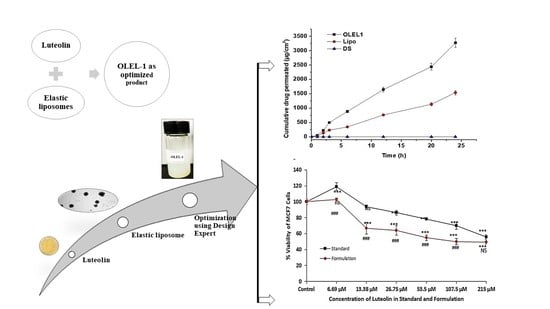
Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Lipid and Surfactant Ratio
2.1.1. Preliminary Study to Select Lipid and Surfactant Ratio
2.1.2. Optimization Using Design Expert
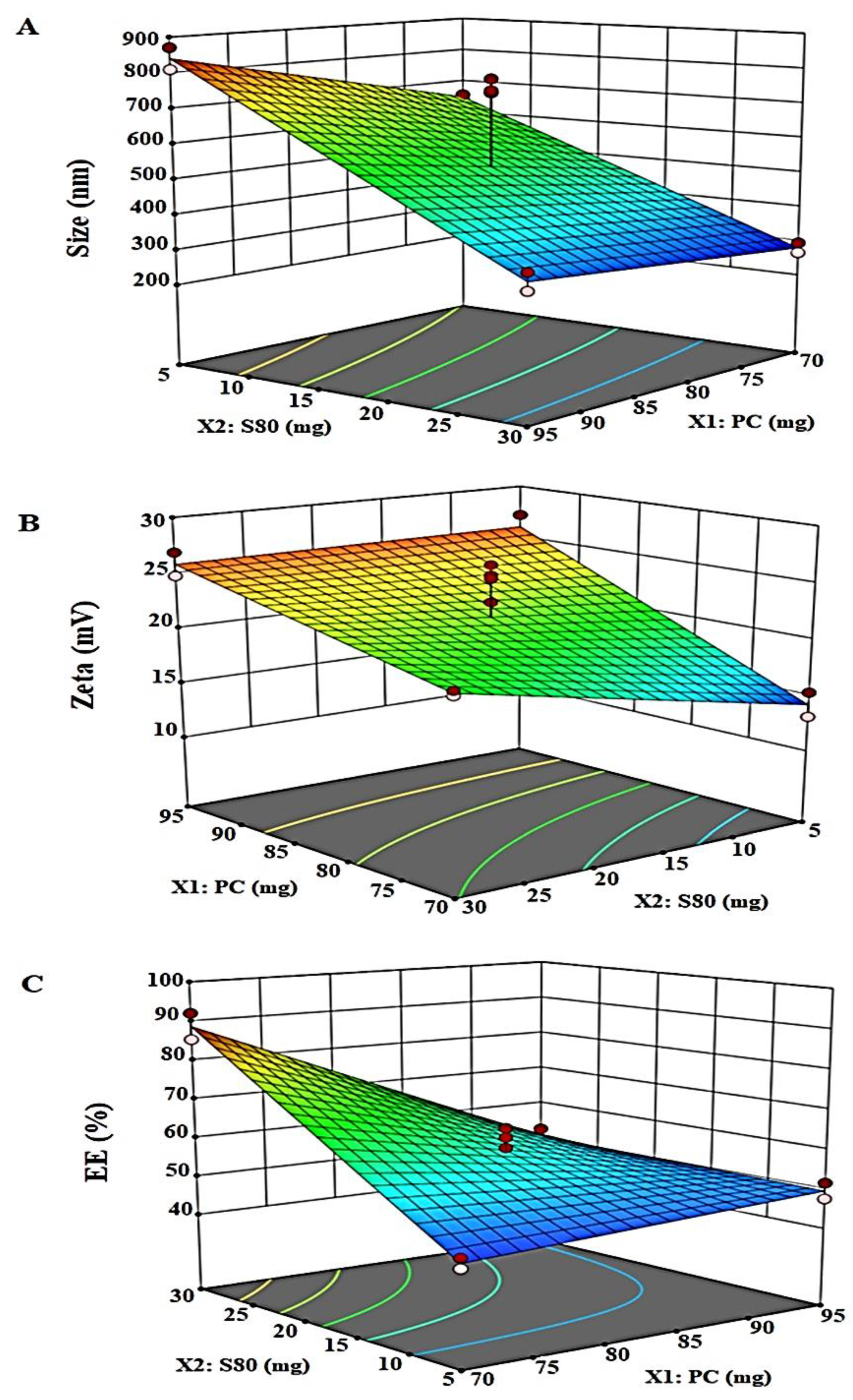
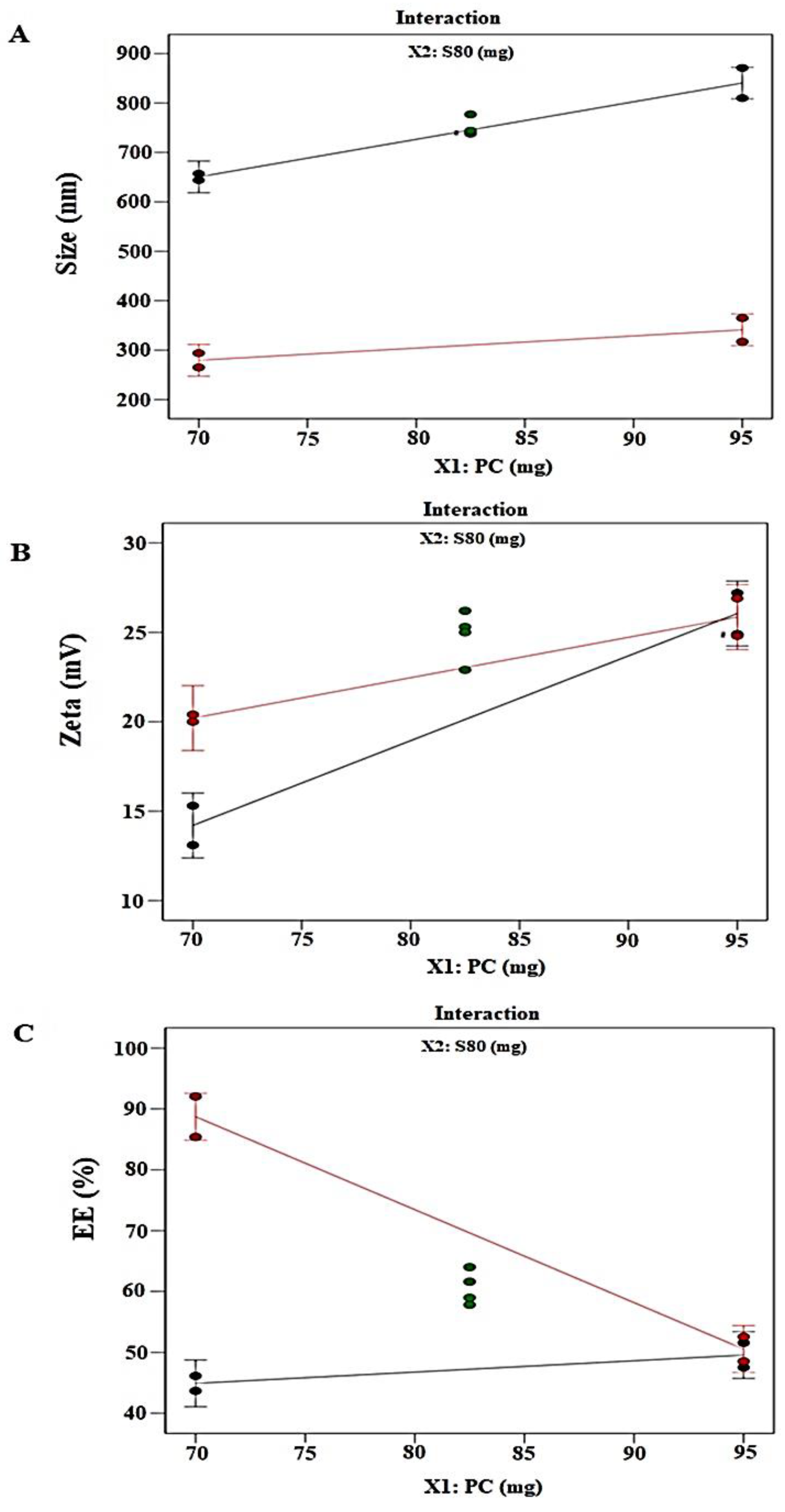
2.1.3. Responses Evaluation
Vesicle Size (nm): Y1
Zeta Potential (ZP): Y2
Percentage Entrapment Efficiency: Y3
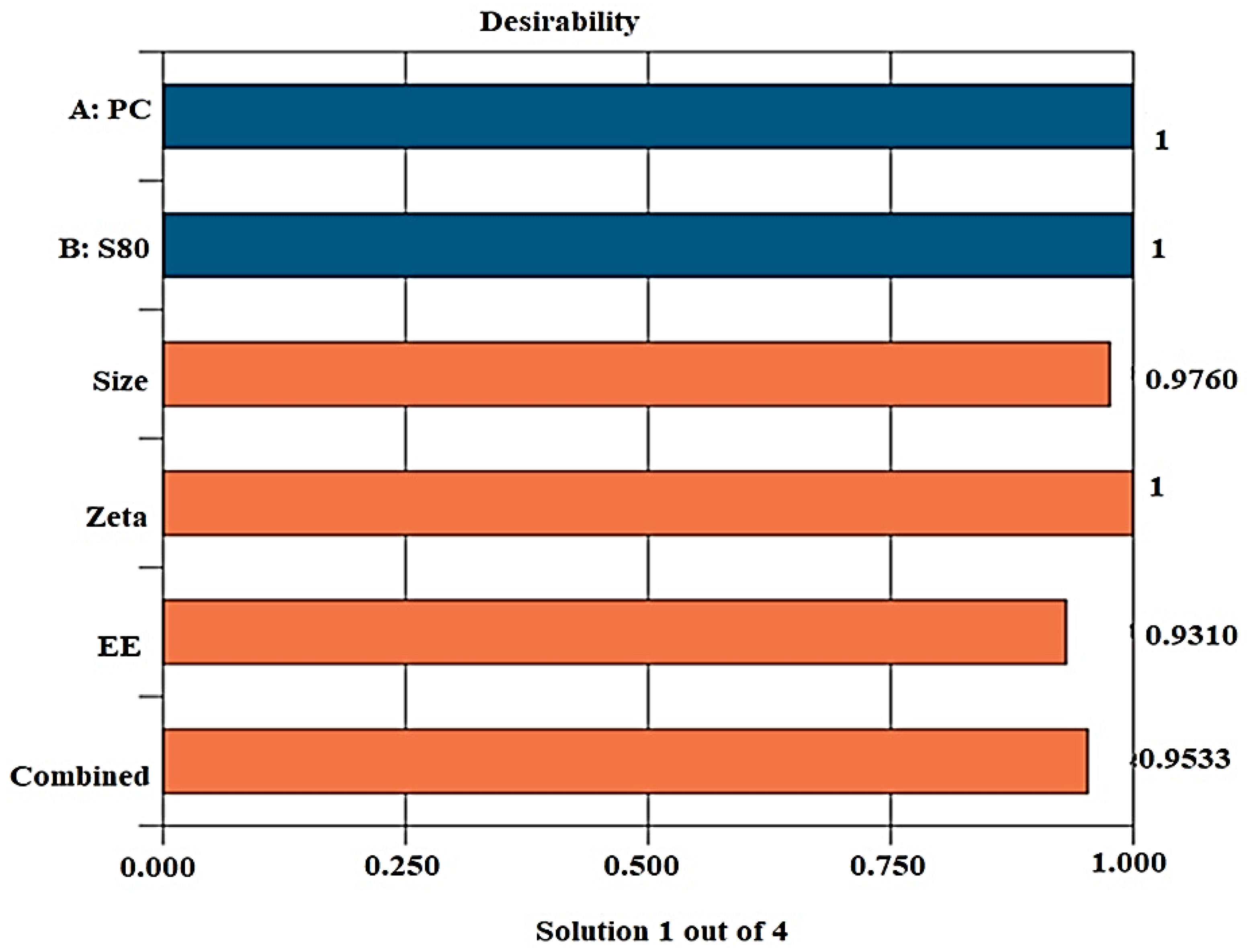
2.1.4. Desirability
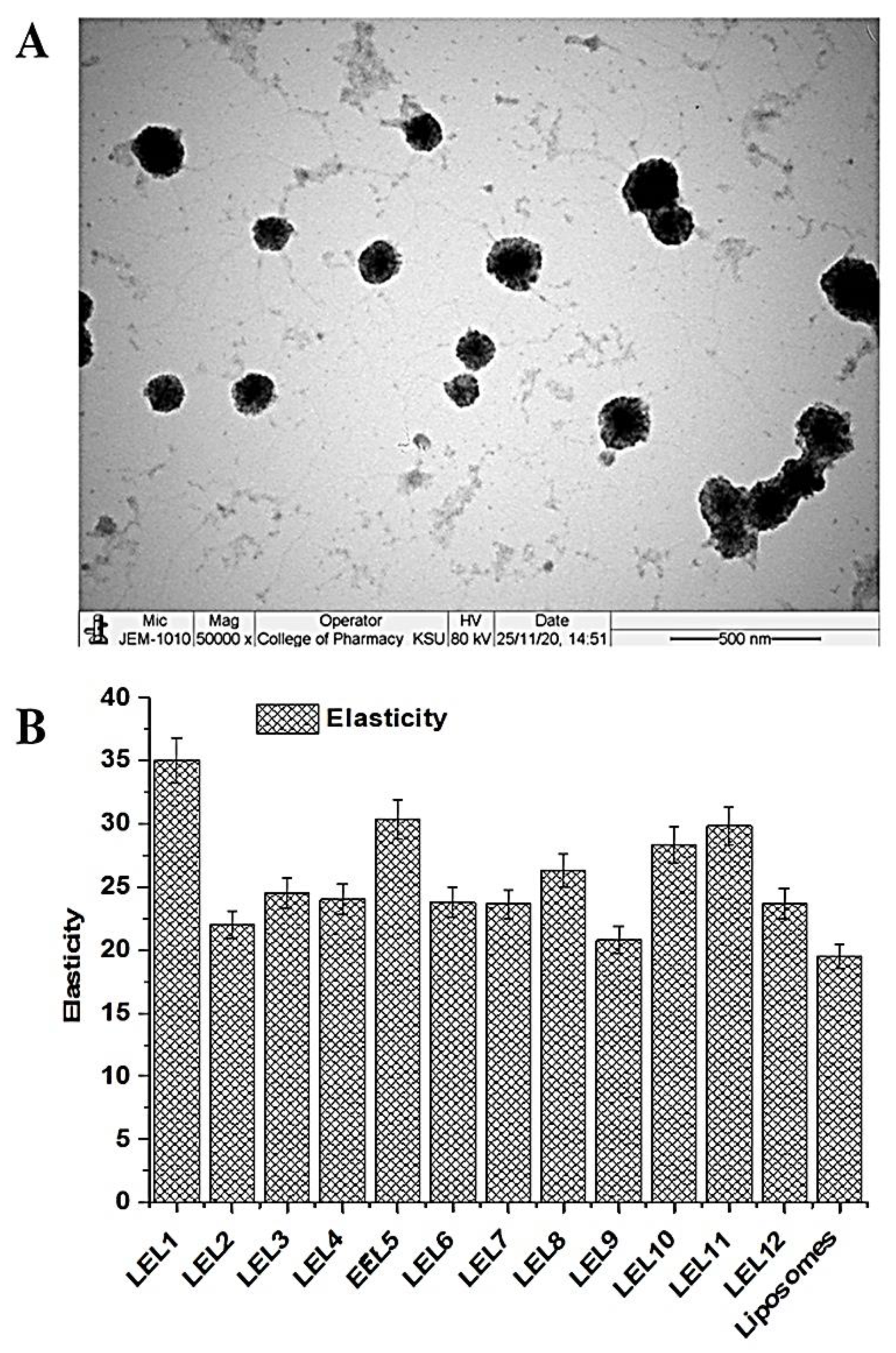
2.1.5. Morphological Assessment
2.1.6. Elasticity
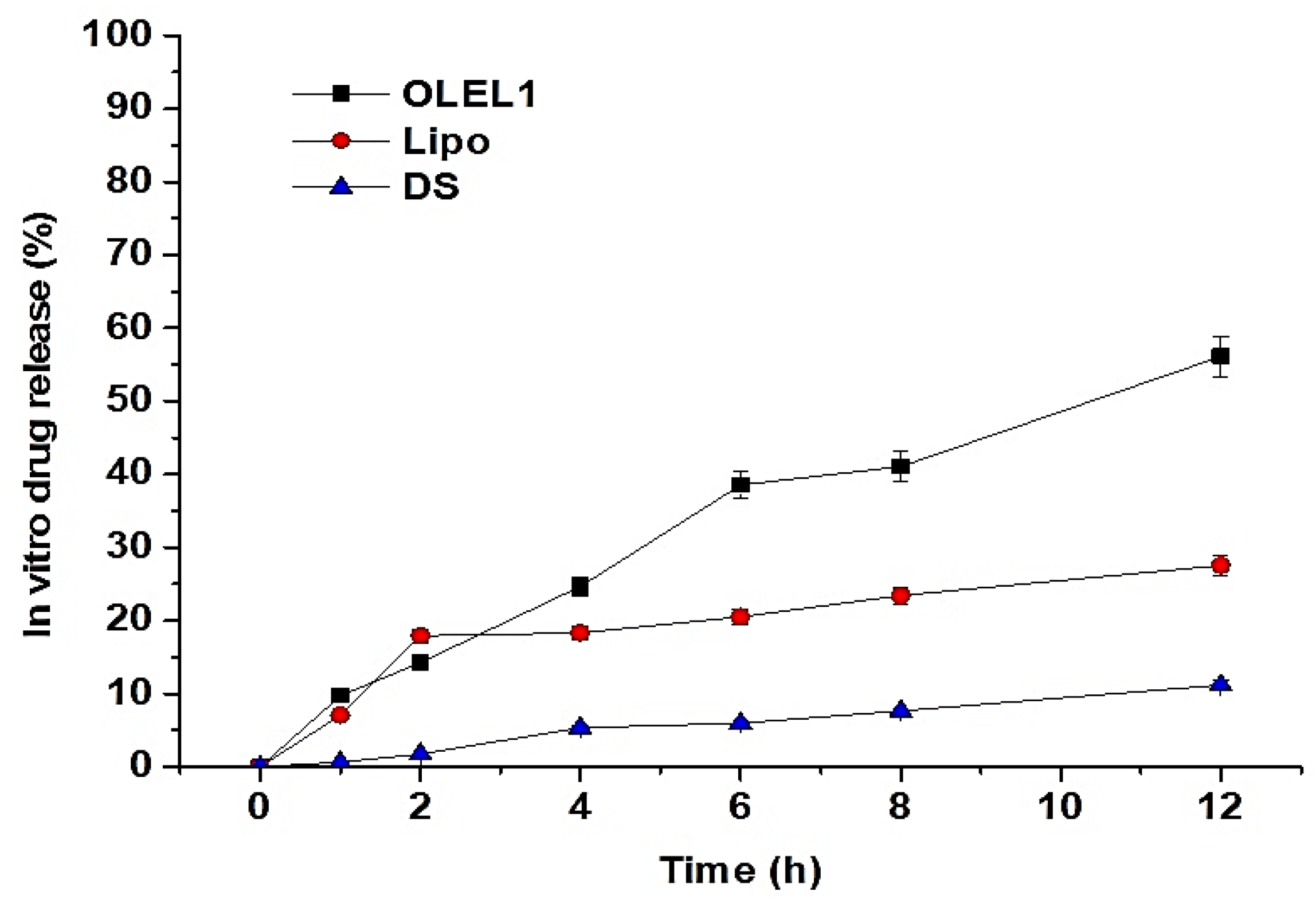
2.1.7. In Vitro Drug Release Study
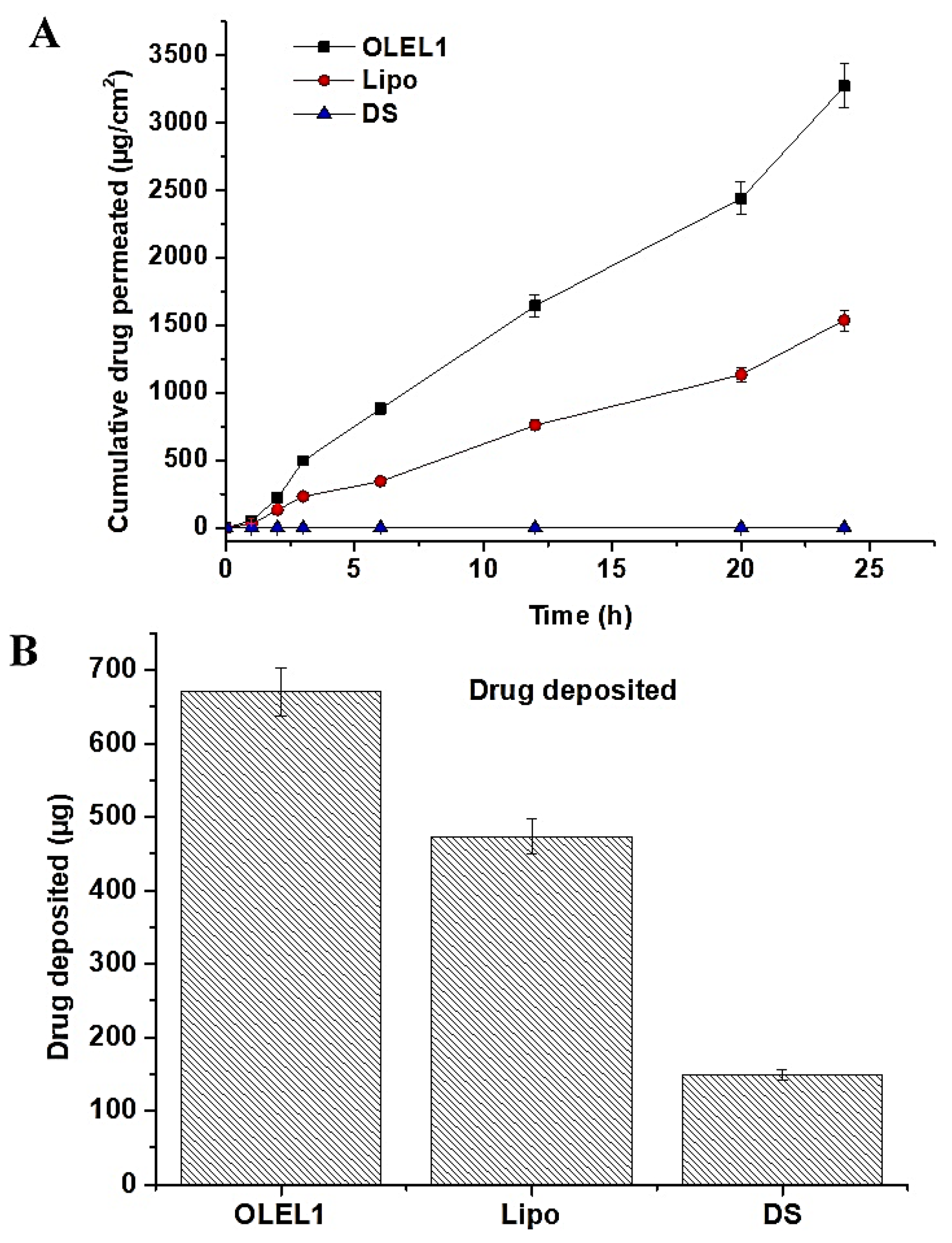
2.1.8. Ex Vivo Permeation and DD Studies across Rat Skin
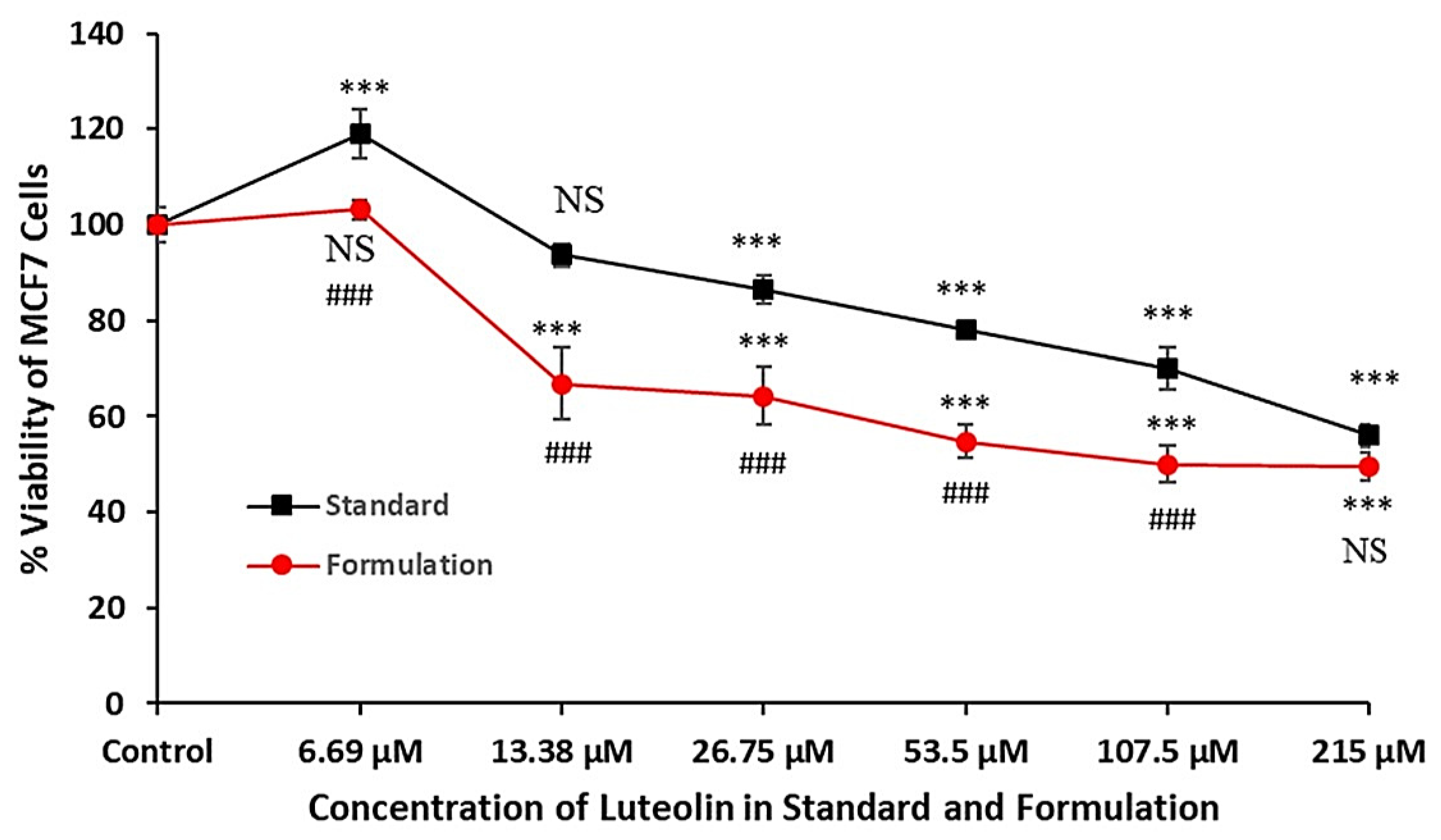
2.1.9. Cytotoxicity Study
3. Materials and Methods
3.1. Materials
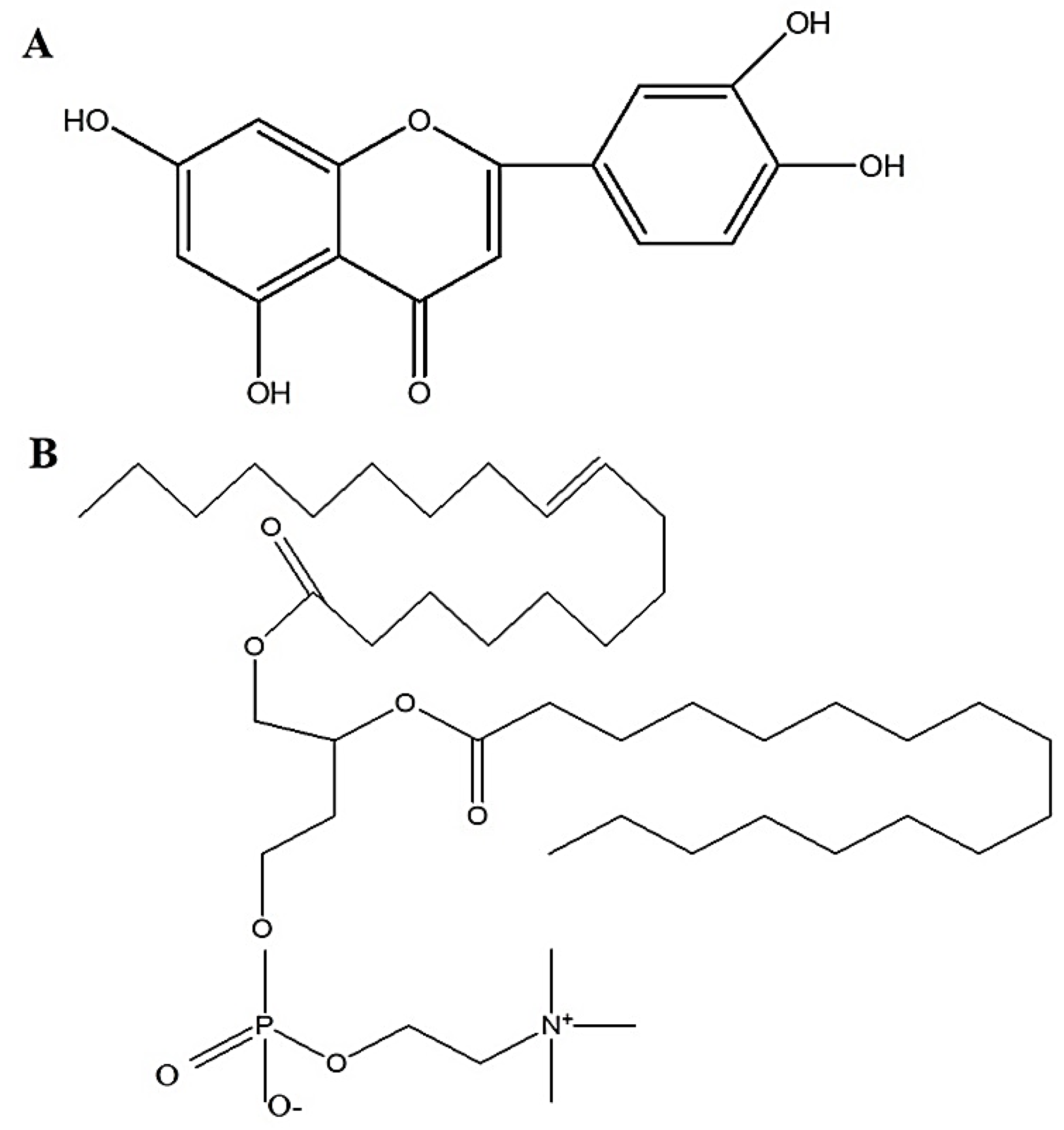
3.2. Preparation of Luteolin-Loaded Elastic Liposomes (LELs) Using Various Surfactants
3.3. Vesicle Size and Size Distribution (Polydispersity Index, PDI)
3.4. Experimental Design Tool (Design Expert®)
3.5. Formulations Characterizations
3.5.1. Vesicle Size and Zeta Potential
3.5.2. Percentage Entrapment Efficiency (% EE)
3.5.3. Desirability Function Parameter and Validation
3.5.4. Morphological Assessment
3.5.5. Elasticity
3.5.6. In Vitro Drug Release (%DR)
3.6. Analytical Method
3.7. Ex Vivo Drug Permeation and Deposition Study
3.8. Cytotoxicity Study Using MCF-7 Cell Lines
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Wani, W.A.; Saleem, K. Cancer scenario in India with future perspectives. Cancer Ther. 2011, 8, 56–70. [Google Scholar]
- Jain, A.S.; Dhawan, V.K.; Sarmento, B.; Nagarsenker, M.S. In vitro and ex vivo evaluations of lipid anti-cancer nanoformulations: Insights and assessment of bioavailability enhancement. AAPS PharmSciTech 2016, 17, 553–571. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- World Health Organization. Fact. Sheet Datail of Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 22 June 2021).
- Seelinger, G.I.; Merfort Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Peng, B.; Yan, W. Solubility of Luteolin in Ethanol + Water Mixed Solvents at Different Temperatures. J. Chem. Eng. Data 2010, 55, 583–585. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, K.; Karlstetter, M.; Ebert, S.; Kraus, D.; Hlawatsch, J.; Walczak, Y.; Moehle, C.; Fuchshofer, R.; Langmann, T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J. Neuroinflamm. 2010, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Süzgeç-Selçuk, S.; Birteksöz, A. Flavonoids of Helichrysum chasmolycicum and its antioxidant and antimicrobial activities. South Afr. J. Bot. 2011, 77, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Li, J.; Yue, J.; Zhang, S.; Yunusi, K. Liposome encapsulated luteolin showed enhanced antitumor efficacy to colorectal carcinoma. Mol. Med. Rep. 2018, 17, 2456–2464. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tu, M.; Sun, S.; Kong, S.; Wang, Y.; Ye, J.; Li, L.; Zeng, S.; Jiang, H. The Exposure of Luteolin Is Much Lower than That of Apigenin in Oral Administration of Flos Chrysanthemi Extract to Rats. Drug Metab. Pharmacokinet. 2012, 27, 162–168. [Google Scholar] [CrossRef]
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanoparticle Res. 2014, 16, 1–10. [Google Scholar]
- Trotta, M.; Peira, E.; Debernardi, F.; Gallarate, M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int. J. Pharm. 2002, 241, 319–327. [Google Scholar] [CrossRef]
- Abidin, L.; Mujeeb, M.; Imam, S.S.; Aqil, M.; Khurana, D. Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: Quality evaluation and anti-arthritic assessment. Drug Deliv. 2016, 23, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Mishra, D.; Garg, M.; Dubey, V.; Jain, S.; Jain, N. Elastic Liposomes Mediated Transdermal Deliveryof an Anti-Hypertensive Agent: Propranolol Hydrochloride. J. Pharm. Sci. 2007, 96, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.; De La Maza, A.; Coderch, L.; López-Iglesias, C.; Wehrli, E.; Parra, J.L. Direct formation of mixed micelles in the solubilization of phospholipid liposomes by Triton X-100. FEBS Lett. 1998, 426, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schätzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1998, 1368, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef] [PubMed]
- Fazel, M.; Daeihamed, M.; Osouli, M.; Almasi, A.; Haeri, A.; Dadashzadeh, S. Preparation, in-vitro characterization and pharmacokinetic evaluation of Brij decorated doxorubicin liposomes as a potential nanocarrier for cancer therapy. IJPR 2018, 17, 33–43. [Google Scholar] [CrossRef]
- Ghosh, S.; Moulik, S. Interfacial and Micellization Behaviors of Binary and Ternary Mixtures of Amphiphiles (Tween-20, Brij-35, and Sodium Dodecyl Sulfate) in Aqueous Medium. J. Colloid Interface Sci. 1998, 208, 357–366. [Google Scholar] [CrossRef]
- Sharma, B.; Rakshit, A.K. Thermodynamics of micellization of a nonionic surfactant: Brij 35 in aquo-sucrose solution. J. Colloid Interface Sci. 1989, 129, 139–144. [Google Scholar] [CrossRef]
- Jain, S.K.; Gupta, Y.; Jain, A.; Rai, K. Enhanced Transdermal Delivery of Acyclovir Sodium via Elastic Liposomes. Drug Deliv. 2008, 15, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Varshosaz, J.; Pardakhty, A.; Hajhashemi, V.I.; Najafabadi, A.R. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003, 10, 251–262. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Tas, C.; Ozkan, Y.; Okyar, A.; Savaser, A. In vitro and ex vivo permeation studies of etodolac from hydrophilic gels and effect of terpenes as enhancers. Drug Deliv. 2007, 14, 453–459. [Google Scholar] [CrossRef]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J. Characterization of flavonoid–biomembrane interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid–membrane interactions: Implications on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Hussain, A.; Samad, A.; Ramzan, M.; Ahsan, M.N.; Rehman, Z.U.; Ahmad, F. Elastic liposome-based gel for topical delivery of 5-fluorouracil: In vitro and in vivo investigation. Drug Deliv. 2016, 23, 1115–1129. [Google Scholar] [CrossRef] [Green Version]
- Yeom, S.; Shin, B.S.; Han, S. An electron spin resonance study of non-ionic surfactant vesicles (niosomes). Chem. Phys. Lipids 2014, 181, 83–89. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G.; Schätzlein, A. Transfersomes-mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo. J. Control Release 1997, 45, 211–226. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2012, 29, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Ita, K.B.; Du Preez, J.; du Plessis, J.; Lane, M.E.; Hadgraft, J. Dermal delivery of selected hydrophilic drugs from elastic liposomes: Effect of phospholipid formulation and surfactants. J. Pharm. Pharmacol. 2007, 59, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Balaguer-Fernández, C.; Femenía-Font, A.; Merino, V.; Cordoba-Diaz, D.; Elorza-Barroeta, M.A.; López-Castellano, A.; Cordoba-Diaz, M. Elastic vesicles of sumatriptan succinate for transdermal administration: Characterization and in vitro permeation studies. J. Liposome Res. 2011, 21, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Gusai, T.; Dhavalkumar, M.; Soniwala, M.; Dudhat, K.; Vasoya, J.; Chavda, J. Formulation and optimization of microsponge-loaded emulgel to improve the transdermal application of acyclovir—a DOE based approach. Drug Deliv. Transl. Res. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, D.; Lakshmi, P.K. Development of ethosomes with taguchi robust design-based studies for transdermal delivery of alfuzosin hydrochloride. Int. Curr. Pharm. J. 2012, 1, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech 2012, 14, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: Statistical optimization, characterization and pharmacokinetic assessment. Int. J. Pharm. 2013, 443, 26–38. [Google Scholar] [CrossRef]
- Mishra, D.; Dubey, V.; Asthana, A.; Saraf, D.; Jain, N. Elastic liposomes mediated transcutaneous immunization against Hepatitis B. Vaccine 2006, 24, 4847–4855. [Google Scholar] [CrossRef]
- Benson, H.A. Elastic Liposomes for Topical and Transdermal Drug Delivery, in Liposomes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 77–86. [Google Scholar]
- Vanić, Ž.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Klarić, D.A.; Bogdanov, A.; Raffai, T.; Virok, D.P.; et al. Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int. J. Nanomed. 2019, 14, 5957–5976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Develop-ment and Evaluations of Transdermally Delivered Luteolin Loaded Cationic Nanoemulsion: In Vitro and Ex Vivo Evaluations. Pharmaceutics 2021, 13, 1218. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Gannu, R.; Vishnu, Y.V.; Kishan, V.; Rao, Y.M. Development of Nitrendipine Transdermal Patches: In vitro and Ex vivo Characterization. Curr. Drug Deliv. 2007, 4, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gannu, R.; Palem, C.R.; Yamsani, V.V.; Yamsani, S.K.; Yamsani, M.R. Enhanced bioavailability of lacidipine via microemulsion based transdermal gels: Formulation optimization, ex vivo and in vivo characterization. Int. J. Pharm. 2010, 388, 231–241. [Google Scholar] [CrossRef]
- AbdelSamie, S.M.; Kamel, A.O.; Sammour, O.; Ibrahim, S. Terbinafine hydrochloride nanovesicular gel: In vitro characterization, ex vivo permeation and clinical investigation. Eur. J. Pharm. Sci. 2016, 88, 91–100. [Google Scholar] [CrossRef]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, K.; Ahmad, F.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Pandey, S.S.; Shah, K.M.; Maulvi, F.A.; Desai, D.T.; Gupta, A.R.; Joshi, S.V.; Shah, D.O. Topical delivery of cyclosporine loaded tailored niosomal nanocarriers for improved skin penetration and deposition in psoriasis: Optimization, ex vivo and animal studies. J. Drug Deliv. Sci. Technol. 2021, 63, 102441. [Google Scholar] [CrossRef]
| Code | PC:S (% w/w) | Surfactant | HLB | Tg (°C) | Vesicle Size (nm) | PDI |
|---|---|---|---|---|---|---|
| F1 | 95:5 * | Span 60 | 4.7 | 53 | 358 ± 16 | 0.62 ± 0.05 |
| F2 | 85:15 | Span 60 | 4.7 | 53 | 284 ± 13 | 0.44 ± 0.03 |
| F3 | 70:30 | Span 60 | 4.7 | 53 | 187 ± 11 | 0.43 ± 0.02 |
| F4 | 95:5 * | Span 80 | 4.3 | −12 | 218 ± 9 | 0.45 ± 0.03 |
| F5 | 85:15 | Span 80 | 4.3 | −12 | 212 ± 9 | 0.30 ± 0.01 |
| F6 | 70:30 | Span 80 | 4.3 | −12 | 170 ± 6 | 0.35 ± 0.02 |
| F7 | 95:5 * | Brij 35 | 16.9 | 40–45 | 385 ± 8 | 0.42 ± 0.03 |
| F8 | 85:15 | Brij 35 | 16.9 | 40–45 | 266 ± 5 | 0.35 ± 0.02 |
| F9 | 70:30 | Brij 35 | 16.9 | 40–45 | 234 ± 6 | 0.45 ± 0.04 |
| Std | Block | Run | Factor 1 X1:PC (mg) | Factor 2 X2:Span 80 (mg) | Response 1 Size (nm) | Response 2 Zeta (mV) | Response 3 EE (%) |
|---|---|---|---|---|---|---|---|
| 5 | Block 1 | 1 | 70 | 30 | 265 | 20.4 | 85.39 |
| 9 | Block 1 | 2 | 82.5 | 17.5 | 739 | 25.3 | 57.82 |
| 1 | Block 1 | 3 | 70 | 5 | 644 | 13.1 | 43.67 |
| 10 | Block 1 | 4 | 82.5 | 17.5 | 738 | 22.9 | 64 |
| 7 | Block 1 | 5 | 95 | 30 | 317 | 26.9 | 52.55 |
| 3 | Block 1 | 6 | 95 | 5 | 871 | 27.2 | 47.53 |
| 11 | Block 2 | 7 | 82.5 | 17.5 | 777 | 26.2 | 58.97 |
| 2 | Block 2 | 8 | 70 | 5 | 657 | 15.3 | 46.13 |
| 12 | Block 2 | 9 | 82.5 | 17.5 | 744 | 25 | 61.61 |
| 8 | Block 2 | 10 | 95 | 30 | 365 | 24.8 | 48.52 |
| 6 | Block 2 | 11 | 70 | 30 | 294 | 20 | 92.06 |
| 4 | Block 2 | 12 | 95 | 5 | 810 | 24.9 | 51.57 |
| Experimental Design and Summary Reports | |||
|---|---|---|---|
| Factors | Range | Goal | |
| X1: PC (mg) | 70–95 | In range | |
| X2: Span 80 (mg) | 5–30 | In range | |
| Responses | |||
| Y1 (nm) as size | 265–871 | Minimum | |
| Y2 (mV) as zeta potential | −13.1––27.2 | Maximum | |
| Y3 (%) as % EE | 43.7–92.1 | Maximum | |
| Regression equations with best fitted model | |||
| Y1 = 527.9 + 62.87X1 − 217.63X2 − 32.12 X1X2 | |||
| Y2 = 21.58 + 4.73X1 + 1.45X2 − 1.55 X1X2 | |||
| Y3 = 58.43 – 8.39X1 + 11.2X2 − 10.71 X1X2 | |||
| Statistical parameters | Y1 | Y2 | Y3 |
| r2 | 0.99 | 0.93 | 0.98 |
| Adjusted r2 | 0.99 | 0.90 | 0.97 |
| Predicted r2 | 0.95 | 0.72 | 0.90 |
| Model f value | 204.16 | 28.69 | 83.67 |
| p value | 0.0001 | 0.0006 | 0.0001 |
| Model | Quadratic | Quadratic | Quadratic |
| SD | 26.15 | 1.48 | 3.14 |
| Mean value | 601.75 | 22.67 | 59.15 |
| % CV | 4.35 | 6.54 | 5.32 |
| PC | Span 80 | Size | Zeta | EE% | |
|---|---|---|---|---|---|
| Predicted | 70 | 30 | 276 | 20.2 | 88.73 |
| Experimental OLEL1 | 70 | 30 | 202 | 22.2 | 92 ± 3.8 |
| Formulations | Jss1 (µg/cm2 h) | TL (mean ± sd) (min) | Pc (mean ± sd) (cm/h) | ER1 |
|---|---|---|---|---|
| OLEL1 | 136.26 ± 7.76 | 2.0 ± 0.01 | 1.36 × 10−2 | 5.61 |
| Lipo | 64.01 ± 0.91 | 4.5 ± 0.03 | 6.4 × 10−3 | 2.63 |
| DS | 24.31 ± 3.38 | 4.0 ± 0.02 | 2.3 × 10−3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamimi, M.A.; Hussain, A.; AlRajhi, M.; Alshehri, S.; Imam, S.S.; Qamar, W. Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations. Pharmaceuticals 2021, 14, 1143. https://doi.org/10.3390/ph14111143
Altamimi MA, Hussain A, AlRajhi M, Alshehri S, Imam SS, Qamar W. Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations. Pharmaceuticals. 2021; 14(11):1143. https://doi.org/10.3390/ph14111143
Chicago/Turabian StyleAltamimi, Mohammad A., Afzal Hussain, Mohammad AlRajhi, Sultan Alshehri, Syed Sarim Imam, and Wajhul Qamar. 2021. "Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations" Pharmaceuticals 14, no. 11: 1143. https://doi.org/10.3390/ph14111143
APA StyleAltamimi, M. A., Hussain, A., AlRajhi, M., Alshehri, S., Imam, S. S., & Qamar, W. (2021). Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations. Pharmaceuticals, 14(11), 1143. https://doi.org/10.3390/ph14111143