A Second Generation Mn-Porphyrin Dimer with a Twisted Linker as a Potential Blood Pool Agent for MRI: Tuning the Geometry and Binding with HSA
Abstract
1. Introduction
2. Results and Discussion
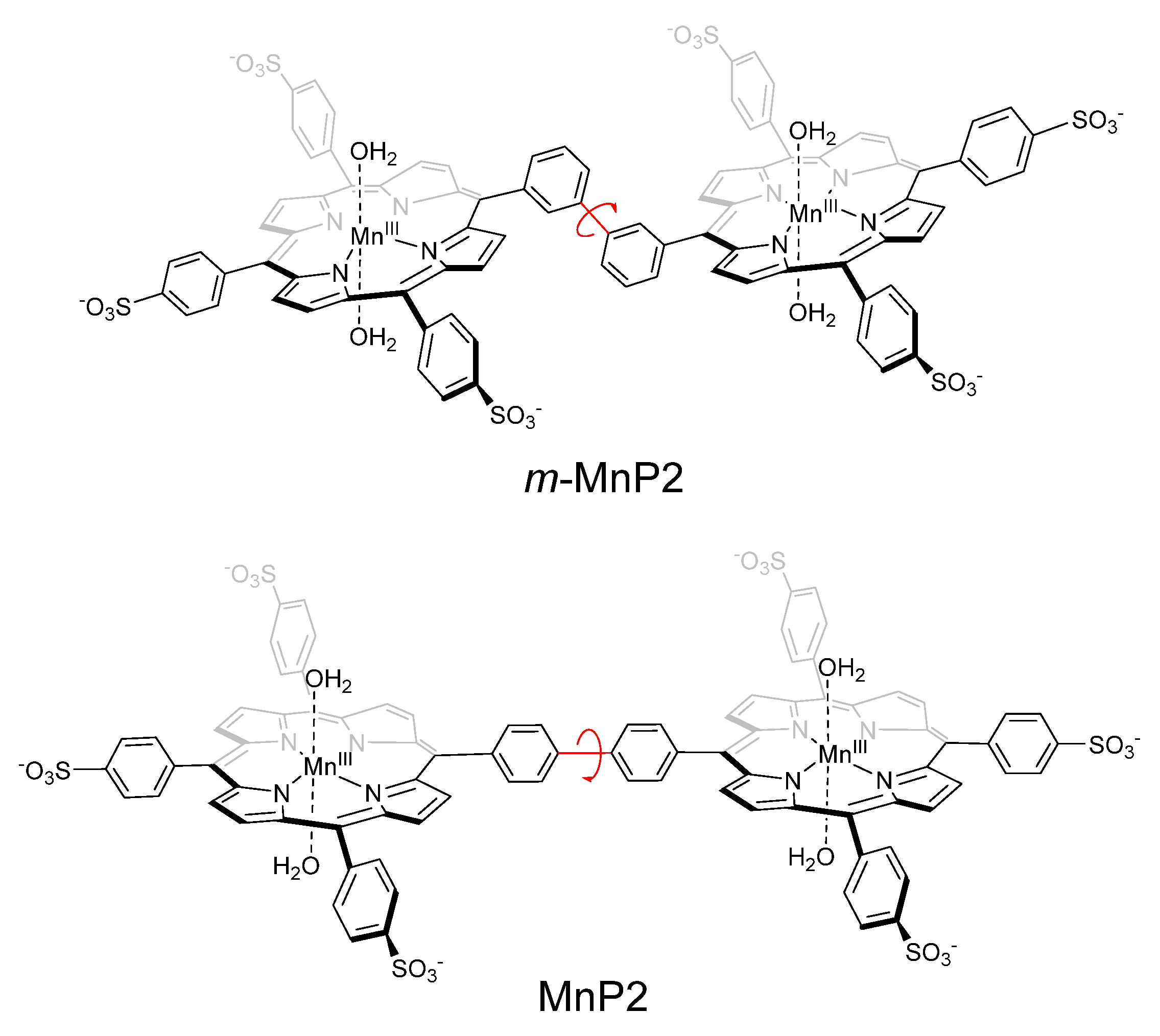
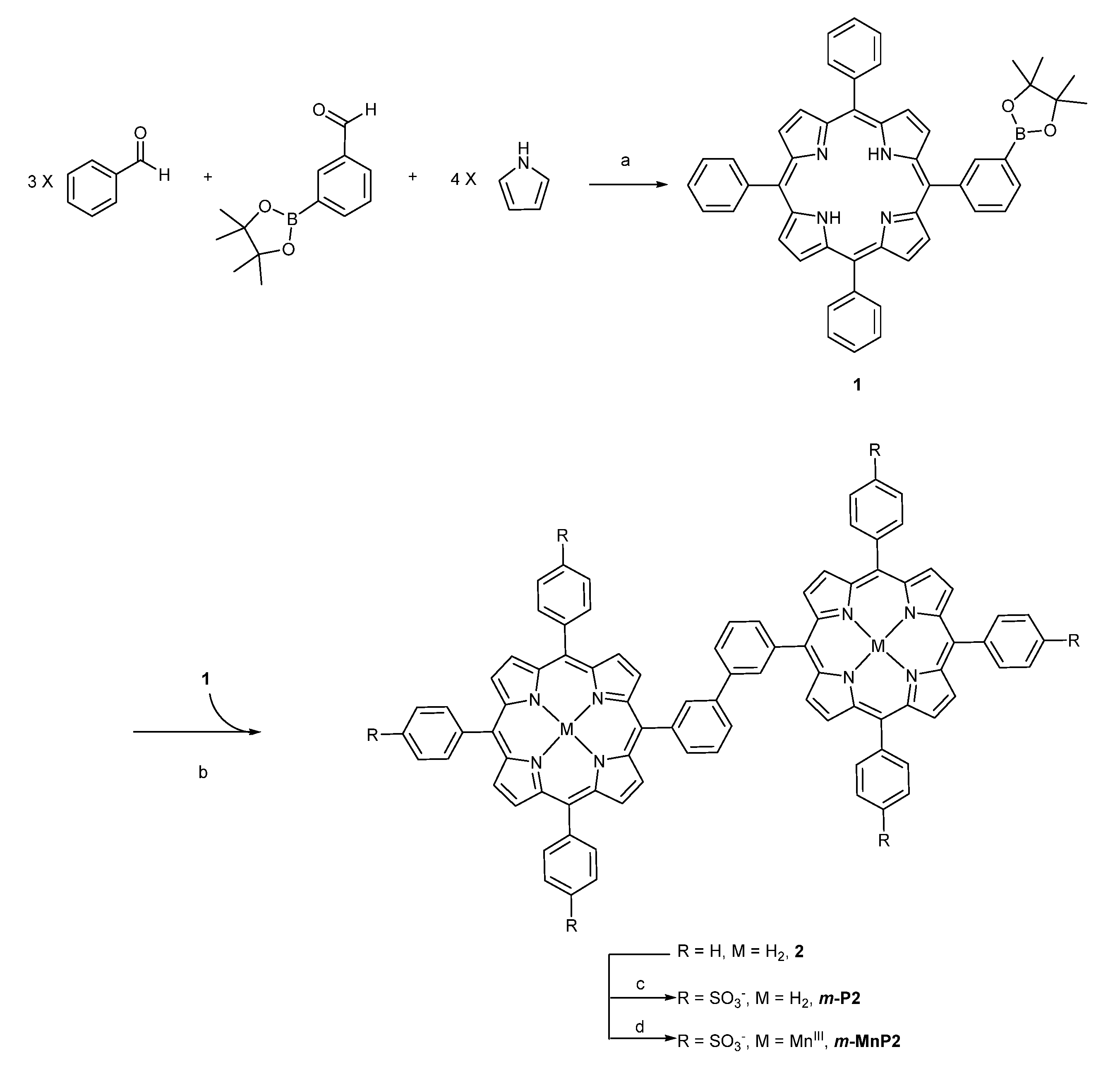
2.1. Synthesis
2.2. Relaxometry Characterization
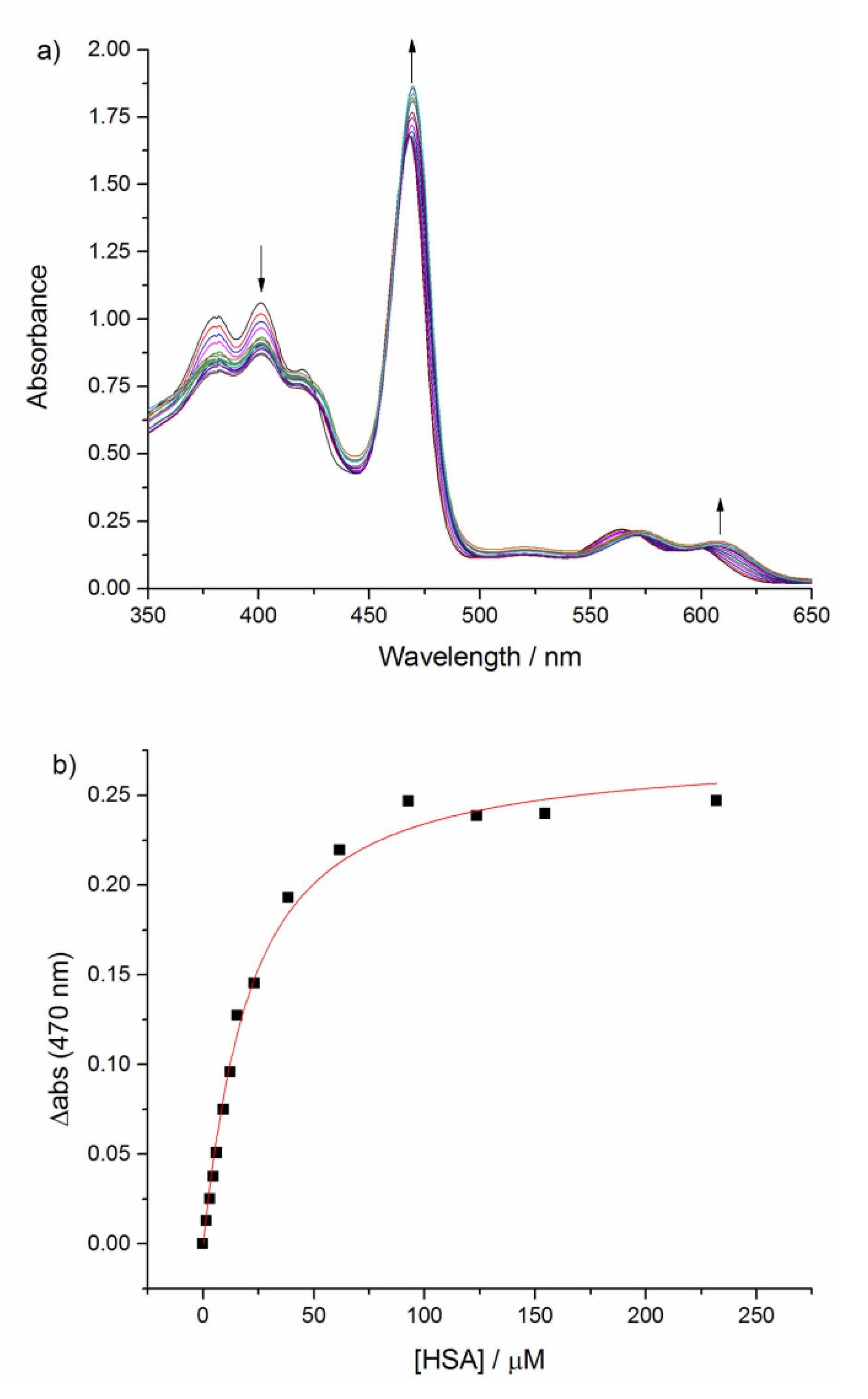
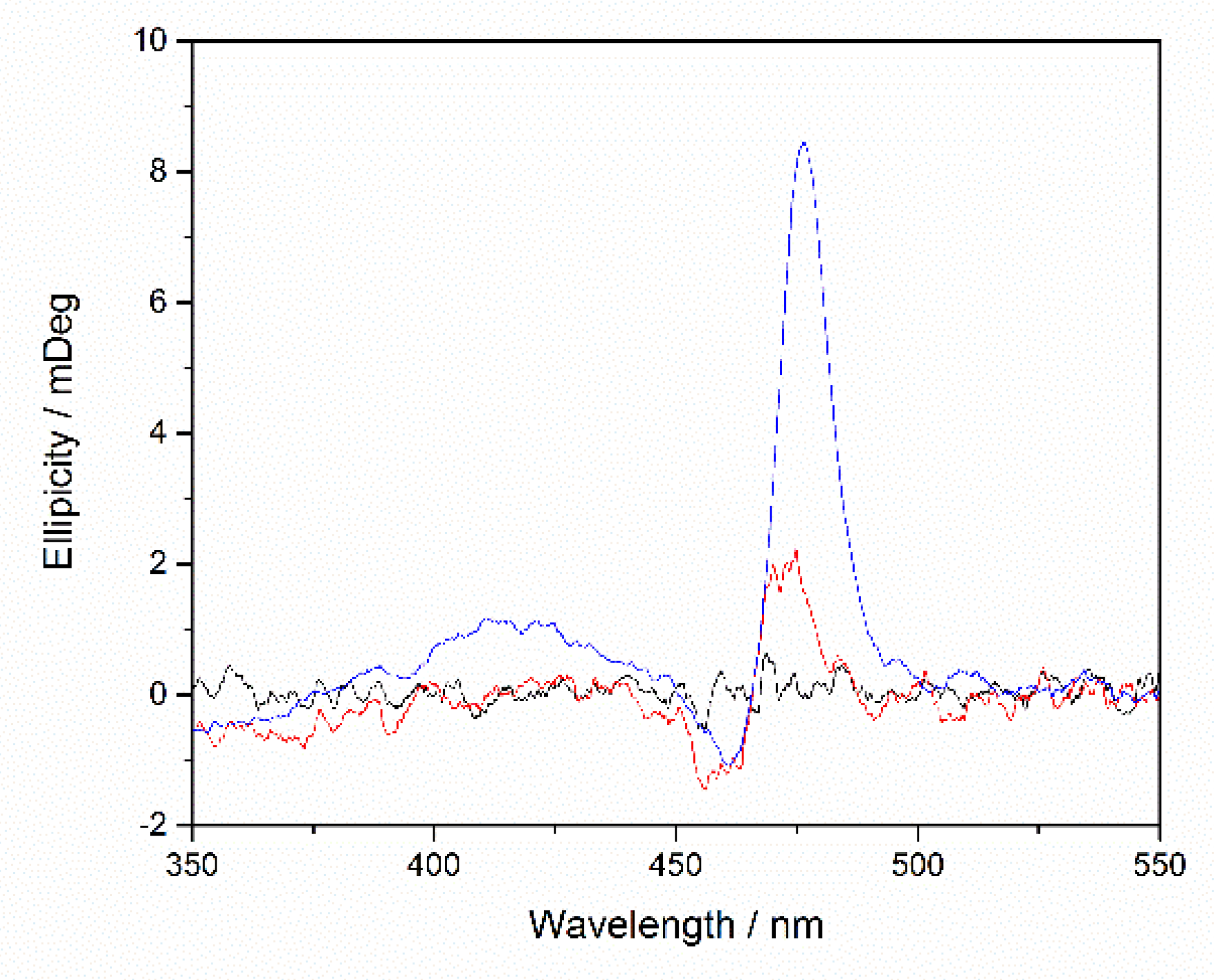
2.3. Binding Affinity with HSA
2.4. Relaxometry Studies with HSA
3. Materials and Methods
Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magnetic Resonance Angiography; Carr, J.C., Carroll, T.J., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Saeed, M.; Wendland, M.F.; Higgins, C.B. Blood Pool MR Contrast Agents for Cardiovascular Imaging. J. Magn. Reson. Imaging 2000, 12, 890–898. [Google Scholar] [CrossRef]
- Lauffer, R.B.; Parmelee, D.J.; Ouellet, H.S.; Dolan, R.P.; Sajiki, H.; Scott, D.M.; Bernard, P.J.; Buchanan, E.M.; Ong, K.Y.; Tyeklár, Z.; et al. MS-325: A Small-Molecule Vascular Imaging Agent for Magnetic Resonance Imaging. Acad. Radiol. 1996, 3, S356–S358. [Google Scholar] [CrossRef]
- Caravan, P.; Cloutier, N.J.; Greenfield, M.T.; McDermid, S.A.; Dunham, S.U.; Bulte, J.W.M.; Amedio, J.C.; Looby, R.J.; Supkowski, R.M.; Horrocks, W.; et al. The Interaction of MS-325 with Human Serum Albumin and Its Effect on Proton Relaxation Rates. J. Am. Chem. Soc. 2002, 124, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Morcos, S.K. Extracellular Gadolinium Contrast Agents: Differences in Stability. Eur. J. Radiol. 2008, 66, 175–179. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-Enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Haedicke, I.E.; Nofiele, J.; Martinez, F.; Beera, K.; Scholl, T.J.; Cheng, H.-L.M.; Zhang, X.-A. Complementary Strategies for Developing Gd-Free High-Field T1 MRI Contrast Agents Based on Mn III Porphyrins. J. Med. Chem. 2014, 57, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Haedicke, I.E.; Li, T.; Zhu, Y.L.K.; Martinez, F.; Hamilton, A.M.; Murrell, D.H.; Nofiele, J.T.; Cheng, H.-L.M.; Scholl, T.J.; Foster, P.J.; et al. An Enzyme-Activatable and Cell-Permeable Mn III-Porphyrin as a Highly Efficient T 1 MRI Contrast Agent for Cell Labeling. Chem. Sci. 2016, 7, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Nofiele, J.T.; Haedicke, I.E.; Zhu, Y.L.K.; Zhang, X.-A.; Cheng, H.-L.M. Gadolinium-Free Extracellular MR Contrast Agent for Tumor Imaging: Gd-Free Contrast Agent for Tumor MRI. J. Magn. Reson. Imaging 2015, 41, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-A.; Lovejoy, K.S.; Jasanoff, A.; Lippard, S.J. Water-Soluble Porphyrins as a Dual-Function Molecular Imaging Platform for MRI and Fluorescence Zinc Sensing. Proc. Natl. Acad. Sci. USA 2007, 104, 10780–10785. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.-A. Manganese-Based Magnetic Resonance Imaging Contrast Agents. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- Raman, F.S.; Nacif, M.S.; Cater, G.; Gai, N.; Jones, J.; Li, D.; Sibley, C.T.; Liu, S.; Bluemke, D.A. 3.0-T Whole-Heart Coronary Magnetic Resonance Angiography: Comparison of Gadobenate Dimeglumine and Gadofosveset Trisodium. Int. J. Cardiovasc. Imaging 2013, 29, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ganesh, T.; Martinez, F.; Lam, J.; Yoon, H.; Macgregor, R.B.; Scholl, T.J.; Cheng, H.-L.M.; Zhang, X.-A. Binding of a Dimeric Manganese Porphyrin to Serum Albumin: Towards a Gadolinium-Free Blood-Pool T 1 MRI Contrast Agent. J. Biol. Inorg. Chem. 2014, 19, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.M.; Haedicke, I.E.; Cheng, W.; Tchouala Nofiele, J.; Zhang, X.-A. Gadolinium-Free T1 Contrast Agents for MRI: Tunable Pharmacokinetics of a New Class of Manganese Porphyrins: Tunable Gadolinium-Free T1 Contrast Agents. J. Magn. Reson. Imaging 2014, 40, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Nofiele, J.T.; Cheng, W.; Haedicke, I.E.; Ganesh, T.; Zhang, X.-A.; Cheng, H.-L.M. Ultrashort Echo Time Magnetic Resonance Imaging of the Lung Using a High-Relaxivity T 1 Blood-Pool Contrast Agent. Mol. Imaging 2014, 13, 7290.2014.00027. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo Reactions Revisited: Synthesis of Tetraphenylporphyrins under Equilibrium Conditions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Punna, S.; Diaz, D.D.; Finn, M.G. Palladium-Catalyzed Homocoupling of Arylboronic Acids and Esters Using Fluoride in Aqueous Solvents. Synlett 2004, 13, 2351–2354. [Google Scholar] [CrossRef]
- Boucher, L.J. Manganese Porphyrin Complexes. Coord. Chem. Rev. 1972, 7, 289–329. [Google Scholar] [CrossRef]
 ), MnTPPS (
), MnTPPS ( ), m-MnP2 (⬛), and MnP2(🔴) in 25 mM pH 7.2 HEPES buffer from 0.23 mT to 3 T at 25 °C. MnP r1 was normalized to per Mn(III) ion.
), m-MnP2 (⬛), and MnP2(🔴) in 25 mM pH 7.2 HEPES buffer from 0.23 mT to 3 T at 25 °C. MnP r1 was normalized to per Mn(III) ion.
 ), MnTPPS (
), MnTPPS ( ), m-MnP2 (⬛), and MnP2(🔴) in 25 mM pH 7.2 HEPES buffer from 0.23 mT to 3 T at 25 °C. MnP r1 was normalized to per Mn(III) ion.
), m-MnP2 (⬛), and MnP2(🔴) in 25 mM pH 7.2 HEPES buffer from 0.23 mT to 3 T at 25 °C. MnP r1 was normalized to per Mn(III) ion.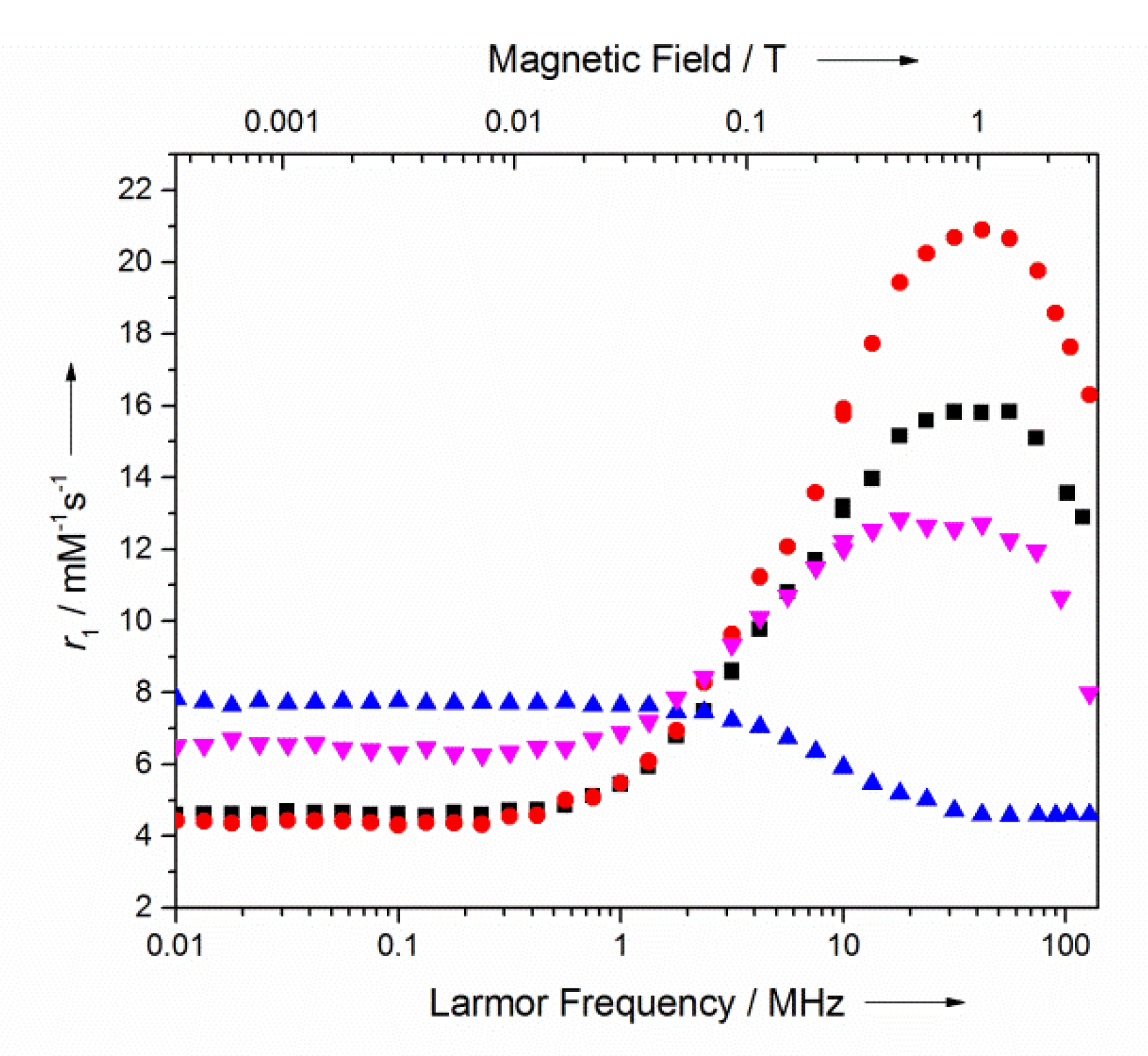
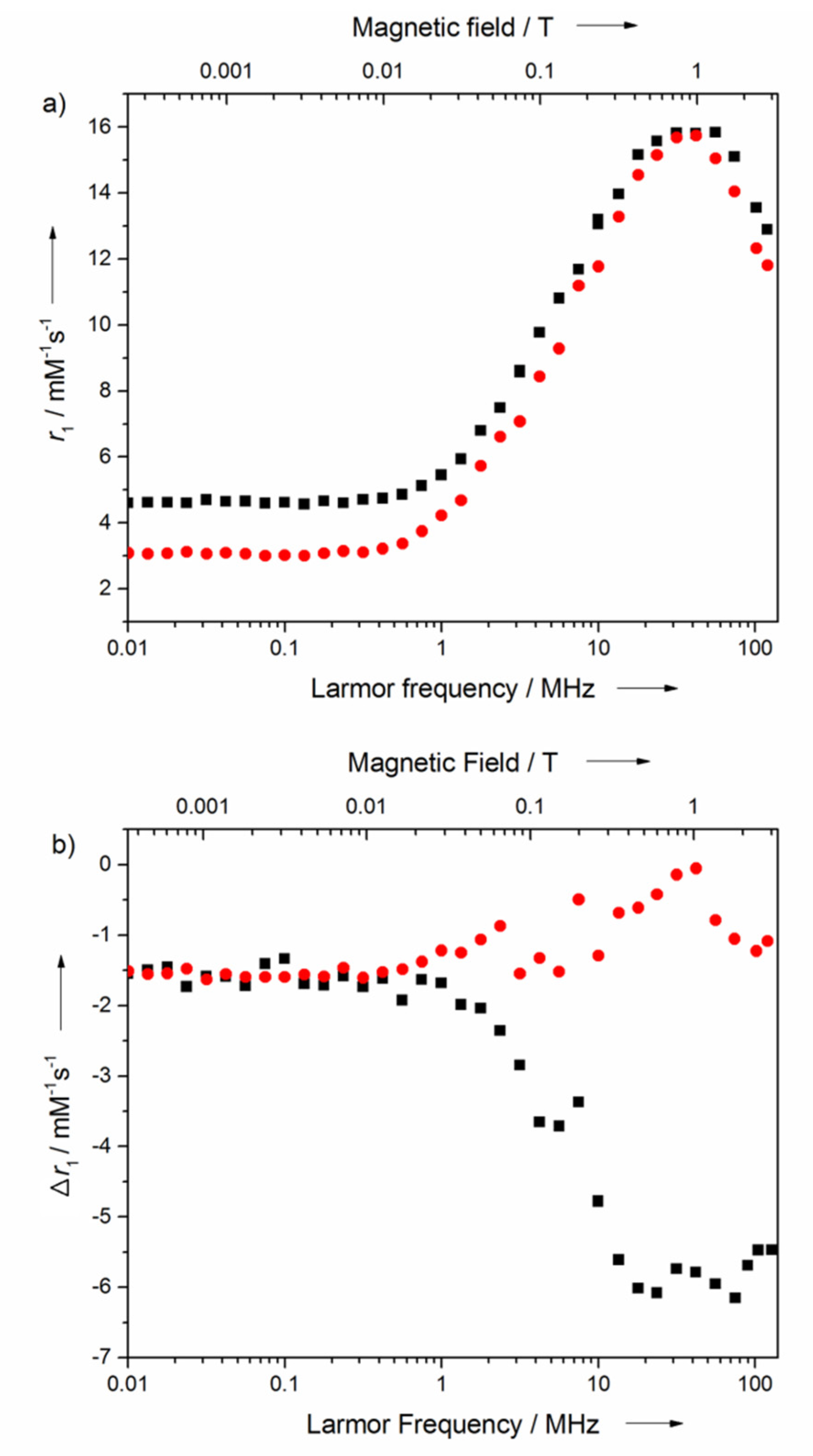

| r1 at 1.6 T (mM−1 s−1) | r1 at 3 T (mM−1 s−1) | |
|---|---|---|
| MnP2 | 20.2 | 14.7 |
| m-MnP2 | 15.4 | 12.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Cheng, W.; Dong, S.; Xu, D.F.; Tang, K.; Zhang, X.-a. A Second Generation Mn-Porphyrin Dimer with a Twisted Linker as a Potential Blood Pool Agent for MRI: Tuning the Geometry and Binding with HSA. Pharmaceuticals 2020, 13, 282. https://doi.org/10.3390/ph13100282
Liu H, Cheng W, Dong S, Xu DF, Tang K, Zhang X-a. A Second Generation Mn-Porphyrin Dimer with a Twisted Linker as a Potential Blood Pool Agent for MRI: Tuning the Geometry and Binding with HSA. Pharmaceuticals. 2020; 13(10):282. https://doi.org/10.3390/ph13100282
Chicago/Turabian StyleLiu, Hanlin, Weiran Cheng, Shili Dong, David Feng Xu, Keith Tang, and Xiao-an Zhang. 2020. "A Second Generation Mn-Porphyrin Dimer with a Twisted Linker as a Potential Blood Pool Agent for MRI: Tuning the Geometry and Binding with HSA" Pharmaceuticals 13, no. 10: 282. https://doi.org/10.3390/ph13100282
APA StyleLiu, H., Cheng, W., Dong, S., Xu, D. F., Tang, K., & Zhang, X.-a. (2020). A Second Generation Mn-Porphyrin Dimer with a Twisted Linker as a Potential Blood Pool Agent for MRI: Tuning the Geometry and Binding with HSA. Pharmaceuticals, 13(10), 282. https://doi.org/10.3390/ph13100282




