Cyclic Oligosaccharides as Active Drugs, an Updated Review
Abstract
1. Introduction
2. Pharmacokinetics, Toxicology, and Regulation
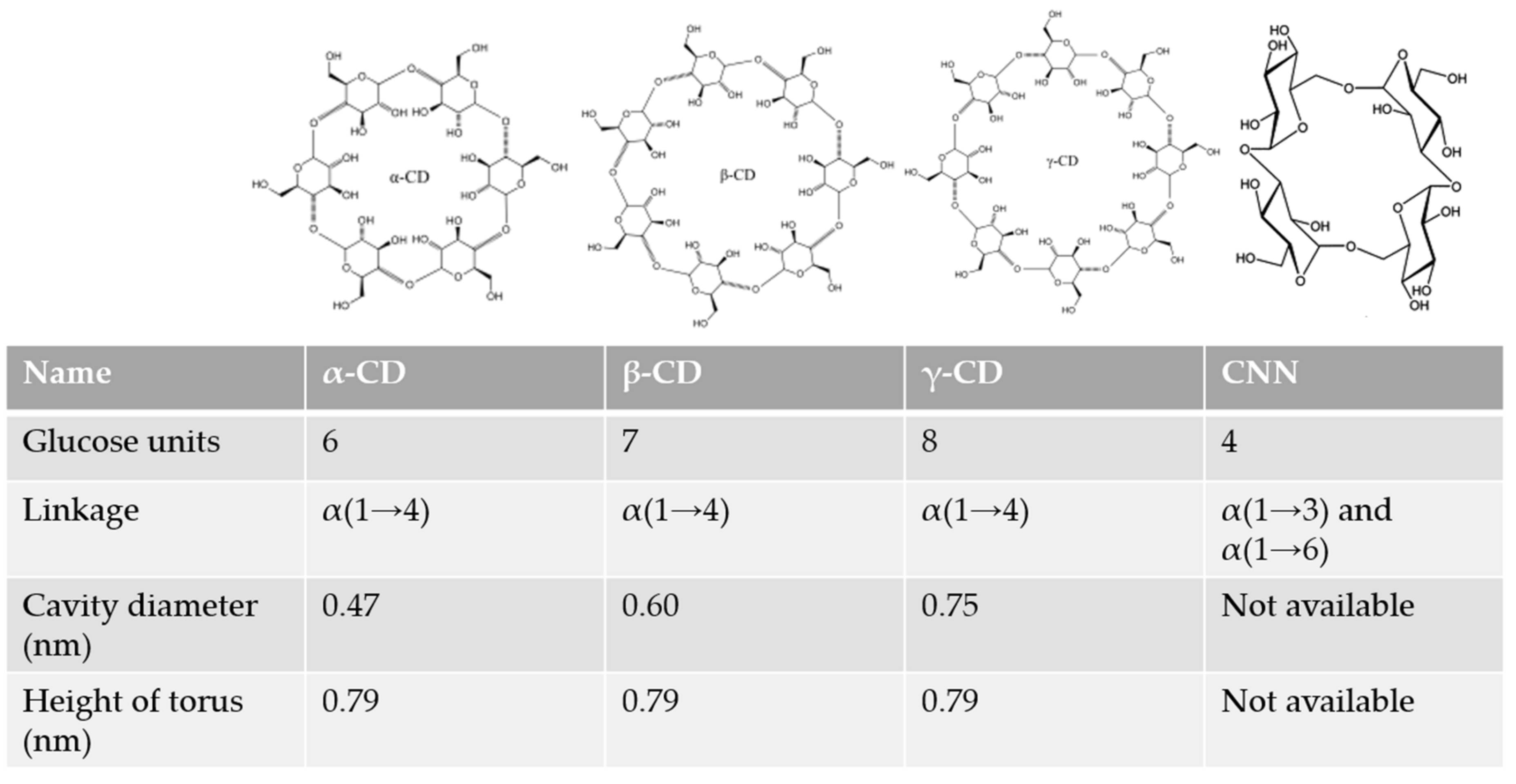
2.1. Cyclodextrins
2.2. Cyclic Nigerosyl-1,6-Nigerose or Cyclotetraglucose
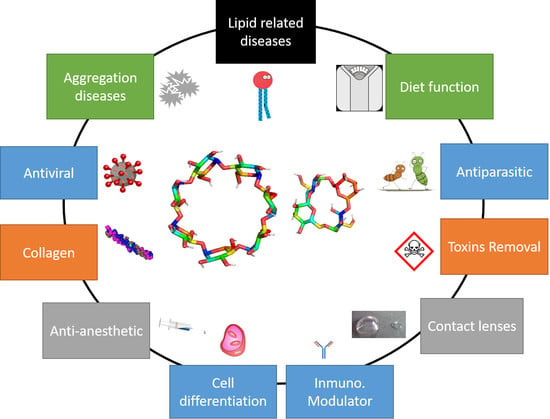
3. Cyclodextrins as Active Drugs
3.1. Lipid-Related Diseases
3.1.1. Niemann-Pick Disease Type C
3.1.2. Atherosclerosis
- (i)
- To inhibit the entry of circulating monocytes into the lesion by inhibiting their adhesion to the endothelium.
- (ii)
- To stimulate cholesterol efflux, and interact with and contribute to the dissolution of cholesterol crystals.
- (iii)
- To decrease the susceptibility of LDL to oxidation.
- (iv)
- To inhibit cholesterol crystal-induced phagocytosis.
- (v)
- To inhibit cholesterol crystal-triggered complement activation.
- (vi)
- To reduce inflammation and production of reactive oxygen species in atherosclerotic lesions.
3.2. Aggregation-Related Diseases
3.2.1. Parkinson Disease
3.2.2. Alzheimer Disease
3.2.3. Huntington’s Disease
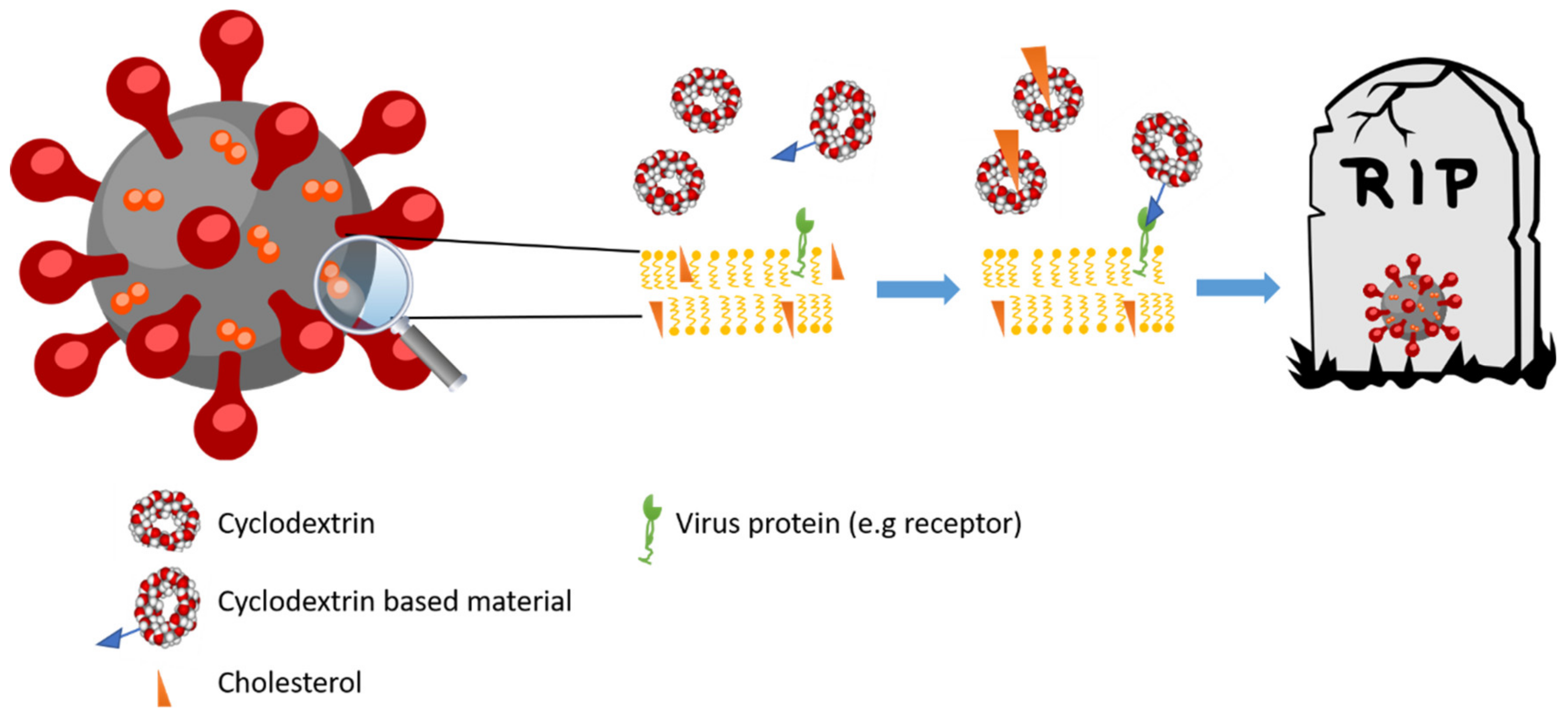
3.3. Antiviral Activity
3.3.1. Influenza Virus
3.3.2. HIV
3.3.3. Coronavirus
3.3.4. Other Viruses
3.4. Antiparasitic Activity
3.5. Anti-Anesthetic Agent
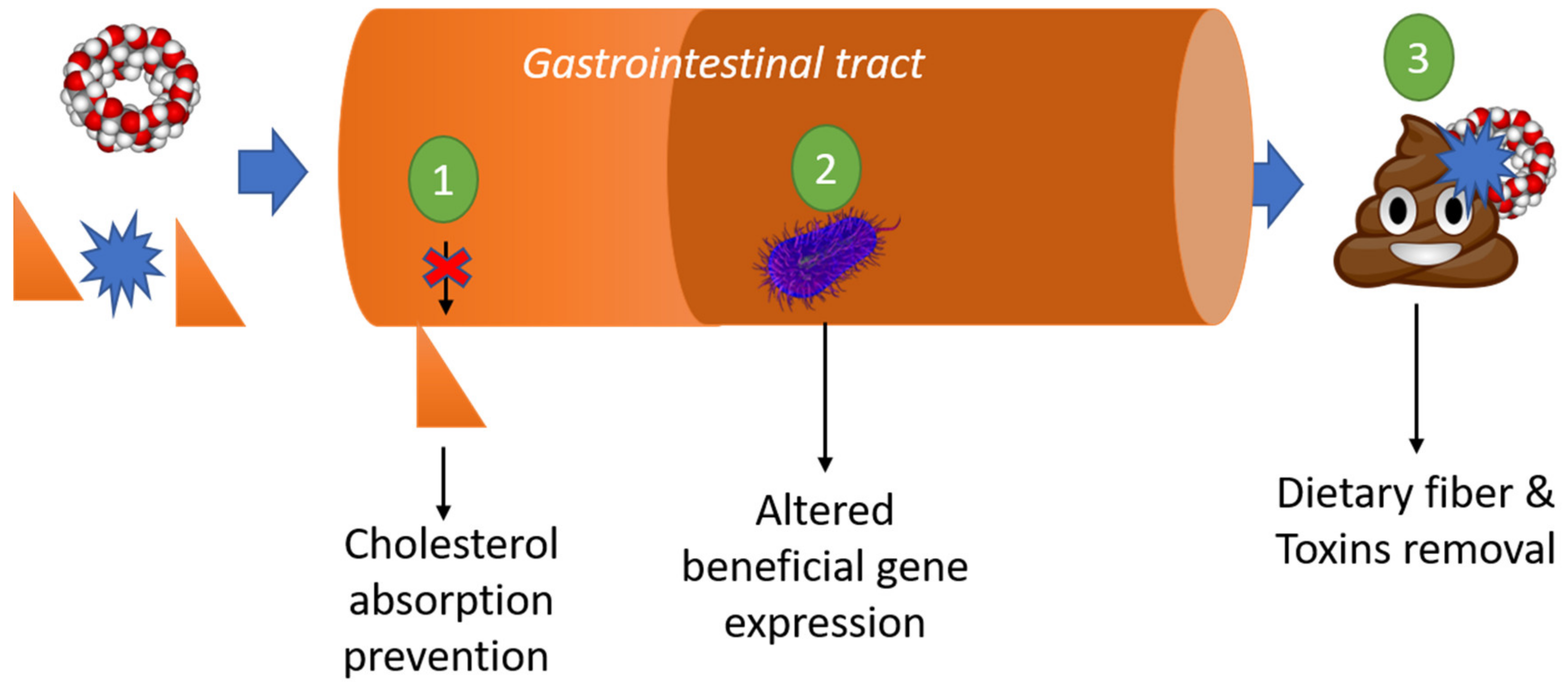
3.6. Dietary Function: Fiber, Prebiotic, and Fat Reduction
3.7. Removal of Organic Toxins
3.8. Collagen
3.9. Cell Differentiation
3.10. Contact Lenses
4. Cyclic Nigerosyl-1,6-Nigerose as Active Drug
4.1. Dietary Function
4.2. Immunological Modulator
5. Critical Perspective of the Structure–Activity Relationship
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villiers, A. Sur la fermentation de la fécule par l’action du ferment butyrique. Comptes Rendus L’Académie Sci. 1891, 112, 536–538. [Google Scholar]
- Pulley, A.O. Studies on the Schardinger dextrins. XI. The isolation of new Scharginger dextrins. Biochem. Biophys. Res. Commun. 1961, 5, 11–15. [Google Scholar] [CrossRef]
- French, D.; Pulley, A.O.; Effenberger, J.A.; Rougvie, M.A.; Abdullah, M. Studies on the Schardinger dextrins: XII. The molecular size and structure of the δ-, ϵ-, ζ-, and η-dextrins. Arch. Biochem. Biophys. 1965, 111, 153–160. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Fourmentin, S.; Crini, G.; Lichtfouse, A. (Eds.) Cyclodextrin Fundamentals, Reactivity and Analysis; Environmental Chemistry for a Sustainable World; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-76158-9. [Google Scholar]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Aggregation of t10,c12 conjugated linoleic Acid in presence of natural and modified cyclodextrins. A physicochemical, thermal and computational analysis. Chem. Phys. Lipids 2017, 204, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Bermejo-Gimeno, M.J.; García-Carmona, F.; López-Nicolás, J.M. Separating and Identifying the Four Stereoisomers of Methyl Jasmonate by RP-HPLC and using Cyclodextrins in a Novel Way. Phytochem. Anal. 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Matencio, A.; Guerrero-Rubio, M.A.; Gandía-Herrero, F.; García-Carmona, F.; López-Nicolás, J.M. Nanoparticles of betalamic acid derivatives with cyclodextrins. Physicochemistry, production characterization and stability. Food Hydrocoll. 2020, 110, 106176. [Google Scholar] [CrossRef]
- Abril-Sánchez, C.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 2019, 219, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Encapsulation of piceatannol, a naturally occurring hydroxylated analogue of resveratrol, by natural and modified cyclodextrins. Food Funct. 2016, 7, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; Manuel López-Nicolás, J. Ellagic acid–borax fluorescence interaction: Application for novel cyclodextrin-borax nanosensors for analyzing ellagic acid in food samples. Food Funct. 2018, 9, 3683–3687. [Google Scholar] [CrossRef] [PubMed]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of Aromatic Chlorinated Pesticides from Aqueous Solution Using β-Cyclodextrin Polymers Decorated with Fe3O4 Nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: Developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr. Polym. 2020, 231, 115763. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Matencio, A.; Hernández-García, S.; García-Carmona, F.; López-Nicolás, J.M. A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers. Polymers 2019, 11, 801. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; Kovanen, P.T.; Xu, S.; Jamialahmadi, T.; Sahebkar, A. Cyclodextrins: Potential therapeutics against atherosclerosis. Pharmacol. Ther. 2020, 214, 107620. [Google Scholar] [CrossRef]
- Bradbrook, G.M.; Gessler, K.; Côté, G.L.; Momany, F.; Biely, P.; Bordet, P.; Pérez, S.; Imberty, A. X-ray structure determination and modeling of the cyclic tetrasaccharide cyclo-{→6)-α-d-Glcp-(1→3)-α-d-Glcp-(1→6)-α-d-Glcp-(1→3)-α-d-Glcp-(1→}. Carbohydr. Res. 2000, 329, 655–665. [Google Scholar] [CrossRef]
- John Marshall, J.; Miwa, I. Kinetic difference between hydrolyses of γ-cyclodextrin by human salivary and pancreatic α-amylases. Biochim. Biophys. Acta (BBA)-Enzym. 1981, 661, 142–147. [Google Scholar] [CrossRef]
- Arima, H.; Motoyama, K.; Irie, T. Recent Findings on Safety Profiles of Cyclodextrins, Cyclodextrin Conjugates, and Polypseudorotaxanes. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 91–122. ISBN 978-0-470-92681-9. [Google Scholar]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia (PhEur.) 9th Edition | EDQM. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition (accessed on 3 April 2019).
- Frijlink, H.W.; Visser, J.; Hefting, N.R.; Oosting, R.; Meijer, D.K.F.; Lerk, C.F. The Pharmacokinetics of β-Cyclodextrin and Hydroxypropyl-β-cyclodextrin in the Rat. Pharm. Res. 1990, 7, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.A.; Ernst, C.C.; Kramer, W.G.; Madden, D.; Lang, E.; Liao, E.; Lacouture, P.G.; Ramaiya, A.; Carr, D.B. Pharmacokinetics of Diclofenac and Hydroxypropyl-β-Cyclodextrin (HPβCD) Following Administration of Injectable HPβCD-Diclofenac in Subjects With Mild to Moderate Renal Insufficiency or Mild Hepatic Impairment. Clin. Pharm. Drug Dev. 2018, 7, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Luke, D.R.; Wood, N.D.; Tomaszewski, K.E.; Damle, B. Pharmacokinetics of sulfobutylether-β-cyclodextrin (SBECD) in subjects on hemodialysis. Nephrol. Dial. Transpl. 2012, 27, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Alcaráz-Gómez, M.A.; García-Carmona, F.; Arias, B.; López-Nicolás, J.M. Application of a simple methodology to analyze Hydroxypropyl-β-Cyclodextrin in urine using HPLC–LS in early Niemann–Pick disease type C patient. J. Chromatogr. B 2018, 1093–1094, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; González-Ramón, A.; García-Carmona, F.; López-Nicolás, J.M. Recent advances in the treatment of Niemann pick disease type C: A mini-review. Int. J. Pharm. 2020, 584, 119440. [Google Scholar] [CrossRef]
- EMA. Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use. In Proceedings of the Committee for Human Medicinal Products (CHMP), London, UK, 9 October 2017; p. 9. [Google Scholar]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Li, P.; Song, J.; Ni, X.; Guo, Q.; Wen, H.; Zhou, Q.; Shen, Y.; Huang, Y.; Qiu, P.; Lin, S.; et al. Comparison in toxicity and solubilizing capacity of hydroxypropyl-β-cyclodextrin with different degree of substitution. Int. J. Pharm. 2016, 513, 347–356. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to alpha cyclodextrin and reduction of post prandial glycaemic responses (ID 2926, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2713. [Google Scholar]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan. Japanese Pharmacopoeia, 17th ed.; Stationery Office Books (TSO): London, UK, 2017; ISBN 978-4-8408-1371-6. [Google Scholar]
- Rajagopalan, G.; Krishnan, C. Functional Oligosaccharides: Production and Action. In Next Generation Biomanufacturing Technologies; Rathinam, N.K., Sani, R.K., Eds.; ACS Symposium Series: Washington, DC, USA, 2019. [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United Nations (Eds.) Evaluation of Certain Food Additives: Seventy-First Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-120956-4. [Google Scholar]
- Joint Expert Committee on Food Additives (Ed.) Safety Evaluation of Certain Food Additives and Contaminants; WHO Food Additives Series; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-166059-4. [Google Scholar]
- Hashimoto, T.; Kurose, M.; Oku, K.; Nishimoto, T.; Chaen, H.; Fukuda, S.; Tsujisaka, Y. Digestibility and Suppressive Effect on Rats’ Body Fat Accumulation of Cyclic Tetrasaccharide. J. Appl. Glycosci. 2006, 53, 233–239. [Google Scholar] [CrossRef]
- Williams, R.O.; Mahaguna, V.; Sriwongjanya, M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-beta-cyclodextrin. Eur. J. Pharm. Biopharm. 1998, 46, 355–360. [Google Scholar] [CrossRef]
- Evans, W.R.H.; Hendriksz, C.J. Niemann–Pick type C disease–The tip of the iceberg? A review of neuropsychiatric presentation, diagnosis and treatment. BJPsych Bull. 2017, 41, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hao, J.; Fujiwara, H.; Xu, M.; Yang, S.; Dai, S.; Long, Y.; Swaroop, M.; Li, C.; Vu, M.; et al. Analytical Characterization of Methyl-β-Cyclodextrin for Pharmacological Activity to Reduce Lysosomal Cholesterol Accumulation in Niemann-Pick Disease Type C1 Cells. Assay Drug Dev. Technol. 2017, 15, 154–166. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Villamandos García, D.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Garatachea, N.; Nogales Gadea, G.; Lucia, A. Niemann-Pick disease treatment: A systematic review of clinical trials. Ann. Transl. Med. 2015, 3, 360. [Google Scholar]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. PNAS 2010, 107, 5477–5482. [Google Scholar] [CrossRef]
- Dai, S.; Dulcey, A.E.; Hu, X.; Wassif, C.A.; Porter, F.D.; Austin, C.P.; Ory, D.S.; Marugan, J.; Zheng, W. Methyl-β-cyclodextrin restores impaired autophagy flux in Niemann-Pick C1-deficient cells through activation of AMPK. Autophagy 2017, 13, 1435–1451. [Google Scholar] [CrossRef]
- Singhal, A.; Szente, L.; Hildreth, J.E.K.; Song, B. Hydroxypropyl-beta and -gamma cyclodextrins rescue cholesterol accumulation in Niemann–Pick C1 mutant cell via lysosome-associated membrane protein 1. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Calias, P. 2-Hydroxypropyl-β-cyclodextrins and the Blood-Brain Barrier: Considerations for Niemann-Pick Disease Type C1. CPD 2018, 23, 6231–6238. [Google Scholar] [CrossRef]
- Camargo, F.; Erickson, R.P.; Garver, W.S.; Hossain, G.S.; Carbone, P.N.; Heidenreich, R.A.; Blanchard, J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001, 70, 131–142. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Chin, J.; Hoffmann, A.; Winston, A.; Stoner, R.; LaGorio, L.; Friedmann, K.; Hernandez, M.; Ory, D.S.; Porter, F.D.; et al. Long-Term Treatment of Niemann-Pick Type C1 Disease with Intrathecal 2-Hydroxypropyl-β-Cyclodextrin. Pediatric Neurol. 2018, 80, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, N.; Ishitsuka, Y.; Fukaura, M.; Yamada, Y.; Nakahara, S.; Ishii, A.; Kondo, Y.; Takeo, T.; Nakagata, N.; Motoyama, K.; et al. In Vitro and In Vivo Evaluation of 6-O-α-Maltosyl-β-Cyclodextrin as a Potential Therapeutic Agent against Niemann-Pick Disease Type C. Int. J. Mol. Sci. 2019, 20, 1152. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Hirai, Y.; Nishiyama, R.; Maeda, Y.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Era, T.; Arima, H. Cholesterol lowering effects of mono-lactose-appended β-cyclodextrin in Niemann–Pick type C disease-like HepG2 cells. Beilstein J. Org. Chem. 2015, 11, 2079–2086. [Google Scholar] [CrossRef]
- Motoyama, K.; Nishiyama, R.; Maeda, Y.; Higashi, T.; Kawaguchi, Y.; Futaki, S.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Era, T.; et al. Cholesterol-lowering Effect of Octaarginine-appended β-Cyclodextrin in Npc1-trap-CHO Cells. Biol. Pharm. Bull. 2016, 39, 1823–1829. [Google Scholar] [CrossRef]
- Puglisi, A.; Yagci, Y. Cyclodextrin-Based Macromolecular Systems as Cholesterol-Mopping Therapeutic Agents in Niemann–Pick Disease Type C. Macromol. Rapid Commun. 2019, 40, 1800557. [Google Scholar] [CrossRef]
- Kulkarni, A.; Caporali, P.; Dolas, A.; Johny, S.; Goyal, S.; Dragotto, J.; Macone, A.; Jayaraman, R.; Fiorenza, M.T. Linear Cyclodextrin Polymer Prodrugs as Novel Therapeutics for Niemann-Pick Type C1 Disorder. Sci. Rep. 2018, 8, 9547. [Google Scholar] [CrossRef]
- Bennett Martin, R.; Sanjay, S.; Owens Gary, K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L.; Terasaka, N.; Pagler, T.; Wang, N. HDL, ABC Transporters, and Cholesterol Efflux: Implications for the Treatment of Atherosclerosis. Cell Metab. 2008, 7, 365–375. [Google Scholar] [CrossRef]
- Montecucco, F.; Lenglet, S.; Carbone, F.; Boero, S.; Pelli, G.; Burger, F.; Roth, A.; Bertolotto, M.; Nencioni, A.; Cea, M.; et al. Treatment with KLEPTOSE® CRYSMEB reduces mouse atherogenesis by impacting on lipid profile and Th1 lymphocyte response. Vasc. Pharmacol. 2015, 72, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Coisne, C.; Hallier-Vanuxeem, D.; Boucau, M.-C.; Hachani, J.; Tilloy, S.; Bricout, H.; Monflier, E.; Wils, D.; Serpelloni, M.; Parissaux, X.; et al. β-Cyclodextrins Decrease Cholesterol Release and ABC-Associated Transporter Expression in Smooth Muscle Cells and Aortic Endothelial Cells. Front. Physiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Zhou, X.; Zhang, W.; Liu, J. Shuttle/sink model composed of β-cyclodextrin and simvastatin-loaded discoidal reconstituted high-density lipoprotein for enhanced cholesterol efflux and drug uptake in macrophage/foam cells. J. Mater. Chem. B 2020, 8, 1496–1506. [Google Scholar] [CrossRef]
- Kritharides, L.; Kus, M.; Brown, A.J.; Jessup, W.; Dean, R.T. Hydroxypropyl-β-cyclodextrin-mediated Efflux of 7-Ketocholesterol from Macrophage Foam Cells. J. Biol. Chem. 1996, 271, 27450–27455. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; Nardo, D.D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, ra50–ra333. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Yu, B.; Peng, X.; Liu, Y.; Wang, A.; Zhao, D.; Pang, D.; OuYang, H.; Tang, X. Cyclodextrin Ameliorates the Progression of Atherosclerosis via Increasing High-Density Lipoprotein Cholesterol Plasma Levels and Anti-inflammatory Effects in Rabbits. J. Cardiovasc. Pharm. 2019, 73, 334–342. [Google Scholar] [CrossRef]
- Pilely, K.; Bakke, S.S.; Palarasah, Y.; Skjoedt, M.-O.; Bartels, E.D.; Espevik, T.; Garred, P. Alpha-cyclodextrin inhibits cholesterol crystal-induced complement-mediated inflammation: A potential new compound for treatment of atherosclerosis. Atherosclerosis 2019, 283, 35–42. [Google Scholar] [CrossRef]
- Amar, M.J.A.; Kaler, M.; Courville, A.B.; Shamburek, R.; Sampson, M.; Remaley, A.T. Randomized double blind clinical trial on the effect of oral α-cyclodextrin on serum lipids. Lipids Health Dis. 2016, 15, 115. [Google Scholar] [CrossRef]
- Sakurai, T.; Sakurai, A.; Chen, Y.; Vaisman, B.L.; Amar, M.J.; Pryor, M.; Thacker, S.G.; Zhang, X.; Wang, X.; Zhang, Y.; et al. Dietary α-cyclodextrin reduces atherosclerosis and modifies gut flora in apolipoprotein E-deficient mice. Mol. Nutr. Food Res. 2017, 61, 1600804. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.; Park, J.-H. Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity. J. Control. Release 2020, 319, 77–86. [Google Scholar] [CrossRef]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. NED 2016, 46, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, P.; Rockenstein, E.; Adame, A.; Ho, G.; Hashimoto, M.; Masliah, E. Effects of the cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synucleinopathy. J. Neurochem. 2006, 98, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Martini-Stoica, H.; Xu, Y.; Ballabio, A.; Zheng, H. The Autophagy–Lysosomal Pathway in Neurodegeneration: A TFEB Perspective. Trends Neurosci. 2016, 39, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, K.; Zeng, Y.; Hancock, T.; Segatori, L. Genetic and Chemical Activation of TFEB Mediates Clearance of Aggregated α-Synuclein. PLoS ONE 2015, 10, e0120819. [Google Scholar] [CrossRef]
- El Kadmiri, N.; Said, N.; Slassi, I.; El Moutawakil, B.; Nadifi, S. Biomarkers for Alzheimer Disease: Classical and Novel Candidates’ Review. Neuroscience 2018, 370, 181–190. [Google Scholar] [CrossRef]
- Finch, C.E.; Cohen, D.M. Aging, Metabolism, and Alzheimer Disease: Review and Hypotheses. Exp. Neurol. 1997, 143, 82–102. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Petrov, D.; Abad, S.; Pedrós, I.; Marin, M.; Olloquequi, J.; Camins, A. Review of the advances in treatment for Alzheimer disease: Strategies for combating β-amyloid protein. Neurologia 2018, 33, 47–58. [Google Scholar] [CrossRef]
- Camilleri, P.; Haskins, N.J.; Hewlett, D.R. β-Cyclodextrin interacts with the Alzheimer amyloid β-A4 peptide. FEBS Lett. 1994, 341, 256–258. [Google Scholar] [CrossRef]
- Danielsson, J.; Jarvet, J.; Damberg, P.; Gräslund, A. Two-Site Binding of β-Cyclodextrin to the Alzheimer Aβ(1−40) Peptide Measured with Combined PFG-NMR Diffusion and Induced Chemical Shifts. Biochemistry 2004, 43, 6261–6269. [Google Scholar] [CrossRef]
- Ren, B.; Jiang, B.; Hu, R.; Zhang, M.; Chen, H.; Ma, J.; Sun, Y.; Jia, L.; Zheng, J. HP-β-cyclodextrin as an inhibitor of amyloid-β aggregation and toxicity. Phys. Chem. Chem. Phys. 2016, 18, 20476–20485. [Google Scholar] [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Gavini, E.; Rassu, G.; Haukvik, T.; Lanni, C.; Racchi, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J. Drug Target. 2009, 17, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Vecchio, G. Synthesis and Evaluation of New Cyclodextrin Derivatives as Amyloid-β Aggregation Inhibitors. ChemistrySelect 2019, 4, 10639–10642. [Google Scholar] [CrossRef]
- Fiumara, F.; Fioriti, L.; Kandel, E.R.; Hendrickson, W.A. Essential Role of Coiled Coils for Aggregation and Activity of Q/N-Rich Prions and PolyQ Proteins. Cell 2010, 143, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Pelassa, I.; Corà, D.; Cesano, F.; Monje, F.J.; Montarolo, P.G.; Fiumara, F. Association of polyalanine and polyglutamine coiled coils mediates expansion disease-related protein aggregation and dysfunction. Hum. Mol. Genet. 2014, 23, 3402–3420. [Google Scholar] [CrossRef]
- Lilliu, E.; Villeri, V.; Pelassa, I.; Cesano, F.; Scarano, D.; Fiumara, F. Polyserine repeats promote coiled coil-mediated fibril formation and length-dependent protein aggregation. J. Struct. Biol. 2018, 204, 572–584. [Google Scholar] [CrossRef]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Del Toro, D.; Xifró, X.; Pol, A.; Humbert, S.; Saudou, F.; Canals, J.M.; Alberch, J. Altered cholesterol homeostasis contributes to enhanced excitotoxicity in Huntington’s disease. J. Neurochem. 2010, 115, 153–167. [Google Scholar] [CrossRef]
- Barman, S.; Nayak, D.P. Lipid Raft Disruption by Cholesterol Depletion Enhances Influenza A Virus Budding from MDCK Cells. J. Virol. 2007, 81, 12169–12178. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Role for Influenza Virus Envelope Cholesterol in Virus Entry and Infection. J. Virol. 2003, 77, 12543–12551. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Gupta, D.; Lal, S.K. Host Lipid Rafts Play a Major Role in Binding and Endocytosis of Influenza A Virus. Viruses 2018, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Si, L.; Meng, K.; Zhou, X.; Zhang, Y.; Zhou, D.; Xiao, S. Inhibition of influenza virus infection by multivalent pentacyclic triterpene-functionalized per-O-methylated cyclodextrin conjugates. Eur. J. Med. Chem. 2017, 134, 133–139. [Google Scholar] [CrossRef]
- Xiao, S.; Si, L.; Tian, Z.; Jiao, P.; Fan, Z.; Meng, K.; Zhou, X.; Wang, H.; Xu, R.; Han, X.; et al. Pentacyclic triterpenes grafted on CD cores to interfere with influenza virus entry: A dramatic multivalent effect. Biomaterials 2016, 78, 74–85. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, S.; Zhou, D.; Sollogoub, M.; Zhang, Y. Design, synthesis and biological evaluation of water-soluble per-O-methylated cyclodextrin-C60 conjugates as anti-influenza virus agents. Eur. J. Med. Chem. 2018, 146, 194–205. [Google Scholar] [CrossRef]
- Liang, S.; Li, M.; Yu, X.; Jin, H.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019, 166, 328–338. [Google Scholar] [CrossRef]
- NIPH Clinical Trials Search A Phase1 Study of Hydroxypropyl-beta-Cyclodextrin(HP-beta-CyD)-Adjuvanted Influenza Split Vaccine. Available online: https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000028530 (accessed on 27 April 2020).
- Kim, S.K.; Yun, C.-H.; Han, S.H. Induction of Dendritic Cell Maturation and Activation by a Potential Adjuvant, 2-Hydroxypropyl-β-Cyclodextrin. Front. Immunol. 2016, 7, 435. [Google Scholar] [CrossRef]
- Onishi, M.; Ozasa, K.; Kobiyama, K.; Ohata, K.; Kitano, M.; Taniguchi, K.; Homma, T.; Kobayashi, M.; Sato, A.; Katakai, Y.; et al. Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J. Immunol. 2015, 194, 2673–2682. [Google Scholar] [CrossRef]
- Graham, D.R.M.; Chertova, E.; Hilburn, J.M.; Arthur, L.O.; Hildreth, J.E.K. Cholesterol Depletion of Human Immunodeficiency Virus Type 1 and Simian Immunodeficiency Virus with β-Cyclodextrin Inactivates and Permeabilizes the Virions: Evidence for Virion-Associated Lipid Rafts. J. Virol. 2003, 77, 8237–8248. [Google Scholar] [CrossRef]
- Liao, Z.; Graham, D.R.; Hildreth, J.E.K. Lipid Rafts and HIV Pathogenesis: Virion-Associated Cholesterol Is Required for Fusion and Infection of Susceptible Cells. AIDS Res. Hum. Retrovir. 2003, 19, 675–687. [Google Scholar] [CrossRef]
- Khanna, K.V.; Whaley, K.J.; Zeitlin, L.; Moench, T.R.; Mehrazar, K.; Cone, R.A.; Liao, Z.; Hildreth, J.E.K.; Hoen, T.E.; Shultz, L.; et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Investig. 2002, 109, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, Z.; Compton, L.; Piatak, M.; Lu, D.; Alvord, W.G.; Lubomirski, M.S.; Hildreth, J.E.K.; Lifson, J.D.; Miller, C.J.; KewalRamani, V.N. Incomplete protection against simian immunodeficiency virus vaginal transmission in rhesus macaques by a topical antiviral agent revealed by repeat challenges. J. Virol. 2008, 82, 6591–6599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matassoli, F.L.; Leão, I.C.; Bezerra, B.B.; Pollard, R.B.; Lütjohann, D.; Hildreth, J.E.K.; Arruda, L.B. de Hydroxypropyl-Beta-Cyclodextrin Reduces Inflammatory Signaling from Monocytes: Possible Implications for Suppression of HIV Chronic Immune Activation. mSphere 2018, 3, e00497-18. [Google Scholar] [CrossRef]
- Mingxue, B.; Chaolumen, B.; Asai, D.; Miyazaki, K.; Yoshida, T. Synthesis and Anti-HIV Activity of Sulfated Oligosaccharide-Branched β-CD. J. Fiber Sci. Technol. 2020, 76, 63–71. [Google Scholar] [CrossRef]
- Mingxue, B.; Chaolumen, B.; Asai, D.; Takemura, H.; Miyazaki, K.; Yoshida, T. Role of a long-chain alkyl group in sulfated alkyl oligosaccharides with high anti-HIV activity revealed by SPR and DLS. Carbohydr. Polym. 2020, 245, 116518. [Google Scholar] [CrossRef]
- Kheat, T.C.; Wen, C.F.; Yogesh, K.M.; Tao, P.; Stinsa, L.; Stinsa, L. Combating Coronavirus: Key Role of Cyclodextrins in Treatment and Prevention | Innovation Hub | Roquette. Available online: https://www.roquette.com:443/en/innovation-hub/expert-opinion/kleptose-combating-coronavirus-key-role-of-cyclodextrins-in-treatment-and-prevention/ (accessed on 10 August 2020).
- Guo, H.; Huang, M.; Yuan, Q.; Wei, Y.; Gao, Y.; Mao, L.; Gu, L.; Tan, Y.W.; Zhong, Y.; Liu, D.; et al. The Important Role of Lipid Raft-Mediated Attachment in the Infection of Cultured Cells by Coronavirus Infectious Bronchitis Virus Beaudette Strain. PLoS ONE 2017, 12, e0170123. [Google Scholar] [CrossRef]
- Li, G.-M.; Li, Y.-G.; Yamate, M.; Li, S.-M.; Ikuta, K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007, 9, 96–102. [Google Scholar] [CrossRef]
- Seddon, A.M. Materials Science in the time of Coronavirus. J. Mater. Sci. 2020, 55, 9145–9147. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, Á.; Garcia-Fandino, R. The Lord of the NanoRings: Cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020, 588, 119689. [Google Scholar] [CrossRef]
- Carro, A.C.; Damonte, E.B. Requirement of cholesterol in the viral envelope for dengue virus infection. Virus Res. 2013, 174, 78–87. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Mosso, C.; Medina, F.; Liprandi, F.; Ludert, J.E.; del Angel, R.M. Antibody-dependent enhancement of dengue virus infection in U937 cells requires cholesterol-rich membrane microdomains. J. Gen. Virol. 2010, 91, 394–403. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Schneider, S.M.; Nicola, A.V. Herpes Simplex Virus 1 Envelope Cholesterol Facilitates Membrane Fusion. Front. Microbiol. 2017, 8, 2383. [Google Scholar] [CrossRef]
- Hambleton, S.; Steinberg, S.P.; Gershon, M.D.; Gershon, A.A. Cholesterol Dependence of Varicella-Zoster Virion Entry into Target Cells. J. Virol. 2007, 81, 7548–7558. [Google Scholar] [CrossRef]
- Shanmugam, S.; Saravanabalaji, D.; Yi, M. Detergent-resistant membrane association of NS2 and E2 during hepatitis C virus replication. J. Virol. 2015, 89, 4562–4574. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, S.; Shen, S.; Guo, H.; Huang, H.; Wei, W. Methyl-β-cyclodextrin inhibits EV-D68 virus entry by perturbing the accumulation of virus particles and ICAM-5 in lipid rafts. Antivir. Res. 2020, 176, 104752. [Google Scholar] [CrossRef]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L.; et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef]
- Zhu, X.; Pandharkar, T.; Werbovetz, K. Identification of new antileishmanial leads from hits obtained by high-throughput screening. Antimicrob. Agents Chemother. 2012, 56, 1182–1189. [Google Scholar] [CrossRef]
- Pucadyil, T.J.; Tewary, P.; Madhubala, R.; Chattopadhyay, A. Cholesterol is required for Leishmania donovani infection: Implications in leishmaniasis. Mol. Biochem. Parasitol. 2004, 133, 145–152. [Google Scholar] [CrossRef]
- Crandall, I.E.; Szarek, W.A.; Vlahakis, J.Z.; Xu, Y.; Vohra, R.; Sui, J.; Kisilevsky, R. Sulfated cyclodextrins inhibit the entry of Plasmodium into red blood cells: Implications for malarial therapy. Biochem. Pharmacol. 2007, 73, 632–642. [Google Scholar] [CrossRef]
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A Novel Concept of Reversing Neuromuscular Block: Chemical Encapsulation of Rocuronium Bromide by a Cyclodextrin-Based Synthetic Host. Angew. Chem. Int. Ed. 2002, 41, 265–270. [Google Scholar] [CrossRef]
- Murphy, G. The Development and Regulatory History of Sugammadex in the United States. Anesth. Patient Saf. Found. 2016, 30, 45–76. [Google Scholar]
- Blobner, M.; Eriksson, L.I.; Scholz, J.; Motsch, J.; Della Rocca, G.; Prins, M.E. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: Results of a randomised, controlled trial. Eur. J. Anaesthesiol. 2010, 27, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Hemmerling, T.M.; Zaouter, C.; Geldner, G.; Nauheimer, D. Sugammadex-A short review and clinical recommendations for the cardiac anesthesiologist. Ann. Card. Anaesth. 2010, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, T.; Mitsuhata, H.; Mertes, P.M. Sugammadex and rocuronium-induced anaphylaxis. J. Anesth. 2016, 30, 290–297. [Google Scholar] [CrossRef]
- Férézou, J.; Riottot, M.; Sérougne, C.; Cohen-Solal, C.; Catala, I.; Alquier, C.; Parquet, M.; Juste, C.; Lafont, H.; Mathé, D.; et al. Hypocholesterolemic action of beta-cyclodextrin and its effects on cholesterol metabolism in pigs fed a cholesterol-enriched diet. J. Lipid Res. 1997, 38, 86–100. [Google Scholar]
- Wagner, E.M.; Jen, K.-L.C.; Artiss, J.D.; Remaley, A.T. Dietary α-cyclodextrin lowers low-density lipoprotein cholesterol and alters plasma fatty acid profile in low-density lipoprotein receptor knockout mice on a high-fat diet. Metabolism 2008, 57, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Burk, J.; Picone, T.; Hendy, C.; Tan, M.M.C.; Caterson, I.D. Effects of alpha-cyclodextrin on cholesterol control and Compound K on glycaemic control in people with pre-diabetes: Protocol for a Phase III randomized controlled trial. Clin. Obes. 2019, 9, e12324. [Google Scholar] [CrossRef]
- Jarosz, P.A.; Fletcher, E.; Elserafy, E.; Artiss, J.D.; Jen, K.-L.C. The effect of α-cyclodextrin on postprandial lipid and glycemic responses to a fat-containing meal. Metab. Clin. Exp. 2013, 62, 1443–1447. [Google Scholar] [CrossRef]
- Nihei, N.; Okamoto, H.; Furune, T.; Ikuta, N.; Sasaki, K.; Rimbach, G.; Yoshikawa, Y.; Terao, K. Dietary α-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. BioFactors 2018, 44, 336–347. [Google Scholar] [CrossRef]
- Poór, M.; Faisal, Z.; Zand, A.; Bencsik, T.; Lemli, B.; Kunsági-Máté, S.; Szente, L. Removal of Zearalenone and Zearalenols from Aqueous Solutions Using Insoluble Beta-Cyclodextrin Bead Polymer. Toxins 2018, 10, 216. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Szabó, Á.; Szente, L.; Poór, M. Extraction of mycotoxin alternariol from red wine and from tomato juice with beta-cyclodextrin bead polymer. J. Mol. Liq. 2020, 319, 114180. [Google Scholar] [CrossRef]
- Varan, C.; Anceschi, A.; Sevli, S.; Bruni, N.; Giraudo, L.; Bilgiç, E.; Korkusuz, P.; İskit, A.B.; Trotta, F.; Bilensoy, E. Preparation and characterization of cyclodextrin nanosponges for organic toxic molecule removal. Int. J. Pharm. 2020, 585, 119485. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Choi, D.-I.; Choi, J.-Y.; Kim, S.-O.; Cho, K.-A.; Lee, J.-B.; Yun, S.-J.; Lee, S.-C. Methyl-β-cyclodextrin up-regulates collagen I expression in chronologically-aged skin via its anti-caveolin-1 activity. Oncotarget 2014, 6, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Wang, X.; Sommerfeld, S.D.; Chae, J.J.; Athanasopoulou, E.-N.; Shores, L.S.; Duan, X.; Amzel, L.M.; Stellacci, F.; Schein, O.; et al. Cyclodextrin Modulated Type I Collagen Self-Assembly to Engineer Biomimetic Cornea Implants. Adv. Funct. Mater. 2018, 28, 1804076. [Google Scholar] [CrossRef]
- Grier, W.K.; Tiffany, A.S.; Ramsey, M.D.; Harley, B.A.C. Incorporating β-cyclodextrin into collagen scaffolds to sequester growth factors and modulate mesenchymal stem cell activity. Acta Biomater. 2018, 76, 116–125. [Google Scholar] [CrossRef]
- Pizzoni, A.; Pizzoni, P. Combination of glycosaminoglycans and cyclodextrins. U.S. Patent 9974803B2, 22 May 2018. [Google Scholar]
- Shi, X.; Li, W.; Liu, H.; Yin, D.; Zhao, J. β-Cyclodextrin induces the differentiation of resident cardiac stem cells to cardiomyocytes through autophagy. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2481–2487. [Google Scholar] [CrossRef]
- Macdonald, S.; Machatha, S.G. Contact Lens Solutions and Kits. U.S. Patent 2019054263W, 9 April 2020. [Google Scholar]
- Oku, K.; Kubota, M.; Fukuda, S.; Miyake, T. Accelerator for mineral absorption and use thereof. U.S. Patent Application 1652527A1, 9 July 2004. [Google Scholar]
- Hino, K.; Kurose, M.; Sakurai, T.; Inoue, S.; Oku, K.; Chaen, H.; Kohno, K.; Fukuda, S. Effect of Dietary Cyclic Nigerosylnigerose on Intestinal Immune Functions in Mice. Biosci. Biotechnol. Biochem. 2006, 70, 2481–2487. [Google Scholar] [CrossRef]
- Tsuruta, T.; Katsumata, E.; Mizote, A.; Jian, H.J.; Muhomah, T.A.; Nishino, N. Cyclic nigerosylnigerose ameliorates DSS-induced colitis with restoration of goblet cell number and increase in IgA reactivity against gut microbiota in mice. Biosci. Microbiota Food Health 2020, 39, 188–196. [Google Scholar] [CrossRef]
- Nakamura, S.; Kunikata, T.; Matsumoto, Y.; Hanaya, T.; Harashima, A.; Nishimoto, T.; Ushio, S. Effects of a non-cyclodextrin cyclic carbohydrate on mouse melanoma cells: Characterization of a new type of hypopigmenting sugar. PLoS ONE 2017, 12, e0186640. [Google Scholar] [CrossRef]
- López, C.A.; de Vries, A.H.; Marrink, S.J. Molecular Mechanism of Cyclodextrin Mediated Cholesterol Extraction. PLoS Comput. Biol. 2011, 7, e1002020. [Google Scholar] [CrossRef]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Guerrero-Rubio, M.A.; Caldera, F.; Cecone, C.; Trotta, F.; García-Carmona, F.; López-Nicolás, J.M. Lifespan extension in Caenorhabditis elegans by oxyresveratrol supplementation in hyper-branched cyclodextrin-based nanosponges. Int. J. Pharm. 2020, 589, 119862. [Google Scholar] [CrossRef] [PubMed]
- Rozema, D.; Gellman, S.H. Artificial chaperone-assisted refolding of carbonic anhydrase B. J. Biol. Chem. 1996, 271, 3478–3487. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matencio, A.; Caldera, F.; Cecone, C.; López-Nicolás, J.M.; Trotta, F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals 2020, 13, 281. https://doi.org/10.3390/ph13100281
Matencio A, Caldera F, Cecone C, López-Nicolás JM, Trotta F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals. 2020; 13(10):281. https://doi.org/10.3390/ph13100281
Chicago/Turabian StyleMatencio, Adrián, Fabrizio Caldera, Claudio Cecone, José Manuel López-Nicolás, and Francesco Trotta. 2020. "Cyclic Oligosaccharides as Active Drugs, an Updated Review" Pharmaceuticals 13, no. 10: 281. https://doi.org/10.3390/ph13100281
APA StyleMatencio, A., Caldera, F., Cecone, C., López-Nicolás, J. M., & Trotta, F. (2020). Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals, 13(10), 281. https://doi.org/10.3390/ph13100281