Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19
Abstract
1. Introduction
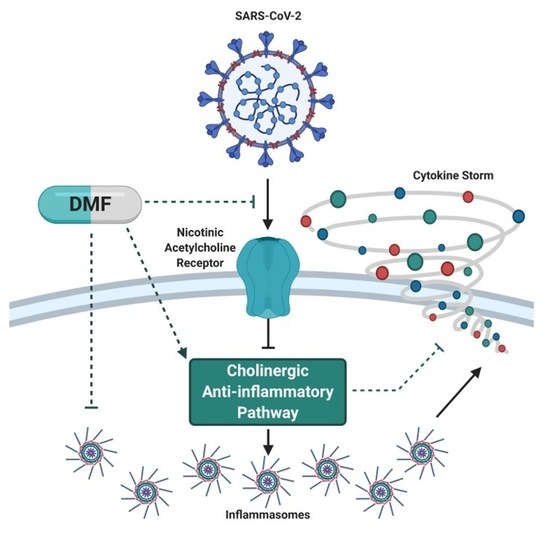
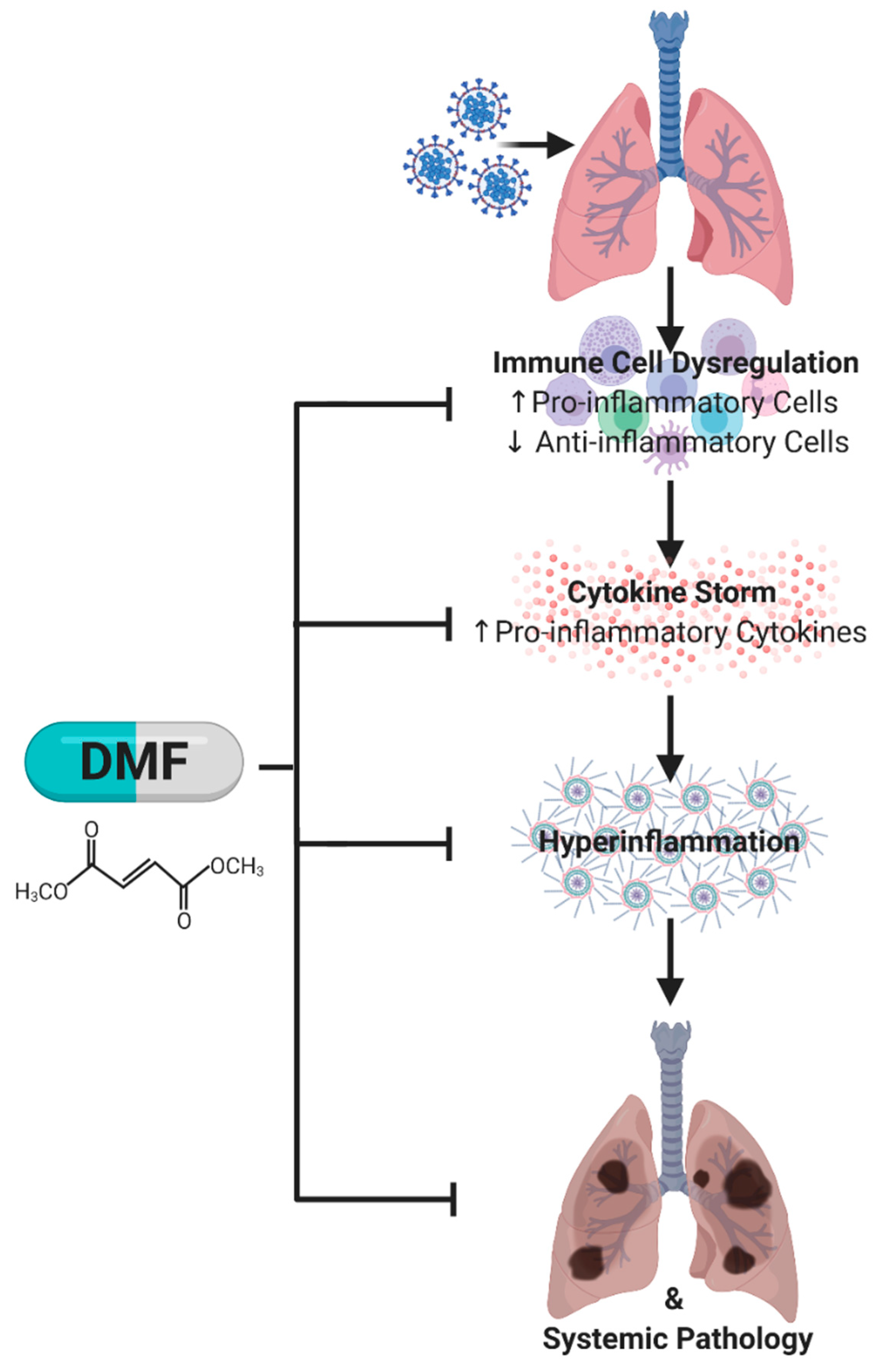
2. Main
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Yagüe, A.J.G.; Lastra, D.; Manda, G.; Rojo, A.I. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Landeck, L.; Asadullah, K.; Amasuno, A.; Pau-Charles, I.; Mrowietz, U. Dimethyl fumarate (DMF) vs. monoethyl fumarate (MEF) salts for the treatment of plaque psoriasis: A review of clinical data. Arch. Dermatol. Res. 2018, 310, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility? Pharmaceuticals 2020, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J.; Kolson, D.L. Dimethyl fumarate modulation of immune and antioxidant responses: Application to HIV therapy. Crit. Rev. Immunol. 2013, 33, 307–359. [Google Scholar] [CrossRef]
- Brück, J.; Glocova, I.; Geisel, J.; Kellerer, C.; Röcken, M.; Ghoreschi, K. Dimethyl fumarate-induced IL-17lowIFN-γlowIL-4+ Th cells protect mice from severe encephalomyelitis. Eur. J. Immunol. 2018, 48, 1588–1591. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Targeting Nrf2 for the treatment of Duchenne Muscular Dystrophy. Redox Biol. 2020, 38, 101803. [Google Scholar] [CrossRef]
- Al-Jaderi, Z.; Maghazachi, A.A. Utilization of Dimethyl Fumarate and Related Molecules for Treatment of Multiple Sclerosis, Cancer, and Other Diseases. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Von Glehn, F.; Dias-Carneiro, R.P.C.; Moraes, A.S.; Farias, A.S.; Silva, V.A.P.G.; Oliveira, F.T.M.; Silva, C.E.B.G.; de Carvalho, F.; Rahal, E.; Baecher-Allan, C.; et al. Dimethyl fumarate downregulates the immune response through the HCA2/GPR109A pathway: Implications for the treatment of multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 46–50. [Google Scholar] [CrossRef]
- Chen, H.; Assmann, J.C.; Krenz, A.; Rahman, M.; Grimm, M.; Karsten, C.M.; Köhl, J.; Offermanns, S.; Wettschureck, N.; Schwaninger, M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J. Clin. Investig. 2014, 124, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.A.; Ogrodnik, M.A.; Plave, A.; Mao-Draayer, Y. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Soin, D.; Ito, K.; Dhib-Jalbut, S. Insight into the mechanism of action of dimethyl fumarate in multiple sclerosis. J. Mol. Med. 2019, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Gesser, B.; Rasmussen, M.K.; Iversen, L. Dimethyl Fumarate Targets MSK1, RSK1, 2 and IKKα/β Kinases and Regulates NF-κB/p65 Activation in Psoriasis: A Demonstration of the Effect on Peripheral Blood Mononuclear Cells, Drawn from Two Patients with Severe Psoriasis Before and After Treatment with Dimethyl Fumarate. Psoriasis Targets Ther. 2020, 10, 1. [Google Scholar]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Klinsing, S.; Posevitz-Fejfár, A.; Wiendl, H.; Klotz, L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3. [Google Scholar] [CrossRef]
- Li, R.; Rezk, A.; Ghadiri, M.; Luessi, F.; Zipp, F.; Li, H.; Giacomini, P.S.; Antel, J.; Bar-Or, A. Dimethyl Fumarate Treatment Mediates an Anti-Inflammatory Shift in B Cell Subsets of Patients with Multiple Sclerosis. J. Immunol. 2017, 198, 691–698. [Google Scholar] [CrossRef]
- Montes Diaz, G.; Fraussen, J.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci. Rep. 2018, 8, 8194. [Google Scholar] [CrossRef]
- Najjar, E.; Staun-Ram, E.; Volkowich, A.; Miller, A. Dimethyl fumarate promotes B cell-mediated anti-inflammatory cytokine profile in B and T cells, and inhibits immune cell migration in patients with MS. J. Neuroimmunol. 2020, 343, 577230. [Google Scholar] [CrossRef]
- Ockenfels, H.; Schultewolter, T.; Ockenfels, G.; Funk, R.; Goos, M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Brit. J. Dermatol. 1998, 139, 390–395. [Google Scholar] [CrossRef]
- Schlöder, J.; Berges, C.; Luessi, F.; Jonuleit, H. Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells. Int. J. Mol. Sci. 2017, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Martin, K.A.; Calabresi, P.A.; Bhargava, P. Dimethyl fumarate alters B-cell memory and cytokine production in MS patients. Ann. Clin. Transl. Neurol. 2017, 4, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Stoof, T.J.; Flier, J.; Sampat, S.; Nieboer, C.; Tensen, C.P.; Boorsma, D.M. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Brit. J. Dermatol. 2001, 144, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Tahvili, S.; Zandieh, B.; Amirghofran, Z. The effect of dimethyl fumarate on gene expression and the level of cytokines related to different T helper cell subsets in peripheral blood mononuclear cells of patients with psoriasis. Int. J. Dermatol. 2015, 54, e254–e260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Q.; Mao, G.; Dowling, C.A.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. J. Immunol. 2017, 198, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Longbrake, E.E.; Ramsbottom, M.J.; Cantoni, C.; Ghezzi, L.; Cross, A.H.; Piccio, L. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult. Scler. J. 2016, 22, 1061–1070. [Google Scholar] [CrossRef]
- Longbrake, E.E.; Cantoni, C.; Chahin, S.; Cignarella, F.; Cross, A.H.; Piccio, L. Dimethyl fumarate induces changes in B-and T-lymphocyte function independent of the effects on absolute lymphocyte count. Mult. Scler. J. 2018, 24, 728–738. [Google Scholar] [CrossRef]
- Michell-Robinson, M.A.; Moore, C.S.; Healy, L.M.; Osso, L.A.; Zorko, N.; Grouza, V.; Touil, H.; Poliquin-Lasnier, L.; Trudelle, A.M.; Giacomini, P.S. Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann. Clin. Transl. Neurol. 2016, 3, 27–41. [Google Scholar] [CrossRef]
- Fleischer, V.; Friedrich, M.; Rezk, A.; Bühler, U.; Witsch, E.; Uphaus, T.; Bittner, S.; Groppa, S.; Tackenberg, B.; Bar-Or, A. Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Mult. Scler. J. 2018, 24, 632–641. [Google Scholar] [CrossRef]
- Lundy, S.K.; Wu, Q.; Wang, Q.; Dowling, C.A.; Taitano, S.H.; Mao, G.; Mao-Draayer, Y. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3. [Google Scholar] [CrossRef]
- Schulze-Topphoff, U.; Varrin-Doyer, M.; Pekarek, K.; Spencer, C.M.; Shetty, A.; Sagan, S.A.; Cree, B.A.; Sobel, R.A.; Wipke, B.T.; Steinman, L. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc. Natl. Acad. Sci. USA 2016, 113, 4777–4782. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Rezk, A.; Li, R.; Evans, A.; Luessi, F.; Zipp, F.; Giacomini, P.S.; Antel, J.; Bar-Or, A. Dimethyl fumarate–induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e340. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Sievers, C.; Bantug, G.; Sanderson, N.; Kappos, L.; Kuhle, J.; Lindberg, R.L.P.; Derfuss, T. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J. Autoimmun. 2018, 86, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Traffehn, S.; Ochs, J.; Häusser-Kinzel, S.; Stephan, S.; Scannevin, R.; Brück, W.; Metz, I.; Weber, M.S. Dimethyl fumarate impairs differentiated B cells and fosters central nervous system integrity in treatment of multiple sclerosis. Brain Pathol. 2019, 29, 640–657. [Google Scholar] [CrossRef]
- Spencer, C.M.; Crabtree-Hartman, E.C.; Lehmann-Horn, K.; Cree, B.A.C.; Zamvil, S.S. Reduction of CD8+ T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e76. [Google Scholar] [CrossRef]
- Staun-Ram, E.; Najjar, E.; Volkowich, A.; Miller, A. Dimethyl fumarate as a first- vs second-line therapy in MS. Focus B Cells 2018, 5, e508. [Google Scholar] [CrossRef]
- Holm Hansen, R.; Højsgaard Chow, H.; Sellebjerg, F.; Rode von Essen, M. Dimethyl fumarate therapy suppresses B cell responses and follicular helper T cells in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2019, 25, 1289–1297. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Fu, J.; Kong, J.; Wang, W.; Wu, M.; Yao, L.; Wang, Z.; Jin, J.; Wu, D.; Yu, X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thromb. Res. 2020, 192, 3–8. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Yin, Y.; Zhang, Y.; Cao, Y.; Lin, X.; Huang, L.; Hoffmann, D.; Lu, M.; Qiu, Y. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Wannick, M.; Assmann, J.C.; Vielhauer, J.F.; Offermanns, S.; Zillikens, D.; Sadik, C.D.; Schwaninger, M. The Immunometabolomic Interface Receptor Hydroxycarboxylic Acid Receptor 2 Mediates the Therapeutic Effects of Dimethyl Fumarate in Autoantibody-Induced Skin Inflammation. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Müller, S.; Behnen, M.; Bieber, K.; Möller, S.; Hellberg, L.; Witte, M.; Hänsel, M.; Zillikens, D.; Solbach, W.; Laskay, T.; et al. Dimethylfumarate Impairs Neutrophil Functions. J. Investig. Dermatol. 2016, 136, 117–126. [Google Scholar] [CrossRef]
- Hoffmann, J.H.O.; Schaekel, K.; Hartl, D.; Enk, A.H.; Hadaschik, E.N. Dimethyl fumarate modulates neutrophil extracellular trap formation in a glutathione- and superoxide-dependent manner. Br. J. Dermatol. 2018, 178, 207–214. [Google Scholar] [CrossRef]
- Giustina, A.D.; Bonfante, S.; Zarbato, G.F.; Danielski, L.G.; Mathias, K.; de Oliveira, A.N.; Garbossa, L.; Cardoso, T.; Fileti, M.E.; De Carli, R.J.; et al. Dimethyl Fumarate Modulates Oxidative Stress and Inflammation in Organs After Sepsis in Rats. Inflammation 2018, 41, 315–327. [Google Scholar] [CrossRef]
- Gillard, G.O.; Collette, B.; Anderson, J.; Chao, J.; Scannevin, R.H.; Huss, D.J.; Fontenot, J.D. DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J. Neuroimmunol. 2015, 283, 74–85. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Nat. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Barshes, N.R.; Goodpastor, S.E.; Goss, J.A. Pharmacologic immunosuppression. Front. Biosci. 2004, 9, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Longbrake, E.E.; Naismith, R.T.; Parks, B.J.; Wu, G.F.; Cross, A.H. Dimethyl fumarate-associated lymphopenia: Risk factors and clinical significance. Mult. Scler. J. Exp. Transl. Clin. 2015, 1, 2055217315596994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Zeng, Z.; Liang, M.; Xue, Y.; Xi, C.; Zhou, M.; Jiang, W. Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis prevents lipopolysaccharide–induced apoptosis of pulmonary microvascular endothelial cells by inhibiting JNK/NF–κB pathways. Sci. Rep. 2015, 5, 8209. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Huang, Y.; Pan, C.; Liu, L.; Qiu, H. Angiotensin-(1-7) attenuates lung fibrosis by way of Mas receptor in acute lung injury. J. Surg. Res. 2013, 185, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, C.-H.; Li, W.; Li, T.; Luo, W.; Huang, S.; Wu, P.-S.; Cai, S.-X.; Li, X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am. J. Respir. Cell Mol. Biol. 2014, 50, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-N.; Yang, X.-H.; Nissen, D.H.; Chen, Y.-Y.; Wang, L.-J.; Wang, J.-H.; Gao, J.-L.; Zhang, L.-Y. Dysregulated renin-angiotensin system contributes to acute lung injury caused by hind-limb ischemia-reperfusion in mice. Shock 2013, 40, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qiu, H.-B.; Yang, Y.; Wang, L.; Ding, H.-M.; Li, H.-P. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharide-induced acute lung injury in rat. Arch. Biochem. Biophys. 2009, 481, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Liu, Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020, 113, 104350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ghosh, A.; Lo, C.-S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.-L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1-7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2017, 159, 836–852. [Google Scholar] [CrossRef]
- Oliveira, A.S.F.; Ibarra, A.A.; Bermudez, I.; Casalino, L.; Gaieb, Z.; Shoemark, D.K.; Gallagher, T.; Sessions, R.B.; Amaro, R.E.; Mulholland, A.J. Simulations support the interaction of the SARS-CoV-2 spike protein with nicotinic acetylcholine receptors and suggest subtype specificity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Farsalinos, K.; Barbouni, A.; Niaura, R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: Could nicotine be a therapeutic option? Int. Emerg. Med. 2020, 15, 845–852. [Google Scholar] [CrossRef]
- Farsalinos, K.; Niaura, R.; Le Houezec, J.; Barbouni, A.; Tsatsakis, A.; Kouretas, D.; Vantarakis, A.; Poulas, K. Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 2020, 7, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.G.; Landi, D.; Monteleone, F.; Mataluni, G.; Albanese, M.; Lauretti, B.; Rocchi, C.; Simonelli, I.; Boffa, L.; Buttari, F.; et al. Treatment with Dimethyl Fumarate Enhances Cholinergic Transmission in Multiple Sclerosis. CNS Drugs 2019, 33, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Mantero, V.; Abate, L.; Basilico, P.; Balgera, R.; Salmaggi, A.; Nourbakhsh, B.; Cordano, C. COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J. Neurol. 2020, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Hashiba, T.; Suzuki, M.; Nagashima, Y.; Suzuki, S.; Inoue, S.; Tsuburai, T.; Matsuse, T.; Ishigatubo, Y. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001, 8, 1499–1507. [Google Scholar] [CrossRef]
- Cummins, N.W.; Weaver, E.A.; May, S.M.; Croatt, A.J.; Foreman, O.; Kennedy, R.B.; Poland, G.A.; Barry, M.A.; Nath, K.A.; Badley, A.D. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice. FASEB J. 2012, 26, 2911–2918. [Google Scholar] [CrossRef]
- Deng, X.; Yasuda, H.; Sasaki, T.; Yamaya, M. Low-Dose Carbon Monoxide Inhibits Rhinovirus Replication in Human Alveolar and Airway Epithelial Cells. Tohoku J. Exp. Med. 2019, 247, 215–222. [Google Scholar] [CrossRef]
- Zhang, A.; Wan, B.; Jiang, D.; Wu, Y.; Ji, P.; Du, Y.; Zhang, G. The Cytoprotective Enzyme Heme Oxygenase-1 Suppresses Pseudorabies Virus Replication in vitro. Front. Microbiol. 2020, 11, 412. [Google Scholar] [CrossRef]
- Gutiérrez-Grobe, Y.; Vitek, L.; Tiribelli, C.; Kobashi-Margáin, R.A.; Uribe, M.; Méndez-Sánchez, N. Biliverdin and heme oxygenase antiviral activity against hepatitis C virus. Ann. Hepatol. 2016, 10, 105–107. [Google Scholar]
- Tseng, C.-K.; Lin, C.-K.; Wu, Y.-H.; Chen, Y.-H.; Chen, W.-C.; Young, K.-C.; Lee, J.-C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016, 6, 32176. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.A.; Cook, D.R.; Chi, A.W.S.; Vance, P.J.; Kolson, L.L.; Wong, B.J.; Jordan-Sciutto, K.L.; Kolson, D.L. Dimethyl Fumarate, an Immune Modulator and Inducer of the Antioxidant Response, Suppresses HIV Replication and Macrophage-Mediated Neurotoxicity: A Novel Candidate for HIV Neuroprotection. J. Immunol. 2011, 187, 5015–5025. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cai, J.; Kostuk, E.W.; Rosenwasser, R.; Iacovitti, L. Fumarate modulates the immune/inflammatory response and rescues nerve cells and neurological function after stroke in rats. J. Neuroinflamm. 2016, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Xiao, J.; Zhai, H.; Hao, J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J. Neuroinflamm. 2016, 13, 97. [Google Scholar] [CrossRef]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight 2018, 3, e120596. [Google Scholar] [CrossRef]
- Drechsler, Y.; Dolganiuc, A.; Norkina, O.; Romics, L.; Li, W.; Kodys, K.; Bach, F.H.; Mandrekar, P.; Szabo, G. Heme Oxygenase-1 Mediates the Anti-Inflammatory Effects of Acute Alcohol on IL-10 Induction Involving p38 MAPK Activation in Monocytes. J. Immunol. 2006, 177, 2592–2600. [Google Scholar] [CrossRef]
- Pitarokoili, K.; Bachir, H.; Sgodzai, M.; Grüter, T.; Haupeltshofer, S.; Duscha, A.; Pedreiturria, X.; Motte, J.; Gold, R. Induction of Regulatory Properties in the Intestinal Immune System by Dimethyl Fumarate in Lewis Rat Experimental Autoimmune Neuritis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Campolo, M.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J. Cell. Mol. Med. 2018, 22, 1081–1094. [Google Scholar] [CrossRef]
- Grzegorzewska, A.P.; Seta, F.; Han, R.; Czajka, C.A.; Makino, K.; Stawski, L.; Isenberg, J.S.; Browning, J.L.; Trojanowska, M. Dimethyl Fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Sci. Rep. 2017, 7, 41605. [Google Scholar] [CrossRef]
- Robles, L.; Vaziri, N.D.; Li, S.; Masuda, Y.; Takasu, C.; Takasu, M.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. Dimethyl Fumarate Protects Pancreatic Islet Cells and Non-Endocrine Tissue in L-Arginine-Induced Chronic Pancreatitis. PLoS ONE 2014, 9, e107111. [Google Scholar] [CrossRef]
- Abdelrahman, R.S.; Abdel-Rahman, N. Dimethyl fumarate ameliorates acetaminophen-induced hepatic injury in mice dependent of Nrf-2/HO-1 pathway. Life Sci. 2019, 217, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Mikulskis, A.; Gold, R.; Fox, R.J.; Dawson, K.T.; Amaravadi, L. Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the Phase 3 DEFINE and CONFIRM studies. Mult. Scler. J. 2017, 23, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.J.; Park, S.; Kim, J.-Y.; Kim, H.-J.; Jeoung, N.H.; Choi, Y.-K.; Go, Y.; Park, K.-G.; Lee, I.-K. Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014, 2, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Brück, J.; Kellerer, C.; Deng, C.; Peng, H.; Rothfuss, O.; Hussain, R.Z.; Gocke, A.R.; Respa, A.; Glocova, I.; et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 2011, 208, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.X.; Lisi, L.; Russo, C.D.; Polak, P.E.; Sharp, A.; Weinberg, G.; Kalinin, S.; Feinstein, D.L. The Anti-Inflammatory Effects of Dimethyl Fumarate in Astrocytes Involve Glutathione and Haem Oxygenase-1. ASN Neuro 2011, 3, AN20100033. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Fang, J.; Chen, Y.; Li, H.; Gao, K. Dimethyl fumarate inhibits the expression and function of hypoxia-inducible factor-1α (HIF-1α). Biochem. Biophys. Res. Commun. 2014, 448, 303–307. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Sandey, M.; Suryawanshi, A.; Cattley, R.C.; Mishra, A. Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function. Immun. Inflamm. Dis. 2019, 7, 201–213. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Sandey, M.; Suryawanshi, A.; Cattley, R.C.; Mishra, A. Dimethyl fumarate attenuates T helper type 2 (Th2)-mediated allergic airway inflammation by modulating dendritic cell function. J. Immunol. 2019, 202, 55.16. [Google Scholar]
- Seidel, P.; Goulet, S.; Hostettler, K.; Tamm, M.; Roth, M. DMF inhibits PDGF-BB induced airway smooth muscle cell proliferation through induction of heme-oxygenase-1. Respir. Res. 2010, 11, 145. [Google Scholar] [CrossRef]
- Seidel, P.; Hostettler, K.E.; Hughes, J.M.; Tamm, M.; Roth, M. Dimethylfumarate inhibits CXCL10 via haem oxygenase-1 in airway smooth muscle. Eur. Respir. J. 2013, 41, 195–202. [Google Scholar] [CrossRef]
- Seidel, P.; Merfort, I.; Hughes, J.M.; Oliver, B.G.G.; Tamm, M.; Roth, M. Dimethylfumarate inhibits NF-κB function at multiple levels to limit airway smooth muscle cell cytokine secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L326–L339. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, Y.A.; Alkhouri, H.; Chen, E.; Lalor, D.J.; Poniris, M.; Henness, S.; Brightling, C.E.; Burgess, J.K.; Armour, C.L.; Ammit, A.J.; et al. Asthmatic airway smooth muscle CXCL10 production: Mitogen-activated protein kinase JNK involvement. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1118–L1127. [Google Scholar] [CrossRef] [PubMed]
- Seidel, P.; Roth, M.; Ge, Q.; Merfort, I.; S’ng, C.T.; Ammit, A.J. IκBα glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur. Respir. J. 2011, 38, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Jawad, M.J.; Ahjel, S.W.; Singh, R.B.; Singh, J.; Awad, S.M.; Hadi, N.R. The Nrf2 Activator (DMF) and Covid-19: Is there a Possible Role? Med. Arch. 2020, 74, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Robles, L.; Vaziri, N.D.; Li, S.; Takasu, C.; Masuda, Y.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. Dimethyl fumarate ameliorates acute pancreatitis in rodent. Pancreas 2015, 44, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, S.; König, V.; Doll, M.; Hailemariam-Jahn, T.; Hrgovic, I.; Zöller, N.; Kaufmann, R.; Kippenberger, S.; Meissner, M. Dimethylfumarate protects against TNF-α-induced secretion of inflammatory cytokines in human endothelial cells. J. Inflamm. 2015, 12, 49. [Google Scholar] [CrossRef]
- McGuire, V.A.; Diez, T.R.-Z.; Emmerich, C.H.; Strickson, S.; Ritorto, M.S.; Sutavani, R.V.; Weiβ, A.; Houslay, K.F.; Knebel, A.; Meakin, P.J. Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci. Rep. 2016, 6, 31159. [Google Scholar] [CrossRef]
- Safavi, F.; Thome, R.; Li, Z.; Zhang, G.-X.; Rostami, A. Dimethyl fumarate suppresses granulocyte macrophage colony-stimulating factor–producing Th1 cells in CNS neuroinflammation. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e729. [Google Scholar] [CrossRef]
- Sangineto, M.; Grabherr, F.; Adolph, T.E.; Grander, C.; Reider, S.; Jaschke, N.; Mayr, L.; Schwärzler, J.; Dallio, M.; Moschen, A.R.; et al. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease. Liver Int. 2020, 40, 1610–1619. [Google Scholar] [CrossRef]
- Wallbrecht, K.; Drick, N.; Hund, A.-C.; Schön, M.P. Downregulation of endothelial adhesion molecules by dimethylfumarate, but not monomethylfumarate, and impairment of dynamic lymphocyte-endothelial cell interactions. Exp. Dermatol. 2011, 20, 980–985. [Google Scholar] [CrossRef]
- Albrecht, P.; Bouchachia, I.; Goebels, N.; Henke, N.; Hofstetter, H.H.; Issberner, A.; Kovacs, Z.; Lewerenz, J.; Lisak, D.; Maher, P.; et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J. Neuroinflamm. 2012, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, S.; Gong, H.; Liu, S.; Lei, L.; Hu, B.; Xu, Y.; Liu, H.; Wu, D. Inhibition of Acute Graft-versus-Host Disease with Retention of Graft-versus-Tumor Effects by Dimethyl Fumarate. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.C.U.; Listopad, J.J.; Rentzsch, C.U.; Igney, F.H.; von Bonin, A.; Hennekes, H.H.; Asadullah, K.; Docke, W.-D.F. Dimethylfumarate Induces Immunosuppression via Glutathione Depletion and Subsequent Induction of Heme Oxygenase 1. J. Investig. Dermatol. 2007, 127, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guerau-de-Arellano, M.; Mehta, V.B.; Yang, Y.; Huss, D.J.; Papenfuss, T.L.; Lovett-Racke, A.E.; Racke, M.K. Dimethyl Fumarate Inhibits Dendritic Cell Maturation via Nuclear Factor κB (NF-κB) and Extracellular Signal-regulated Kinase 1 and 2 (ERK1/2) and Mitogen Stress-activated Kinase 1 (MSK1) Signaling. J. Biol. Chem. 2012, 287, 28017–28026. [Google Scholar] [CrossRef]
- Müller, S.; Smatlik, N.; Burian, M.; Ghoreschi, K.; Röcken, M.; Yazdi, A.S. Differential induction of ATF3 and HO-1 in myeloid cells and keratinocytes via Dimethylfumarate or Cyclosporine A. J. Dermatol. Sci. 2017, 87, 246–251. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Zhou, W.; Zhang, X.; Lu, P.; Du, Q.; Tao, L.; Ding, Y.; Wang, Y.; Hu, R. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem. Pharmacol. 2016, 112, 37–49. [Google Scholar] [CrossRef]
- Singh, N.; Saha, L.; Kumari, P.; Singh, J.; Bhatia, A.; Banerjee, D.; Chakrabarti, A. Effect of dimethyl fumarate on neuroinflammation and apoptosis in pentylenetetrazol kindling model in rats. Brain Res. Bull. 2019, 144, 233–245. [Google Scholar] [CrossRef]
- Wierinckx, A.; Brevé, J.; Mercier, D.; Schultzberg, M.; Drukarch, B.; Van Dam, A.-M. Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J. Neuroimmunol. 2005, 166, 132–143. [Google Scholar] [CrossRef]
- Wilms, H.; Sievers, J.; Rickert, U.; Rostami-Yazdi, M.; Mrowietz, U.; Lucius, R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflamm. 2010, 7, 30. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, K.; Zhang, P.; Abdulla, F.; Nguyen, P.; Killeen, T.; Xu, P.; O’Sullivan, G.; Nath, K.A.; et al. Control of Oxidative Stress and Inflammation in Sickle Cell Disease with the Nrf2 Activator Dimethyl Fumarate. Antioxid. Redox Signal. 2017, 26, 748–762. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, G.; Zhang, J.; Ting, S.-M.; Gonzales, N.; Aronowski, J. Dimethyl Fumarate Protects Brain from Damage Produced by Intracerebral Hemorrhage by Mechanism Involving Nrf2. Stroke 2015, 46, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Blewett, M.M.; Xie, J.; Zaro, B.W.; Backus, K.M.; Altman, A.; Teijaro, J.R.; Cravatt, B.F. Chemical proteomic map of dimethyl fumarate–sensitive cysteines in primary human T cells. Sci. Signal. 2016, 9, rs10. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.Z.M.; Chiarotto, G.B.; Bernardes, D.; Kempe, P.R.G.; Oliveira, A.L.R. Neuroprotection by dimethyl fumarate following ventral root crush in C57BL/6J mice. Brain Res. Bull. 2020, 164, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Galloway, D.A.; Williams, J.B.; Moore, C.S. Effects of fumarates on inflammatory human astrocyte responses and oligodendrocyte differentiation. Ann. Clin. Transl. Neurol. 2017, 4, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Pace, B.; Gupta, D.; Sturtevant, S.; Li, B.; Makala, L.; Brittain, J.; Moore, N.; Vieira, B.F.; Thullen, T.; et al. Dimethyl fumarate increases fetal hemoglobin, provides heme detoxification, and corrects anemia in sickle cell disease. JCI Insight 2017, 2, e96409. [Google Scholar] [CrossRef]
- Ragab, D.; Abdallah, D.M.; El-Abhar, H.S. The dual reno-and neuro-protective effects of dimethyl fumarate against uremic encephalopathy in a renal ischemia/reperfusion model. Pharmacol. Rep. 2020, 72, 969–983. [Google Scholar] [CrossRef]
- Takasu, C.; Vaziri, N.D.; Li, S.; Robles, L.; Vo, K.; Takasu, M.; Pham, C.; Farzaneh, S.H.; Shimada, M.; Stamos, M.J.; et al. Treatment with dimethyl fumarate ameliorates liver ischemia/reperfusion injury. World J. Gastroenterol. 2017, 23, 4508–4516. [Google Scholar] [CrossRef]
- Seidel, P.; Merfort, I. Inhibition of NF-κB and AP-1 by dimethylfumarate correlates with down-regulated IL-6 secretion and proliferation in human lung fibroblasts. Swiss Med. Wkly. 2010, 140. [Google Scholar] [CrossRef]
- Zarbato, G.F.; de Souza Goldim, M.P.; Giustina, A.D.; Danielski, L.G.; Mathias, K.; Florentino, D.; de Oliveira Junior, A.N.; da Rosa, N.; Laurentino, A.O.; Trombetta, T.; et al. Dimethyl Fumarate Limits Neuroinflammation and Oxidative Stress and Improves Cognitive Impairment After Polymicrobial Sepsis. Neurotox. Res. 2018, 34, 418–430. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, G.; Wang, Y.; Lin, X.; Zhou, J.; Wang, Z.; Suo, N. Dimethyl fumarate protects nucleus pulposus cells from inflammation and oxidative stress and delays the intervertebral disc degeneration. Exp. Ther. Med. 2020, 20, 269. [Google Scholar] [CrossRef]
- Förster, A.; Preussner, L.M.; Seeger, J.M.; Rabenhorst, A.; Kashkar, H.; Mrowietz, U.; Hartmann, K. Dimethylfumarate induces apoptosis in human mast cells. Exp. Dermatol. 2013, 22, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.R.; Rademaker, M.; Ravensbergen, B.; Rea, D.; van der Plas, M.J.A.; Thio, B.; Walding, A.; van Dissel, J.T.; Nibbering, P.H. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur. J. Immunol. 2004, 34, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lückel, C.; Picard, F.; Raifer, H.; Campos Carrascosa, L.; Guralnik, A.; Zhang, Y.; Klein, M.; Bittner, S.; Steffen, F.; Moos, S.; et al. IL-17+ CD8+ T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat. Commun. 2019, 10, 5722. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Ou, P.; Rousso, C.; Bergeron, A.; Krishnan, R.; Pikor, L.; Chen, A.; Keller, B.A.; Ilkow, C.; Bell, J.C.; et al. Dimethyl fumarate potentiates oncolytic virotherapy through NF-κB inhibition. Sci. Transl. Med. 2018, 10, eaao1613. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | Age Range (years) | Length of DMF Treatment | Additional Medication during DMF Treatment | Effect on T & B Cells | Ref |
|---|---|---|---|---|---|
| 15 (7F/8M) | 24–54 (median 40.7) | 6 m | - | T cells: ↓ Th1 & Th17 cells, ↑ CD4 and CD8 naïve cells, ↓ CD4 and CD8 memory cells, ↓ CD8 cells | [16] |
| 13 (11F/2M) | 20–60 (median 41) | Not stated | - | B cells: ↓ B cell number, ↓ memory B cells, ↑ naïve B cells, ↓ pro-inflammatory B cells (GM-CSF+, IL-6+, TNF-α+), ↓ pro-inflammatory co-stimulatory molecules (CD80+) | [17] |
| 20 (16F/4M) | 43 ± 8 | 4–6 m | - | T cells: ↑ Th2/Th1Th17 ratio, ↓ memory T cells, ↑ naïve T cells, ↓ CD4 and CD8 cells, ↓ pro-inflammatory T cells (IFN-γ+), ↑ anti-inflammatory T cells (IL-4+) B cells: ↓ B cell number | [25] |
| 18 (14F/4M) | 43 ± 9 | 18–26 m | - | T cells: ↓ Th1 & Th17 cells, ↑ Th2 cells, ↑Th2/Th1Th17 ratio, ↓ CD4 and CD8 cells, ↓ memory T cells, ↑ naïve T cells, ↓ pro-inflammatory T cells (IFN-γ+, IL-17+), ↑ anti-inflammatory T cells (IL-4+) B cells: ↓ B cell number | |
| 18 (13F/5M) | 43.9 ± 10.8 | 6 m | - | B cells: ↓ memory B cells, ↑ naïve B cells, ↓ pro-inflammatory B cells (GM-CSF+, IL-6+, TNF-α+) | [22] |
| 24 (21F/3M) | 24–63 (median 44.6) | ≥6 m | - | T cells: ↓ Th1 cells, ↓ CD4 and CD8 memory cells, ↑ CD4 and CD8 naïve cells | [26] |
| 43 (31F/12M) | 46±11 | 15 ± 9 m | - | T cells: ↓ CD8 memory cells, ↑ CD8 naïve cells, ↓ pro-inflammatory T cells (GM-CSF+, IFN-γ+, TNF-α+), B cells: ↓ pro-inflammatory co-stimulatory molecules (CD80+) | [27] |
| 13 (8F/5M) | Female: 31–58 (median 46.5) Male:33–57 (median 35) | 4–6 m | One patient tapered off steroids for first 6 weeks of DMF treatment | B cells: ↓ B cell number, ↓ memory B cells | [30] |
| 13 (11F/2M) | 20–60 (median 41) | 0–12 m | - | T cells: ↓ CD4 and CD8 cells, ↓ memory T cells, ↑ naïve T cells, ↓ pro-inflammatory T cells (IFN-γ+) | [32] |
| 20 (11F/9M) | 26–60 (median 41) | 0–12 m | - | T cells: ↓ T cell number, ↓ CD4 and CD8 cells, ↑ anti-inflammatory Treg cells, ↓ memory T cells, ↑ naïve T cells | [33] |
| 25 (48% F/52% M) | 35.4 ± 11.1 | At least 3 m | - | T cells: ↓ CD8 cells B cells: ↓ memory B cells, ↓ pro-inflammatory B cells (IL-6+, TNF-α+), ↓ pro-inflammatory co-stimulatory molecules (CD 40+, CD69+, CD80+, CD86+) | [34] |
| 35 (71.4% F/28.6% M) | 21–67 (mean 46.1) | 0–12 m | - | T cells: ↓ T cell number, ↓ CD4 and CD8 cells B cells: ↓ B cell number | [35] |
| 51 (35F/16M) | 34.8 ± 10.8 | 6 m | Methylprednisone-treated patient samples collected 4 weeks after last administration | T cells: ↓ T cell number, ↓ CD4 and CD8 cells B cells: ↓ B cell number | [29] |
| 43 (28F/15M) | 38 ± 2 | 15 w | - | T cells: ↑ transitional T cells B cells: ↓ memory B cells, ↑ naïve B cells, ↑ anti-inflammatory B cells (IL-4+, IL-10+, TGF-β+), ↓ pro-inflammatory co-stimulatory molecules (CD69+, CD80+, CD86+) | [36] |
| 21 | 25–50 (median 37) | 12 m | - | T cells: ↓ T cell number, ↑ transitional T cells B cells: ↓ B cell number, ↓ memory B cells, ↑ naïve B cells, | [37] |
| Elevated Cytokines in COVID-19 Patients | Effect of DMF | Model/Disease |
|---|---|---|
| G-CSF [40,61,62] | ↓ | Murine splenocytes [59], Human primary ASMCs [110] |
| GM-CSF [40,61,62] | ↓ | Human RRMS PBMCs [16,17,18,22], Human Psoriatic PBMCs [24], Murine splenocytes [59,115], Human UVECs [116], Murine BMDMs [117], Murine EAE [118] |
| Gro-1α [62] | ↓ | Human keratinocytes & PBMCs [23], Murine hepatic injury & Kupffer cells [119], Human UVECs [120] |
| IFN-γ [40,61,62] | ↓ | Murine EAE [8,104,118], Human RRMS PBMCs [16,18,19,21,25], Human psoriatic keratinocytes [20], Human psoriatic PBMCs [24], Murine ischaemic stroke model [93], Murine EAN & macrophage cell line [94], Human psoriatic T cells [104], Murine splenocytes [115,121], Murine BMDCs & allogeneic splenic T cell co-culture [122], Human PBMCs [123], Murine BMDCs [124] |
| IL-1α [61,62] | ↓ | Murine splenocytes [115], Primary human keratinocytes & PBMCs [125] |
| IL-1β [40,61,62] | ↓ | Murine splenocytes [59], Murine ischaemic stroke model [93], Murine hepatic injury & Kupffer cells [119], Primary human keratinocytes & PBMCs [125], Murine colitis model [126], Murine epilepsy model [127], Primary murine microglial & astroglial co-cultures [128,129], Murine SCD model [130], Murine intracerebral hemorrhage models [131] |
| IL-2 [40,61,62] | ↓ | Murine EAE [8], Murine splenocytes [121], Murine BMDCs & allogeneic splenic T cell co-culture [122], Human PBMCs [123], Primary human & murine T cells [132] |
| IL-4 [40,61] | ↑ | Murine EAE [8,104], Human PBMCs [24], Human RRMS PBMCs [25], Murine EAN & macrophage cell line [94], Human psoriatic T cells [104], Murine spinal cord damage model [133] |
| IL-6 [3,40,61,62] | ↓ | Human RRMS PBMCs [17,21,22], Human psoriatic keratinocytes [20], Murine splenocytes [59,115,121], Murine EAN & macrophage cell line [94], Primary human asthmatic ASMCs [111], Human UVECs [116,120], Murine BMDMs [117], Murine BMDCs & allogeneic splenic T cell co-culture [122], Human PBMCs [123], Murine BMDCs [124], Primary human keratinocytes & PBMCs [125], Murine colitis model [126], Murine epilepsy model [127], Primary murine microglial & astroglial co-cultures [128,129], Primary human & murine astrocyte cultures [134], Murine & primate SCD models [130,135], Murine renal/liver I/R injury model [136,137], Primary human lung fibroblasts [138], Murine experimental sepsis [139], Murine IDD model [140] |
| IL-8 [3,40,61] | ↓ | Human keratinocytes & PBMCs [23], Murine osteoblastic cells [106], Human UVECs [120], Murine IDD model [140], Human mast cell line & primary CBDMCs [141] |
| IL-10 [40,61,62] | ↑ | Human RRMS PBMCs [30], Murine EAN & macrophage cell line [94], Human psoriatic T cells & murine EAE [104], Murine intracerebral hemorrhage models [131] |
| IL-12p40 [61,62] | ↓ | Human psoriatic T cells & murine EAE [104], Human PBMCs [123], Murine BMDCs [124], Murine primary microglia [142] |
| IL-12p70 [62] | ↓ | Murine ischaemic stroke model [93], Human PBMCs [123,143] |
| IL-13 [40,61,62] | ↓ | Murine BMDMs [117], Murine splenocytes [115] |
| IL-17 [40] | ↓ | Murine EAE [8,104], Human PBMCs [24], Human RRMS PBMCs [18,25,144], Murine ischaemic stroke model [93], Murine EAN & macrophage cell line [94], Human psoriatic T cells [104], Murine splenocytes [121], Murine BMDCs [124] |
| IL-18 [61,62] | ↓ | Murine SCD model [130], Murine renal I/R injury model [136] |
| IP-10 [40,62] | ↓ | Human keratinocytes & PBMCs [23], Murine splenocytes [59], Murine ischaemic stroke model [93], Human primary ASMCs [110,112], Primary human & murine astrocyte cultures [134], Human tumour biopsies, cancer cell lines & oncolytic viruses [145] |
| MCP-1 [3,40,61] | ↓ | Murine splenocytes [59], Human HIV-infected monocyte-derived macrophages [92], Human UVECs [116,120], Primary human & murine astrocyte cultures [134], Primary murine microglia [142] |
| MCP-3 [62] | ↓ | Murine liver I/R injury model [137] |
| MIG [3,61,62] | ↓ | Human keratinocytes & PBMCs [23] |
| MIP-1α [40,61,62] | ↓ | Murine splenocytes [59] |
| MIP-1β [62] | ↓ | Murine splenocytes [59] |
| PDGF-BB [40,61,62] | ↓ | Human UVECs [115] |
| RANTES [61] | ↓ | Murine splenocytes [59], Murine ischaemic stroke model [93], Primary human asthmatic ASMCs [111], Primary human ASMCs [113], Human UVECs [116] |
| TNF-α [40,61] | ↓ | Human RRMS PBMCs [16,17,19,21,22], Murine splenocytes [59,115], Human HIV-infected monocyte-derived macrophages [92], Murine EAN & macrophage cell line [94], Murine hepatotoxicity model [101], Murine BMDMs [117], Murine hepatic injury & Kupffer cells [119], Murine BMDCs & allogeneic splenic T cell co-culture [122], Healthy human PBMCs [123], Murine colitis model [126], Murine epilepsy model [127], Primary murine microglial & astroglial co-cultures [128,129], Murine & primate SCD models [135], Murine renal/liver I/R injury model [136,137], Murine experimental sepsis [139], Primary murine microglia [142] |
| TNF-β [61,62] | ↓ | Murine BMDMs [117] |
| VEGF [40] | ↓ | Murine ischaemic stroke model [93], Murine osteoblastic cells [106], Murine splenocytes [115], Murine liver I/R injury model [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timpani, C.A.; Rybalka, E. Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19. Pharmaceuticals 2021, 14, 15. https://doi.org/10.3390/ph14010015
Timpani CA, Rybalka E. Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19. Pharmaceuticals. 2021; 14(1):15. https://doi.org/10.3390/ph14010015
Chicago/Turabian StyleTimpani, Cara A., and Emma Rybalka. 2021. "Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19" Pharmaceuticals 14, no. 1: 15. https://doi.org/10.3390/ph14010015
APA StyleTimpani, C. A., & Rybalka, E. (2021). Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19. Pharmaceuticals, 14(1), 15. https://doi.org/10.3390/ph14010015