus J. An Update on the Anticancer Activity of Xanthone Derivatives: A Review. Pharmaceuticals. 2021; 14(11):1144. https://doi.org/10.3390/ph14111144
Chicago/Turabian StyleKurniawan, Yehezkiel Steven, Krisfian Tata Aneka Priyangga, Jumina, Harno Dwi Pranowo, Eti Nurwening Sholikhah, Abdul Karim Zulkarnain, Hana Anisa Fatimi, and Jeffry Julianus. 2021. "An Update on the Anticancer Activity of Xanthone Derivatives: A Review" Pharmaceuticals 14, no. 11: 1144. https://doi.org/10.3390/ph14111144
APA StyleKurniawan, Y. S., Priyangga, K. T. A., Jumina, Pranowo, H. D., Sholikhah, E. N., Zulkarnain, A. K., Fatimi, H. A., & Julianus, J. (2021). An Update on the Anticancer Activity of Xanthone Derivatives: A Review. Pharmaceuticals, 14(11), 1144. https://doi.org/10.3390/ph14111144






