Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis
Abstract
1. Introduction
2. Results
2.1. Bacteriophage Host Range: Spot Test and Plaque Assay
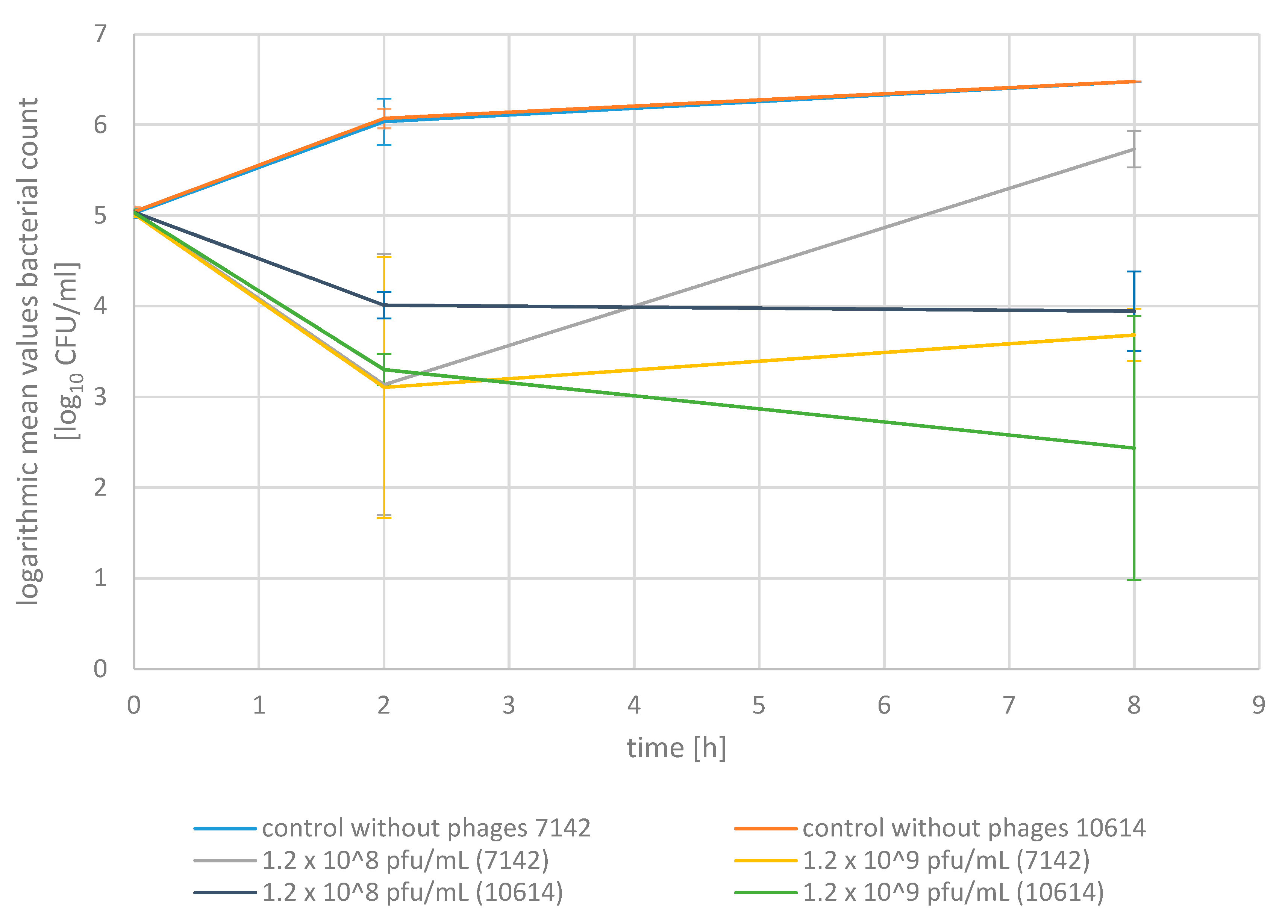
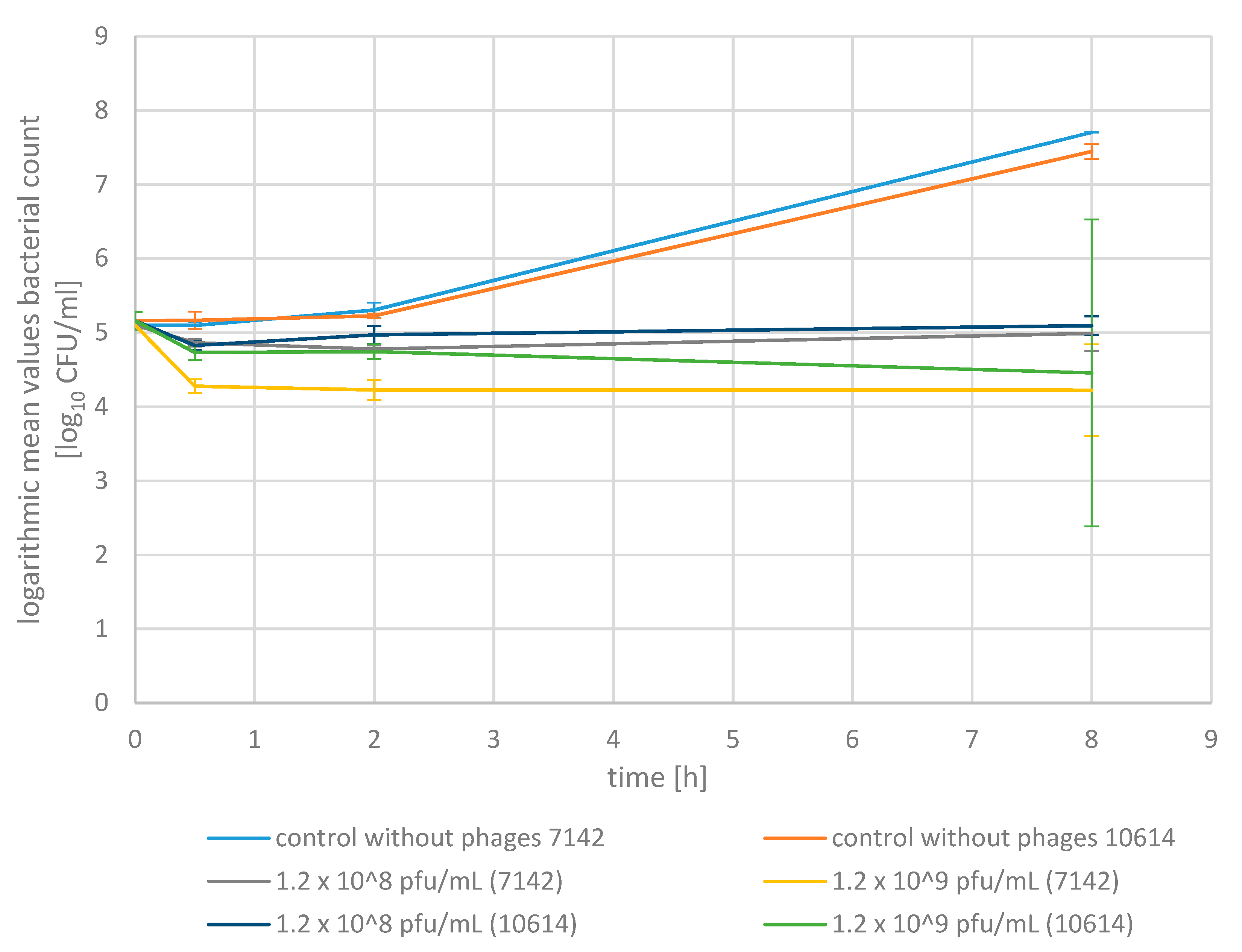
2.2. Phage Bactericidal Activity in Pasteurized Milk
2.3. Phage Bactericidal Activity in Raw Milk
3. Discussion
4. Materials and Methods
4.1. S. aureus Strains and Culture Methods
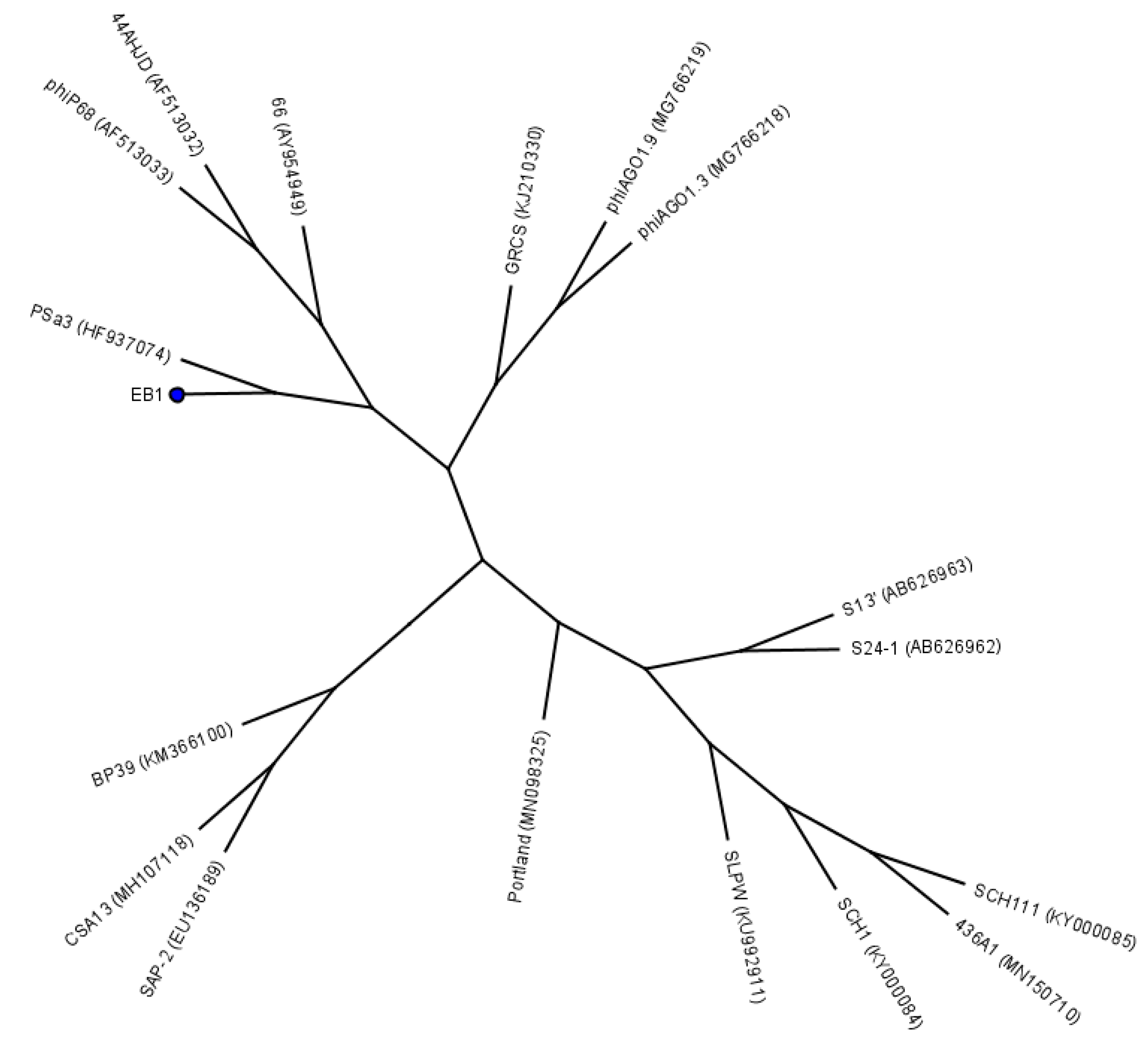
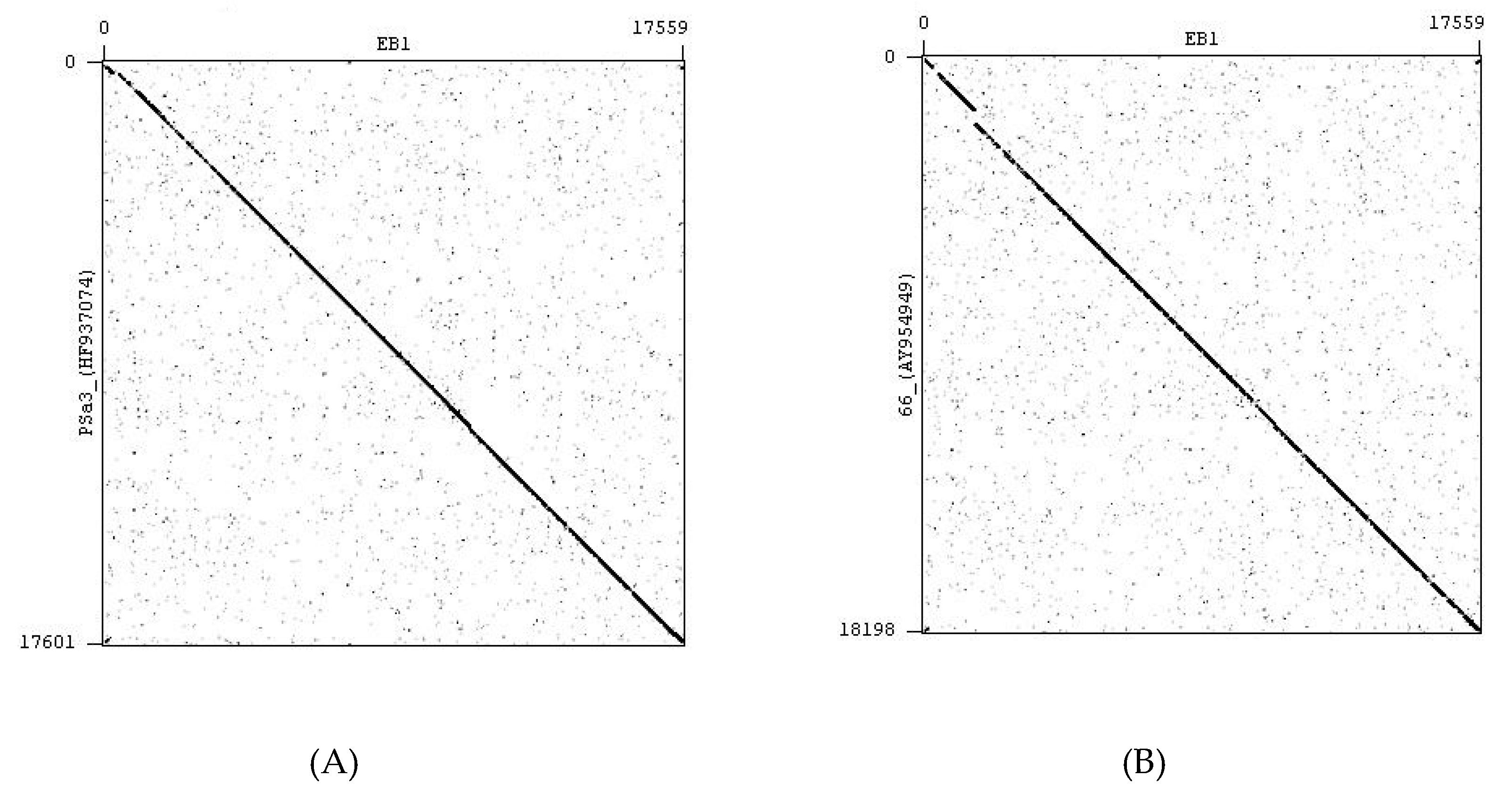
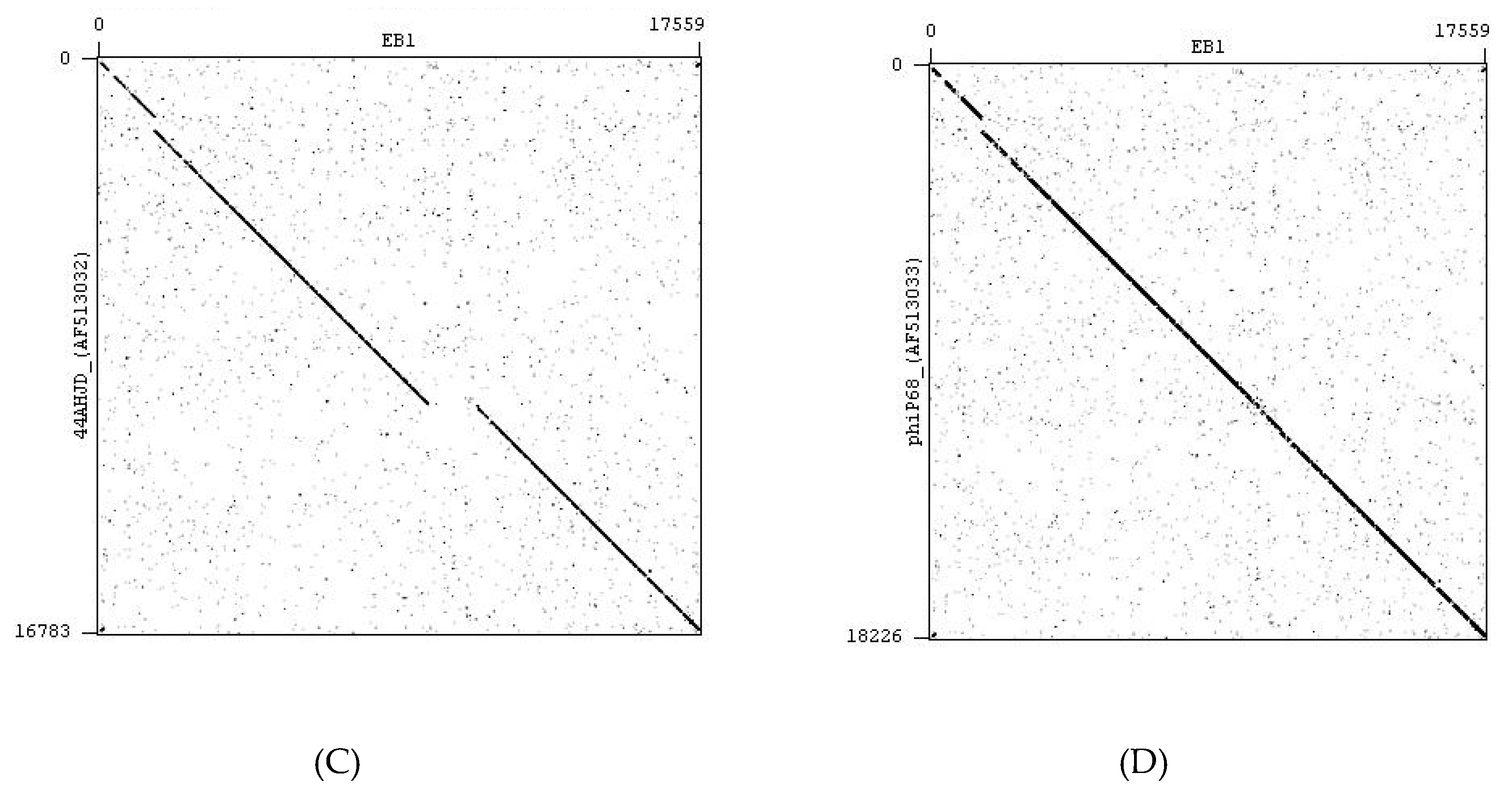
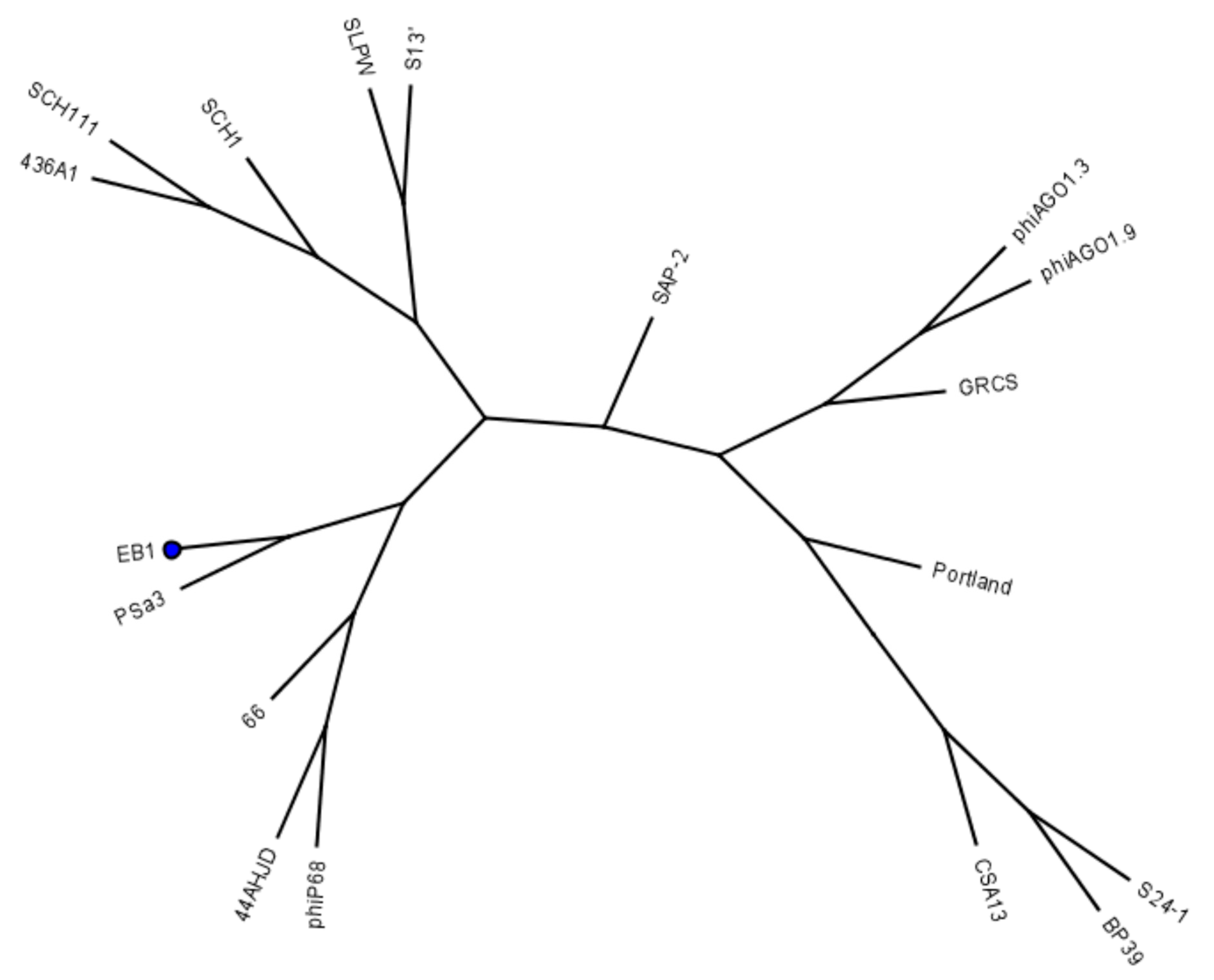
4.2. Bacteriophages
4.3. Bacteriophage Propagation
4.4. Spot Test
4.5. Plaque Assay
4.6. Phage Bactericidal Activity in Pasteurized Milk
4.7. Phage Bactericidal Activity in Raw Milk
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- IDF. Economic Consequences of Mastitis; Bulletin No 394; International Dairy Federation: Brussels, Belgium, 2005. [Google Scholar]
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Sol, J.; Sampimon, O.C.; Barkema, H.W.; Schukken, Y.H. Factors Associated with Cure after Therapy of Clinical Mastitis Caused by Staphylococcus aureus. J. Dairy Sci. 2000, 83, 278–284. [Google Scholar] [CrossRef]
- Barkema, H.; Schukken, Y.; Zadoks, R. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
- Tenhagen, B.-A.; Köster, G.; Wallmann, J.; Heuwieser, W. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J. Dairy Sci. 2006, 89, 2542–2551. [Google Scholar] [CrossRef]
- Grieger, A.-S.; Zoche-Golob, V.; Paduch, J.-H.; Hoedemaker, M.; Krömker, V. Rezidivierende klinische Mastitiden bei Milchkühen–Bedeutung und Ursachen. Tierärztliche Prax. Ausg. G Großtiere Nutztiere 2014, 42, 156–162. [Google Scholar] [CrossRef]
- Schönborn, S.; Krömker, V. Detection of the biofilm component polysaccharide intercellular adhesin in Staphylococcus aureus infected cow udders. Vet. Microbiol. 2016, 196, 126–128. [Google Scholar] [CrossRef]
- GVA. Guidelines for Combating Bovine Mastitis as a Stock Problem, 5th ed.; German Veterinary Association: Gießen, Germany, 2012. [Google Scholar]
- GERMAP. GERMAP 2015–Bericht Über den Antibiotikaverbrauch und die Verbreitung von Antibiotikaresistenzen in der Human-und Veterinärmedizin in Deutschland; Bundesamt für Verbraucherschutz: Berlin, Germany, 2016; ISBN 978-3-9818383-0-5.
- Dingwell, R.T.; Leslie, K.E.; Duffield, T.F.; Schukken, Y.H.; DesCoteaux, L.; Keefe, G.P.; Bagg, R. Efficacy of intramammary tilmicosin and risk factors for cure of Staphylococcus aureus infection in the dry period. J. Dairy Sci. 2003, 86, 159–168. [Google Scholar] [CrossRef]
- Nickerson, S.C.; Owens, W.E.; Fox, L.K.; Scheifinger, C.C.; Shryock, T.R.; Spike, T.E. Comparison of tilmicosin and cephapirin as therapeutics for Staphylococcus aureus mastitis at dry-off. J. Dairy Sci. 1999, 82, 696–703. [Google Scholar] [CrossRef]
- Steele, N.; McDougall, S. Effect of prolonged duration therapy of subclinical mastitis in lactating dairy cows using penethamate hydriodide. N. Z. Vet. J. 2014, 62, 38–46. [Google Scholar] [CrossRef]
- Linder, M.; Paduch, J.H.; Grieger, A.S.; Mansion-de Vries, E.; Knorr, N.; Zinke, C.; Krömker, V. Heilungsraten chronischer subklinischer Staphylococcus aureus-Mastitiden nach antibiotischer Therapie bei laktierenden Milchkühen. Cure rates of chronic subclinical Staphylococcus aureus mastitis in lactating dairy cows after antibiotic therapy. Berl. Münch. Tierärztl. Wschr. 2013, 6, 291–296. [Google Scholar] [CrossRef]
- Owens, W.; Nickerson, S.; Ray, C. Efficacy of parenterally or intramammarily administered tilmicosin or ceftiofur against Staphylococcus aureus mastitis during lactation. J. Dairy Sci. 1999, 82, 645–647. [Google Scholar] [CrossRef]
- Spohr, M.; Rau, J.; Friedrich, A.; Klittich, G.; Fetsch, A.; Guerra, B.; Hammerl, J.A.; Tenhagen, B.-A. Methicillin-Resistant Staphylococcus aureus (MRSA) in Three Dairy Herds in Southwest Germany. Zoonoses Public Health 2011, 58, 252–261. [Google Scholar] [CrossRef]
- Kreausukon, K.; Fetsch, A.; Kraushaar, B.; Alt, K.; Muller, K.; Kromker, V.; Zessin, K.H.; Kasbohrer, A.; Tenhagen, B.A. Prevalence, antimicrobial resistance, and molecular characterization of methicillin-resistant Staphylococcus aureus from bulk tank milk of dairy herds. J. Dairy Sci. 2012, 95, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Hart, T.; Cars, O.; Streulens, M.; Helmuth, R.; Huovinen, P.; Sprenger, M. Antimicrobial resistance. BMJ 1998, 317, 609–610. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Matsuzaki, S.; Yasuda, M.; Nishikawa, H.; Kuroda, M.; Ujihara, T.; Shuin, T.; Shen, Y.; Jin, Z.; Fujimoto, S.; Nasimuzzaman, M.D.; et al. Experimental Protection of Mice against Lethal Staphylococcus aureus Infection by Novel Bacteriophage ϕMR11. J. Infect. Dis. 2003, 187, 613–624. [Google Scholar] [CrossRef]
- Wills, Q.F.; Kerrigan, C.; Soothill, J.S. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob. Agents Chemother. 2005, 49, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; Parlato, M.; Borriello, G.; Salvatore, P.; Iannelli, D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 2007, 51, 2765–2773. [Google Scholar] [CrossRef]
- Hobbs, Z.; Abedon, S.T. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Carlton, R.M. Phage therapy: Past history and future prospects. Arch. Immunol. Ther. Exp. Engl. Ed. 1999, 47, 267–274. [Google Scholar] [CrossRef]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H.; Canchaya, C.; Hardt, W.-D. Phages and the evolution of bacterial pathogens: From genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Krömker, V. Kurzes Lehrbuch Milchkunde und Milchhygiene, 1st ed.; Stuttgart: Parey, Germany, 2007; pp. 10–12. [Google Scholar]
- O’Flaherty, S.; Coffey, A.; Meaney, W.J.; Fitzgerald, G.F.; Ross, R.P. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 2005, 41, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Sabour, P.M.; Leslie, K.E.; Griffiths, M.W. Bovine whey proteins inhibit the interaction of Staphylococcus aureus and bacteriophage K. J. Appl. Microbiol. 2006, 101, 377–386. [Google Scholar] [CrossRef]
- Garcia, P.; Madera, C.; Martinez, B.; Rodriguez, A.; Evaristo Suarez, J. Prevalence of bacteriophages infecting Staphylococcus aureus in dairy samples and their potential as biocontrol agents. J. Dairy Sci. 2009, 92, 3019–3026. [Google Scholar] [CrossRef]
- Arber, W. Host-controlled modification of bacteriophage. Annu. Rev. Microbiol. 1965, 19, 365–378. [Google Scholar] [CrossRef]
- Abedon, S.T. Lysis from without. Bacteriophage 2011, 1, 46–49. [Google Scholar] [CrossRef]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar] [CrossRef]
- Scholl, D.; Adhya, S.; Merril, C. Escherichia coli K1′s capsule is a barrier to bacteriophage T7. Appl. Environ. Microbiol. 2005, 71, 4872–4874. [Google Scholar] [CrossRef]
- Xia, G.; Corrigan, R.M.; Winstel, V.; Goerke, C.; Grundling, A.; Peschel, A. Wall teichoic Acid-dependent adsorption of staphylococcal siphovirus and myovirus. J. Bacteriol. 2011, 193, 4006–4009. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gerlach, D.; Du, X.; Larsen, J.; Stegger, M.; Kühner, P.; Peschel, A.; Xia, G.; Winstel, V. An accessory wall teichoic acid glycosyltransferase protects Staphylococcus aureus from the lytic activity of Podoviridae. Sci. Rep. 2015, 5, 17219. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Takeuchi, I.; Osada, K.; Azam, A.H.; Asakawa, H.; Miyanaga, K.; Tanji, Y. The Presence of Two Receptor-Binding Proteins Contributes to the Wide Host Range of Staphylococcal Twort-Like Phages. Appl. Environ. Microbiol. 2016, 82, 5763–5774. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Xia, G.; Luhachack, L.G.; Campbell, J.; Meredith, T.C.; Chen, C.; Winstel, V.; Gekeler, C.; Irazoqui, J.E.; Peschel, A. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. USA 2012, 109, 18909–18914. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Martinez, B.; Obeso, J.M.; Lavigne, R.; Lurz, R.; Rodriguez, A. Functional genomic analysis of two Staphylococcus aureus phages isolated from the dairy environment. Appl. Environ. Microbiol. 2009, 75, 7663–7673. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Ross, R.P.; Flynn, J.; Meaney, W.J.; Fitzgerald, G.F.; Coffey, A. Isolation and characterization of two anti-staphylococcal bacteriophages specific for pathogenic Staphylococcus aureus associated with bovine infections. Lett. Appl. Microbiol. 2005, 41, 482–486. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Edwards, R.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. Genome of Staphylococcal Phage K: A New Lineage of Myoviridae Infecting Gram-Positive Bacteria with a Low G+C Content. J. Bacteriol. 2004, 186, 2862–2871. [Google Scholar] [CrossRef]
- Harris, L.G.; Foster, S.J.; Richards, R.G. An introduction to staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur. Cells Mater. 2002, 4, 39–60. [Google Scholar] [CrossRef]
- Krömker, V.F.J.; Klocke, D. Shedding patterns of Staphylococcus aureus in quarter foremilk samples of cows with known S. aureus infections. Tieraerztliche Prax. Ausg. Grosstiere Nutztiere 2008, 36, 389–392. [Google Scholar]
- Iwano, H.; Inoue, Y.; Takasago, T.; Kobayashi, H.; Furusawa, T.; Taniguchi, K.; Fujiki, J.; Yokota, H.; Usui, M.; Tanji, Y. Bacteriophage ΦSA012 has a broad host range against Staphylococcus aureus and effective lytic capacity in a mouse mastitis model. Biology 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zou, W.; Zhang, M.; Xu, L.; Liu, F.; Li, X.; Wang, L.; Xu, Y. Evaluation of phage therapy in the treatment of Staphylococcus aureus-induced mastitis in mice. Folia Microbiol. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional value and technological suitability of milk from various animal species used for dairy production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Ross, R.P.; Meaney, W.; Fitzgerald, G.F.; Elbreki, M.F.; Coffey, A. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microbiol. 2005, 71, 1836–1842. [Google Scholar] [CrossRef]
- Kraushaar, B.; Thanh, M.D.; Hammerl, J.A.; Reetz, J.; Fetsch, A.; Hertwig, S. Isolation and characterization of phages with lytic activity against methicillin-resistant Staphylococcus aureus strains belonging to clonal complex 398. Arch. Virol. 2013, 158, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Vybiral, D.; Takáč, M.; Loessner, M.; Witte, A.; von Ahsen, U.; Bläsi, U. Complete nucleotide sequence and molecular characterization of two lytic Staphylococcus aureus phages: 44AHJD and P68. FEMS Microbiol. Lett. 2003, 219, 275–283. [Google Scholar] [CrossRef]
- Kwan, T.; Liu, J.; DuBow, M.; Gros, P.; Pelletier, J. The complete genomes and proteomes of 27 Staphylococcus aureus bacteriophages. Proc. Natl. Acad. Sci. USA 2005, 102, 5174–5179. [Google Scholar] [CrossRef]
- Krumsiek, J.; Arnold, R.; Rattei, T. Gepard: A rapid and sensitive tool for creating dotplots on genome scale. Bioinformatics 2007, 23, 1026–1028. [Google Scholar] [CrossRef]
- Takáč, M.; Bläsi, U. Phage P68 virion-associated protein 17 displays activity against clinical isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 2934–2940. [Google Scholar] [CrossRef]
- Hrebík, D.; Štveráková, D.; Škubník, K.; Füzik, T.; Pantůček, R.; Plevka, P. Structure and genome ejection mechanism of Staphylococcus aureus phage P68. Sci. Adv. 2019, 5, eaaw7414. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- German Institute for Standardization (Deutsches Institut für Normung). Microbiological Milk Testing—Determination of the Bacterial Count–Part 5: Spatula Method; DIN 10192-5:1995-05; German Institute for Standardization: Berlin, Germany, 1995. [Google Scholar]
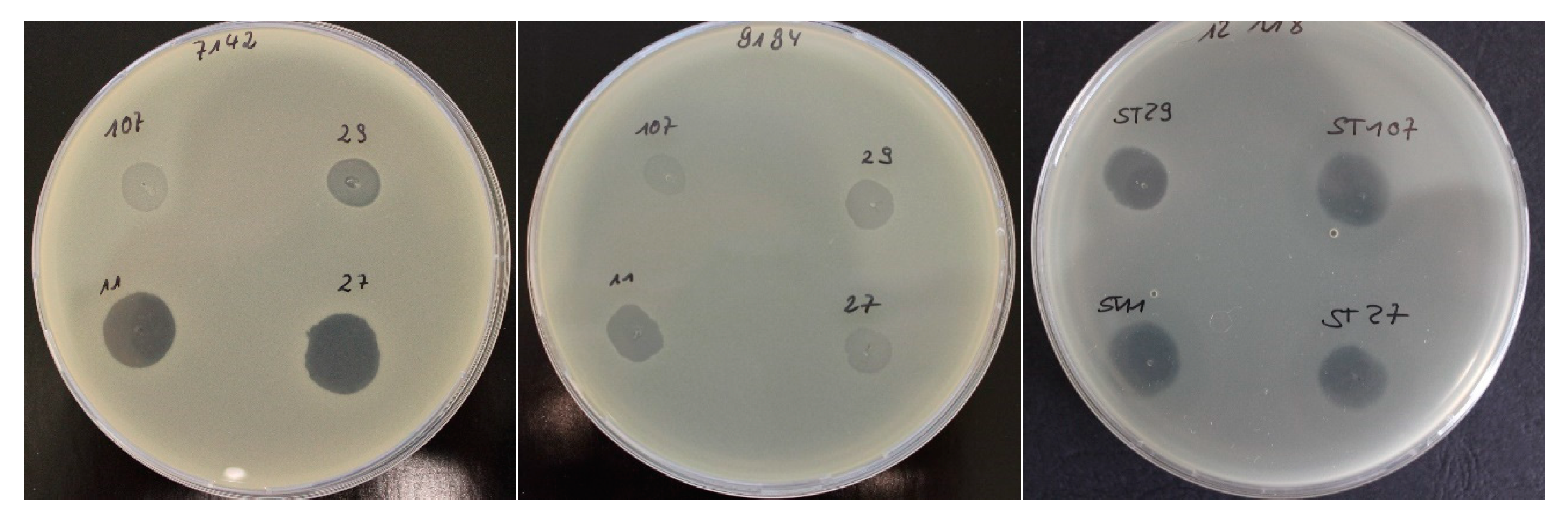
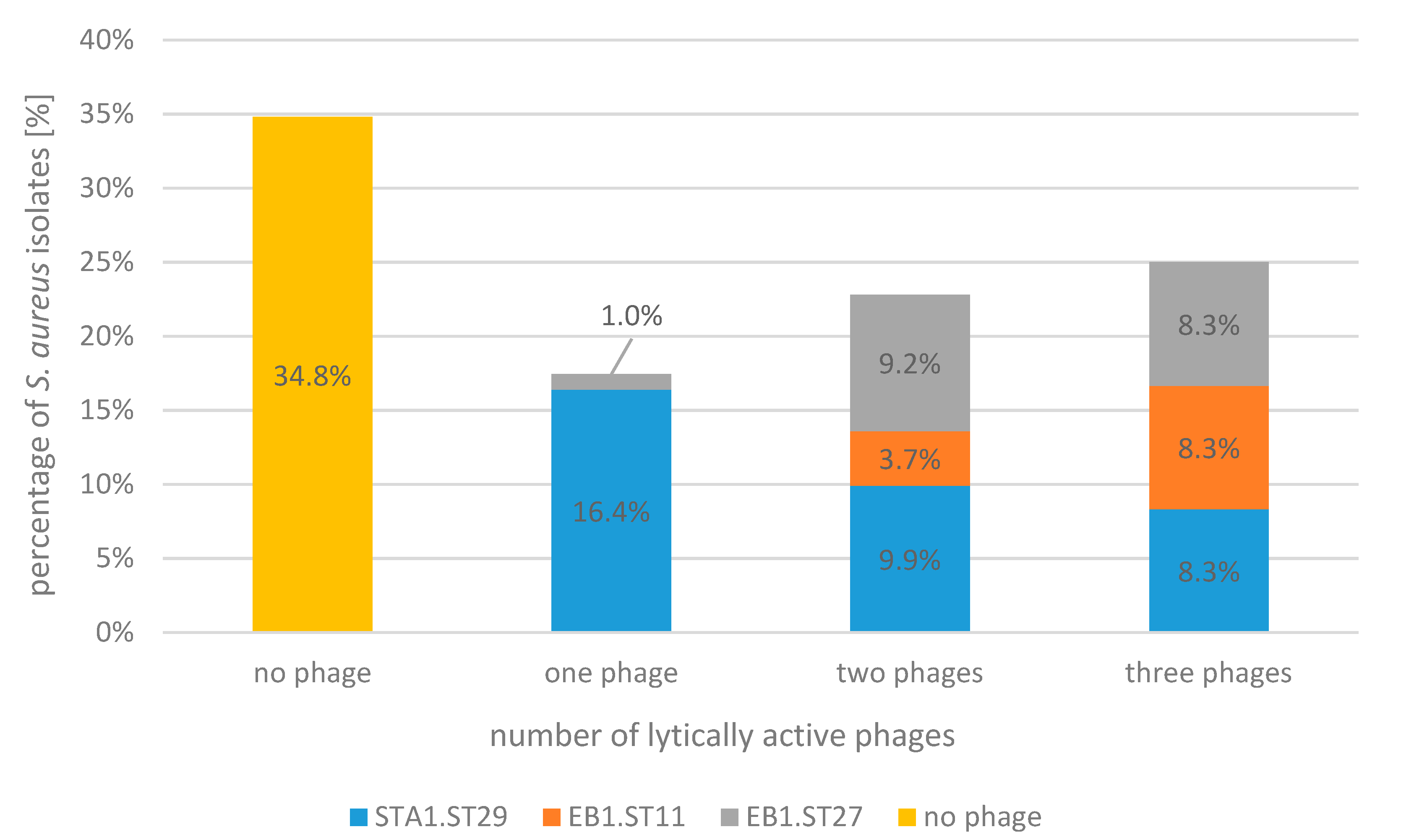
| Spot test/relative EOP | ||||||
|---|---|---|---|---|---|---|
| STA1.ST29 | EB1.ST11 | EB1.ST27 | ||||
| S. aureus isolates | spot test a | EOP b,c | spot test a | EOP b,d | spot test a | EOP b,e |
| 5208 | +/− | + | 6.67 × 10−5 | + | 4.93 × 10−5 | |
| 5209 | +/− | + | 1.20 × 10−4 | + | 8.42 × 10−6 | |
| 5210 | + | 3.88 × 10−5 | + | 5.70 × 10−4 | +/− | |
| 5213 | +/− | +/− | − | |||
| 5214 | + | 6.95 × 10−2 | + | 1.15 × 10−7 | + | 1.76 × 10−5 |
| 5219 | +/− | − | − | |||
| 5220 | +/− | − | − | |||
| 5221 | +/− | +/− | − | |||
| 5222 | +/− | +/− | − | |||
| 5223 | +/− | +/− | − | |||
| 5224 | +/− | +/− | − | |||
| 5225 | +/− | +/− | − | |||
| 5226 | +/− | +/− | − | |||
| 5228 | +/− | +/− | − | |||
| 5233 | +/− | +/− | − | |||
| 5235 | +/− | +/− | − | |||
| 7135 | + | 9.07 × 10−3 | + | 5.97 × 10−9 | + | 1.24 × 10−6 |
| 7142 | + | 1.23 × 10−2 | + | 7.13 × 10−4 | + | 1.79 × 10−2 |
| 8046 | + | 4.05 × 10−1 | + | 2.74 × 10−6 | + | 5.86 × 10−5 |
| 9184 | + | 5.20 × 10−1 | + | 1.58 × 10−6 | + | 7.57 × 10−5 |
| 9185 | +/− | +/− | − | |||
| 9192 | +/− | +/− | − | |||
| 9194 | +/− | +/− | +/− | |||
| 9210 | +/− | + | 2.32 × 10−4 | + | 2.74 × 10−4 | |
| 9220 | +/− | +/− | +/− | |||
| 9226 | +/− | +/− | − | |||
| 9234 | +/− | +/− | − | |||
| 9240 | +/− | +/− | − | |||
| 9242 | +/− | +/− | − | |||
| 9264 | + | 1.95 × 10−1 | + | 2.53 × 10−4 | + | 4.70 × 10−5 |
| 9267 | + | 1.81 × 10−1 | +/− | + | 5.59 × 10−6 | |
| 9271 | +/− | +/− | − | |||
| 9276 | +/− | +/− | − | |||
| 9833 | + | 1.12 × 10−1 | +/− | + | 9.87 × 10−8 | |
| 9854 | + | 9.51 × 10−3 | +/− | +/− | ||
| 9858 | + | 1.43 × 100 | +/− | + | 6.58 × 10−7 | |
| 9899 | + | 1.55 × 10−2 | +/− | +/− | ||
| 9931 | + | 3.05 × 10−2 | +/− | +/− | ||
| 9996 | + | 1.33 × 10−2 | +/− | + | 3.29 × 10−7 | |
| 9999 | + | 6.90 × 10−2 | + | 9.18 × 10−8 | + | 1.72 × 10−6 |
| 10048 | + | 1.16 × 10−2 | +/− | +/− | ||
| 10141 | + | 1.34 × 10−2 | +/− | + | 3.29 × 10−7 | |
| 10154 | + | 1.32 × 10−2 | +/− | + | 6.58 × 10−7 | |
| 10170 | + | 3.78 × 10−3 | + | 1.05 × 10−7 | + | 5.26 × 10−6 |
| 10172 | +/− | − | − | |||
| 10201 | + | 9.76 × 10−3 | +/− | + | 3.29 × 10−7 | |
| 10237 | + | 1.57 × 10−2 | +/− | +/− | ||
| 10366 | + | 1.23 × 10−2 | +/− | +/− | ||
| 10451 | +/− | − | − | |||
| 10455 | + | 2.93 × 10−4 | +/− | − | ||
| 10490 | + | 5.44 × 10−4 | − | − | ||
| 10538 | + | 4.39 × 10−3 | +/− | +/− | ||
| 10574 | + | 1.03 × 10−2 | − | + | 2.39 × 10−5 | |
| 10614 | + | 3.93 × 10−2 | + | 1.34 × 10−3 | + | 3.85 × 10−3 |
| 10621 | + | 5.56 × 10−2 | − | + | 5.92 × 10−5 | |
| 10644 | + | 2.40 × 10−2 | − | +/− | ||
| 10645 | + | 2.03 × 10−1 | − | + | 2.39 × 10−5 | |
| 10647 | + | 1.46 × 10−4 | − | + | 1.43 × 10−2 | |
| 10649 | + | 7.44 × 10−6 | + | 2.57 × 10−7 | + | 1.54 × 10−4 |
| 10678 | +/− | − | − | |||
| 10682 | +/− | − | − | |||
| 10693 | + | 1.46 × 10−4 | + | 2.28 × 10−6 | + | 5.17 × 10−5 |
| 10754 | + | 6.37 × 10−6 | + | 1.22 × 10−6 | + | 1.66 × 10−5 |
| 10811 | + | 8.54 × 10−3 | +/− | + | 6.58 × 10−7 | |
| 10856 | + | 4.88 × 10−5 | +/− | + | 7.89 × 10−4 | |
| 10934 | + | 1.68 × 10−3 | +/− | |||
| 10939 | + | 9.76 × 10−5 | +/− | +/− | ||
| 10940 | + | 3.41 × 10−4 | − | − | ||
| 12101 | + | 2.80 × 10−3 | +/− | − | ||
| 12104 | + | 1.00 × 10−2 | + | 5.95 × 10−6 | +/− | |
| 12105 | + | 1.06 × 10−2 | + | 1.19 × 10−4 | + | 2.39 × 10−3 |
| 12108 | +/− | − | + | 7.50 × 10−6 | ||
| 12109 | + | 1.30 × 10−2 | + | 1.34 × 10−5 | + | 9.54 × 10−5 |
| 12110 | + | 4.32 × 10−4 | + | 3.73 × 10−5 | + | 3.29 × 10−6 |
| 12111 | +/− | +/− | − | |||
| 12112 | +/− | +/− | − | |||
| 12113 | + | 1.93 × 10−2 | + | 3.27 × 10−5 | + | 6.91 × 10−7 |
| 12114 | + | 9.76 × 10−4 | + | 8.65 × 10−7 | − | |
| 12115 | + | 6.34 × 10−4 | + | 2.49 × 10−6 | + | 5.92 × 10−6 |
| 12116 | + | 3.27 × 10−2 | +/− | + | ||
| 12117 | + | 1.52 × 10−2 | + | 1.25 × 10−7 | + | 1.02 × 10−5 |
| 12118 | + | 2.63 × 10−2 | + | 7.49 × 10−6 | + | 1.91 × 10−5 |
| 12119 | + | 8.88 × 10−5 | + | 2.11 × 10−7 | + | 1.61 × 10−6 |
| 12120 | +/− | +/− | − | |||
| 12121 | +/− | +/− | +/− | |||
| 12122 | + | 2.03 × 10−1 | + | 9.01 × 10−5 | + | 3.81 × 10−3 |
| 12123 | + | 8.56 × 10−3 | + | 6.33 × 10−6 | + | 4.27 × 10−4 |
| 12124 | +/− | +/− | − | |||
| 12125 | + | 2.46 × 10−3 | + | 6.54 × 10−3 | + | 4.64 × 10−2 |
| 12126 | + | 6.39 × 10−3 | + | 1.58 × 10−6 | + | 3.03 × 10−6 |
| 12128 | +/− | +/− | − | |||
| 12134 | + | 2.78 × 10−3 | − | − | ||
| Phage | Phage Origin | Details | Propagation Strain (S. aureus) | Strain Origin |
|---|---|---|---|---|
| STA1.ST29 | sewage water | Myovirus; related to phage K | ST29 | human isolate |
| STA1.ST107 | sewage water | Myovirus; related to phage K | ST107 | pig farm |
| EB1.ST11 | pig manure | Podovirus related to phage PSa3 | ST11 | mastitis milk sample |
| EB1.ST27 | pig manure | Podovirus related to phage PSa3 | ST27 | mastitis milk sample |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titze, I.; Lehnherr, T.; Lehnherr, H.; Krömker, V. Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis. Pharmaceuticals 2020, 13, 35. https://doi.org/10.3390/ph13030035
Titze I, Lehnherr T, Lehnherr H, Krömker V. Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis. Pharmaceuticals. 2020; 13(3):35. https://doi.org/10.3390/ph13030035
Chicago/Turabian StyleTitze, Isabel, Tatiana Lehnherr, Hansjörg Lehnherr, and Volker Krömker. 2020. "Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis" Pharmaceuticals 13, no. 3: 35. https://doi.org/10.3390/ph13030035
APA StyleTitze, I., Lehnherr, T., Lehnherr, H., & Krömker, V. (2020). Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis. Pharmaceuticals, 13(3), 35. https://doi.org/10.3390/ph13030035






