Targeted Protein Degradation by Chimeric Compounds using Hydrophobic E3 Ligands and Adamantane Moiety
Abstract
1. Introduction
2. Results and Discussion
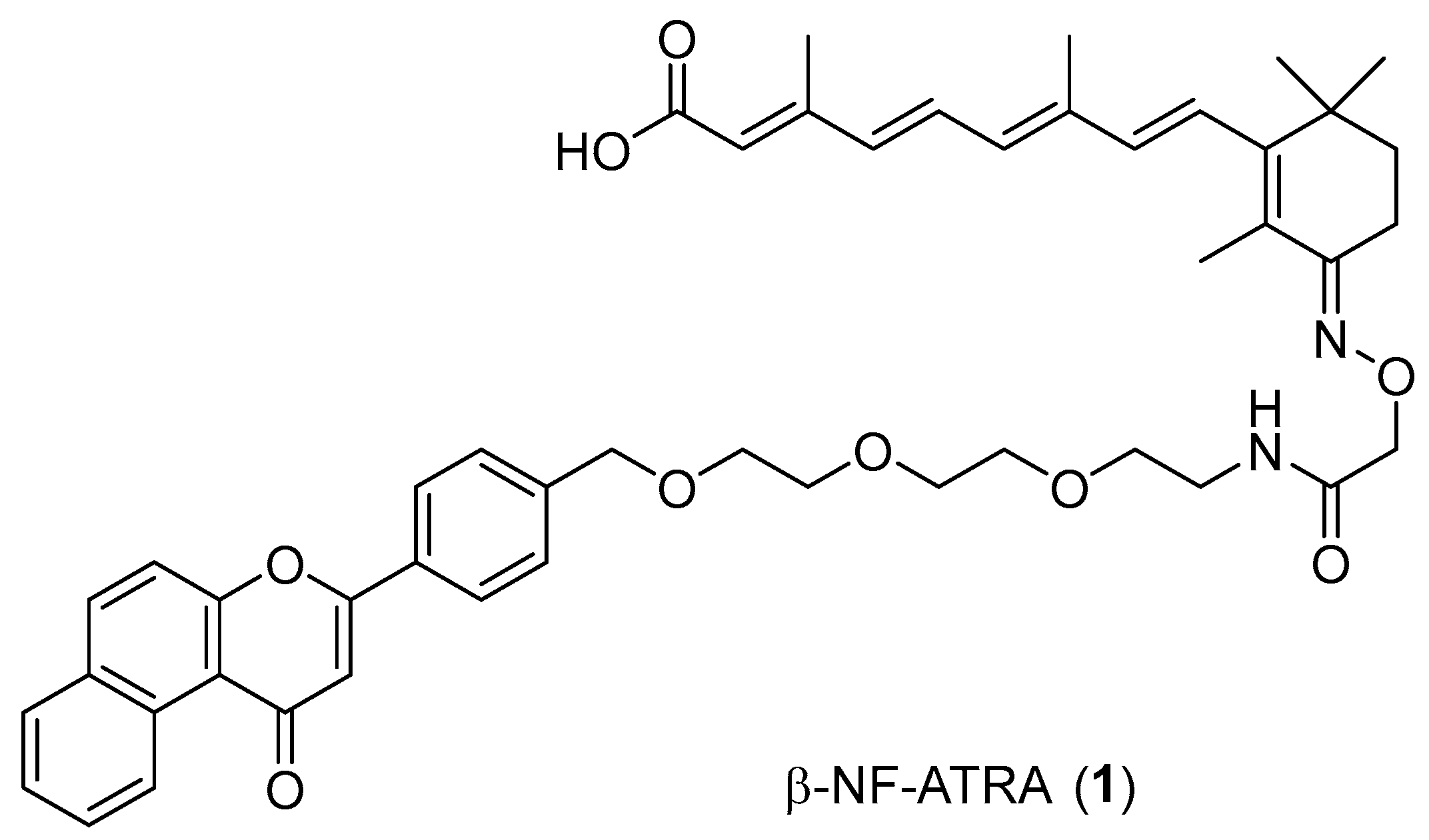
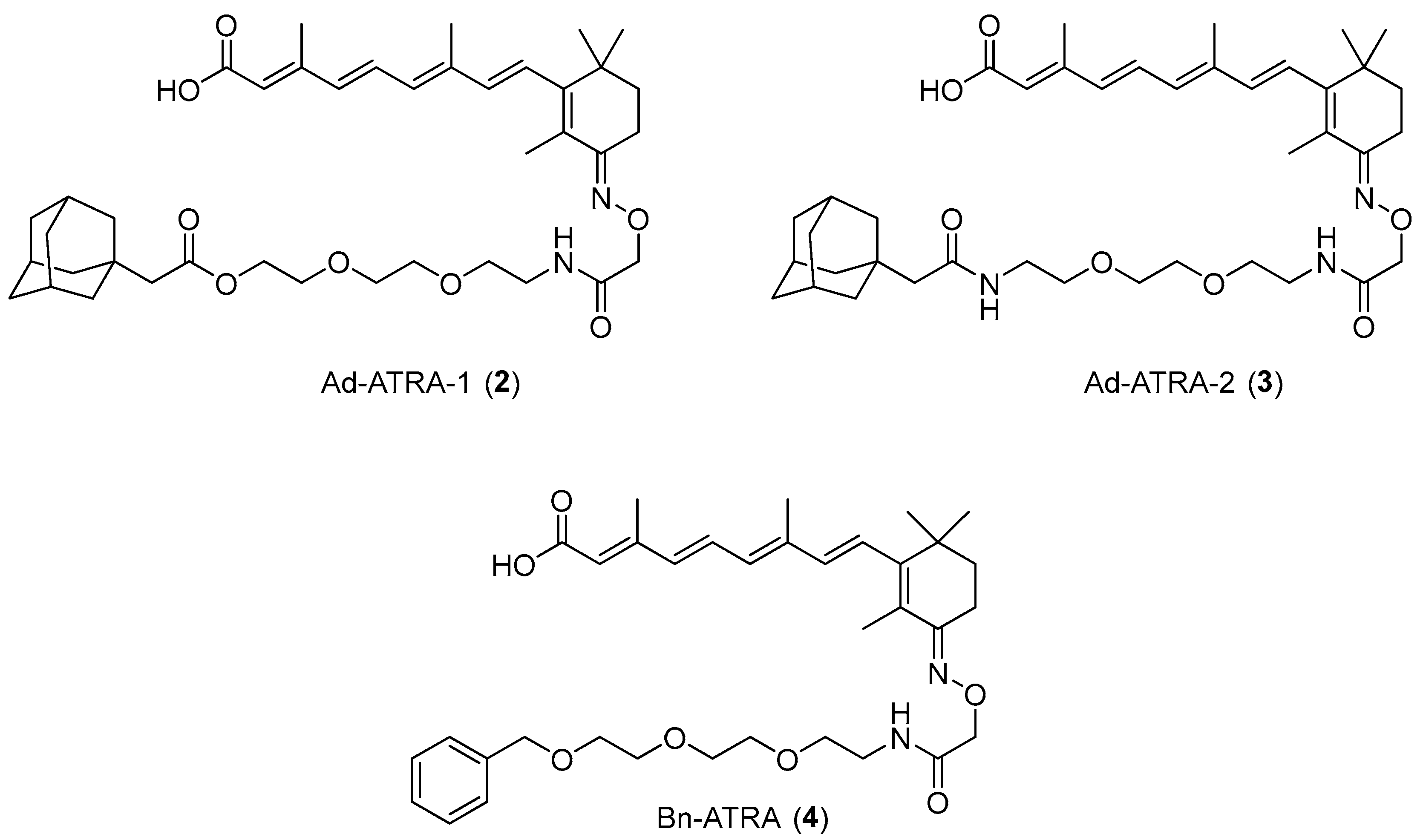
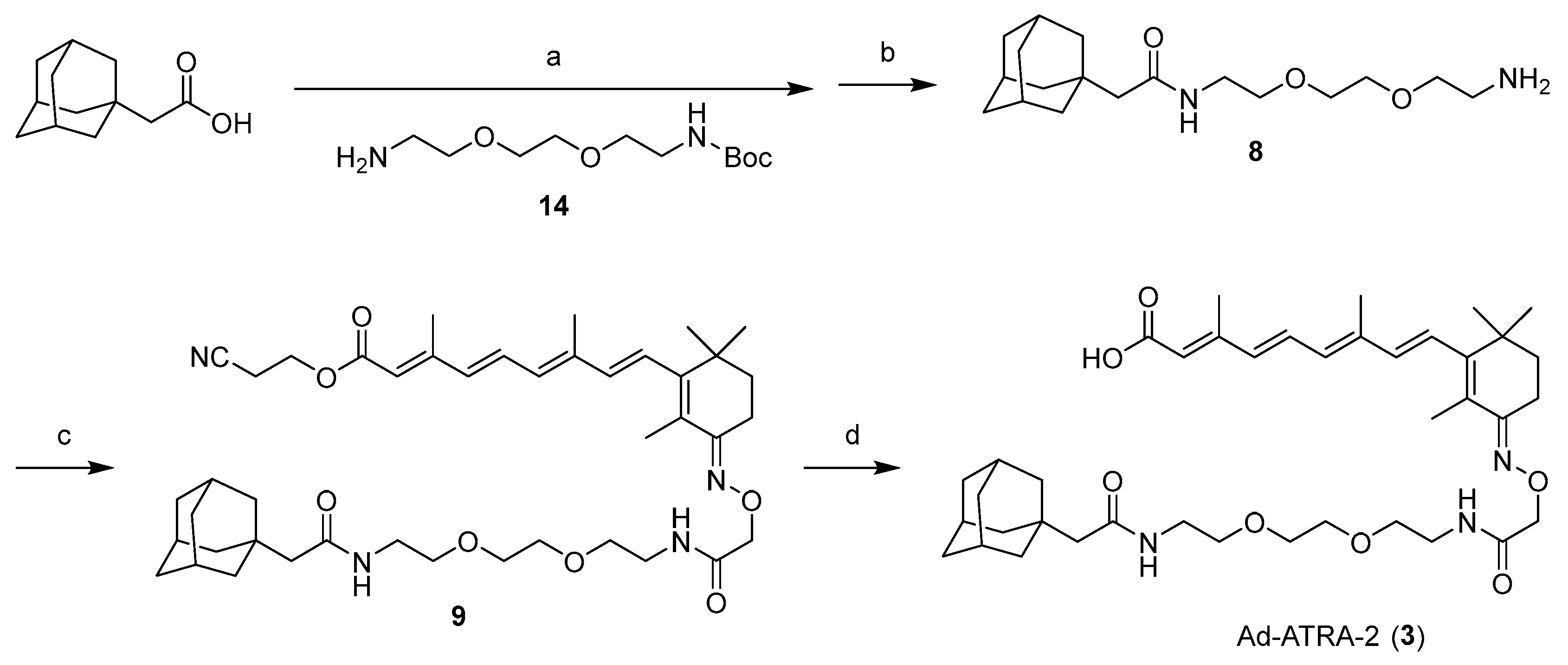
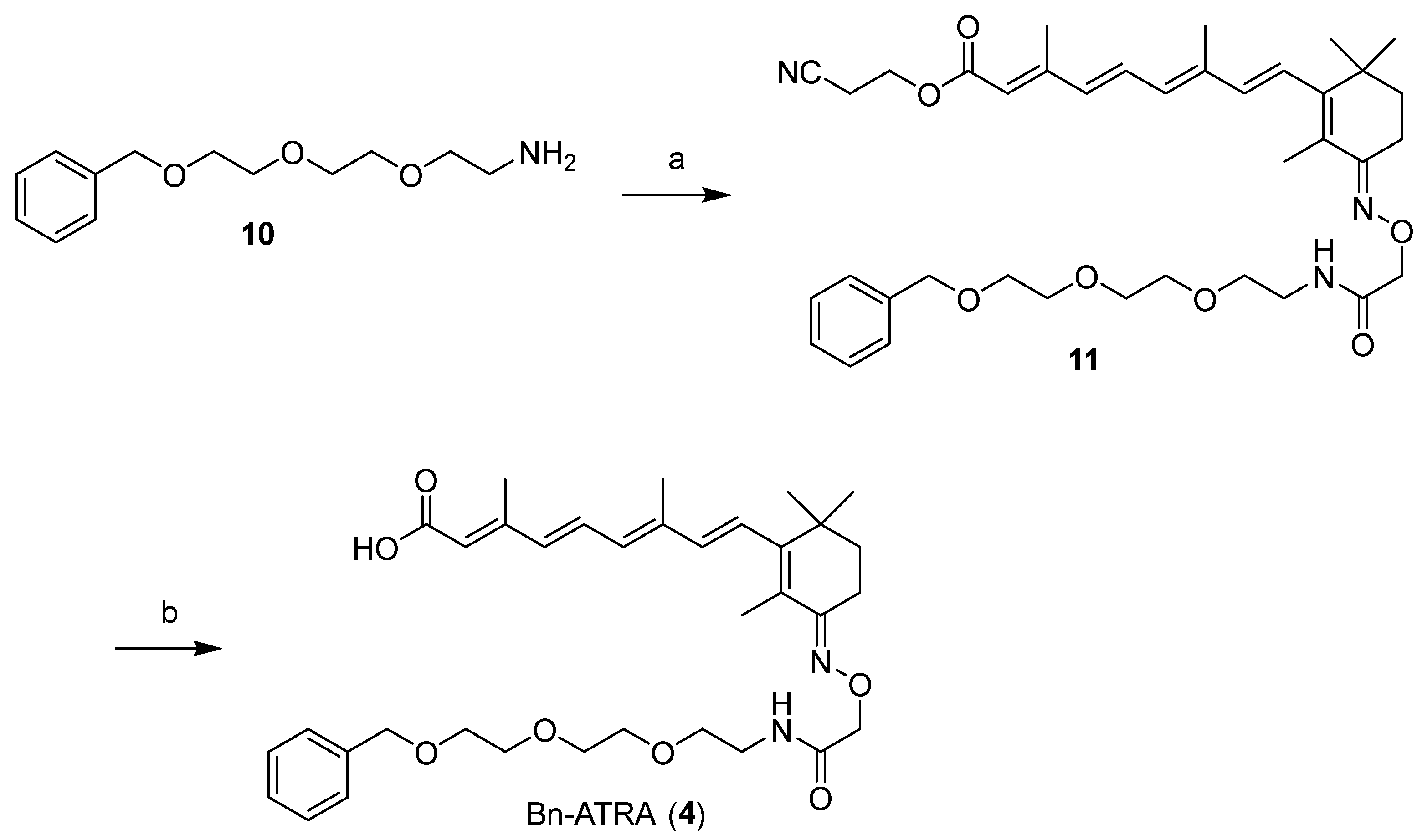
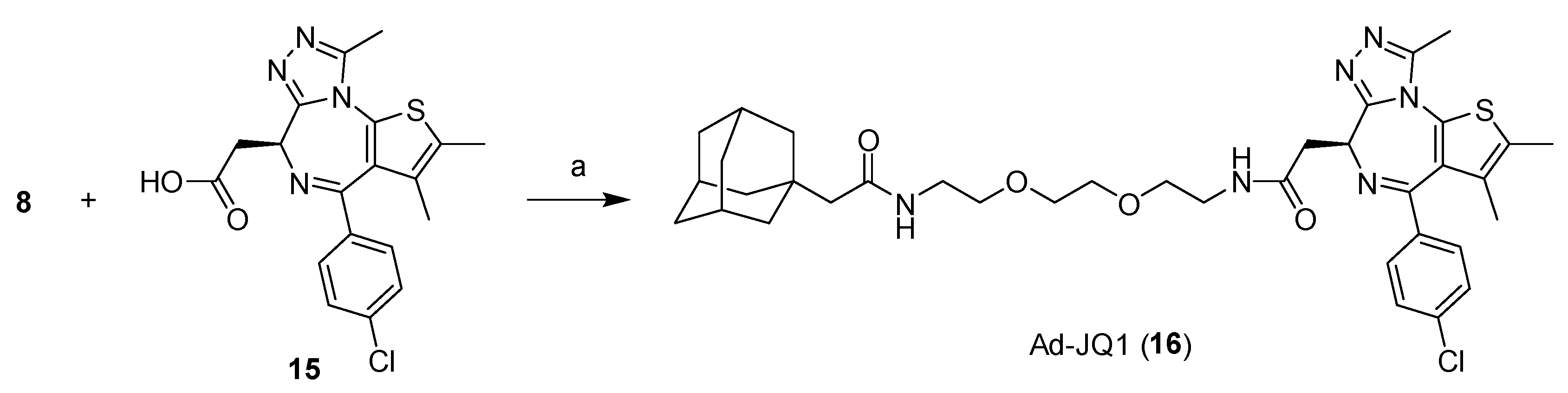
2.1. Design and Synthesis of Compounds
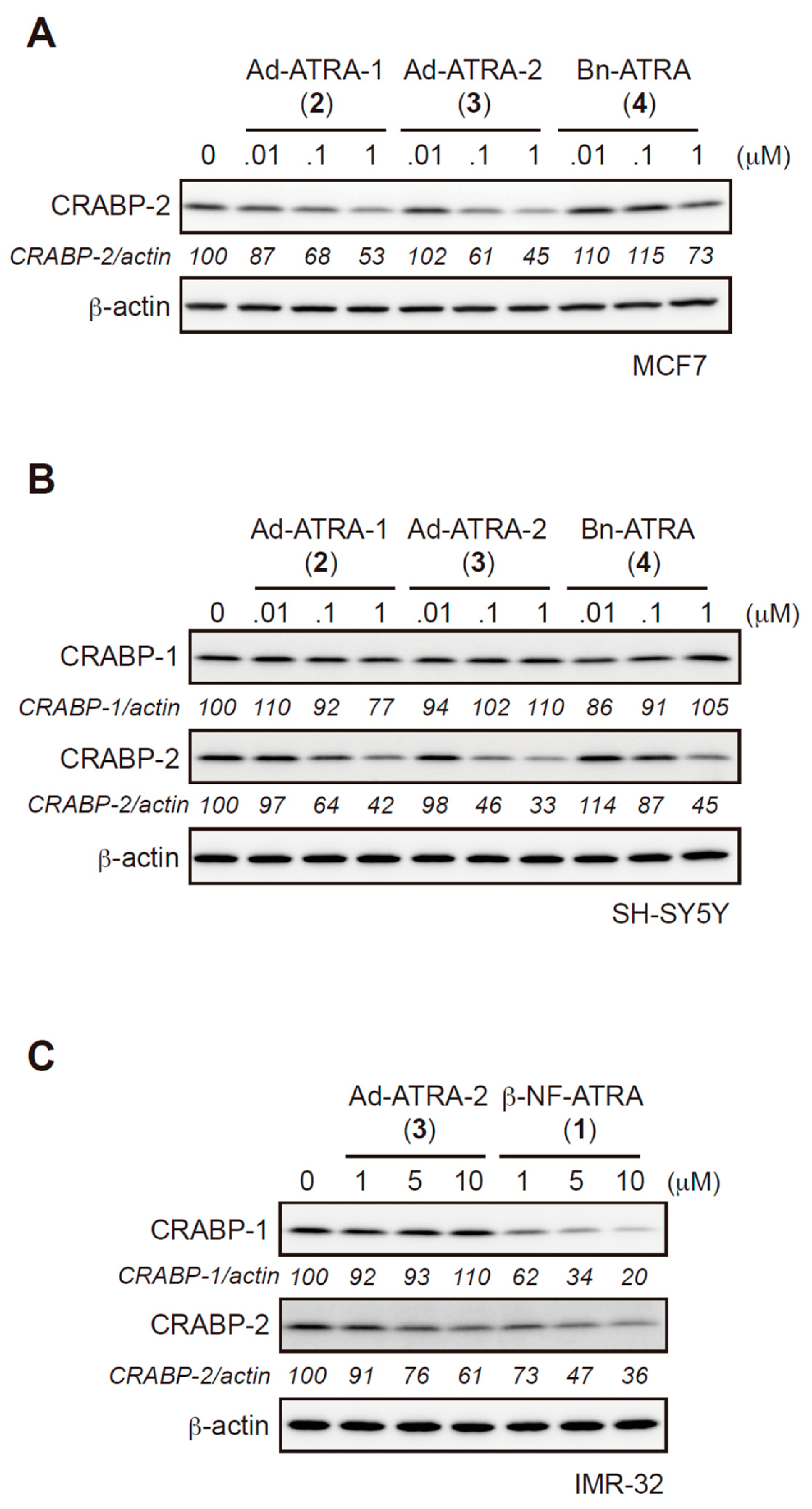
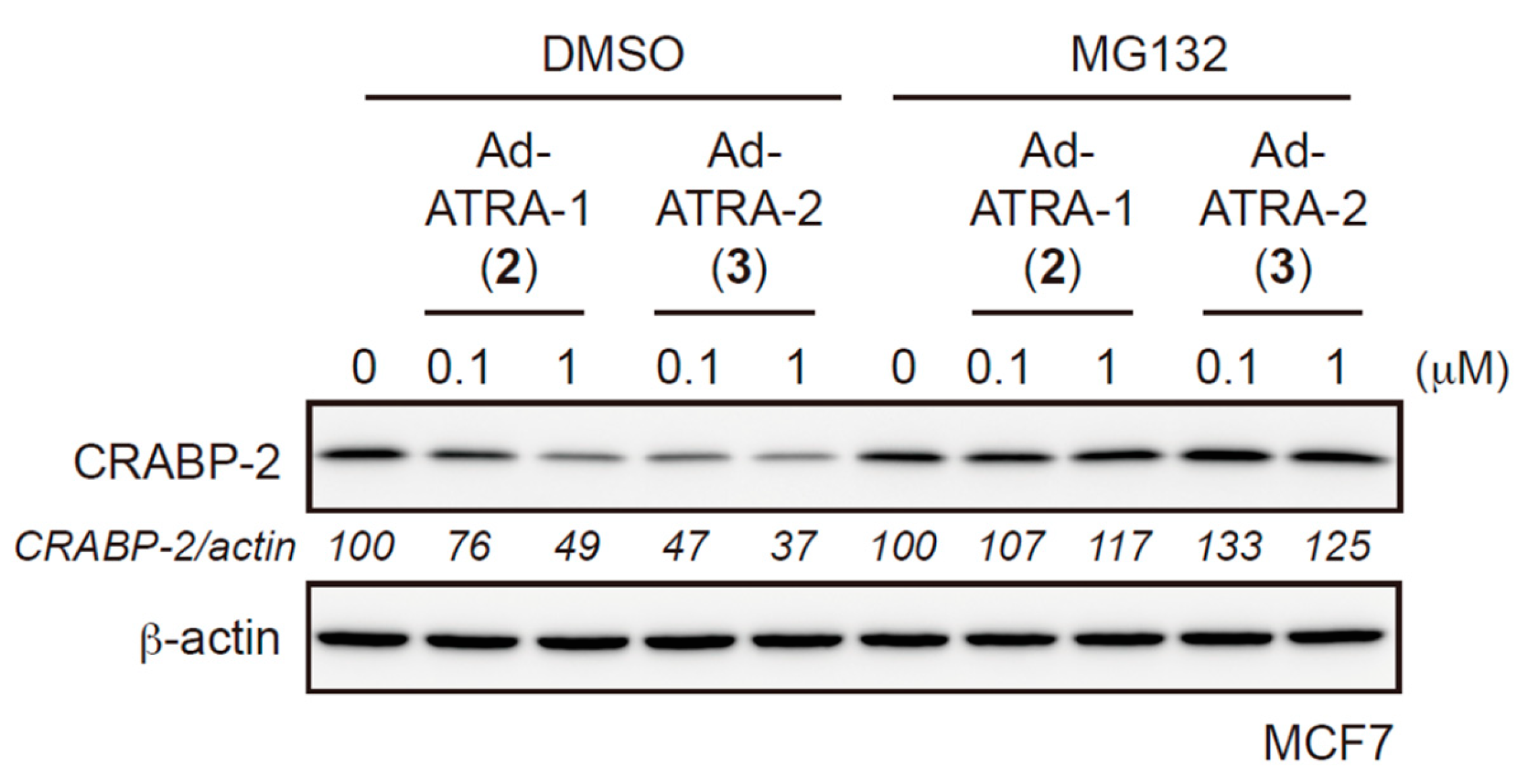
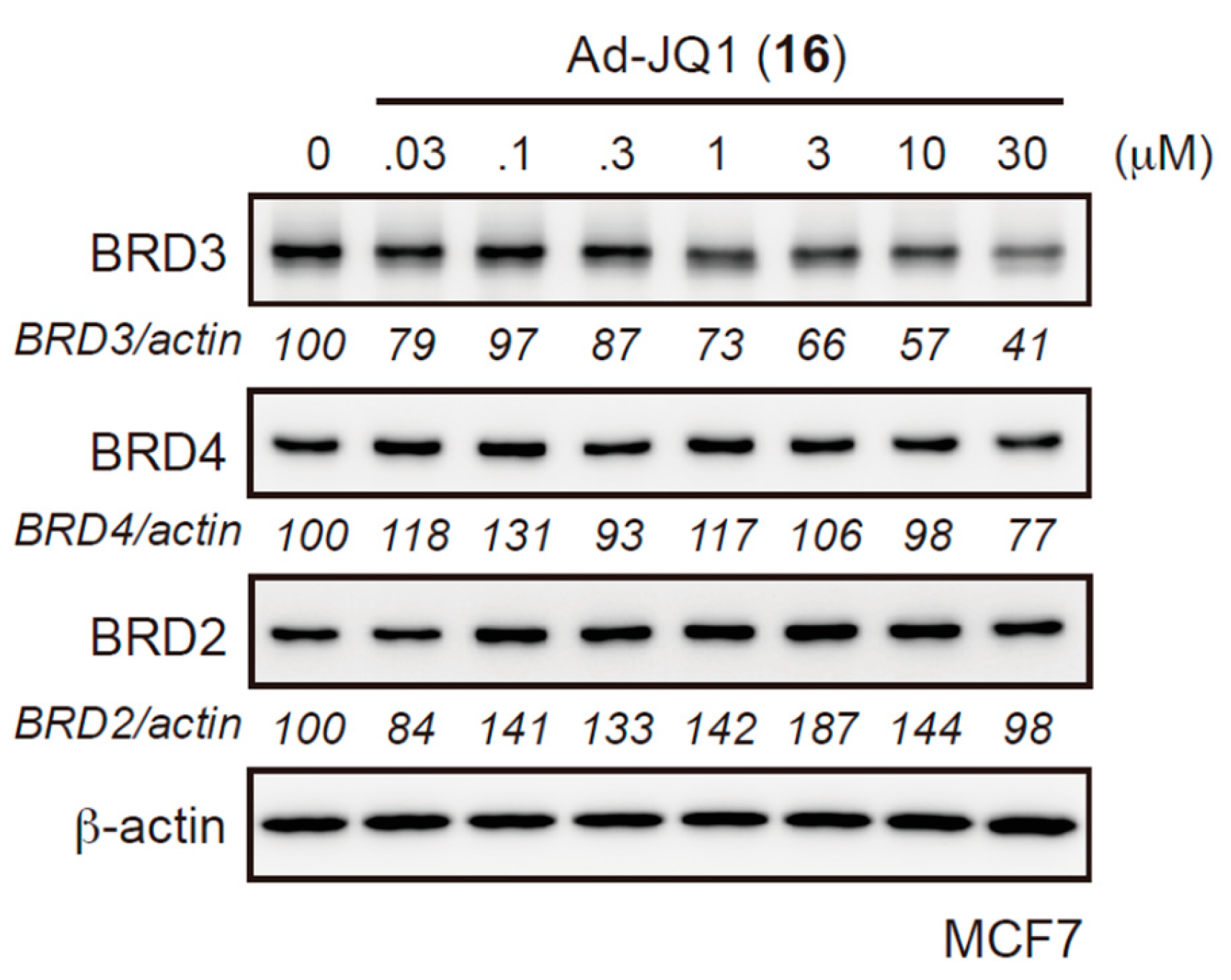
2.2. Evaluation of Protein Degradation Activity
3. Materials and Methods
3.1. Cell Culture
3.2. Western Blotting
3.3. Cell Viability Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PROTAC | proteolysis-targeting chimeras |
| SNIPER | specific and nongenetic inhibitors of apoptosis protein [IAP]-dependent protein erasers |
| AhR | aryl hydrocarbon receptor |
| β-NF | β-naphthoflavone |
| ATRA | all-trans retinoic acid |
| CRABPs | cellular retinoic acid-binding proteins |
| ITE | 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester |
| HyT | hydrophobic tagging |
| Ad | adamantane |
| HATU | 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-Oxide Hexafluorophosphate |
| DIPEA | N,N-diisopropylethylamine |
| DMF | N,N-dimethylformamide |
| TBAF | tetrabutylammonium Fluoride |
| THF | tetrahydrofuran |
| HBTU | 1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-Oxide Hexafluorophosphate |
| TLC | thin-layer chromatography |
| UV | ultra-violet |
| NMR | nuclear magnetic resonance |
| TMS | tetramethylsilane |
| IT-TOF MS | ion trap-time-of-flight mass spectrometry |
| calcd | caluculated |
| DMAP | N,N-dimethyl-4-aminopyridine |
References
- Ohoka, N.; Shibata, N.; Hattori, T.; Naito, M. Protein Knockdown Technology: Application of Ubiquitin Ligase to Cancer Therapy. Curr. Cancer Drug Targets 2016, 16, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)-Past, present and future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Okuhira, K.; Motoi, H.; Ohno, A.; Shoda, T.; Fukuhara, K.; Okuda, H.; Naito, M.; Kurihara, M. Design and synthesis of estrogen receptor degradation inducer based on a protein knockdown strategy. Bioorg. Med. Chem. Lett. 2012, 22, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, D.P.; Mares, A.; Smith, I.E.; Ko, E.; Campos, S.; Miah, A.H.; Mulholland, K.E.; Routly, N.; Buckley, D.L.; Gustafson, J.L.; et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 2015, 11, 611–617. [Google Scholar] [CrossRef]
- Ohoka, N.; Okuhira, K.; Ito, M.; Nagai, K.; Shibata, N.; Hattori, T.; Ujikawa, O.; Shimokawa, K.; Sano, O.; Koyama, R.; et al. In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs). J. Biol. Chem. 2017, 292, 4556–4570. [Google Scholar] [CrossRef]
- Shibata, N.; Nagai, K.; Morita, Y.; Ujikawa, O.; Ohoka, N.; Hattori, T.; Koyama, R.; Sano, O.; Imaeda, Y.; Nara, H.; et al. Development of Protein Degradation Inducers of Androgen Receptor by Conjugation of Androgen Receptor Ligands and Inhibitor of Apoptosis Protein Ligands. J. Med. Chem. 2018, 61, 543–575. [Google Scholar] [CrossRef]
- Salami, J.; Alabi, S.; Willard, R.R.; Vitale, N.J.; Wang, J.; Dong, H.; Jin, M.; McDonnell, D.P.; Crew, A.P.; Neklesa, T.K.; et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun. Biol. 2018, 1, 100. [Google Scholar] [CrossRef]
- Shibata, N.; Shimokawa, K.; Nagai, K.; Ohoka, N.; Hattori, T.; Miyamoto, N.; Ujikawa, O.; Sameshima, T.; Nara, H.; Cho, N.; et al. Pharmacological difference between degrader and inhibitor against oncogenic BCR-ABL kinase. Sci. Rep. 2018, 8, 13549. [Google Scholar] [CrossRef]
- Raina, K.; Lu, J.; Qian, Y.; Altieri, M.; Gordon, D.; Rossi, A.M.; Wang, J.; Chen, X.; Dong, H.; Siu, K.; et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 7124–7129. [Google Scholar] [CrossRef]
- Winter, G.E.; Buckley, D.L.; Paulk, J.; Roberts, J.M.; Souza, A.; Dhe-Paganon, S.; Bradner, J.E. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015, 348, 1376–1381. [Google Scholar] [CrossRef]
- McCoull, W.; Cheung, T.; Anderson, E.; Barton, P.; Burgess, J.; Byth, K.; Cao, Q.; Castaldi, M.P.; Chen, H.; Chiarparin, E.; et al. Development of a Novel B-Cell Lymphoma 6 (BCL6) PROTAC To Provide Insight into Small Molecule Targeting of BCL6. ACS Chem. Biol. 2018, 13, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ishikawa, M.; Naito, M.; Hashimoto, Y. Protein knockdown using methyl bestatin-ligand hybrid molecules: Design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J. Am. Chem. Soc. 2010, 132, 5820–5826. [Google Scholar] [CrossRef] [PubMed]
- Okuhira, K.; Ohoka, N.; Sai, K.; Nishimaki-Mogami, T.; Itoh, Y.; Ishikawa, M.; Hashimoto, Y.; Naito, M. Specific degradation of CRABP-II via cIAP1-mediated ubiquitylation induced by hybrid molecules that crosslink cIAP1 and the target protein. FEBS Lett. 2011, 585, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Bondeson, J.C.; Lewis, J.H.; Mannarino, E.G.; Friesen, S.A.; Wagar, M.M.; Balboni, T.A.; Alexander, B.M.; Arvold, N.D.; Sher, D.J.; et al. Evaluation of initial setup accuracy and intrafraction motion for spine stereotactic body radiation therapy using stereotactic body frames. Pract. Radiat. Oncol. 2016, 6, e17–e24. [Google Scholar] [CrossRef]
- Toure, M.; Crews, C.M. Small-Molecule PROTACS: New Approaches to Protein Degradation. Angew. Chem. Int. Ed. Engl. 2016, 55, 1966–1973. [Google Scholar] [CrossRef]
- Ohoka, N.; Tsuji, G.; Shoda, T.; Fujisato, T.; Kurihara, M.; Demizu, Y.; Naito, M. Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins. ACS Chem. Biol. 2019, 14, 2822–2832. [Google Scholar] [CrossRef]
- Ohtake, F.; Takeyama, K.; Matsumoto, T.; Kitagawa, H.; Yamamoto, Y.; Nohara, K.; Tohyama, C.; Krust, A.; Mimura, J.; Chambon, P.; et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 2003, 423, 545–550. [Google Scholar] [CrossRef]
- Soucy, T.A.; Smith, P.G.; Rolfe, M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin. Cancer Res. 2009, 15, 3912–3916. [Google Scholar] [CrossRef]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef]
- Lai, A.C.; Crews, C.M. Induced protein degradation: An emerging drug discovery paradigm. Nat. Rev. Drug Discov. 2017, 16, 101–114. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Small-Molecule Modulation of Protein Homeostasis. Chem. Rev. 2017, 117, 11269–11301. [Google Scholar] [CrossRef]
- Cromm, P.M.; Crews, C.M. Targeted Protein Degradation: From Chemical Biology to Drug Discovery. Cell Chem. Biol. 2017, 24, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Neklesa, T.K.; Tae, H.S.; Schneekloth, A.R.; Stulberg, M.J.; Corson, T.W.; Sundberg, T.B.; Raina, K.; Holley, S.A.; Crews, C.M. Small-molecule hydrophobic tagging-induced degradation of HaloTag fusion proteins. Nat. Chem. Biol. 2011, 7, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Long, M.J.; Gollapalli, D.R.; Hedstrom, L. Inhibitor mediated protein degradation. Chem. Biol. 2012, 19, 629–637. [Google Scholar] [CrossRef]
- Xie, T.; Lim, S.M.; Westover, K.D.; Dodge, M.E.; Ercan, D.; Ficarro, S.B.; Udayakumar, D.; Gurbani, D.; Tae, H.S.; Riddle, S.M.; et al. Pharmacological targeting of the pseudokinase Her3. Nat. Chem. Biol. 2014, 10, 1006–1012. [Google Scholar] [CrossRef]
- Gustafson, J.L.; Neklesa, T.K.; Cox, C.S.; Roth, A.G.; Buckley, D.L.; Tae, H.S.; Sundberg, T.B.; Stagg, D.B.; Hines, J.; McDonnell, D.P.; et al. Small-Molecule-Mediated Degradation of the Androgen Receptor through Hydrophobic Tagging. Angew. Chem. Int. Ed. Engl. 2015, 54, 9659–9662. [Google Scholar] [CrossRef]
- Canaria, C.A.; Smith, J.O.; Yu, C.J.; Fraser, S.E.; Lansford, R. New syntheses for 11-(mercaptoundecyl)triethylene glycol and mercaptododecyltriethyleneoxy biotin amide. Tetrahedron Lett. 2005, 46, 4813–4816. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Liu, X.; Qu, F.; Xiao, M.; Zhang, Y.; Charles, L.; Zhang, C.C.; Peng, L. Cooperative binding and self-assembling behavior of cationic low molecular-weight dendrons with RNA molecules. Org. Biomol. Chem. 2006, 4, 581–585. [Google Scholar] [CrossRef]
- Suthagar, K.; Fairbanks, A.J. A new way to do an old reaction: Highly efficient reduction of organic azides by sodium iodide in the presence of acidic ion exchange resin. Chem. Commun. (Camb.) 2017, 53, 713–715. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoda, T.; Ohoka, N.; Tsuji, G.; Fujisato, T.; Inoue, H.; Demizu, Y.; Naito, M.; Kurihara, M. Targeted Protein Degradation by Chimeric Compounds using Hydrophobic E3 Ligands and Adamantane Moiety. Pharmaceuticals 2020, 13, 34. https://doi.org/10.3390/ph13030034
Shoda T, Ohoka N, Tsuji G, Fujisato T, Inoue H, Demizu Y, Naito M, Kurihara M. Targeted Protein Degradation by Chimeric Compounds using Hydrophobic E3 Ligands and Adamantane Moiety. Pharmaceuticals. 2020; 13(3):34. https://doi.org/10.3390/ph13030034
Chicago/Turabian StyleShoda, Takuji, Nobumichi Ohoka, Genichiro Tsuji, Takuma Fujisato, Hideshi Inoue, Yosuke Demizu, Mikihiko Naito, and Masaaki Kurihara. 2020. "Targeted Protein Degradation by Chimeric Compounds using Hydrophobic E3 Ligands and Adamantane Moiety" Pharmaceuticals 13, no. 3: 34. https://doi.org/10.3390/ph13030034
APA StyleShoda, T., Ohoka, N., Tsuji, G., Fujisato, T., Inoue, H., Demizu, Y., Naito, M., & Kurihara, M. (2020). Targeted Protein Degradation by Chimeric Compounds using Hydrophobic E3 Ligands and Adamantane Moiety. Pharmaceuticals, 13(3), 34. https://doi.org/10.3390/ph13030034




