A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications
Abstract
1. Introduction
IOL Modification to Prevent Adverse Outcomes in Cataract Surgery
2. Results
3. Discussion
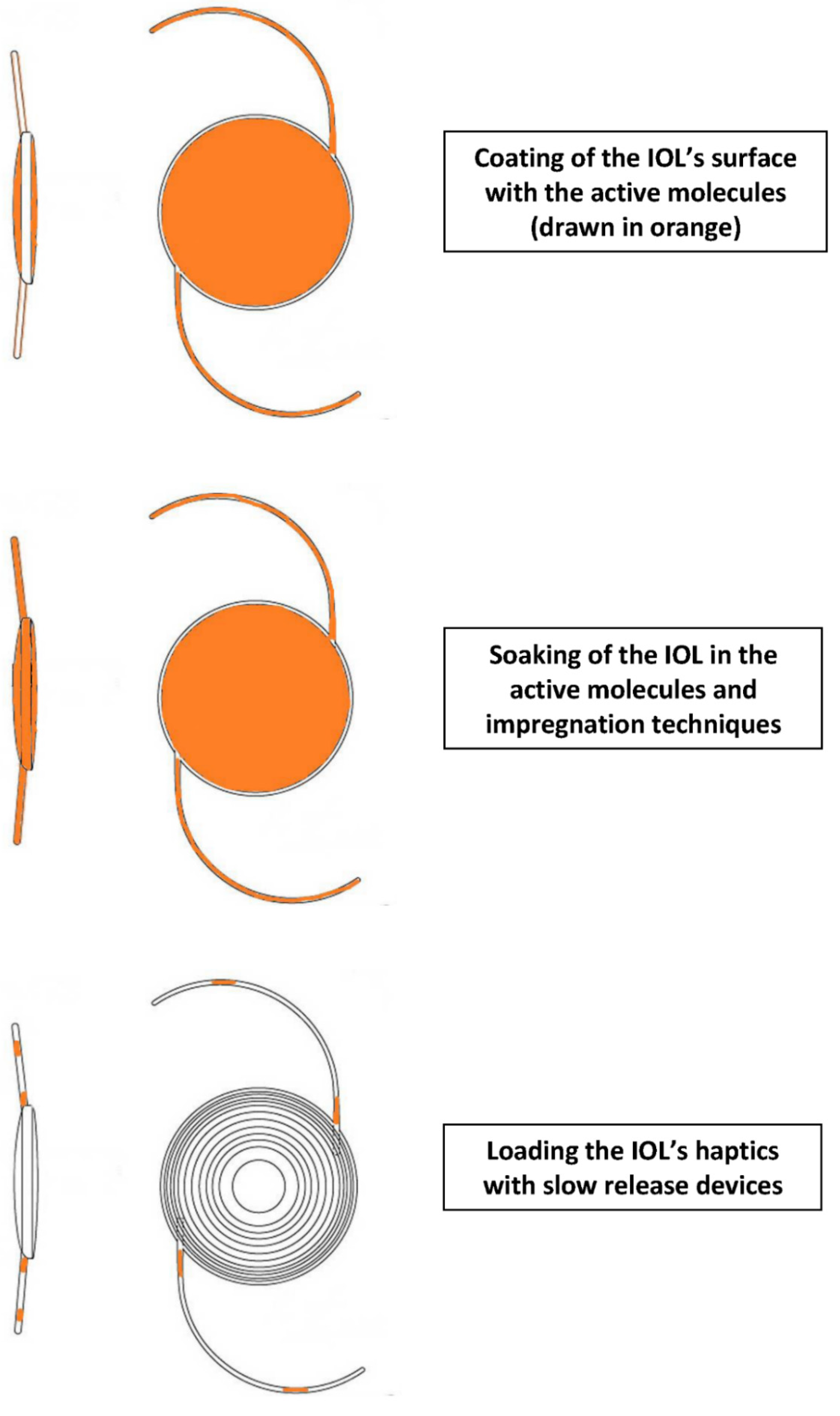
3.1. Studies That Present New Improvements in Modalities Designed to Assist the Introduction of Active Molecules into the Eye via an IOL
3.2. Studies That Examine the Therapeutic Impact of Specific Drugs in the Prevention of Adverse Outcomes Following IOL Implantation
3.3. Alternatives to Medication against PCO and Infection
3.4. Apparent Lack of Progress in Heparin IOL Surface Modification
3.5. Lack of Clinical Studies Currently Underway for a Commercially-Viable IOL Drug Delivery System
3.6. Alternative Strategies for PCO Prevention
3.7. Commercially Available Alternatives to IOL Drug Delivery
3.8. Overview of the Results and the Course of the Field
3.9. Limitations of this Review
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Addo, E.; Bamiro, O.A.; Siwale, R. Anatomy of the eye and common diseases affecting the eye. In Ocular Drug Delivery: Advances, Challenges and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 11–25. [Google Scholar]
- Kanski, J.J.; Bowling, B. Clinical Ophthalmology: A Systematic Approach, 8th ed.; Elsevier Health Sciences (Elsevier): Amsterdam, The Netherlands, 2015. [Google Scholar]
- Solebo, A.L.; Rahi, J.S. Epidemiology of congenital cataract. In Congenital Cataract; Springer: Berlin, Germany, 2017; pp. 15–25. [Google Scholar]
- Morrison, D.G.; Umfress, A.C. Secondary and acquired cataracts. In Pediatric Cataract Surgery and Iol Implantation; Springer: Berlin, Germany, 2020; pp. 51–60. [Google Scholar]
- Davis, G. The evolution of cataract surgery. Mo. Med. 2016, 113, 58–62. [Google Scholar] [PubMed]
- Apple, D.J.; Sims, J. Harold ridley and the invention of the intraocular lens. Surv. Ophthalmol. 1996, 40, 279–292. [Google Scholar] [CrossRef]
- Tetz, M.; Jorgensen, M.R. New hydrophobic iol materials and understanding the science of glistenings. Curr. Eye Res. 2015, 40, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Nowakowska, D.; Brzozowska, A.; Reibaldi, M.; Avitabile, T.; Bucolo, C.; Murabito, P.; Chisari, C.; Nowomiejska, K.; Rejdak, R. Pain following the use of anesthesia formulation among individuals undergoing cataract surgery: A randomized controlled trial. Front. Pharmacol. 2020, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Mylona, I.; Dermenoudi, M.; Glynatsis, M.; Ziakas, N.; Tsinopoulos, I. Development of a reliable preoperative risk stratification system for phacoemulsification. J. Cataract Refract. Surg. 2020, 46, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Longo, A.; Avitabile, T.; Nowomiejska, K.; Gagliano, C.; Tripodi, S.; Choragiewicz, T.; Kaminska, A.; Figus, M.; Posarelli, C.; et al. Five-year follow-up of secondary iris-claw intraocular lens implantation for the treatment of aphakia: Anterior chamber versus retropupillary implantation. PLoS ONE 2019, 14, e0214140. [Google Scholar] [CrossRef]
- Wormstone, I.M.; Wormstone, Y.M.; Smith, A.J.O.; Eldred, J.A. Posterior capsule opacification: What’s in the bag? Prog. Retin. Eye Res. 2020, 100905. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, K.; Li, J.; Huang, Y.; Zhu, S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysis. Medicine (Baltimore) 2017, 96, e8301. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Dana, M.R.; Christen, W.G.; Glynn, R.J. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology 1998, 105, 1213–1221. [Google Scholar] [CrossRef]
- Wesolosky, J.D.; Tennant, M.; Rudnisky, C.J. Rate of retinal tear and detachment after neodymium: Yag capsulotomy. J. Cataract Refract. Surg. 2017, 43, 923–928. [Google Scholar] [CrossRef]
- Knight, P.M.; Link, W.J. Surface modification of intraocular lenses to reduce corneal endothelial damage. J. Am. Intra-Ocul. Implant 1979, 5, 123–130. [Google Scholar] [CrossRef]
- Nishi, O.; Nishi, K.; Yamada, Y.; Mizumoto, Y. Effect of indomethacin-coated posterior chamber intraocular lenses on postoperative inflammation and posterior capsule opacification. J. Cataract Refract. Surg. 1995, 21, 574–578. [Google Scholar] [CrossRef]
- Tetz, M.R.; Ries, M.W.; Lucas, C.; Stricker, H.; Völcker, H. Inhibition of posterior capsule opacification by an intraocular-lens-bound sustained drug delivery system: An experimental animal study and literature review. J. Cataract Refract. Surg. 1996, 22, 1070–1078. [Google Scholar] [CrossRef]
- An, J.A.; Kasner, O.; Samek, D.A.; Lévesque, V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J. Cataract Refract. Surg. 2014, 40, 1857–1861. [Google Scholar] [CrossRef]
- Helary, G.; Yammine, P.; Migonney, V. Surface modification of hydrogel intraocular lenses to prevent cell proliferation. J. Appl. Biomater. Biomech. 2004, 2, 183–189. [Google Scholar]
- Bozukova, D.; Pagnoulle, C.; De Pauw-Gillet, M.-C.; Desbief, S.; Lazzaroni, R.; Ruth, N.; Jérôme, R.; Jérôme, C. Improved performances of intraocular lenses by poly (ethylene glycol) chemical coatings. Biomacromolecules 2007, 8, 2379–2387. [Google Scholar] [CrossRef]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef]
- González-Chomón, C.; Concheiro, A.; Alvarez-Lorenzo, C. Drug-eluting intraocular lenses. Materials 2011, 4, 1927–1940. [Google Scholar] [CrossRef]
- Verma, L.; Chakravarti, A. Prevention and management of postoperative endophthalmitis: A case-based approach. Indian J. Ophthalmol. 2017, 65, 1396–1402. [Google Scholar] [CrossRef]
- Chirila, T.; Harkin, D. Biomaterials and Regenerative Medicine in Ophthalmology; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Siqueira, R.C.; Ribeiro Filho, E.; Fialho, S.L.; Lucena, L.R.; Maia Filho, A.; Haddad, A.; Jorge, R.; Scott, I.U.; da Silva Cunha, A. Pharmacokinetic and toxicity investigations of a new intraocular lens with a dexamethasone drug delivery system: A pilot study. Ophthalmologica 2006, 220, 338–342. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Artigas, J.M.; García-Domene, M.C.; Navea, A.; Botella, P.; Fernández, E. Intra-ocular lens optical changes resulting from the loading of dexamethasone. Biomed. Opt. Express 2017, 8, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Bouledjouidja, A.; Masmoudi, Y.; Sergent, M.; Trivedi, V.; Meniai, A.; Badens, E. Drug loading of foldable commercial intraocular lenses using supercritical impregnation. Int. J. Pharm. 2016, 500, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Bouledjouidja, A.; Masmoudi, Y.; Li, Y.; He, W.; Badens, E. Supercritical impregnation and optical characterization of loaded foldable intraocular lenses using supercritical fluids. J. Cataract Refract. Surg. 2017, 43, 1343–1349. [Google Scholar] [CrossRef]
- Gudnason, K.; Sigurdsson, S.; Snorradottir, B.S.; Masson, M.; Jonsdottir, F. A numerical framework for drug transport in a multi-layer system with discontinuous interlayer condition. Math. Biosci. 2018, 295, 11–23. [Google Scholar] [CrossRef]
- Han, Y.; Tang, J.; Xia, J.; Wang, R.; Qin, C.; Liu, S.; Zhao, X.; Chen, H.; Lin, Q. Anti-adhesive and antiproliferative synergistic surface modification of intraocular lens for reduced posterior capsular opacification. Int. J. Nanomed. 2019, 14, 9047. [Google Scholar] [CrossRef]
- Karamitsos, A.; Lamprogiannis, L.; Karagkiozaki, V.; Laskarakis, A.; Papadopoulou, L.; Fatouros, D.; Ziakas, N.; Logothetidis, S.; Tsinopoulos, I. Design, characterisation and drug release study of polymeric, drug-eluting single layer thin films on the surface of intraocular lenses. IET Nanobiotechnol. 2020, 14, 501–507. [Google Scholar] [CrossRef]
- Kassumeh, S.A.; Wertheimer, C.M.; von Studnitz, A.; Hillenmayer, A.; Priglinger, C.; Wolf, A.; Mayer, W.J.; Teupser, D.; Holdt, L.M.; Priglinger, S.G.; et al. Poly(lactic-co-glycolic) acid as a slow-release drug-carrying matrix for methotrexate coated onto intraocular lenses to conquer posterior capsule opacification. Curr. Eye Res. 2018, 43, 702–708. [Google Scholar] [CrossRef]
- Lamprogiannis, L.; Karamitsos, A.; Karagkiozaki, V.; Tsinopoulos, I.; Gioti, M.; Fatouros, D.G.; Dimitrakos, S.; Logothetidis, S. Design and fabrication of drug-eluting polymeric thin films for applications in ophthalmology. IET Nanobiotechnol. 2018, 12, 1074–1079. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Serro, A.P.; Colaço, R.; Chauhan, A. Drug delivery to the eye anterior chamber by intraocular lenses: An in vivo concentration estimation model. Eur. J. Pharm. Biopharm. 2018, 133, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Vieira, A.P.; Guiomar, A.J.; Alves, P.; Másson, M. Utilization of tbdms chitosan for synthesis of photoactive chitosan derivatives and application in photografting on ophthalmic lens material. React. Funct. Polym. 2020, 153, 104600. [Google Scholar] [CrossRef]
- Tan, D.W.; Lim, S.G.; Wong, T.T.; Venkatraman, S.S. Sustained antibiotic-eluting intra-ocular lenses: A new approach. PLoS ONE 2016, 11, e0163857. [Google Scholar] [CrossRef]
- Topete, A.; Pinto, C.A.; Barroso, M.H.; Saraiva, J.A.; Barahona, I.; Saramago, B.; Serro, A.P. High hydrostatic pressure (hhp) as sterilization method for drug-loaded intraocular lenses. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Vieira, A.P.; Pimenta, A.F.; Silva, D.; Gil, M.H.; Alves, P.; Coimbra, P.; Mata, J.L.; Bozukova, D.; Correia, T.R.; Correia, I.J. Surface modification of an intraocular lens material by plasma-assisted grafting with 2-hydroxyethyl methacrylate (hema), for controlled release of moxifloxacin. Eur. J. Pharm. Biopharm. 2017, 120, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, G.-J.; Guo, Y.-T.; Zhou, L.; Du, J.; He, H. Preparation of novel biodegradable phema hydrogel for a tissue engineering scaffold by microwave-assisted polymerization. Asian Pac. J. Trop. Dis. 2014, 7, 136–140. [Google Scholar] [CrossRef]
- Garty, S.; Shirakawa, R.; Warsen, A.; Anderson, E.M.; Noble, M.L.; Bryers, J.D.; Ratner, B.D.; Shen, T.T. Sustained antibiotic release from an intraocular lens–hydrogel assembly for cataract surgery. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6109–6116. [Google Scholar] [CrossRef]
- Anderson, E.M.; Noble, M.L.; Garty, S.; Ma, H.; Bryers, J.D.; Shen, T.T.; Ratner, B.D. Sustained release of antibiotic from poly (2-hydroxyethyl methacrylate) to prevent blinding infections after cataract surgery. Biomaterials 2009, 30, 5675–5681. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Simplicio, A.L.; Vega-Gonzalez, A.; Subra-Paternault, P.; Coimbra, P.; Gil, M.; de Sousa, H.C.; Duarte, C.M. Impregnation of an intraocular lens for ophthalmic drug delivery. Curr. Drug Deliv. 2008, 5, 102–107. [Google Scholar] [CrossRef]
- González-Chomón, C.; Braga, M.E.; de Sousa, H.C.; Concheiro, A.; Alvarez-Lorenzo, C. Antifouling foldable acrylic iols loaded with norfloxacin by aqueous soaking and by supercritical carbon dioxide technology. Eur. J. Pharm. Biopharm. 2012, 82, 383–391. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Wang, Y.; Liu, S.; Zhao, X.; Chen, H.; Lin, Q. Drug eluting intraocular lens surface modification for pco prevention. Colloids Interface Sci. Commun. 2018, 24, 40–44. [Google Scholar] [CrossRef]
- Filipe, H.P.; Bozukova, D.; Pimenta, A.; Vieira, A.P.; Oliveira, A.S.; Galante, R.; Topete, A.; Masson, M.; Alves, P.; Coimbra, P.; et al. Moxifloxacin-loaded acrylic intraocular lenses: In vitro and in vivo performance. J. Cataract Refract. Surg. 2019, 45, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Kassumeh, S.; Kueres, A.; Hillenmayer, A.; von Studnitz, A.; Elhardt, C.; Ohlmann, A.; Priglinger, S.G.; Wertheimer, C.M. Development of a drug-eluting intraocular lens to deliver epidermal growth factor receptor inhibitor gefitinib for posterior capsule opacification prophylaxis. Eur. J. Ophthalmol. 2019, 1120672119891042. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Serro, A.; Saramago, B. Dual drug delivery from intraocular lens material for prophylaxis of endophthalmitis in cataract surgery. Int. J. Pharm. 2019, 558, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Tang, J.; Ding, X.; Filipe, H.P.; Saraiva, J.A.; Serro, A.P.; Lin, Q.; Saramago, B. Dual drug delivery from hydrophobic and hydrophilic intraocular lenses: In-vitro and in-vivo studies. J. Control. Release 2020, 326, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, C.; Brandlhuber, U.; Kook, D.; Mayer, W.J.; Laubichler, P.; Wolf, A.; Kampik, A.; Eibl-Lindner, K. Erufosine, a phosphoinositide-3-kinase inhibitor, to mitigate posterior capsule opacification in the human capsular bag model. J. Cataract Refract. Surg. 2015, 41, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, C.; Kassumeh, S.; Piravej, N.P.; Nilmayer, O.; Braun, C.; Priglinger, C.; Luft, N.; Wolf, A.; Mayer, W.J.; Priglinger, S.G.; et al. The intraocular lens as a drug delivery device: In vitro screening of pharmacologic substances for the prophylaxis of posterior capsule opacification. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6408–6418. [Google Scholar] [CrossRef]
- Wertheimer, C.; Kueres, A.; Siedlecki, J.; Braun, C.; Kassumeh, S.; Wolf, A.; Mayer, W.; Priglinger, C.; Priglinger, S.; Eibl-Lindner, K. The intraocular lens as a drug delivery device for an epidermal growth factor–receptor inhibitor for prophylaxis of posterior capsule opacification. Acta Ophthalmol. 2018, 96, e874–e882. [Google Scholar] [CrossRef]
- Kleinmann, G.; Apple, D.J.; Chew, J.; Hunter, B.; Stevens, S.; Larson, S.; Mamalis, N.; Olson, R.J. Hydrophilic acrylic intraocular lens as a drug-delivery system for fourth-generation fluoroquinolones. J. Cataract Refract. Surg. 2006, 32, 1717–1721. [Google Scholar] [CrossRef]
- Dawes, L.J.; Illingworth, C.D.; Wormstone, I.M. A fully human in vitro capsular bag model to permit intraocular lens evaluation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 23–29. [Google Scholar] [CrossRef]
- Liegl, R.; Wertheimer, C.; Kernt, M.; Docheva, D.; Kampik, A.; Eibl-Lindner, K.H. Attenuation of human lens epithelial cell spreading, migration and contraction via downregulation of the pi3k/akt pathway. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, C.; Liegl, R.; Kernt, M.; Mayer, W.; Docheva, D.; Kampik, A.; Eibl-Lindner, K.H. Egf receptor inhibitor erlotinib as a potential pharmacological prophylaxis for posterior capsule opacification. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, C.; Siedlecki, J.; Kook, D.; Mayer, W.J.; Wolf, A.; Klingenstein, A.; Kampik, A.; Eibl-Lindner, K. Egfr inhibitor gefitinib attenuates posterior capsule opacification in vitro and in the ex vivo human capsular bag model. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, N.; Perdue, N.R.; Matsushima, H.; Sage, E.H.; Yan, Q.; Clark, J.I. An in vitro model of posterior capsular opacity: Sparc and tgf-β2 minimize epithelial-to-mesenchymal transition in lens epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4679–4687. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Song, Y.; Lim, H.; Lee, S.H.; Lee, H.K.; Lee, E.; Choi, B.G.; Lee, J.J.; Im, S.G.; Lee, K.G. Antibacterial nanopillar array for an implantable intraocular lens. Adv. Healthc. Mater. 2020, 9, 2000447. [Google Scholar] [CrossRef]
- Farukhi, M.A.; Werner, L.; Kohl, J.C.; Gardiner, G.L.; Ford, J.R.; Cole, S.C.; Vasavada, S.A.; Noristani, R.; Mamalis, N. Evaluation of uveal and capsule biocompatibility of a single-piece hydrophobic acrylic intraocular lens with ultraviolet-ozone treatment on the posterior surface. J. Cataract Refract. Surg. 2015, 41, 1081–1087. [Google Scholar] [CrossRef]
- Krall, E.M.; Arlt, E.M.; Jell, G.; Strohmaier, C.; Moussa, S.; Dexl, A.K. Prospective randomized intraindividual comparison of posterior capsule opacification after implantation of an iol with and without heparin surface modification. J. Refract. Surg. 2015, 31, 466–472. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, X.; Wang, B.; Shen, C.; Tang, J.; Han, Y.; Chen, H. Hydrated polysaccharide multilayer as an intraocular lens surface coating for biocompatibility improvements. J. Mater. Chem. B 2015, 3, 3695–3703. [Google Scholar] [CrossRef]
- Lin, Q.K.; Xu, X.; Wang, Y.; Wang, B.; Chen, H. Antiadhesive and antibacterial polysaccharide multilayer as iol coating for prevention of postoperative infectious endophthalmitis. Int. J. Polym. Mater. 2017, 66, 97–104. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Hu, X.-F.; Zhao, Y.; Gao, Y.-J.; Yang, C.; Qiao, S.-L.; Wang, Y.; Yang, P.-P.; Yan, J.; Sui, X.-C.; et al. Photothermal ring integrated intraocular lens for high-efficient eye disease treatment. Adv. Mater. 2017, 29, 1701617. [Google Scholar] [CrossRef]
- Magin, C.M.; May, R.M.; Drinker, M.C.; Cuevas, K.H.; Brennan, A.B.; Reddy, S.T. Micropatterned protective membranes inhibit lens epithelial cell migration in posterior capsule opacification model. Transl. Vis. Sci. Technol. 2015, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yu, S.; Kang, Y.; Zhang, D.; Wu, S.; Zhang, J.; Xiong, Y.; Li, M.; Zhang, J.; Wang, J.; et al. Cuins/zns quantum dots modified intraocular lens for photothermal therapy of posterior capsule opacification. Exp. Eye Res. 2020, 108282. [Google Scholar] [CrossRef] [PubMed]
- Syed Hussain, S.; Donempudi, S.; Tammishetti, S.; Garikapati, K.R.; Bhadra, M.P. Cell adhesion resistant, uv curable, polymer zinc oxide nanocomposite materials for intraocular lens application. Polym. Adv. Technol. 2018, 29, 1234–1241. [Google Scholar] [CrossRef]
- Tan, X.; Zhan, J.; Zhu, Y.; Cao, J.; Wang, L.; Liu, S.; Wang, Y.; Liu, Z.; Qin, Y.; Wu, M. Improvement of uveal and capsular biocompatibility of hydrophobic acrylic intraocular lens by surface grafting with 2-methacryloyloxyethyl phosphorylcholine-methacrylic acid copolymer. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Viveiros, M.M.; Soares, R.T.; Omodei, M.S.; Rainho, C.A.; Padovani, C.R.; Cruz, N.; Schellini, S.A.; Rodrigues, A.C. Adhesion study of cultured human lens capsule cells on hydrophilic intraocular lenses coated with polyethylene glycol. J. Cataract Refract. Surg. 2015, 41, 1478–1483. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Lin, Q.; Jin, T.; Shen, C.; Tang, J.; Han, Y.; Chen, H. Surface modification of intraocular lenses with hyaluronic acid and lysozyme for the prevention of endophthalmitis and posterior capsule opacification. RSC Adv. 2015, 5, 3597–3604. [Google Scholar] [CrossRef]
- Xu, X.; Tang, J.-M.; Han, Y.-M.; Wang, W.; Chen, H.; Lin, Q.-K. Surface pegylation of intraocular lens for pco prevention: An in vivo evaluation. J. Biomater. Appl. 2016, 31, 68–76. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Kuang, Z.; Jin, Y.; Pang, S.; Wang, Y.; Lin, D.; Chen, H.; Qian, S.; Wang, B.; et al. Bionic antibacterial modification of iol through si-raft polymerization of p(toeac-co-mpc) brushes to prevent pco and endophthalmitis. Polym. Test. 2020, 88, 106553. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, Y.; Jiao, Y.; Zhai, Z. Biological performance of functionalized biomedical polymers for potential applications as intraocular lens. J. Biomed. Mater. Res. A 2016, 104, 1961–1967. [Google Scholar] [CrossRef]
- Matsushima, H.; Iwamoto, H.; Mukai, K.; Obara, Y. Active oxygen processing for acrylic intraocular lenses to prevent posterior capsule opacification. J. Cataract Refract. Surg. 2006, 32, 1035–1040. [Google Scholar] [CrossRef]
- Krall, E.M.; Arlt, E.-M.; Jell, G.; Strohmaier, C.; Bachernegg, A.; Emesz, M.; Grabner, G.; Dexl, A.K. Intraindividual aqueous flare comparison after implantation of hydrophobic intraocular lenses with or without a heparin-coated surface. J. Cataract Refract. Surg. 2014, 40, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Spalton, D.J.; Russell, S.L.; Evans-Gowing, R.; Eldred, J.A.; Wormstone, I.M. Effect of total lens epithelial cell destruction on intraocular lens fixation in the human capsular bag. J. Cataract Refract. Surg. 2014, 40, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, S.; Hwang, F.S.; Kim, J.; LeClair, B.; Yoon, E.; Pham, M.; Rauser, M.E. Comparative analysis of intravitreal triamcinolone acetonide–moxifloxacin versus standard perioperative eyedrops in cataract surgery. J. Cataract Refract. Surg. 2019, 45, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Lipnitzki, I.; Eliahu, S.B.; Marcovitz, A.L.; Ezov, N.; Kleinmann, G. Intraocular concentration of moxifloxacin after intracameral injection combined with presoaked intraocular lenses. J. Cataract Refract. Surg. 2014, 40, 639–643. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59. [Google Scholar] [CrossRef]

| Authors | Type of Study | Aim | Procedure | Outcome |
|---|---|---|---|---|
| Artigas et al. [27] | In vitro, three-way comparison study | Evaluation of dexamethasone-loaded IOL’s optical properties | Dexamethasone loading by soaking the IOL in a polymer matrix | Drug loading impairs modulation transfer function and spectral transmission; they recover after the drug is released |
| Bouledjouidja et al. [28] | In vitro single-group experimental study | Development of supercritical impregnation for IOL drug loading | IOL supercritical impregnation with ciprofloxacin (CIP) and dexamethasone 21-phosphate disodium (DXP) | Higher affinity for DXP compared to CIP, highest DXP impregnation yields were obtained in the presence of ethanol as a co-solvent, unlike with CIP |
| Bouledjouidja et al. [29] | In vitro single-group experimental study | Examination of optical properties in IOLs loaded with supercritical impregnation | IOL supercritical impregnation with ciprofloxacin (CIP) and dexamethasone 21-phosphate disodium (DXP) | Supercritical impregnation does not damage the optical properties of IOLs |
| Gudnason et al. [30] | In vitro comparison of simulated and experimental data | Evaluation of a mathematical model to estimate the concentration of drug released from drug-loaded IOLs via in vitro calculations verified against in vivo measurements | The authors compared their simulated moxifloxacin (MFX) release data to experimental data from MFX-loaded IOLs | The authors managed to explain three release curves, corresponding to different thicknesses of IOLs. |
| Han et al. [31] | In vitro observations and in vivo single-group experimental study | Manufacture and evaluation of an antiproliferative drug: doxorubicin (DOX)-incorporated chitosan (CHI) nanoparticle on cellular adhesion, proliferation and migration, in vivo evaluation of PCO inhibitory effect | Drug-loaded multilayer fabrication and coating onto IOL by multi-stage injection, in vivo cell migration experiment, in vivo PCO inhibition in rabbit eyes for up to two months | In vitro cell adhesion was reduced, cell migration and proliferation were remarkably inhibited and PCO formation after drug-eluting IOL implantation was significantly inhibited. |
| Karamitsos et al. [32] | In vitro observational study | Design, development and study of the properties of a novel polymeric, drug-eluting thin film and its application on an IOL with dexamethasone (DXM) | Examination of the initial durability of the IOLs during the spinning process and of structural and optical properties of the modified IOLs. A drug release study run for 8 weeks | There was acceptable optical transparency of the lenses regardless of the deposition of the drug-eluting films on their surface. The drug release study demonstrated gradual DXM release over the selected period. |
| Kassumeh et al. [33] | In vitro and ex-vivo experimental three-group study | Determine the feasibility of methotrexate (MTX) loaded biomatrices sprayed on IOLs | Evaluation for IOL growth-inhibiting properties, release kinetics of MTX and its toxicity on corneal endothelial cells | Significant benefits compared to controls on all parameters with no difference in toxicity |
| Lamprogiannis et al. [34] | In vitro observational study | Design, development, characterization, and drug release of one- and two-layered thin films based on organic polymers and DXM | Examination of the opacity and rate of DXM release over a six-week period. | The single-layer thin films demonstrated a sufficient encapsulation of dexamethasone and appropriate release of the therapeutic substance over a six-week period. |
| Ongkasin et al. [35] | In vitro observations and ex vivo case-control study | Application of supercritical impregnation technology to load acrylic IOLs with MTX to produce a sustained drug delivery device to mitigate PCO | In vitro assessment of drug release kinetic employed to appropriately load the IOLs. Measurement of time to full coverage of the optical axis with PCO cells | No statistically significant variation in the duration required for a full cell coverage of the posterior capsule ex vivo. Reduction in fibrosis by inhibiting epithelial-mesenchymal transformation. |
| Pimenta et al. [36] | In vitro observations and in vivo multiple-group experimental study | Evaluation of a mathematical model to estimate the concentration of drug released from drug-loaded IOLs via in vitro calculations verified with in vivo experiments | Partition and effective diffusivity values were determined for MFX, levofloxacin, diclofenac and ketorolac in hydrophilic acrylic IOLs and in silicone hydrogel. | The hydrophilic acrylic material presented promising results, especially for MFX and diclofenac controlled-release |
| Sahariah et al. [37] | In vitro experimental study | Manufacture and evaluation of three chitosan derivatives for suitability as a drug carrier for MFX | Photografting and photocrosslinking of three polymethacrylate copolymers and in vitro comparison of drug release profiles | The authors reported an optimized procedure for synthesizing chitosan derivatives of high molecular weight and determined the best performing derivative in terms of highest amount of released drug. |
| Tan et al. [38] | In vitro experimental study | Evaluation of loading IOLs with a fully-degradable polymer depot with levofloxacin or MFX against infection. | The effects of drug loading and solvent type on drug release and film morphology were investigated using cast films. | A slower-evaporating solvent tetrahydrofuran and a lower drug loading percentage led to better surface morphology and lower initial release burst compared to dichloromethane and higher drug loading percentages. |
| Topete et al. [39] | In vitro case-control study | Assessment of sterilization of a drug-loaded IOL by high hydrostatic pressure (HHP) | Comparative study of the effectiveness of HHP against gamma radiation and steam heat on IOLs loaded with MFX or an anti-inflammatory drug | Gamma radiation degraded the drugs while steam heat cannot be applied to temperature-sensitive drugs. However, HHP sterilized highly contaminated and also enhanced drug loading and did not affect significantly the hydrogel properties. |
| Vieira et al. [40] | In vitro observational study | Manufacture and evaluation of the effects and properties of MFX-loading on pHEMA-silicone IOLs | Characterization of MFX-loaded IOLs and study of antibacterial, cytotoxic and storage properties | The MFX-loaded IOLs were effective against S. aureus and S. epidermidis without cytotoxicity. While there was no case of drug loss during three months of storage, an increase in storage time lead to an increase in the amount of MFX released and to an increase in its release duration |
| Authors | Type of Study | Aim | Procedure | Outcome |
|---|---|---|---|---|
| Filipe et al. [47] | In vitro observations and in vivo case-control experimental study | To assess IOLs loaded with moxifloxacin (MFX) for drug release activity, cytotoxicity and efficacy against bacterial infection. | The activity of the released drug was tested in vitro while in vivo cytotoxicity and efficacy was evaluated comparing the effects of topical MFX drops (control) and MFX-loaded IOLs | The presence of MFX in the IOLs had little effect on the evaluated physical properties and did not induce cytotoxicity. In vitro drug release experiments showed that the IOLs provided controlled release of MFX for ~2 weeks with less variability than controls. |
| Kassumeh et al. [48] | In vitro observations and ex vivo case-control study | Evaluation of loading IOLs with gefitinib against the development of PCO. | In vitro observations of rates of LEC growth in an anterior segment model, followed by in vivo experiment to determine drug release and biocompatibility in a human capsular bag model | Coated IOLS attenuated PCO cell growth with a constant drug release over the first ten days, without any reduction in cell viability of corneal endothelial cells and with a related decrease in fibronectin expression. |
| Topete et al. [49] | In vitro multiple-group observational study | To assess the possibility of loading IOLs with a combination of an antibiotic and an anti-inflammatory drug, either MFX + ketorolac (KTL) or MFX + diclofenac | Drug-loaded IOLs were assessed for their optical and mechanical properties and the time duration of effective dosing was calculated | Simultaneous drug loading improved the release profiles with no adverse impact on the optical and mechanical properties. The most effective combination was a loading with MFX + KTL, effective against S. aureus and S. epidermidis for up to 15 days and against inflammation for at least 16 days. |
| Topete et al. [50] | In vitro observations and in vivo case-control experimental study | Evaluation of loading IOLs with MFX + KTL against the development of infection and inflammation. | In vitro drug release tests and the antimicrobial activity of the released antibiotic was determined while the in vivo performance and safety of both hydrophobic and hydrophilic lenses was compared to that of ophthalmic drops. | The developed IOLs were able to release MFX and KTL at therapeutic levels, in a sustained way, unlike eye drops prophylaxis. No PCO signs were detected and histological analyses demonstrated biocompatibility of these devices. |
| Wertheimer et al. [51] | Ex vivo and in vitro case-control experimental study | Evaluation of whether erufosine alone or erufosine-loaded IOLs can inhibit growth of LEC | Tissue from cadaver eyes that underwent sham surgery was exposed to erufosine while IOLs soaked with erufosine were implanted in capsular bags | Erufosine as a single therapeutic agent increased the time until confluence of the capsular bag, but not significantly compared with the control. When IOLs were soaked with erufosine, a long-term prophylactic effect was observed in this organ model for PCO |
| Wertheimer et al. [52] | Ex vivo multi-group comparative study following in vitro study | Evaluation of various substances for loading on IOLs for PCO prevention | In vitro screening of potential candidate substances for toxicity and testing for effect on PCO with IOLs in vitro and ex vivo | Long-term inhibitory effects in the human capsular bag model were observed for caffeic acid phenethyl ester and methotrexate IOLs. Only methotrexate and disulfiram were not toxic. Methotrexate was released constantly for two weeks |
| Wertheimer et al. [53] | Ex vivo and in vitro case-control experimental study | Evaluation of whether erlotinib-loaded IOLs can prevent PCO while not being toxic | Tissue from cadaver eyes that underwent sham surgery was exposed to erlotinib while IOLs soaked with erlotinib were implanted in capsular bags | Modified IOLs mitigated cell growth in the anterior segment model without short-term corneal toxicity while there was no toxicity on corneal endothelial cells. Erlotinib was released constantly from IOL. |
| Authors | Type of Study | Aim | Procedure | Outcome |
|---|---|---|---|---|
| Choi et al. [60] | In vitro experimental study | To assess the feasibility of a integrating a polymeric nanopillar array (NPA) onto an IOL to prevent infection, decrease the adhesivity of LEC and prevent PCO without cytotoxicity | In vitro comparison of an NPA coated with cross-linked ionic polymer thin film (pVD) to a bare NPA as to the anti-bacterial efficiency. | The pVD-coated NPA exhibited excellent anti-bacterial efficiency >99.6%. The bare NPA only showed 51% efficiency stemming from the topological bacteria-killing effect |
| Farukhi et al. [61] | In vivo case-control experimental study | To assess the effectiveness of treating an IOL with ultraviolet–ozone (UV–O3) on the posterior surface for prevention of PCO | The UV–O3 treated IOL was compared for effectiveness to an identical untreated IOL in a rabbit model | Treatment of an IOL with UV–O3 appears to prevent PCO with no signs of untoward inflammation or toxicity or any other difference in histopathologic findings between study eyes and control eyes. |
| Krall et al. [62] | In vivo case-control human clinical study | To assess the performance of a heparin surface modified IOL (HSM-IOL) for benefit against PCO to an uncoated IOL (UC-IOL) 1 year after implantation. | The heparin surface modified IOL was compared for effectiveness to an identical untreated IOL in human patients | Patients were compared in PCO grading, straylight measurement, distance visual acuities, flare in the anterior chamber, and mesopic and photopic contrast sensitivity. There were no statistically significant differences between the groups. |
| Lin et al. [63] | In vitro observations and in vivo single-group experimental study | Design, development and study of the properties of a novel hyaluronic acid (HA)/chitosan (CHI) polyelectrolyte multilayer for IOL application | In vitro observations of protein adsorption and LEC adhesion/ proliferation followed by in vivo experiment after IOL implantation | In vitro results showed no reduction of the optical properties and inhibition of LEC adhesion and proliferation while in vivo ocular implantation results showed good biocompatibility and reduction of PCO. |
| Lin et al. [64] | In vitro observations and in vivo single-group experimental study | Further research of a previously presented HA/CHI polyelectrolyte multilayer for antibacterial and anti-acute inflammatory properties | In vitro antibacterial adhesion test followed by in vivo experiment after IOL implantation | In vitro results showed that antibacterial activity is increasing with an increased number of layers while the modified IOLs demonstrated a marked reduction of inflammation compared to unmodified IOLs |
| Lin et al. [65] | In vitro observations and in vivo case-control experimental study | To assess the feasibility of grafting a nanostructure photothermal ring onto an IOL (nano-IOL) to prevent infection, decrease the adhesivity of LEC and prevent PCO without cytotoxicity | In vitro examination of the properties of the components of the nanostructure and of the nano-IOL. The nano-IOL was compared in vivo to a regular IOL as to LEC proliferation | The nano-IOL demonstrated good biocompatibility, region-confined photothermal effect and no toxicity. PCO occurrence after surgery was 30% in eyes with nano-IOLs compared to 100% of eyes with untreated IOLs |
| Magin et al. [66] | In vitro observations and controlled study | To determine the feasibility of employing sharklet-micropatterned protective membrane (PM) implanted in combination with a posterior chamber IOL to improve resistance to PCO. | In vitro assessment of several microtopographies in a modified scratch-wound test to assess LEC migration, best performing was compared to an un-patterned PM and an IOL without any PM | An IOL with a PM fitted with the best-performing micropatterned design had reduced LEC migration by 50% compared with the IOL-only condition. IOLs with simple PMs did not differ in LEC migration compared with the IOL only condition. |
| Mao et al. [67] | In vitro observational and case-control group study | To assess the properties of carboxylated CuInS/ZnS quantum dots (ZCIS QDs) and their potential as LEC-antiproliferative surface materials to IOLs | Fabrication, characterization and in vitro examination of photothermal properties of ZCIS QDs. In vitro case-control examination of biocompatibility | The results indicated that combining QDs-IOLs and NIR irradiation achieves photothermal killing effects on LECs limited to the specific region with no cytotoxicity. |
| Syed Hussain et al. [68] | In vitro observational study | To assess the properties of zinc oxide nanocomposite resins and their potential as LEC-antiproliferative surface materials to IOLs | Films made with the resins underwent an in vitro cytotoxicity test, a morphological study of attached cells and a fibroblast adherence assay. | An extensive presentation of the films’ properties concluded that they resisted fibroblast attachment, filtered harmful UV light and had appropriate visible light transparency, glass transition temperatures, mechanical strength, and biocompatibility |
| Tan et al. [69] | In vitro observations and in vivo case-control experimental study | To assess the feasibility of grafting a hydrophilic copolymer P(MPC-MAA) onto an IOL to reduce inflammation, decrease the adhesivity of LEC and prevent ACO and PCO without cytotoxicity | The assessment of the characteristics of the copolymer P(MPC-MAA) in vitro was followed by an in vivo examination in a case-control experimental design | P(MPC-MAA) modification significantly reduced postoperative inflammation and ACO, but did not affect PCO. |
| Viveiros et al. [70] | Ex vivo case-control experimental study | To evaluate the adhesion of LEC on IOLs coated with polyethylene glycol (PEG). | An ex vivo case-control experimental design to compare rate of adhesion for LECs | PEG-coated IOLs was effective in inhibiting cell adhesion |
| Wang et al. [71] | In vitro observational study | To assess the feasibility of grafting a Hyaluronic acid–lysozyme (HA–lysozyme) composite coating onto an IOL to decrease the adhesivity of LEC and S. aureus and prevent PCO | HA-IOLs and HA-lysozyme IOLs underwent surface characterization, cytotoxicity assays and an antibacterial test | Adherence of S. aureus and LECs on IOLs with HA or HA–lysozyme coating was significantly reduced while the bactericidal activity of HA–lysozyme coatings was effective against S. aureus |
| Xu et al. [72] | In vitro observations and in vivo case-control experimental study | To assess the performance of hydrophilic polyethylene glycol (PEG) as an IOL surface graft to inhibit LEC adhesion. | In vitro examination of the properties of the PEGylated IOLs that were subsequently compared to plain IOLs in an in vivo case-control study | PEGylated IOLs retained their optical properties with inhibited LEC initial adhesion. They presented good in vivo biocompatibility, and effective prevention of PCO. |
| Zhang et al. [73] | In vitro observations and in vivo case-control experimental study | To assess the feasibility of grafting a bionic zwitterionic polymer onto an IOL to resist nonspecific proteins, bacterial and LEC adhesion | In vitro examination of the properties of the polymer and description of the forming of P(TOEAC-co-MPC) brushes. The brushes were prepared onto IOLs and compared to plain IOLs | The P(TOEAC-co-MPC) brushes showed excellent antibacterial and antibiofilm abilities, good biocompatibility while the in vivo study confirmed that they effectively prevented PCO and endophthalmitis |
| Zheng et al. [74] | In vitro observations and in vivo case-control experimental study | To assess the feasibility of grafting a hirudin polymer onto an IOL to resist inflammation, bacterial and LEC adhesion | In vitro examination of the properties of the polymer. Hirudin-IOLs were compared to plain IOLs in an in vivo case-control study | Grafting hirudin to the IOL surface led to better resistance to cell adhesion than a pure amination process and reduced in vivo the incidence of cell aggregation and inflammation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylona, I.; Tsinopoulos, I. A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications. Pharmaceuticals 2020, 13, 448. https://doi.org/10.3390/ph13120448
Mylona I, Tsinopoulos I. A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications. Pharmaceuticals. 2020; 13(12):448. https://doi.org/10.3390/ph13120448
Chicago/Turabian StyleMylona, Ioanna, and Ioannis Tsinopoulos. 2020. "A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications" Pharmaceuticals 13, no. 12: 448. https://doi.org/10.3390/ph13120448
APA StyleMylona, I., & Tsinopoulos, I. (2020). A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications. Pharmaceuticals, 13(12), 448. https://doi.org/10.3390/ph13120448





