Multifunctional Delivery Systems for Peptide Nucleic Acids
Abstract
:1. Introduction
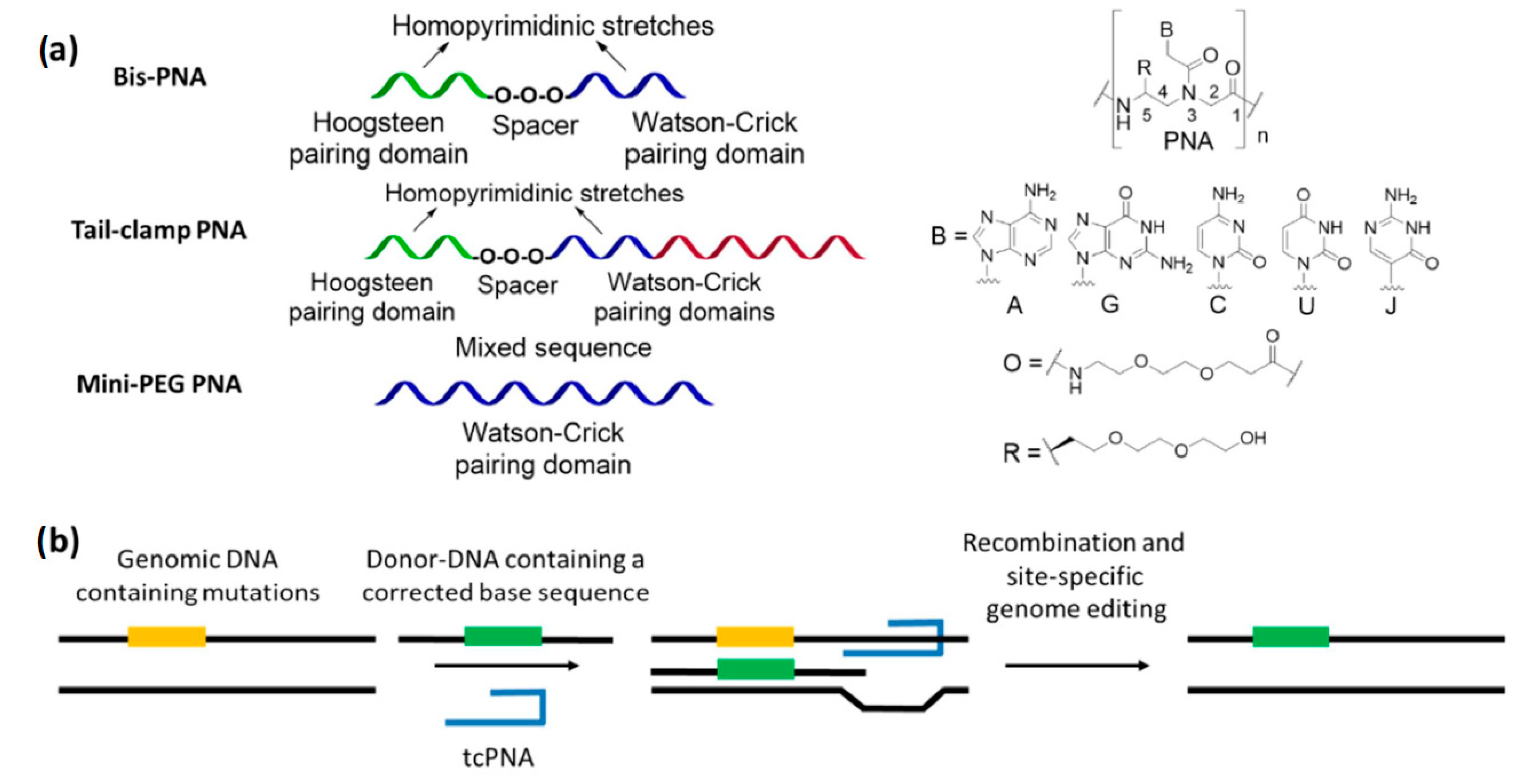
1.1. Peptide Nucleic Acids and Their Uses
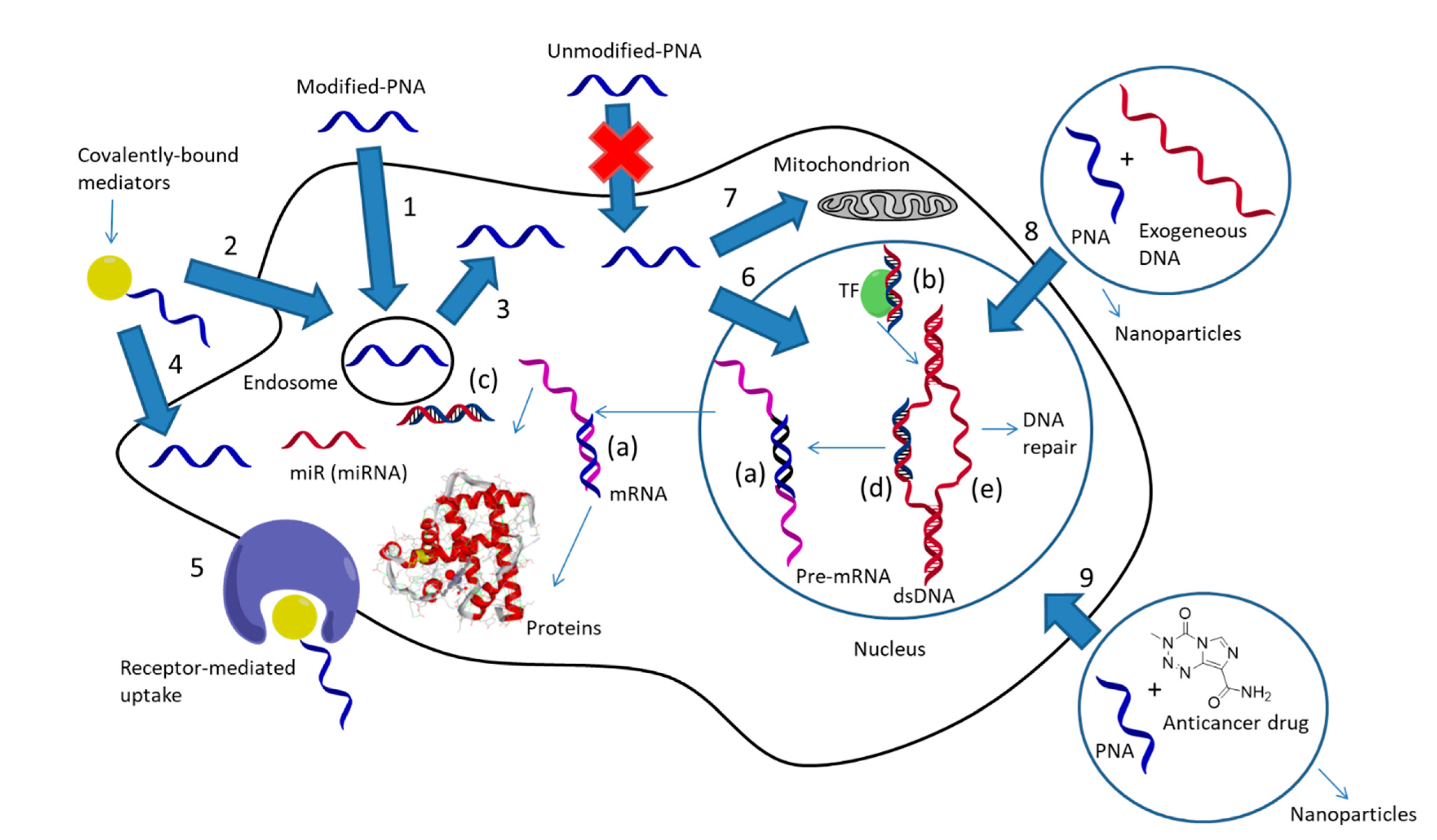
1.2. Approaches for Cellular PNA Delivery and PNA-Mediated Delivery
1.3. PNA Localization
2. PNA Conjugates
2.1. Small Molecule Ligand Conjugation
2.2. Peptide Conjugation
3. Lipid and Liposome-Based Approaches
4. Polymer Nanoparticles and Carriers
4.1. Polylactic Co-Glycolic Acids (PLGA)
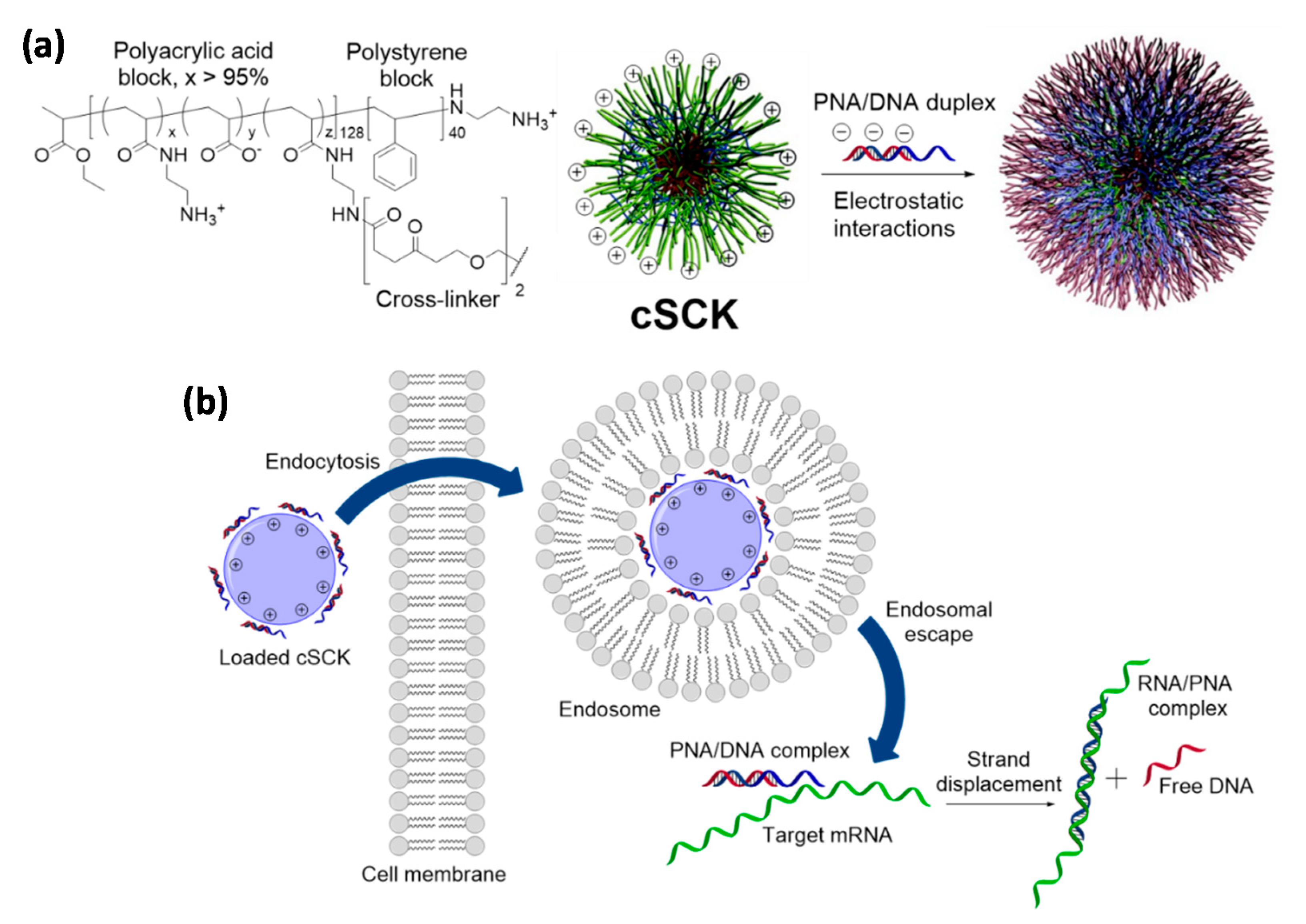
4.2. Cationic Shell-Cross-Linked Knedel-Like (cSCK) Nanoparticles
4.3. Natural Occurring Biopolymers
5. Inorganic Nanocarriers
5.1. Zeolite Nanocrystals
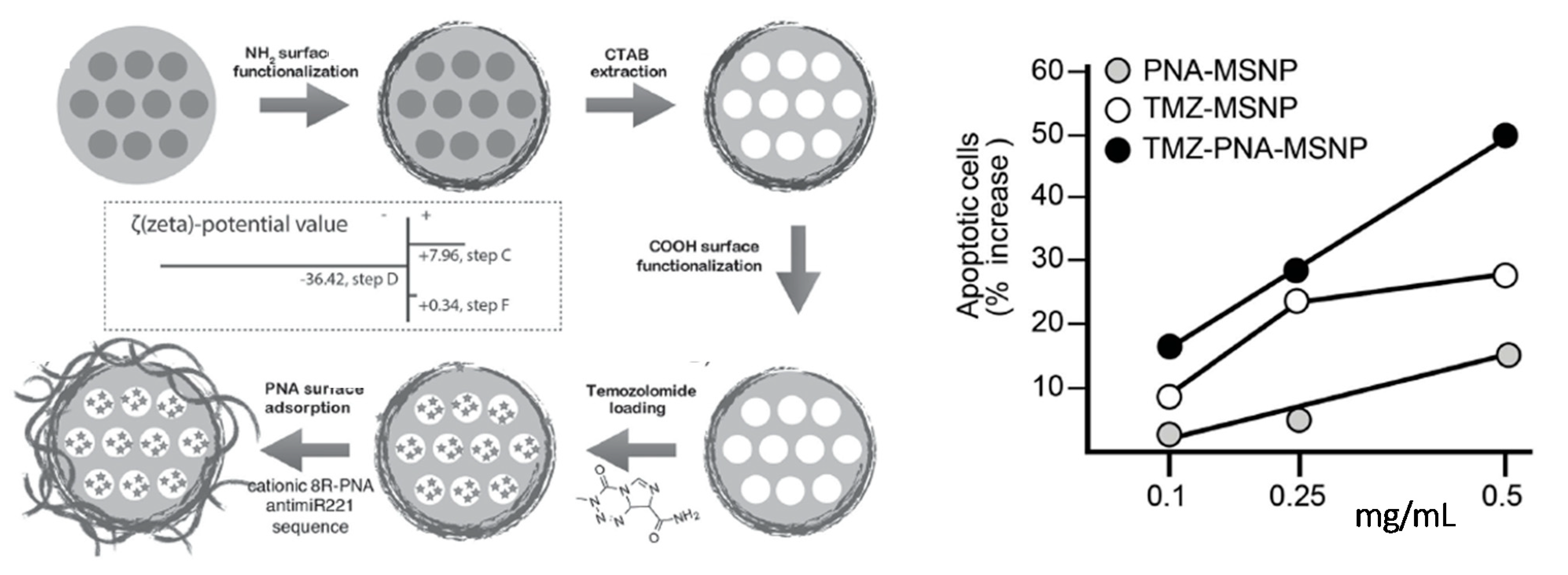
5.2. Mesoporous Silica Nanoparticles (MSNPs)
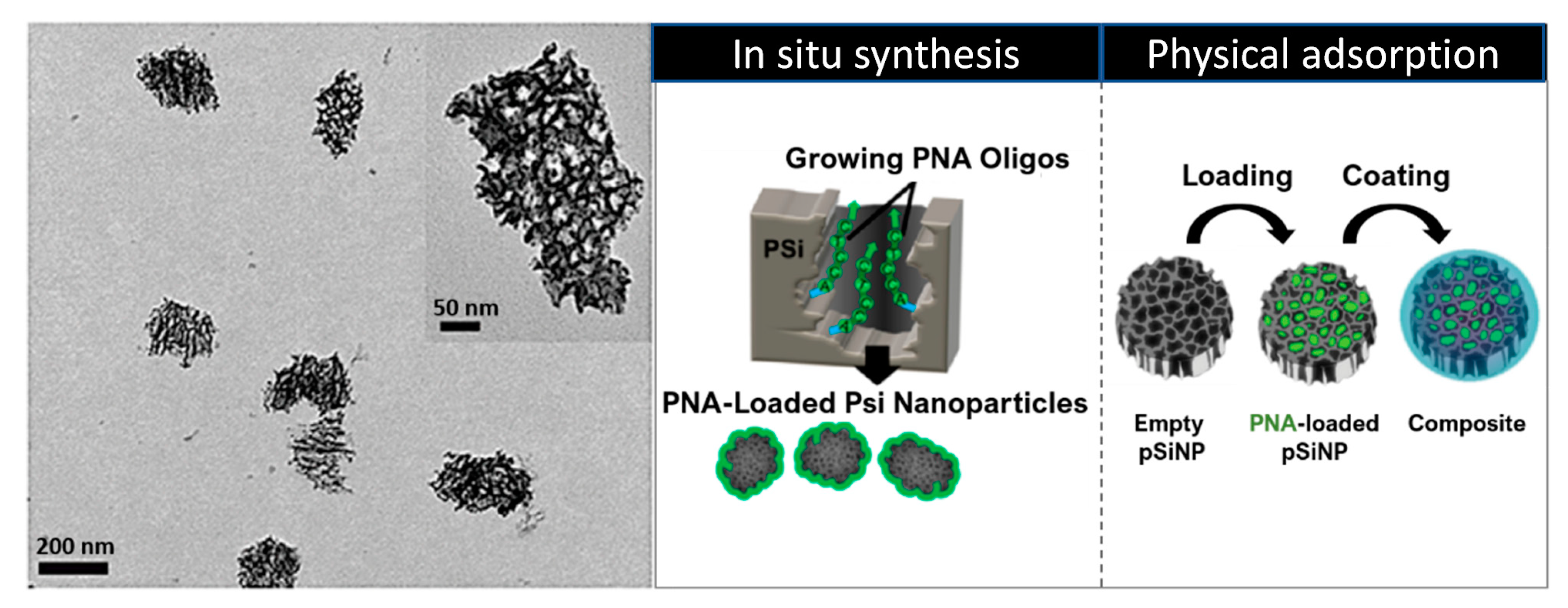
5.3. Porous Silicon
5.4. Miscellaneous Inorganic Nanocarriers
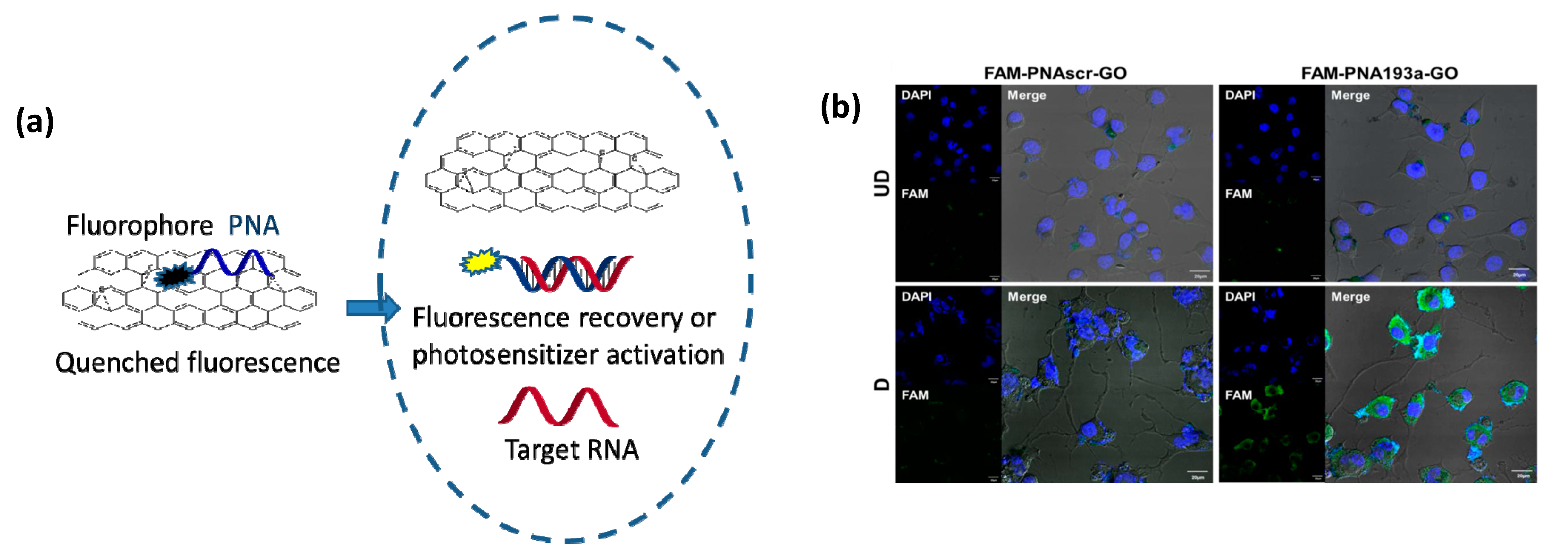
6. Carbon-Based Nanocarriers
7. Supramolecular Multifunctional Systems
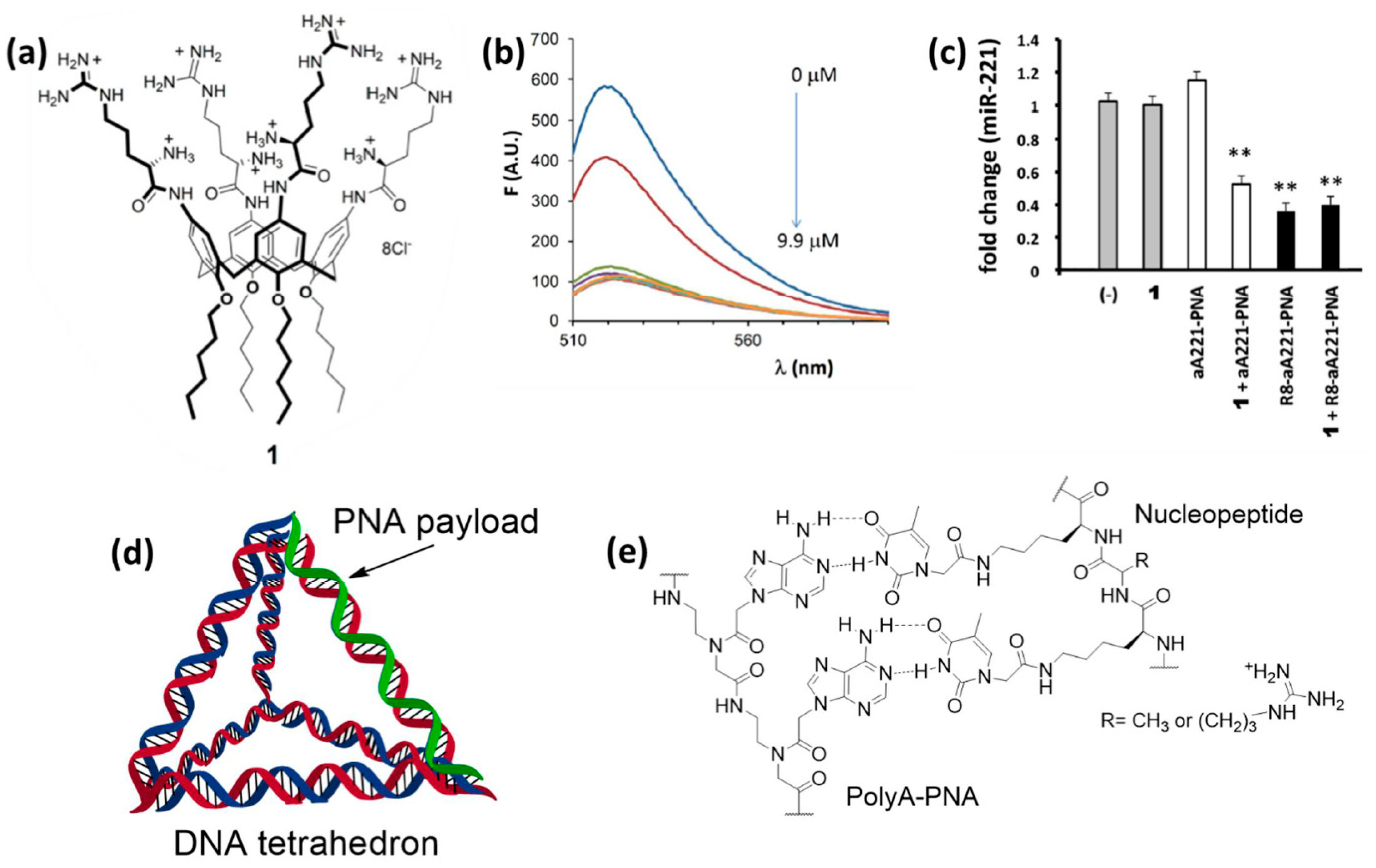
7.1. Peptide, Protein, and PNA Nanoparticles
7.2. Cationic Calixarenes
7.3. DNA Nanostructures and Nucleopeptides
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nielsen, P.; Egholm, M.; Berg, R.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nat. Cell Biol. 1993, 365, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.C.; A Thomson, S.; Veal, J.M.; Davis, D.G. NMR solution structure of a peptide nucleic acid complexed with RNA. Science 1994, 265, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Wittung, P.; Nielsen, P.; Nordén, B.; Wittung-Stafshede, P. Direct Observation of Strand Invasion by Peptide Nucleic Acid (PNA) into Double-Stranded DNA. J. Am. Chem. Soc. 1996, 118, 7049–7054. [Google Scholar] [CrossRef]
- Nielsen, P.E. Targeting Double Stranded DNA with Peptide Nucleic Acid (PNA). Curr. Med. Chem. 2001, 8, 545–550. [Google Scholar] [CrossRef]
- Saarbach, J.; Sabale, P.M.; Winssinger, N. Peptide nucleic acid (PNA) and its applications in chemical biology, diagnostics, and therapeutics. Curr. Opin. Chem. Biol. 2019, 52, 112–124. [Google Scholar] [CrossRef]
- Nielsen, P.E. Gene Targeting and Expression Modulation by Peptide Nucleic Acids (PNA). Curr. Pharm. Des. 2010, 16, 3118–3123. [Google Scholar] [CrossRef]
- Nielsen, P.E. Peptide Nucleic Acids (PNA) in Chemical Biology and Drug Discovery. Chem. Biodivers. 2010, 7, 786–804. [Google Scholar] [CrossRef]
- Dong, B.; Nie, K.; Shi, H.; Chao, L.; Ma, M.; Gao, F.; Liang, B.; Chen, W.; Long, M.; Liu, Z. Film-Spotting chiral miniPEG-γPNA array for BRCA1 gene mutation detection. Biosens. Bioelectron. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Zhang, N.; Appella, D.H. Colorimetric Detection of Anthrax DNA with a Peptide Nucleic Acid Sandwich-Hybridization Assay. J. Am. Chem. Soc. 2007, 129, 8424–8425. [Google Scholar] [CrossRef]
- D’Agata, R.; Bellassai, N.; Allegretti, M.; Rozzi, A.; Korom, S.; Manicardi, A.; Melucci, E.; Pescarmona, E.; Corradini, R.; Giacomini, P.; et al. Direct plasmonic detection of circulating RAS mutated DNA in colorectal cancer patients. Biosens. Bioelectron. 2020, 170, 112648. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pearse, A.; Liu, Y.; Taylor, R.E. Modular self-assembly of gamma-modified peptide nucleic acids in organic solvent mixtures. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Swenson, C.S.; Heemstra, J.M. Peptide nucleic acids harness dual information codes in a single molecule. Chem. Commun. 2020, 56, 1926–1935. [Google Scholar] [CrossRef]
- Totsingan, F.; Marchelli, R.; Corradini, R. Molecular Computing by PNA. Artif. DNA PNA XNA 2011, 2, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidov, V.V.; Potaman, V.N.; Frank-Kamenetskil, M.; Egholm, M.; Buchard, O.; Sönnichsen, S.H.; Nlelsen, P.E. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem. Pharmacol. 1994, 48, 1310–1313. [Google Scholar] [CrossRef]
- Wittung, P.; Nielsen, P.E.; Buchardt, O.; Egholm, M.; Norde, B. DNA-like double helix formed by peptide nucleic acid. Nat. Cell Biol. 1994, 368, 561–563. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Giuffrida, M.C.; Spoto, G. Peptide Nucleic Acid-Based Biosensors for Cancer Diagnosis. Molecules 2017, 22, 1951. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Oh, B.-K.; Choi, J.-W. Application of peptide nucleic acid towards development of nanobiosensor arrays. Bioelectrochemistry 2010, 79, 153–161. [Google Scholar] [CrossRef]
- Tedeschi, T.; Sforza, S.; Dossena, A.; Corradini, R.; Marchelli, R. Lysine-based peptide nucleic acids (PNAs) with strong chiral constraint: Control of helix handedness and DNA binding by chirality. Chirality 2005, 17, S196–S204. [Google Scholar] [CrossRef]
- Sacui, I.; Hsieh, W.-C.; Manna, A.; Sahu, B.; Ly, D.H. Gamma Peptide Nucleic Acids: As Orthogonal Nucleic Acid Recognition Codes for Organizing Molecular Self-Assembly. J. Am. Chem. Soc. 2015, 137, 8603–8610. [Google Scholar] [CrossRef]
- Liu, Y.; Braasch, D.A.; Nulf, C.J.; Corey, D.R. Efficient and Isoform-Selective Inhibition of Cellular Gene Expression by Peptide Nucleic Acids†. Biochemistry 2004, 43, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, G.D.; Arzumanov, A.; Abes, R.; Yin, H.; Wood, M.J.A.; LeBleu, B.; Gait, M.J. Improved cell-penetrating peptide–PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008, 36, 6418–6428. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Betts, C.A.; Saleh, A.F.; Ivanova, G.D.; Lee, H.; Seow, Y.; Kim, D.; Gait, M.J.; Wood, M.J.A. Optimization of Peptide Nucleic Acid Antisense Oligonucleotides for Local and Systemic Dystrophin Splice Correction in the mdx Mouse. Mol. Ther. 2010, 18, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Kim, S.K.; Yoon, J.W. Antisense peptide nucleic acids as a potential anti-infective agent. J. Microbiol. 2019, 57, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, A.; Nielsen, P.E. Potent Antibacterial Antisense Peptide–Peptide Nucleic Acid Conjugates Against Pseudomonas aeruginosa. Nucleic Acid Ther. 2012, 22, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Good, L.; Stach, J.E.M. Synthetic RNA Silencing in Bacteria? Antimicrobial Discovery and Resistance Breaking. Front. Microbiol. 2011, 2, 185. [Google Scholar] [CrossRef] [Green Version]
- Cutrona, G.; Carpaneto, E.M.; Ulivi, M.; Roncella, S.; Landt, O.; Ferrarini, M.; Boffa, L.C. Effects in live cells of a c-myc anti-gene PNA linked to a nuclear localization signal. Nat. Biotechnol. 2000, 18, 300–303. [Google Scholar] [CrossRef]
- Tonelli, R.; Purgato, S.; Camerin, C.; Fronza, R.; Bologna, F.; Alboresi, S.; Franzoni, M.; Corradini, R.; Sforza, S.; Faccini, A.; et al. Anti-gene peptide nucleic acid specifically inhibits MYCN expression in human neuroblastoma cells leading to cell growth inhibition and apoptosis. Mol. Cancer Ther. 2005, 4, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Corey, D.R. Inhibiting Gene Expression with Peptide Nucleic Acid (PNA)−Peptide Conjugates That Target Chromosomal DNA†. Biochemistry 2007, 46, 7581–7589. [Google Scholar] [CrossRef] [Green Version]
- Fabani, M.M.; Abreu-Goodger, C.; Williams, D.; Lyons, P.A.; Torres, A.G.; Smith, K.G.C.; Enright, A.J.; Gait, M.J.; Vigorito, E. Efficient inhibition of miR-155 function in vivo by peptide nucleic acids. Nucleic Acids Res. 2010, 38, 4466–4475. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.N.; Liu, C.; A Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nat. Cell Biol. 2015, 518, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambari, R.; Fabbri, E.; Borgatti, M.; Lampronti, I.; Finotti, A.; Brognara, E.; Bianchi, N.; Manicardi, A.; Marchelli, R.; Corradini, R. Targeting microRNAs involved in human diseases: A novel approach for modification of gene expression and drug development. Biochem. Pharmacol. 2011, 82, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Fabani, M.M.; Gait, M.J. miR-122 targeting with LNA/2’-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA 2007, 14, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgatti, M.; Lampronti, I.; Romanelli, A.; Pedone, C.; Saviano, M.; Bianchi, N.; Mischiati, C.; Gambari, R. Transcription Factor Decoy Molecules Based on a Peptide Nucleic Acid (PNA)-DNA Chimera Mimicking Sp1 Binding Sites. J. Biol. Chem. 2002, 278, 7500–7509. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, H.; Nielsen, P.E. Antisense Properties of Duplex- and Triplex-Forming PNAs. Nucleic Acids Res. 1996, 24, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Hanvey, J.C.; Peffer, N.J.; E Bisi, J.; A Thomson, S.; Cadilla, R.; A Josey, J.; Ricca, D.J.; Hassman, C.F.; A Bonham, M.; Au, K.G.; et al. Antisense and antigene properties of peptide nucleic acids. Science 1992, 258, 1481–1485. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, A.; Puri, N. Peptide nucleic acids: Advanced tools for biomedical applications. J. Biotechnol. 2017, 259, 148–159. [Google Scholar] [CrossRef]
- Economos, N.G.; Oyaghire, S.; Quijano, E.; Ricciardi, A.S.; Saltzman, W.M.; Glazer, P.M. Peptide Nucleic Acids and Gene Editing: Perspectives on Structure and Repair. Molecules 2020, 25, 735. [Google Scholar] [CrossRef] [Green Version]
- Koppelhus, U.; E Nielsen, P. Cellular delivery of peptide nucleic acid (PNA). Adv. Drug Deliv. Rev. 2003, 55, 267–280. [Google Scholar] [CrossRef]
- Gupta, A.; Bahal, R.; Gupta, M.; Glazer, P.M.; Saltzman, W.M. Nanotechnology for delivery of peptide nucleic acids (PNAs). J. Control. Release 2016, 240, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Malik, S.; Asmara, B.; Moscato, Z.; Mukker, J.K.; Bahal, R. Advances in Nanoparticle-based Delivery of Next Generation Peptide Nucleic Acids. Curr. Pharm. Des. 2019, 24, 5164–5174. [Google Scholar] [CrossRef] [PubMed]
- Manicardi, A.; Rozzi, A.; Korom, S.; Corradini, R. Building on the peptide nucleic acid (PNA) scaffold: A biomolecular engineering approach. Supramol. Chem. 2017, 29, 784–795. [Google Scholar] [CrossRef]
- Adlerz, L.; Soomets, U.; Holmlund, L.; Viirlaid, S.; Langel, Ü.; Iverfeldt, K. Down-regulation of amyloid precursor protein by peptide nucleic acid oligomer in cultured rat primary neurons and astrocytes. Neurosci. Lett. 2003, 336, 55–59. [Google Scholar] [CrossRef]
- Good, L.; Sandberg, R.; Larsson, O.; Nielsen, P.E.; Wahlestedt, C. Antisense PNA effects in Escherichia coli are limited by the outer-membrane LPS layer. Microbiology 2000, 146, 2665–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschman, S.Z.; Chen, C.W. Peptide nucleic acids stimulate gamma interferon and inhibit the replication of the human immunodeficiency virus. J. Investig. Med. 1996, 44, 347–351. [Google Scholar] [PubMed]
- Wang, G.; Xu, X.; Pace, B.; Dean, D.A.; Glazer, P.M.; Chan, P.; Goodman, S.R.; Shokolenko, I. Peptide nucleic acid (PNA) binding-mediated induction of human-globin gene expression. Nucleic Acids Res. 1999, 27, 2806–2813. [Google Scholar] [CrossRef] [Green Version]
- Doyle, D.F.; Braasch, D.A.; Janowski, B.A.; Corey, D.R. Inhibition of Gene Expression Inside Cells by Peptide Nucleic Acids: Effect of mRNA Target Sequence, Mismatched Bases, and PNA Length. Biochemistry 2001, 40, 53–64. [Google Scholar] [CrossRef]
- Faruqi, A.F.; Egholm, M.; Glazer, P.M. Peptide nucleic acid-targeted mutagenesis of a chromosomal gene in mouse cells. Proc. Natl. Acad. Sci. USA 1998, 95, 1398–1403. [Google Scholar] [CrossRef] [Green Version]
- Mitra, R.; Ganesh, K.N. Aminomethylene Peptide Nucleic Acid (am-PNA): Synthesis, Regio-/Stereospecific DNA Binding, And Differential Cell Uptake of (α/γ,R/S)am-PNA Analogues. J. Org. Chem. 2012, 77, 5696–5704. [Google Scholar] [CrossRef]
- Kumar, P.; Jain, D.R. Cγ-Aminopropylene peptide nucleic acid (amp-PNA): Chiral cationic PNAs with superior PNA:DNA/RNA duplex stability and cellular uptake. Tetrahedron 2015, 71, 3378–3384. [Google Scholar] [CrossRef]
- Delgado, E.; Bahal, R.; Yang, J.; Lee, J.M.; Ly, D.H.; Monga, S.P. β-Catenin Knockdown in Liver Tumor Cells by a Cell Permeable Gamma Guanidine-based Peptide Nucleic Acid. Curr. Cancer Drug Targets 2013, 13, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Dragulescu-Andrasi, A.; Rapireddy, S.; He, G.; Bhattacharya, B.; Hyldig-Nielsen, J.J.; Zon, G.; Ly, D.H. Cell-Permeable Peptide Nucleic Acid Designed to Bind to the 5‘-Untranslated Region of E-cadherin Transcript Induces Potent and Sequence-Specific Antisense Effects. J. Am. Chem. Soc. 2006, 128, 16104–16112. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Sahu, B.; Rapireddy, S.; Bahal, R.; Wheeler, S.E.; Procopio, E.M.; Kim, J.; Joyce, S.C.; Contrucci, S.; Wang, Y.; et al. Antitumor Effects of EGFR Antisense Guanidine-Based Peptide Nucleic Acids in Cancer Models. ACS Chem. Biol. 2012, 8, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Wang, M.; Du, L.; Fisher, G.W.; Waggoner, A.; Ly, D.H. Novel Binding and Efficient Cellular Uptake of Guanidine-Based Peptide Nucleic Acids (GPNA). J. Am. Chem. Soc. 2003, 125, 6878–6879. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Sacui, I.; Chenna, V.; Lathrop, K.L.; Thomas, S.M.; Zon, G.; Livak, K.J.; Ly, D.H. Synthesis of Conformationally Preorganized and Cell-Permeable Guanidine-Based gamma-Peptide Nucleic Acids (gamma GPNAs). J. Org. Chem. 2009, 74, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Sforza, S.; Tedeschi, T.; Corradini, R.; Marchelli, R. Induction of Helical Handedness and DNA Binding Properties of Peptide Nucleic Acids (PNAs) with Two Stereogenic Centres. Eur. J. Org. Chem. 2007, 2007, 5879–5885. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kittaka, A. Chiral Peptide Nucleic Acids with a Substituent in the N-(2-Aminoethy) glycine Backbone. Molecules 2012, 18, 287–310. [Google Scholar] [CrossRef] [Green Version]
- Corradini, R.; Sforza, S.; Tedeschi, T.; Totsingan, F.; Manicardi, A.; Marchelli, R. Peptide Nucleic Acids with a Structurally Biased Backbone. Updated Review and Emerging Challenges. Curr. Top. Med. Chem. 2011, 11, 1535–1554. [Google Scholar] [CrossRef]
- Uğurlu, Ö.; Barlas, F.B.; Evran, S.; Timur, S. The cell-penetrating YopM protein-functionalized quantum dot-plasmid DNA conjugate as a novel gene delivery vector. Plasmid 2020, 110, 102513. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Artemov, D. Theranostic Pretargeting Drug Delivery and Imaging Platforms in Cancer Precision Medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Jarreau, C.; Welch, M.J.; Taylor, J.-S.A. Nucleic acid-directed self-assembly of multifunctional gold nanoparticle imaging agents. Biomater. Sci. 2013, 1, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Abes, S.; Williams, D.; Prevot, P.; Thierry, A.; Gait, M.J.; LeBleu, B. Endosome trapping limits the efficiency of splicing correction by PNA-oligolysine conjugates. J. Control. Release 2006, 110, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.F.; Arzumanov, A.; Abes, R.; Owen, D.; LeBleu, B.; Gait, M.J. Synthesis and Splice-Redirecting Activity of Branched, Arginine-Rich Peptide Dendrimer Conjugates of Peptide Nucleic Acid Oligonucleotides. Bioconjugate Chem. 2010, 21, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Peschke, P.; Pipkorn, R.; Lampel, S.; Wachsmuth, M.; Waldeck, W.; Friedrich, E.; Debus, J. A Biological Transporter for the Delivery of Peptide Nucleic Acids (PNAs) to the Nuclear Compartment of Living Cells. J. Mol. Biol. 2002, 318, 237–243. [Google Scholar] [CrossRef]
- Chen, S.-S.; Tu, X.-Y.; Xie, L.-X.; Xiong, L.-P.; Song, J.; Ye, X.-Q. Peptide nucleic acids targeting mitochondria enhances sensitivity of lung cancer cells to chemotherapy. Am. J. Transl. Res. 2018, 10, 2940–2948. [Google Scholar]
- Lamla, M.; Seliger, H.; Kaufmann, D. Differences in uptake, localization, and processing of PNAs modified by COX VIII pre-sequence peptide and by triphenylphoshonium cation into mitochondria of tumor cells. Drug Deliv. 2010, 17, 263–271. [Google Scholar] [CrossRef]
- McMahon, B.M.; Mays, D.; Lipsky, J.; Stewart, J.A.; Fauq, A.; Richelson, E. Pharmacokinetics and Tissue Distribution of a Peptide Nucleic Acid After Intravenous Administration. Antisense Nucleic Acid Drug Dev. 2002, 12, 65–70. [Google Scholar] [CrossRef]
- Ren, J.; Shen, S.; Wang, D.; Xi, Z.; Guo, L.; Pang, Z.; Qian, Y.; Sun, X.; Jiang, X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 2012, 33, 3324–3333. [Google Scholar] [CrossRef]
- Ljungstrøm, T.; Knudsen, H.; Nielsen, P.E. Cellular uptake of adamantyl conjugated peptide nucleic acids. Bioconjugate Chem. 1999, 10, 965–972. [Google Scholar] [CrossRef]
- Muratovska, A.; Lightowlers, R.N.; Taylor, R.W.; Turnbull, D.M.; Smith, R.A.J.; Wilce, J.A.; Martin, S.T.W.; Murphy, M.P. Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: Implications for mitochondrial DNA replication, expression and disease. Nucleic Acids Res. 2001, 29, 1852–1863. [Google Scholar] [CrossRef] [Green Version]
- Biessen, E.A.L.; Sliedregt-Bol, K.; Chr’T Hoen, P.A.; Prince, P.; Van Der Bilt, E.; Valentijn, A.R.P.M.; Meeuwenoord, N.J.; Princen, H.M.; Bijsterbosch, M.K.; Van Der Marel, G.A.; et al. Design of a Targeted Peptide Nucleic Acid Prodrug to Inhibit Hepatic Human Microsomal Triglyceride Transfer Protein Expression in Hepatocytes†. Bioconjugate Chem. 2002, 13, 295–302. [Google Scholar] [CrossRef]
- Van Rossenberg, S.M.W.; Sliedregt-Bol, K.M.; Prince, P.; Van Berkel, T.J.C.; Van Boom, J.H.; Van Der Marel, G.A.; Biessen, E.A.L. A Targeted Peptide Nucleic Acid to Down-Regulate Mouse Microsomal Triglyceride Transfer Protein Expression in Hepatocytes. Bioconjugate Chem. 2003, 14, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Bhingardeve, P.; Madhanagopal, B.R.; Naick, H.; Jain, P.; Manoharan, M.; Ganesh, K.N. Receptor-Specific Delivery of Peptide Nucleic Acids Conjugated to Three Sequentially Linked N-Acetyl Galactosamine Moieties into Hepatocytes. J. Org. Chem. 2020, 85, 8812–8824. [Google Scholar] [CrossRef] [PubMed]
- Gabas, I.M.; Nielsen, P.E. Effective Cellular Delivery of Antisense Peptide Nucleic Acid by Conjugation to Guanidinylated Diaminobutanoic Acid-Based Peptide Dendrons. Biomacromolecules 2019, 21, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Równicki, M.; Wojciechowska, M.; Wierzba, A.J.; Czarnecki, J.; Bartosik, D.; Gryko, D.; Trylska, J. Vitamin B12 as a carrier of peptide nucleic acid (PNA) into bacterial cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Równicki, M.; Dąbrowska, Z.; Wojciechowska, M.; Wierzba, A.J.; Maximova, K.; Gryko, D.; Trylska, J. Inhibition of Escherichia coli Growth by Vitamin B12–Peptide Nucleic Acid Conjugates. ACS Omega 2019, 4, 819–824. [Google Scholar] [CrossRef]
- Wierzba, A.J.; Maximova, K.; Wincenciuk, A.; Równicki, M.; Wojciechowska, M.; Nexo, E.; Trylska, J.; Gryko, D. Does a Conjugation Site Affect Transport of Vitamin B 12 –Peptide Nucleic Acid Conjugates into Bacterial Cells? Chem. A Eur. J. 2018, 24, 18772–18778. [Google Scholar] [CrossRef]
- Shen, G.; Fang, H.; Song, Y.; Bielska, A.A.; Wang, Z.; Taylor, J.-S.A. Phospholipid Conjugate for Intracellular Delivery of Peptide Nucleic Acids. Bioconjugate Chem. 2009, 20, 1729–1736. [Google Scholar] [CrossRef] [Green Version]
- Zorko, M.; Langel, U. Cell-penetrating peptides: Mechanism and kinetics of cargo delivery. Adv. Drug Deliv. Rev. 2005, 57, 529–545. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Pooga, M.; Soomets, U.; Hällbrink, M.; Valkna, A.; Saar, K.; Rezaei, K.; Kahl, U.; Hao, J.-X.; Xu, X.-J.; Wiesenfeld-Hallin, Z.; et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 1998, 16, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.A.; Lin, S.S.; Hegan, D.C.; Krause, D.S.; Glazer, P.M. Targeted Gene Modification of Hematopoietic Progenitor Cells in Mice Following Systemic Administration of a PNA-peptide Conjugate. Mol. Ther. 2012, 20, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Bruchez, M.P.; Armitage, B.A. Closing the Loop: Constraining TAT Peptide by γPNA Hairpin for Enhanced Cellular Delivery of Biomolecules. Bioconjugate Chem. 2018, 29, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Zoonens, M.; Reshetnyak, Y.K.; Engelman, N.M. Bilayer Interactions of pHLIP, a Peptide that Can Deliver Drugs and Target Tumors. Biophys. J. 2008, 95, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gait, M.J.; Arzumanov, A.A.; McClorey, G.; Godfrey, C.; Betts, C.; Hammond, S.; Wood, M.J. Cell-Penetrating Peptide Conjugates of Steric Blocking Oligonucleotides as Therapeutics for Neuromuscular Diseases from a Historical Perspective to Current Prospects of Treatment. Nucleic Acid Ther. 2019, 29, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gambari, R.; Gasparello, J.; Fabbri, E.; Borgatti, M.; Tamanini, A.; Finotti, A. Peptide Nucleic Acids for MicroRNA Targeting. In Peptide Nucleic Acids: Methods and Protocols; Nielsen, P.E., Ed.; Springer: New York, NY, USA, 2020; pp. 199–215. ISBN 978-1-0716-0243-0. [Google Scholar]
- Wojciechowska, M.; Równicki, M.; Mieczkowski, A.; Miszkiewicz, J.; Trylska, J. Antibacterial Peptide Nucleic Acids—Facts and Perspectives. Molecules 2020, 25, 559. [Google Scholar] [CrossRef] [Green Version]
- Goltermann, L.; Nielsen, P.E. PNA Antisense Targeting in Bacteria: Determination of Antibacterial Activity (MIC) of PNA-Peptide Conjugates. In Peptide Nucleic Acids: Methods and Protocols; Nielsen, P.E., Ed.; Springer: New York, NY, USA, 2020; pp. 231–239. ISBN 978-1-0716-0243-0. [Google Scholar]
- Fabbri, E.; Tamanini, A.; Jakova, T.; Gasparello, J.; Manicardi, A.; Corradini, R.; Sabbioni, G.; Finotti, A.; Borgatti, M.; Lampronti, I.; et al. A Peptide Nucleic Acid against MicroRNA miR-145-5p Enhances the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Calu-3 Cells. Molecules 2017, 23, 71. [Google Scholar] [CrossRef] [Green Version]
- Brognara, E.; Fabbri, E.; Aimi, F.; Manicardi, A.; Bianchi, N.; Finotti, A.; Breveglieri, G.; Borgatti, M.; Corradini, R.; Marchelli, R.; et al. Peptide nucleic acids targeting miR-221 modulate p27Kip1 expression in breast cancer MDA-MB-231 cells. Int. J. Oncol. 2012, 41, 2119–2127. [Google Scholar] [CrossRef] [Green Version]
- Ndeboko, B.; Ramamurthy, N.; Lemamy, G.J.; Jamard, C.; Nielsen, P.E.; Cova, L. Role of Cell-Penetrating Peptides in Intracellular Delivery of Peptide Nucleic Acids Targeting Hepadnaviral Replication. Mol. Ther. Nucleic Acids 2017, 9, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Ndeboko, B.; Hantz, O.; Lemamy, G.J.; Cova, L. Developments in Cell-Penetrating Peptides as Antiviral Agents and as Vehicles for Delivery of Peptide Nucleic Acid Targeting Hepadnaviral Replication Pathway. Biomolecules 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, W.B.; Guha, S.; Wimley, W.C. Synthetic molecular evolution of hybrid cell penetrating peptides. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soudah, T.; Mogilevsky, M.; Karni, R.; Yavin, E. CLIP6-PNA-Peptide Conjugates: Non-Endosomal Delivery of Splice Switching Oligonucleotides. Bioconjugate Chem. 2017, 28, 3036–3042. [Google Scholar] [CrossRef]
- Lundin, P.; Johansson, H.; Guterstam, P.; Holm, T.; Hansen, M.; Langel, Ü.; El Andaloussi, S. Distinct Uptake Routes of Cell-Penetrating Peptide Conjugates. Bioconjugate Chem. 2008, 19, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.E. Addressing the challenges of cellular delivery and bioavailability of peptide nucleic acids (PNA). Q. Rev. Biophys. 2005, 38, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Brock, R.; Fotin-Mleczek, M. Endocytosis and Cationic Cell-Penetrating Peptides—A Merger of Concepts and Methods. Curr. Pharm. Des. 2005, 11, 3613–3628. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Simmons, C.G.; Kathiriya, I.S.; Corey, D.R. Cellular delivery of peptide nucleic acids and inhibition of human telomerase. Chem. Biol. 1999, 6, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.M.; Kim, M.H.; Yu, G.S.; Um, B.H.; Park, H.K.; Lee, H.-I.; Lee, K.T.; Suh, Y.D.; Choi, J.S. Enhanced splicing correction effect by an oligo-aspartic acid–PNA conjugate and cationic carrier complexes. J. Control. Release 2014, 175, 54–62. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, H.J. PEGylated DC-Chol/DOPE cationic liposomes containing KSP siRNA as a systemic siRNA delivery Carrier for ovarian cancer therapy. Biochem. Biophys. Res. Commun. 2018, 503, 1716–1722. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Yu, B.; Wang, X.; Lu, Y.; Schmidt, C.R.; Lee, R.J.; Lee, L.J.; Jacob, S.T.; Ghoshal, K. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.; Accardo, A.; Ringhieri, P.; Morelli, G.; Saviano, M.; Montagner, G.; Fabbri, E.; Gallerani, E.; Gambari, R.; Romanelli, A. Incorporation of Naked Peptide Nucleic Acids into Liposomes Leads to Fast and Efficient Delivery. Bioconjugate Chem. 2015, 26, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Ringhieri, P.; Avitabile, C.; Saviano, M.; Morelli, G.; Romanelli, A.; Accardo, A. The influence of liposomal formulation on the incorporation and retention of PNA oligomers. Colloids Surfaces B Biointerfaces 2016, 145, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.K.; Mattern-Schain, S.I.; Best, M.D.; Kirkpatrick, S.S.; Freeman, M.B.; Grandas, O.H.; Mountain, D.J. Improving the efficacy of liposome-mediated vascular gene therapy via lipid surface modifications. J. Surg. Res. 2017, 219, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, M.; Shiraishi, T.; Nielsen, P.E. Enzyme-Triggered Release of the Antisense Octaarginine-PNA Conjugate from Phospholipase A2 Sensitive Liposomes. ACS Appl. Bio Mater. 2020, 3, 1018–1025. [Google Scholar] [CrossRef]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Tasies, L.P.F.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef] [Green Version]
- Grijalvo, S.; Puras, G.; Zarate, J.; Sainz-Ramos, M.; Al Qtaish, N.; Lopez-Mendez, T.B.; Mashal, M.; Attia, N.; Díaz, D.D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Rad, A.T.; Malik, S.; Yang, L.; Oberoi-Khanuja, T.K.; Nieh, M.; Bahal, R. A universal discoidal nanoplatform for the intracellular delivery of PNAs. Nanoscale 2019, 11, 12517–12529. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Fields, R.J.; Quijano, E.; McNeer, N.A.; Caputo, C.; Bahal, R.; Anandalingam, K.; Egan, M.E.; Glazer, P.M.; Saltzman, W.M. Modified Poly (lactic-co-glycolic Acid) Nanoparticles for Enhanced Cellular Uptake and Gene Editing in the Lung. Adv. Heal. Mater. 2014, 4, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Fong, P.M.; Lu, J.; Russell, K.S.; Booth, C.J.; Saltzman, W.M.; Fahmy, T.M. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.J.; Saltzman, W.M. Enhanced siRNA delivery into cells by exploiting the synergy between targeting ligands and cell-penetrating peptides. Biomaterials 2011, 32, 6194–6203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynn, D.M.; Langer, R. Degradable Poly (β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Lynn, D.M.; Amiji, M.M.; Langer, R. pH-responsive polymer microspheres: Rapid release of encapsulated material within the range of intracellular pH. Angew. Chem. Int. Ed. 2001, 40, 1707–1710. [Google Scholar] [CrossRef]
- Little, S.R.; Lynn, D.M.; Ge, Q.; Anderson, D.G.; Puram, S.V.; Chen, J.; Eisen, H.N.; Langer, R.S. From The Cover: Poly-amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl. Acad. Sci. USA 2004, 101, 9534–9539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vlerken, L.E.; Duan, Z.; Little, S.R.; Seiden, M.V.; Amiji, M. Biodistribution and Pharmacokinetic Analysis of Paclitaxel and Ceramide Administered in Multifunctional Polymer-Blend Nanoparticles in Drug Resistant Breast Cancer Model. Mol. Pharm. 2008, 5, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Fields, R.J.; Cheng, C.J.; Quijano, E.; Weller, C.; Kristofik, N.; Duong, N.; Hoimes, C.; Egan, M.E.; Saltzman, W.M. Surface modified poly (β amino ester)-containing nanoparticles for plasmid DNA delivery. J. Control Release 2012, 164, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.J.; Saltzman, W.M. Polymer Nanoparticle-Mediated Delivery of MicroRNA Inhibition and Alternative Splicing. Mol. Pharm. 2012, 9, 1481–1488. [Google Scholar] [CrossRef] [Green Version]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhaas, J.B.; Saltzman, W.M.; Slack, F.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, E1695–E1704. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Fiori, M.E.; Albini, S.; Cifaldi, L.; Giovinazzi, S.; Forloni, M.; Boldrini, R.; Donfrancesco, A.; Federici, V.; Giacomini, P.; et al. Antagomir-17-5p Abolishes the Growth of Therapy-Resistant Neuroblastoma through p21 and BIM. PLoS ONE 2008, 3, e2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Reinhardt, F.; Pan, E.; Soutschek, J.; Bhat, B.; Marcusson, E.G.; Teruya-Feldstein, J.; Bell, G.W.; Weinberg, R.A. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol. 2010, 28, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Quijano, E.; Liu, Y.; Bahal, R.; Scanlon, S.E.; Song, E.; Hsieh, W.-C.; Braddock, D.E.; Ly, D.H.; Saltzman, W.M.; et al. Anti-tumor Activity of miniPEG-γ-Modified PNAs to Inhibit MicroRNA-210 for Cancer Therapy. Mol. Ther. Nucleic Acids 2017, 9, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, S.; Doyen, J.; Parks, S.K.; Bertero, T.; Paye, A.; Cardinaud, B.; Gounon, P.; Lacas-Gervais, S.; Noël, A.; Pouysségur, J.; et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013, 4, e544. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Huang, X.; Cao, H.; Christman-Skieller, C.; Bennewith, K.; Le, Q.-T.; Koong, A.C. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl. Oncol. 2010, 3, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.; Furong, W.; Li, B. Multiple functions of hypoxia-regulated miR-210 in cancer. J. Exp. Clin. Cancer Res. 2014, 33, 50. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.; Sacui, I.; Rapireddy, S.; Zanotti, K.J.; Bahal, R.; Armitage, A.A.; Ly, D.H. Synthesis and characterization of conformationally peptide nucleic acids with superior hybridization properties and water solubility. J. Org. Chem. 2011, 76, 5614–5627. [Google Scholar] [CrossRef] [Green Version]
- Dragulescu-Andrasi, A.; Rapireddy, S.; Frezza, B.M.; Gayathri, C.; Gil, R.R.; Ly, D.H. A Simple γ-Backbone Modification Preorganizes Peptide Nucleic Acid into a Helical Structure. J. Am. Chem. Soc. 2006, 128, 10258–10267. [Google Scholar] [CrossRef]
- Bahal, R.; McNeer, N.A.; Ly, D.H.; Saltzman, W.M.; Glazer, P.M. Nanoparticle for delivery of antisense γPNA oligomers targeting CCR5. Artif. DNA PNA XNA 2013, 4, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Samson, M.; Libert, F.; Doranz, B.J.; Rucker, J.; Liesnard, C.; Farber, C.-M.; Saragosti, S.; Lapouméroulie, C.; Cognaux, J.; Forceille, C.; et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nat. Cell Biol. 1996, 382, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.S.; Quijano, E.; Putman, R.; Saltzman, W.M.; Glazer, P.M. Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing. Molecules 2018, 23, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleifman, E.B.; Bindra, R.; Leif, J.; Del Campo, J.; Rogers, F.A.; Uchil, P.D.; Kutsch, O.; Shultz, L.D.; Kumar, P.; Greiner, D.L.; et al. Targeted Disruption of the CCR5 Gene in Human Hematopoietic Stem Cells Stimulated by Peptide Nucleic Acids. Chem. Biol. 2011, 18, 1189–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaihatsu, K.; Shah, R.H.; Zhao, X.; Corey, D.R. Extending Recognition by Peptide Nucleic Acids (PNAs): Binding to Duplex DNA and Inhibition of Transcription by Tail-Clamp PNA−Peptide Conjugates†. Biochemicals 2003, 42, 13996–14003. [Google Scholar] [CrossRef]
- Bentin, T.; Larsen, H.J.; Nielsen, P.E. Combined Triplex/Duplex Invasion of Double-Stranded DNA by “Tail-Clamp” Peptide Nucleic Acid†. Biochemicals 2003, 42, 13987–13995. [Google Scholar] [CrossRef]
- Bahal, R.; Sahu, B.; Rapireddy, S.; Lee, C.-M.; Ly, D.H. Sequence-Unrestricted, Watson-Crick Recognition of Double Helical B-DNA by (R)-MiniPEG-γPNAs. ChemBioChem 2011, 13, 56–60. [Google Scholar] [CrossRef]
- Yeh, J.I.; Shivachev, B.; Rapireddy, S.; Crawford, M.J.; Gil, R.R.; Du, S.; Madrid, M.; Ly, D.H. Crystal Structure of Chiral γPNA with Complementary DNA Strand: Insights into the Stability and Specificity of Recognition and Conformational Preorganization. J. Am. Chem. Soc. 2010, 132, 10717–10727. [Google Scholar] [CrossRef] [Green Version]
- Bahal, R.; Quijano, E.; McNeer, N.A.; Liu, Y.; Bhunia, D.C.; Lopez-Giraldez, F.; Fields, R.J.; Saltzman, W.M.; Ly, D.H.; Glazer, P.M. Single-Stranded γPNAs for In Vivo Site-Specific Genome Editing via Watson-Crick Recognition. Curr. Gene Ther. 2014, 14, 331–342. [Google Scholar] [CrossRef]
- Chin, J.Y.; Kuan, J.Y.; Lonkar, P.S.; Krause, D.S.; Seidman, M.M.; Peterson, K.R.; Nielsen, P.E.; Kole, R.; Glazer, P.M. Correction of a splice-site mutation in the beta-globin gene stimulated by triplex-forming peptide nucleic acids. Proc. Natl. Acad. Sci. USA 2008, 105, 13514–13519. [Google Scholar] [CrossRef] [Green Version]
- A McNeer, N.; Chin, J.Y.; Schleifman, E.B.; Fields, R.J.; Glazer, P.M.; Saltzman, W.M. Nanoparticles Deliver Triplex-forming PNAs for Site-specific Genomic Recombination in CD34+ Human Hematopoietic Progenitors. Mol. Ther. 2011, 19, 172–180. [Google Scholar] [CrossRef]
- McNeer, N.A.; Schleifman, E.B.; Cuthbert, A.; A Brehm, M.; Jackson, A.; Cheng, C.; Anandalingam, K.; Kumar, P.; Shultz, L.D.; Greiner, D.L.; et al. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene Ther. 2012, 20, 658–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, A.S.; Bahal, R.; Farrelly, J.S.; Quijano, E.; Bianchi, A.H.; Luks, V.L.; Putman, R.; López-Giráldez, F.; Coşkun, S.; Song, E.; et al. In utero nanoparticle delivery for site-specific genome editing. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; A Paxton, W.; Choe, S.; Ceradini, D.; Martin, S.R.; Horuk, R.; E MacDonald, M.; Stuhlmann, H.; A Koup, R.; Landau, N.R. Homozygous Defect in HIV-1 Coreceptor Accounts for Resistance of Some Multiply-Exposed Individuals to HIV-1 Infection. Cell 1996, 86, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Schleifman, E.B.; McNeer, N.A.; Jackson, A.; Yamtich, J.; A Brehm, M.; Shultz, L.D.; Greiner, D.L.; Kumar, P.; Saltzman, W.M.; Glazer, P.M. Site-specific Genome Editing in PBMCs With PLGA Nanoparticle-delivered PNAs Confers HIV-1 Resistance in Humanized Mice. Mol. Ther. Nucleic Acids 2013, 2, e135. [Google Scholar] [CrossRef]
- E Perez, E.; Wang, J.; Miller, J.C.; Jouvenot, Y.; A Kim, K.; Liu, O.; Wang, N.; Lee, G.; Bartsevich, V.V.; Lee, Y.-L.; et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic Fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef]
- McNeer, N.A.; Anandalingam, K.; Fields, R.J.; Caputo, C.; Kopic, S.; Gupta, A.; Quijano, E.; Polikoff, L.; Kong, Y.; Bahal, R.; et al. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat. Commun. 2015, 6, 6952. [Google Scholar] [CrossRef]
- Van Nostrum, C.F. Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter 2011, 7, 3246–3259. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Zhang, K.; Shen, G.; Wooley, K.L.; Taylor, J.-S.A. Cationic Shell-Cross-Linked Knedel-like (cSCK) Nanoparticles for Highly Efficient PNA Delivery. Mol. Pharm. 2009, 6, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.L.; Becker, M.L.; Li, X.; Taylor, J.-S.A.; Wooley, K.L. PNA-directed solution- and surface-assembly of shell crosslinked (SCK) nanoparticle conjugates. Soft Matter 2005, 1, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fang, H.; Wang, Z.; Taylor, J.-S.A.; Wooley, K.L. Cationic shell-crosslinked knedel-like nanoparticles for highly efficient gene and oligonucleotide transfection of mammalian cells. Biomaterials 2009, 30, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Cho, M.-J.; Kole, R. Up-Regulation of Luciferase Gene Expression with Antisense Oligonucleotides: Implications and Applications in Functional Assay Development†. Biochemicals 1998, 37, 6235–6239. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’H, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Hosogi, S.; Iwasaki, Y.; Yamada, T.; Komatani-Tamiya, N.; Hiramatsu, A.; Kohno, Y.; Ueda, M.; Arimoto, T.; Marunaka, Y. Effect of inducible nitric oxide synthase on apoptosis in Candida-induced acute lung injury. Biomed. Res. 2008, 29, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S. The effects of nitric oxide in acute lung injury. Vasc. Pharmacol. 2005, 43, 390–403. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Wooley, K.L.; Taylor, J.-S.A. Imaging mRNA Expression in Live Cells via PNA·DNA Strand Displacement-Activated Probes. J. Nucleic Acids 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, H.; Demidov, V.V.; Gildea, B.D.; Fiandaca, M.J.; Coull, J.C.; Frank-Kamenetskii, M.D. PNA Beacons for Duplex DNA. Antisense Nucleic Acid Drug Dev. 2001, 11, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S. Imaging intracellular RNA distribution and dynamics in living cells. Nat. Methods 2009, 6, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Shen, Y.; Pollack, K.A.; Taylor, J.-S.A.; Wooley, K.L. Dual Peptide Nucleic Acid- and Peptide-Functionalized Shell Cross-Linked Nanoparticles Designed to Target mRNA toward the Diagnosis and Treatment of Acute Lung Injury. Bioconjugate Chem. 2012, 23, 574–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Shrestha, R.; Ibricevic, A.; Gunsten, S.P.; Welch, M.J.; Wooley, K.L.; Brody, S.L.; Taylor, J.-S.A.; Liu, Y. Antisense peptide nucleic acid-functionalized cationic nanocomplex for in vivo mRNA detection. Interface Focus 2013, 3, 20120059. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Huang, S.; Yu, L.; Wang, Y.; Chen, H.; Wang, D. Self-assembled nanoparticles for cellular delivery of peptide nucleic acid using amphiphilic N,N,N-trimethyl-O-alkyl chitosan derivatives. J. Mater. Sci. Mater. Electron. 2018, 29, 114. [Google Scholar] [CrossRef] [PubMed]
- Lülf, H.; Bertucci, A.; Septiadi, D.; Corradini, R.; De Cola, L. Multifunctional Inorganic Nanocontainers for DNA and Drug Delivery into Living Cells. Chem. A Eur. J. 2014, 20, 10900–10904. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Lülf, H.; Septiadi, D.; Manicardi, A.; Corradini, R.; De Cola, L. Intracellular Delivery of Peptide Nucleic Acid and Organic Molecules Using Zeolite-L Nanocrystals. Adv. Heal. Mater. 2014, 3, 1812–1817. [Google Scholar] [CrossRef]
- Bertucci, A. Alessandro Bertucci Hybrid Organic-Inorganic Interfaces for Biomedical Applications. Ph.D. Thesis, University of Parma, Parma, Italy, University of Strasbourg, Strasburg, France, March 2015. [Google Scholar]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Climent, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Maquieira, Á.; Amorós, P. Controlled Delivery Using Oligonucleotide-Capped Mesoporous Silica Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7281–7283. [Google Scholar] [CrossRef]
- Mackowiak, S.A.; Schmidt, A.; Weiss, V.; Argyo, C.; Von Schirnding, C.; Bein, T.; Bräuchle, C. Targeted Drug Delivery in Cancer Cells with Red-Light Photoactivated Mesoporous Silica Nanoparticles. Nano Lett. 2013, 13, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.A.; Argyo, C.; Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 8693–8699. [Google Scholar] [CrossRef]
- Sauer, A.M.; Schlossbauer, A.; Ruthardt, N.; Cauda, V.A.; Bein, T.; Braäuchle, C. Role of Endosomal Escape for Disulfide-Based Drug Delivery from Colloidal Mesoporous Silica Evaluated by Live-Cell Imaging. Nano Lett. 2010, 10, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Li, P.-Z.; Nguyen, K.T.; Wang, X.; Luo, Z.; Zhang, H.; Tan, N.S.; Zhao, Y. Biocompatible, Uniform, and Redispersible Mesoporous Silica Nanoparticles for Cancer-Targeted Drug Delivery In Vivo. Adv. Funct. Mater. 2013, 24, 2450–2461. [Google Scholar] [CrossRef]
- Kruk, M.; Cao, L. Pore Size Tailoring in Large-Pore SBA-15 Silica Synthesized in the Presence of Hexane. Langmuir 2007, 23, 7247–7254. [Google Scholar] [CrossRef]
- Moeller, K.; Müller, K.; Engelke, H.; Bräuchle, C.; Wagner, E.; Bein, T. Highly efficient siRNA delivery from core–shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale 2016, 8, 4007–4019. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-H.; Tsai, P.-H.; Chen, W.; Chiou, S.-H.; Mou, C.-Y. Dual delivery of siRNA and plasmid DNA using mesoporous silica nanoparticles to differentiate induced pluripotent stem cells into dopaminergic neurons. J. Mater. Chem. B 2017, 5, 3012–3023. [Google Scholar] [CrossRef]
- Bertucci, A.; Prasetyanto, E.A.; Septiadi, D.; Manicardi, A.; Brognara, E.; Gambari, R.; Corradini, R.; De Cola, L. Combined Delivery of Temozolomide and Anti-miR221 PNA Using Mesoporous Silica Nanoparticles Induces Apoptosis in Resistant Glioma Cells. Small 2015, 11, 5687–5695. [Google Scholar] [CrossRef]
- Ma, X.; Devi, G.; Qu, Q.; Toh, D.-F.K.; Chen, G.; Zhao, Y. Intracellular Delivery of Antisense Peptide Nucleic Acid by Fluorescent Mesoporous Silica Nanoparticles. Bioconjugate Chem. 2014, 25, 1412–1420. [Google Scholar] [CrossRef]
- Prasetyanto, E.A.; Bertucci, A.; Septiadi, D.; Corradini, R.; Castro-Hartmann, P.; De Cola, L. Breakable Hybrid Organosilica Nanocapsules for Protein Delivery. Angew. Chem. Int. Ed. 2016, 55, 3323–3327. [Google Scholar] [CrossRef]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef] [PubMed]
- Canham, L.T. Silicon quantum wire array fabricaiton by electrochemical. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Canham, L.T. Bioactive Silicon Structure Fabrication through Nanoetching Techniques. Adv. Mater. 1995, 7, 1033–1037. [Google Scholar] [CrossRef]
- Bertucci, A.; Kim, K.-H.; Kang, J.; Zuidema, J.M.; Lee, S.H.; Kwon, E.J.; Kim, D.; Howell, S.B.; Ricci, F.; Ruoslahti, E.; et al. Tumor-Targeting, MicroRNA-Silencing Porous Silicon Nanoparticles for Ovarian Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23926–23937. [Google Scholar] [CrossRef] [Green Version]
- Beavers, K.R.; Mares, J.W.; Swartz, C.M.; Zhao, Y.; Weiss, S.M.; Duvall, C.L. In Situ Synthesis of Peptide Nucleic Acids in Porous Silicon for Drug Delivery and Biosensing. Bioconjugate Chem. 2014, 25, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Beavers, K.R.; Werfel, T.A.; Shen, T.; Kavanaugh, T.E.; Kilchrist, K.V.; Mares, J.W.; Fain, J.S.; Wiese, C.B.; Vickers, K.C.; Weiss, S.M.; et al. Porous Silicon and Polymer Nanocomposites for Delivery of Peptide Nucleic Acids as Anti-MicroRNA Therapies. Adv. Mater. 2016, 28, 7984–7992. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Kang, J.; Joo, J.; Kwon, E.J.; Skalak, M.; Hussain, S.; She, Z.-G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Self-Sealing Porous Silicon-Calcium Silicate Core-Shell Nanoparticles for Targeted siRNA Delivery to the Injured Brain. Adv. Mater. 2016, 28, 7962–7969. [Google Scholar] [CrossRef]
- Kwon, E.J.; Skalak, M.; Bertucci, A.; Braun, G.; Ricci, F.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Porous Silicon Nanoparticle Delivery of Tandem Peptide Anti-Infectives for the Treatment ofPseudomonas aeruginosaLung Infections. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef] [Green Version]
- Kelly, I.B.; Fletcher, R.B.; McBride, J.R.; Weiss, S.M.; Duvall, C.L. Tuning Composition of Polymer and Porous Silicon Composite Nanoparticles for Early Endosome Escape of Anti-microRNA Peptide Nucleic Acids. ACS Appl. Mater. Interfaces 2020, 12, 39602–39611. [Google Scholar] [CrossRef]
- Evans, B.C.; Fletcher, B.; Kilchrist, K.V.; Dailing, E.A.; Mukalel, A.J.; Colazo, J.M.; Oliver, M.; Cheung-Flynn, J.; Brophy, C.M.; Tierney, J.W.; et al. An anionic, endosome-escaping polymer to potentiate intracellular delivery of cationic peptides, biomacromolecules, and nanoparticles. Nat. Commun. 2019, 10, 5012–5019. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hu, P.; Xu, Y.; Bao, Q.; Ni, D.; Wei, C.; Shi, J. Efficient Gene Therapy of Pancreatic Cancer via a Peptide Nucleic Acid (PNA)-Loaded Layered Double Hydroxides (LDH) Nanoplatform. Small 2020, 16, e1907233. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Guerrini, A.; Cauteruccio, S.; Thakare, P.; Dova, D.; Orsini, F.; Arosio, P.; Carrara, C.; Sangregorio, C.; Lascialfari, A.; et al. Superparamagnetic iron oxide nanoparticles functionalized by peptide nucleic acids. RSC Adv. 2017, 7, 15500–15512. [Google Scholar] [CrossRef] [Green Version]
- Prencipe, G.; Maiorana, S.; Verderio, P.; Colombo, M.; Fermo, P.; Caneva, E.; Prosperi, D.; Licandro, E. Magnetic peptide nucleic acids for DNA targeting. Chem. Commun. 2009, 6017–6019. [Google Scholar] [CrossRef]
- Ghaffari, E.; Rezatofighi, S.E.; Ardakani, M.R.; Rastegarzadeh, S. Delivery of antisense peptide nucleic acid by gold nanoparticles for the inhibition of virus replication. Nanomedicine 2019, 14, 1827–1840. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.-J.; Min, D.-H. Emerging Approaches for Graphene Oxide Biosensor. Anal. Chem. 2016, 89, 232–248. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, J.; Park, H.; Chung, S.; Hwang, D.W.; Lee, D.S. Graphene-oxide quenching-based molecular beacon imaging of exosome-mediated transfer of neurogenic miR-193a on microfluidic platform. Biosens. Bioelectron. 2019, 126, 647–656. [Google Scholar] [CrossRef]
- Ryoo, S.-R.; Lee, J.; Yeo, J.; Na, H.-K.; Kim, Y.-K.; Jang, H.; Lee, J.H.; Han, S.W.; Lee, Y.; Kim, V.N.; et al. Quantitative and Multiplexed MicroRNA Sensing in Living Cells Based on Peptide Nucleic Acid and Nano Graphene Oxide (PANGO). ACS Nano 2013, 7, 5882–5891. [Google Scholar] [CrossRef]
- Hwang, D.W.; Choi, Y.R.; Kim, H.; Park, H.Y.; Kim, K.W.; Kim, M.Y.; Park, C.-K.; Lee, D. Graphene oxide-quenching-based fluorescence in situ hybridization (G-FISH) to detect RNA in tissue: Simple and fast tissue RNA diagnostics. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 162–172. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.; Na, H.-K.; Min, D.-H. MicroRNA-Responsive Drug Release System for Selective Fluorescence Imaging and Photodynamic Therapy In Vivo. Adv. Heal. Mater. 2016, 5, 2386–2395. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Baek, Y.M.; Kim, H.-M.; Jun, B.-H.; Kim, D.-E. Polyethylene Glycol-Engrafted Graphene Oxide as Biocompatible Materials for Peptide Nucleic Acid Delivery into Cells. Bioconjugate Chem. 2018, 29, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Kim, H.Y.; Li, F.; Park, J.Y.; Kim, D.; Park, J.H.; Han, H.S.; Byun, J.W.; Lee, Y.-S.; Jeong, J.M.; et al. In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy. Biomaterials 2017, 121, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, Q.; Ju, H. A peptide nucleic acid-functionalized carbon nitride nanosheet as a probe for in situ monitoring of intracellular microRNA. Analyst 2015, 140, 4245–4252. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, C.; Girard, H.A.; Falck, C.; Paget, V.; Simic, V.; Ugolin, N.; Bergonzo, P.; Chevillard, S.; Arnault, J.C. Peptide nucleic acid–nanodiamonds: Covalent and stable conjugates for DNA targeting. RSC Adv. 2014, 4, 3566–3572. [Google Scholar] [CrossRef] [Green Version]
- Arayachukiat, S.; Seemork, J.; Pan-In, P.; Amornwachirabodee, K.; Sangphech, N.; Sansureerungsikul, T.; Sathornsantikun, K.; Vilaivan, C.; Shigyou, K.; Pienpinijtham, P.; et al. Bringing Macromolecules into Cells and Evading Endosomes by Oxidized Carbon Nanoparticles. Nano Lett. 2015, 15, 3370–3376. [Google Scholar] [CrossRef]
- Tarvirdipour, S.; Huang, X.; Mihali, V.; Schoenenberger, C.-A.; Palivan, C.G. Peptide-Based Nanoassemblies in Gene Therapy and Diagnosis: Paving the Way for Clinical Application. Molecules 2020, 25, 3482. [Google Scholar] [CrossRef]
- Macadangdang, B.; Zhang, N.; Lund, P.E.; Marple, A.H.; Okabe, M.; Gottesman, M.M.; Appella, D.H.; Kimchi-Sarfaty, C. Inhibition of Multidrug Resistance by SV40 Pseudovirion Delivery of an Antigene Peptide Nucleic Acid (PNA) in Cultured Cells. PLoS ONE 2011, 6, e17981. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef]
- Morris, M.C.; Chaloin, L.; Choob, M.; Archdeacon, J.; Heitz, F.; Divita, G. Combination of a new generation of PNAs with a peptide-based carrier enables efficient targeting of cell cycle progression. Gene Ther. 2004, 11, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Galli, V.; Sadhu, K.K.; Masi, D.; Saarbach, J.; Roux, A.; Winssinger, N. Caprin-1 Promotes Cellular Uptake of Nucleic Acids with Backbone and Sequence Discrimination. Helv. Chim. Acta 2019, 103, 1900255. [Google Scholar] [CrossRef]
- Valero, J.; Shiraishi, T.; De Mendoza, J.; Nielsen, P.E. Cellular Antisense Activity of PNA-Oligo (bicycloguanidinium) Conjugates Forming Self-Assembled Nanoaggregates. ChemBioChem 2015, 16, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, M.; Shiraishi, T.; Nielsen, P.E. Cooperative Cellular Uptake and Activity of Octaarginine Antisense Peptide Nucleic acid (PNA) Conjugates. Biomolecules 2019, 9, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, F.; Dudič, M.; Donofrio, G.; Rivetti, C.; Baldini, L.; Casnati, A.; Cellai, A.S.; Ungaro, R. DNA Condensation and Cell Transfection Properties of Guanidinium Calixarenes: Dependence on Macrocycle Lipophilicity, Size, and Conformation. J. Am. Chem. Soc. 2006, 128, 14528–14536. [Google Scholar] [CrossRef]
- Gasparello, J.; Lomazzi, M.; Papi, C.; D’Aversa, E.; Sansone, F.; Casnati, A.; Donofrio, G.; Gambari, R.; Finotti, A. Efficient Delivery of MicroRNA and AntimiRNA Molecules Using an Argininocalix[4]arene Macrocycle. Mol. Ther. Nucleic Acids 2019, 18, 748–763. [Google Scholar] [CrossRef] [Green Version]
- Gasparello, J.; Manicardi, A.; Casnati, A.; Corradini, R.; Gambari, R.; Finotti, A.; Sansone, F. Efficient cell penetration and delivery of peptide nucleic acids by an argininocalix[4]arene. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tomassi, S.; Ieranò, C.; Mercurio, M.E.; Nigro, E.; Daniele, A.; Russo, R.; Chambery, A.; Baglivo, I.; Pedone, P.V.; Rea, G.; et al. Cationic nucleopeptides as novel non-covalent carriers for the delivery of peptide nucleic acid (PNA) and RNA oligomers. Bioorganic Med. Chem. 2018, 26, 2539–2550. [Google Scholar] [CrossRef]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA Origami: Scaffolds for Creating Higher Order Structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 2011, 3, 103–113. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.-Z.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2018, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Chen, B.-C.; Han, J.-C.; Wei, L.; Pan, X.-B. Delivery of cell-penetrating peptide-peptide nucleic acid conjugates by assembly on an oligonucleotide scaffold. Sci. Rep. 2015, 5, 17640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, S.L.; Wang, S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials 2008, 29, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Readman, J.B.; Dickson, G.; Coldham, N.G. Tetrahedral DNA Nanoparticle Vector for Intracellular Delivery of Targeted Peptide Nucleic Acid Antisense Agents to Restore Antibiotic Sensitivity in Cefotaxime-ResistantEscherichia coli. Nucleic Acid Ther. 2017, 27, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Readman, J.B.; Dickson, G.; Coldham, N.G. Translational Inhibition of CTX-M Extended Spectrum β-Lactamase in Clinical Strains of Escherichia coli by Synthetic Antisense Oligonucleotides Partially Restores Sensitivity to Cefotaxime. Front. Microbiol. 2016, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, W.; Zhu, Y.; Shi, S.; Li, Q.-S.; Mao, C.; Zhao, D.; Shi, J.; Li, W.; Wang, L.; et al. Inhibiting Methicillin-Resistant Staphylococcus aureus by Tetrahedral DNA Nanostructure-Enabled Antisense Peptide Nucleic Acid Delivery. Nano Lett. 2018, 18, 5652–5659. [Google Scholar] [CrossRef] [PubMed]
- Haydon, D.J.; Stokes, N.R.; Ure, R.; Galbraith, G.; Bennett, J.M.; Brown, D.R.; Baker, P.J.; Barynin, V.V.; Rice, D.W.; Sedelnikova, S.E.; et al. An Inhibitor of FtsZ with Potent and Selective Anti-Staphylococcal Activity. Science 2008, 321, 1673–1675. [Google Scholar] [CrossRef]
- Mercurio, M.E.; Tomassi, S.; Gaglione, M.; Russo, R.; Chambery, A.; Lama, S.; Stiuso, P.; Cosconati, S.; Novellino, E.; Di Maro, S.; et al. Switchable Protecting Strategy for Solid Phase Synthesis of DNA and RNA Interacting Nucleopeptides. J. Org. Chem. 2016, 81, 11612–11625. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpi, S.; Cancelli, U.; Neri, M.; Corradini, R. Multifunctional Delivery Systems for Peptide Nucleic Acids. Pharmaceuticals 2021, 14, 14. https://doi.org/10.3390/ph14010014
Volpi S, Cancelli U, Neri M, Corradini R. Multifunctional Delivery Systems for Peptide Nucleic Acids. Pharmaceuticals. 2021; 14(1):14. https://doi.org/10.3390/ph14010014
Chicago/Turabian StyleVolpi, Stefano, Umberto Cancelli, Martina Neri, and Roberto Corradini. 2021. "Multifunctional Delivery Systems for Peptide Nucleic Acids" Pharmaceuticals 14, no. 1: 14. https://doi.org/10.3390/ph14010014
APA StyleVolpi, S., Cancelli, U., Neri, M., & Corradini, R. (2021). Multifunctional Delivery Systems for Peptide Nucleic Acids. Pharmaceuticals, 14(1), 14. https://doi.org/10.3390/ph14010014




