Citrus hystrix Extracts Protect Human Neuronal Cells against High Glucose-Induced Senescence
Abstract
1. Introduction
2. Results
2.1. Antioxidant Properties and Total Phenolic and Flavonoid Contents
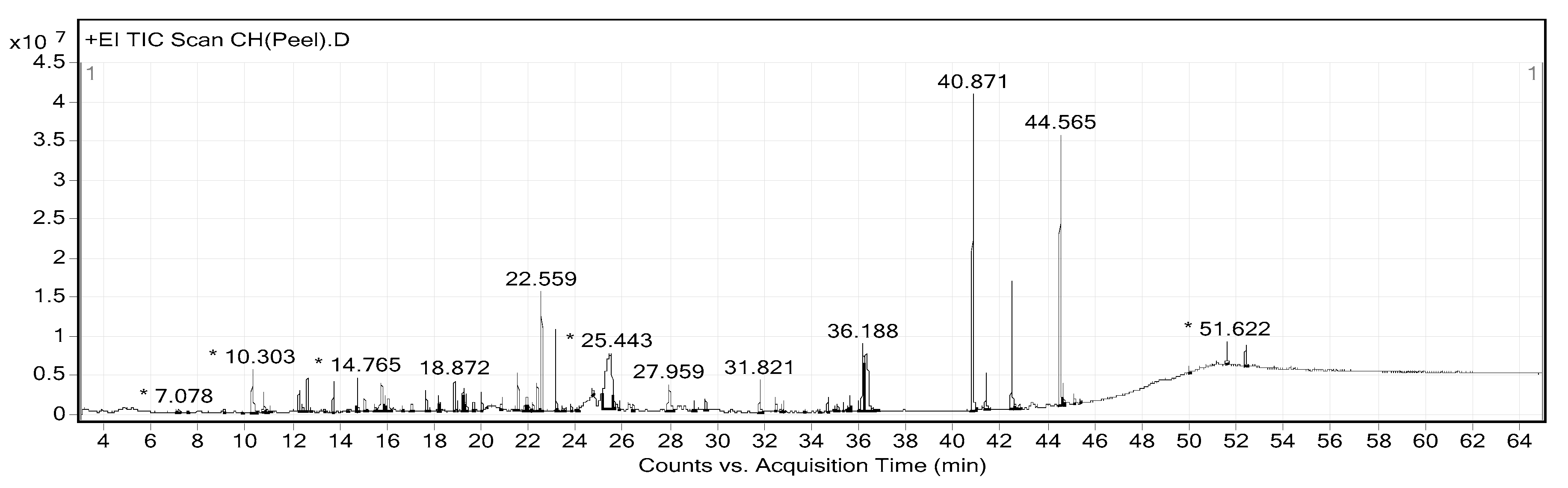
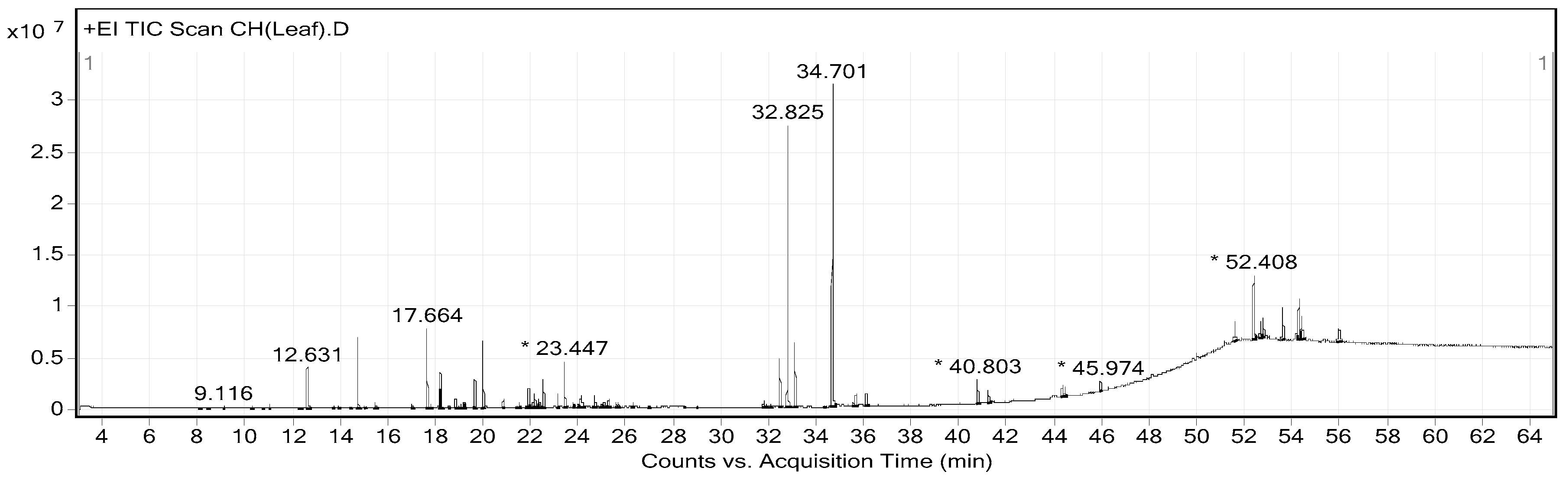
2.2. Phytochemical Constituents of CHP and CHL
2.3. Neuronal Senescent Model
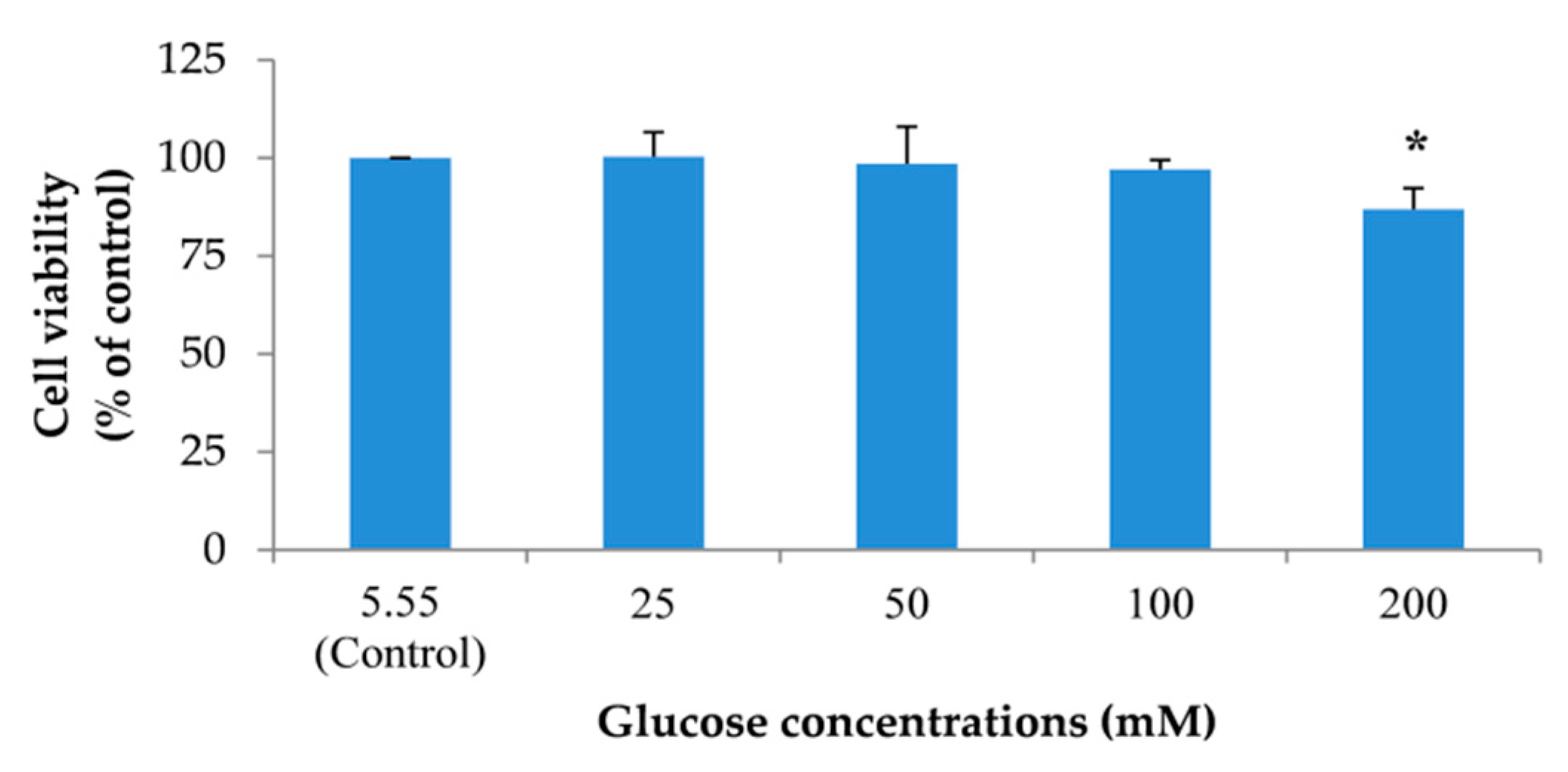
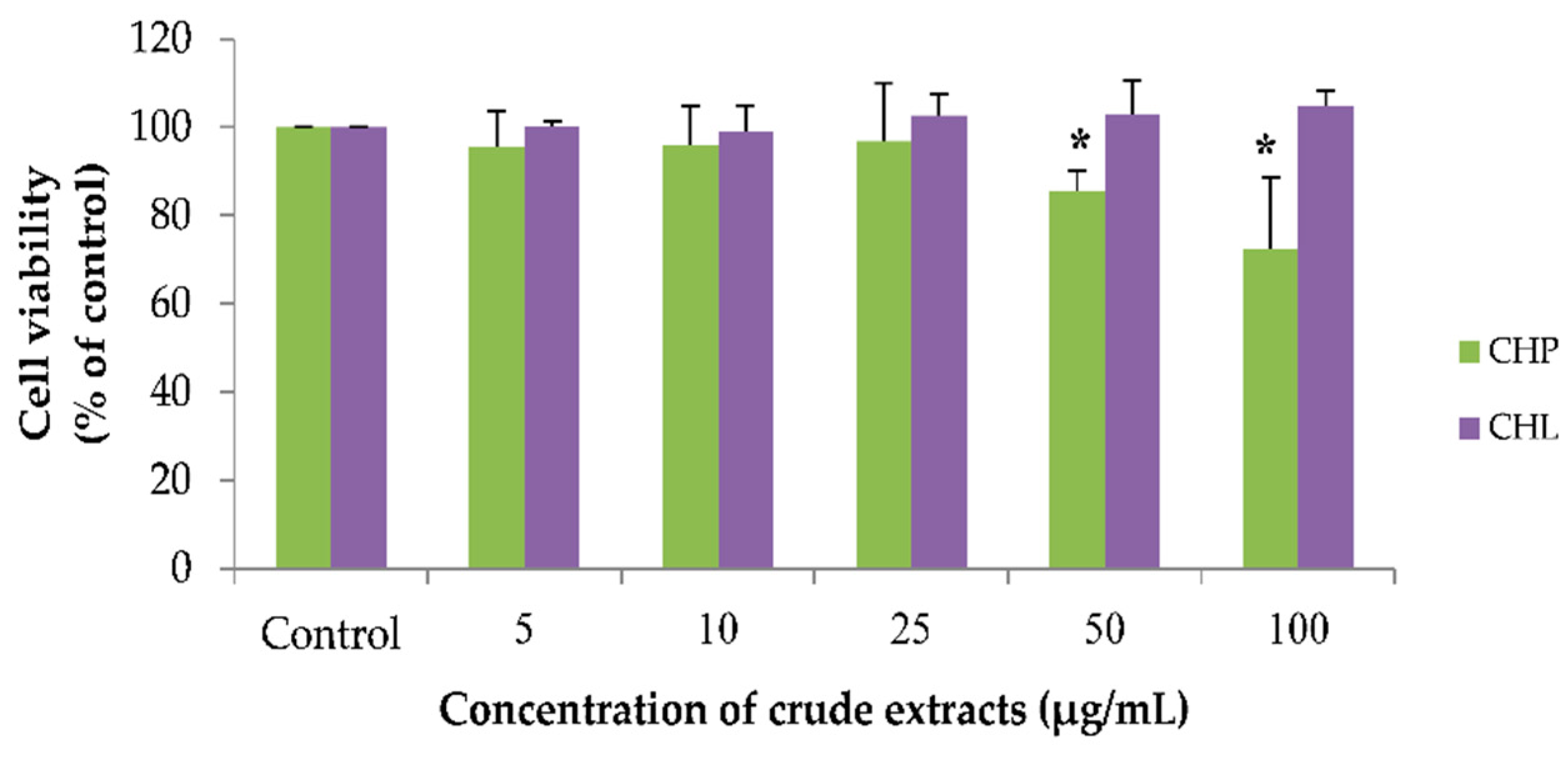
2.4. Effects of the Extracts on Cell Viability
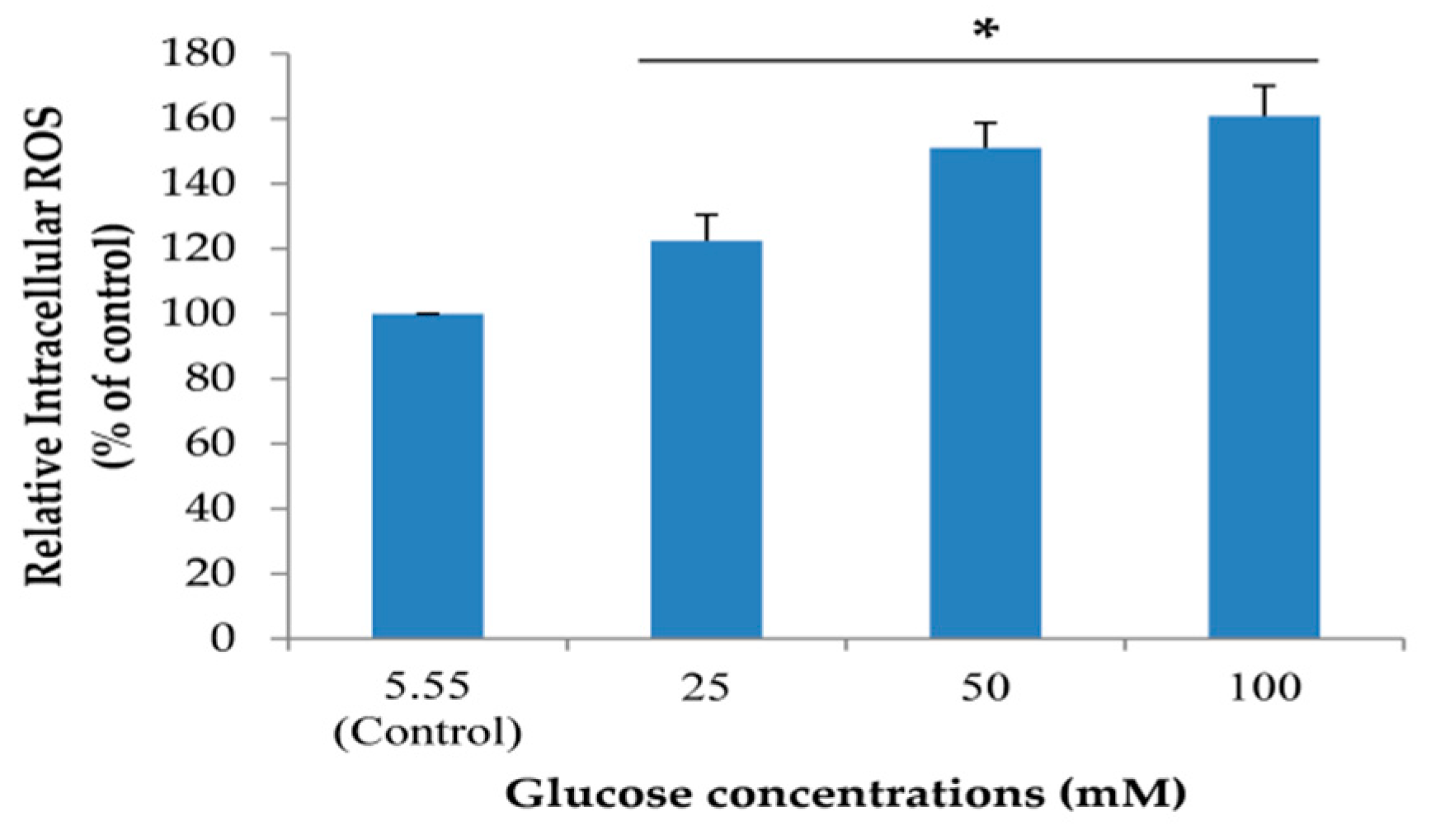
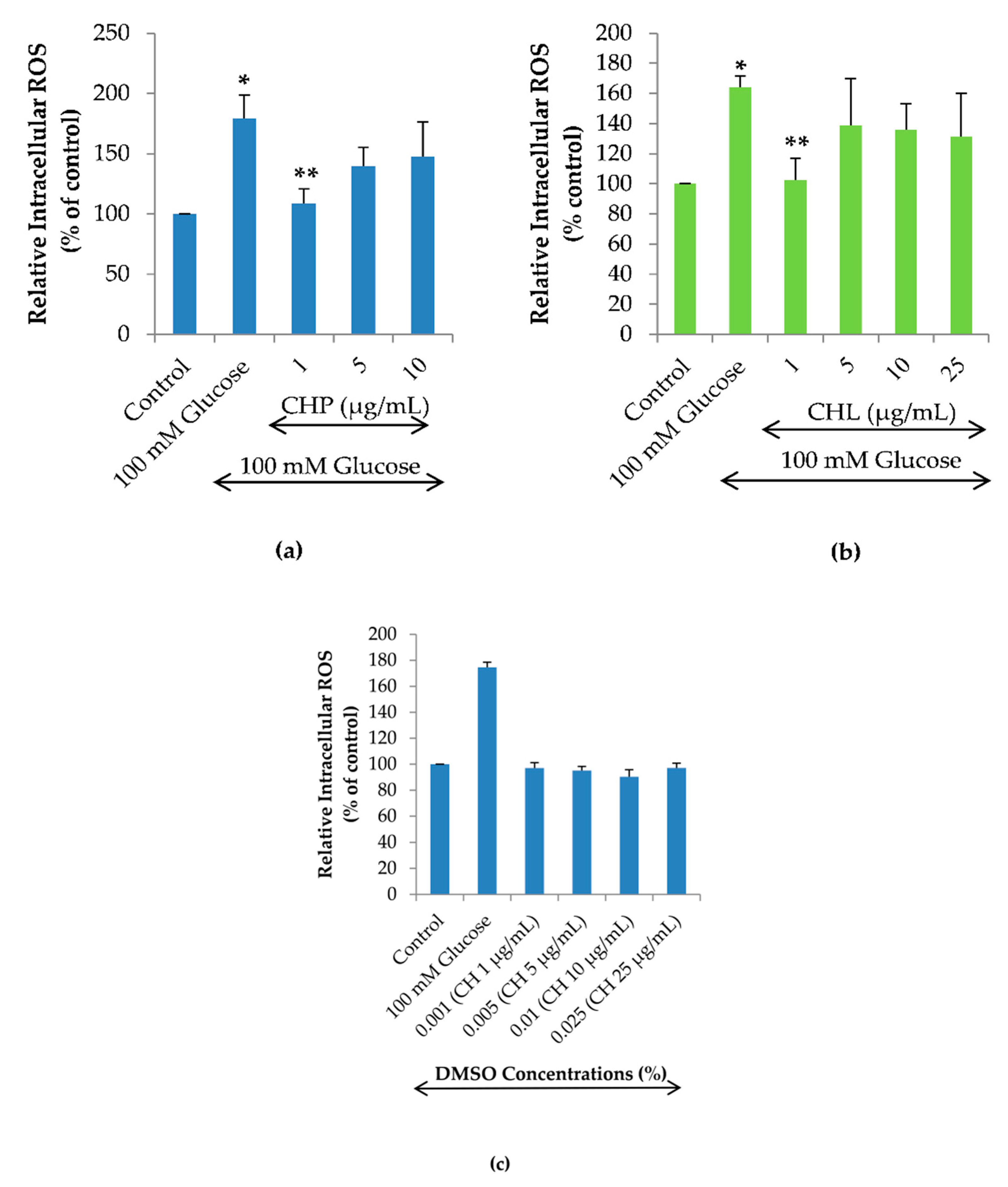
2.5. Effects of the Extracts on Intracellular ROS Reduction
2.6. Effects of the Extracts on Cell Cycle
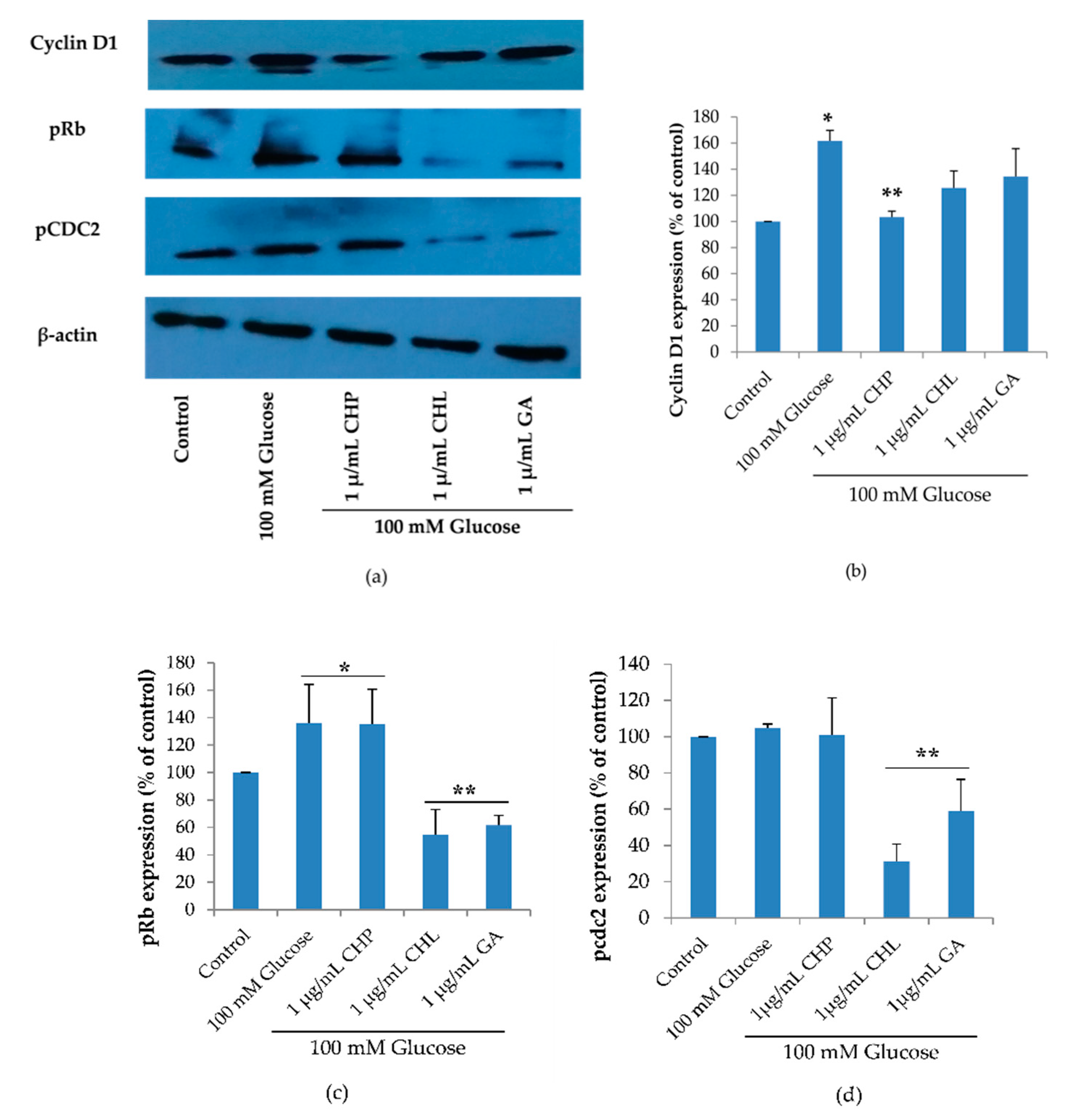
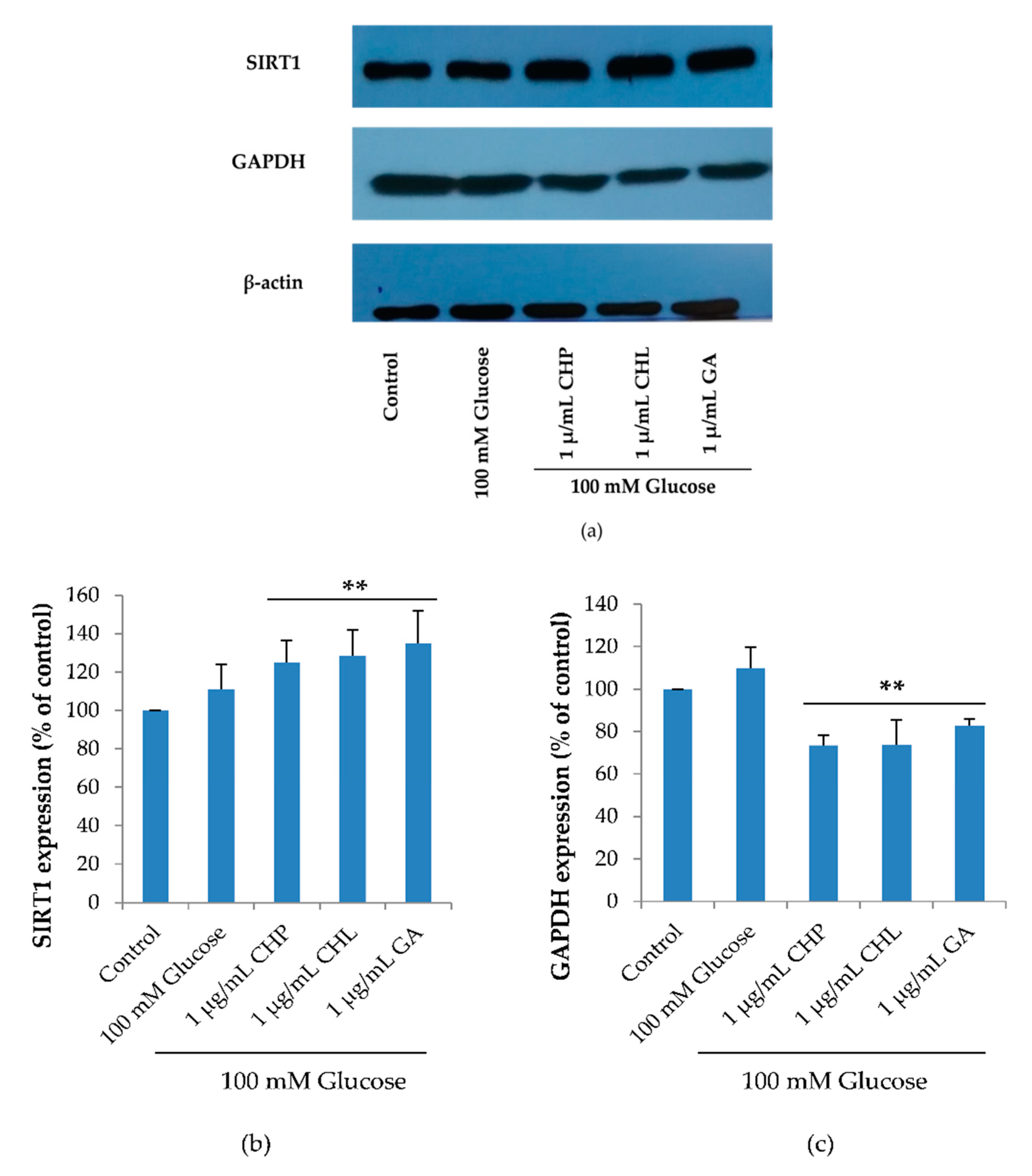
2.7. Effect of the Extracts on Cell Cycle-Associated Protein and SIRT1 Expression
3. Discussion
3.1. A Biochemical Perspective
- Therapeutic agents with high lipid solubility can penetrate cells more rapidly than therapeutic agents with water solubility. So, lipid or water solubility should be assessed.
- Most of the therapeutic agents are available as weak acids or weak bases. Thus the pH of therapeutic agents should be addressed appropriately.
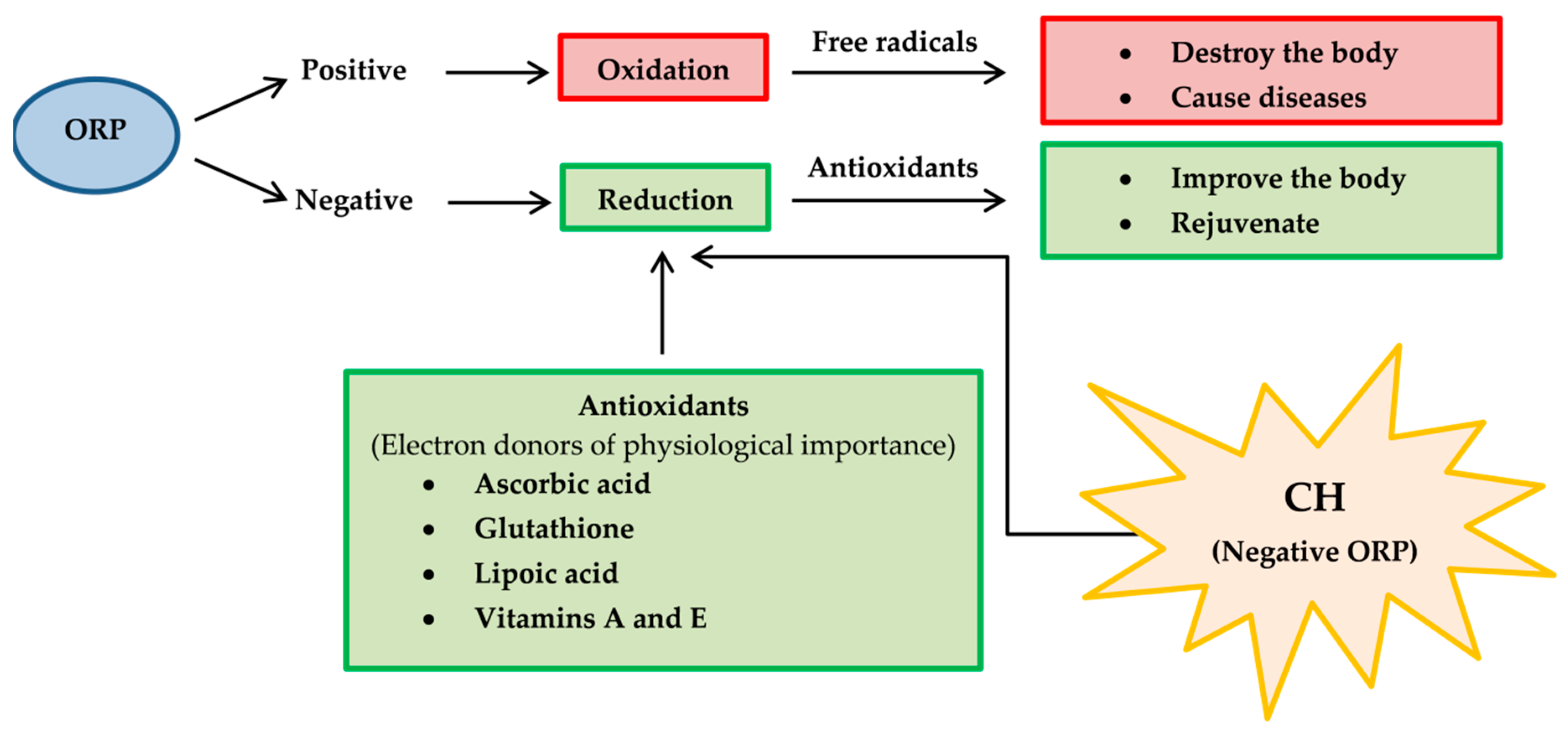
- Oxidation-reduction potential (ORP) [133], also known as redox, should be determined because it reflects a molecule’s ability to oxidize or reduce another molecule. Reducers have negative ORP and oxidizers have positive ORP. Typically, all organs in our body have negative OPR (−10 to −250 mV of ORP). Oxidizers can oxidize another molecule, which causes it to lose electrons. Oxidizers become free radicals after accepting radicals and cause many diseases. On the other hand, free radicals can be neutralized with reducers and antioxidants, causing body improvement and rejuvenation. For medicinal benefits, CH or their major active constituents should have negative ORP and play a role as a reducer or antioxidant. Therefore, the proposed application of redox reactions is useful for CH determination in pharmaceuticals (Figure 11).
- Oxygen partial pressure (PO2) [134]: PO2 is the force exerted by oxygen. In the human body (highly aerobic organism), oxygen plays a role in energy production. Therefore, oxygen supply at the tissue must match metabolic demand. PO2 is useful to maintain homeostasis (the balance between oxygen delivery and its consumption) within organ and tissue. Each organ and tissue has its PO2 requirements in order to function correctly. PO2 is useful in predicting oxygen and oxygen movement will move from a higher PO2 area to a lower PO2 area. Typically, PO2 in tissue is low because oxygen is used in cellular respiration. When PO2 in tissue increased by several factors such as stress, anesthesia, tumor and diabetes, oxygen availability is low or hypoxia. In consideration of CH’s pharmacology, the importance of hypoxia as below is concerned [135].
- Hypoxia may alter the therapeutic effectiveness and metabolism of CH.
- Hypoxia may alter cellular function.
- Hypoxia may potentiate or mitigate CH-induced toxicity.
- CH may potentiate or protect against hypoxia-induced pathology.
- CH may alter the relative coupling of blood flow and energy metabolism in an organ.
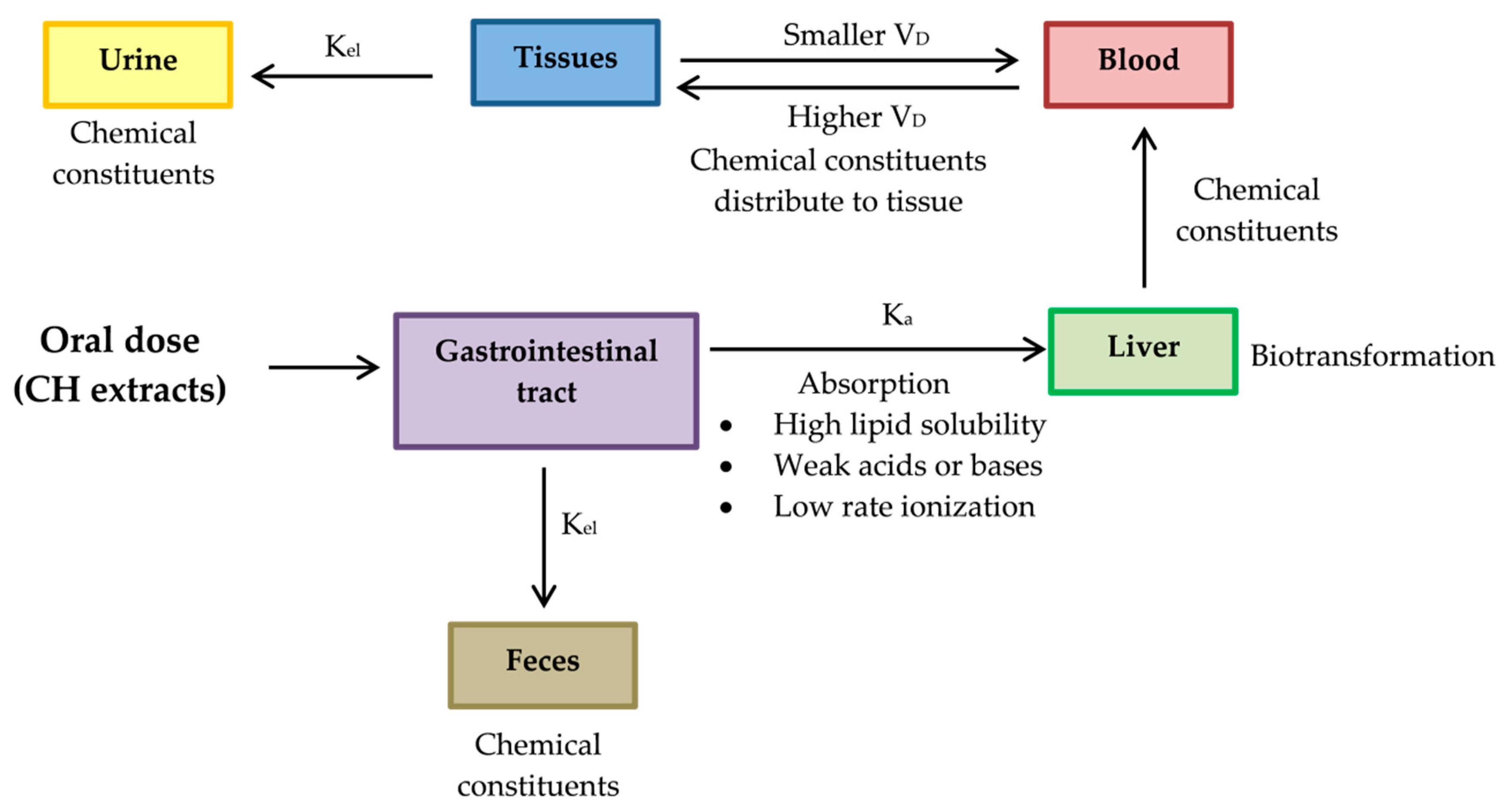
3.2. A Pharmacokinetic Perspective
- Absorption rate constant (Ka) should be determined for a chemical compound of CH. The compound investigated should have high Ka, so its characteristics should be high lipid solubility, weak acids or weak bases and low rate ionization.
- Constant elimination (Kel) is a value that describes the rate at which an active compound of CH is removed from the human system. Its value is affected by all processes such as distribution, biotransformation and excretion.
- Volume of distribution (VD) represents the distribution of a compound of CH in body tissues rather than the plasma. If VD is higher than the total body water, it indicates a greater amount of tissue distribution. A smaller VD means a compound remains in the plasma than CH distribution in tissues [137]. A compound studied for medicinal benefits should have higher VD, so it should be characterized by high lipid solubility, low rates of ionization and low plasma protein binding capabilities.
- Biotransformation represents the chemical alteration process of therapeutic agents in the body.
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Extraction
4.3. Gas Chromatograph–Mass Spectrometer (GC-MS) Analysis
4.4. Antioxidant Determination
4.4.1. Folin–Ciocalteu Phenol Assay (FCP)
4.4.2. Total Flavonoid of Determination
4.4.3. Radical Scavenging Activity Assays
4.5. Cell Line
4.6. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium (MTT) Assay
4.7. Reactive Oxygen Species (ROS) Assay
4.8. Cell Cycle Assay by Flow Cytometer
4.9. Protein Expression by Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Chen, Y.; Wang, C.Y.; Tang, Y.Y.; Huang, H.L.; Kang, X.; Li, X.; Xie, Y.R.; Tang, X.Q. Hydrogen sulfide inhibits high glucose-induced neuronal senescence by improving autophagic flux via up-regulation of SIRT1. Front. Mol. Neurosci. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Fan, F.; Liu, T.; Wang, X.; Ren, D.; Liu, H.; Zhang, P.; Wang, Z.; Liu, N.; Li, Q.; Tu, Y.; et al. ClC-3 expression and its association with hyperglycemia induced HT22 hippocampal neuronal cell apoptosis. J. Diabetes Res. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Sima, A.A.; Kamiya, H.; Li, Z.G. Insulin, C-peptide, hyperglycemia, and central nervous system complications in diabetes. Eur. J. Pharmacol. 2004, 490, 187–197. [Google Scholar] [CrossRef]
- Reno, C.M.; Tanoli, T.; Bree, A.; Daphna-Iken, R.; Cui, C.; Maloney, S.E.; Wozniak, D.F.; Fisher, S.J. Antecedent glycemic control reduces severe hypoglycemia-induced neuronal damage in diabetic rats. Am. J. Physiol. Metab. 2013, 304, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s Disease: Mechanisms and Nutritional Aspects. Clin. Nutr. Res. 2018, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ke, Z.; Gorbunova, V.; Seluanov, A. Replicatively senescent cells are arrested in G1 and G2 phases. Aging 2012, 4, 431–435. [Google Scholar] [CrossRef]
- Stein, G.H.; Dulic, V. Origins of G1 arrest in senescent human fibroblasts. BioEssays 1995, 17, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, C.; Giordano, A. RB and cell cycle progress. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.D.; Phillips, R.A.; Gallie, B.L. Cumulative Effect of Phosphorylation of pRB on Regulation of E2F Activity. Mol. Cell. Boil. 1999, 19, 3246–3256. [Google Scholar] [CrossRef]
- Draetta, G.; Luca, F.; Westendorf, J.; Brizuela, L.; Ruderman, J.; Beach, D. Cdc2 protein kinases complexed with both cyclin A and B: Vidence for proteolytic inactivation of MPF. Cell 1989, 56, 829–838. [Google Scholar] [CrossRef]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, P.F.; Fouladdel, S.; Ghaffari, S.M.; Amin, G.; Azizi, E. Induction of G1 cell cycle arrest and cyclin D1 down-regulation in response to pericarp extract of Baneh in human breast cancer T47D cells. DARU J. Pharm. Sci. 2012, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Masamha, C.P.; Benbrook, D.M. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009, 69, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Surtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lamichane, S.; Baek, S.H.; Kim, Y.J.; Park, J.H.; Lamichane, B.D.; Jang, W.B.; Ji, S.T.; Lee, N.K.; Dehua, L.; Kim, D.Y.; et al. MHY2233 attenuates replicative cellular senescence in human endothelial progenitor cells via SIRT1 signaling. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Vanhoutte, P.M. SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Lett. 2010, 585, 986–994. [Google Scholar] [CrossRef]
- Liu, T.F.; McCall, C.E. Deacetylation by SIRT1 reprograms inflammation and cancer. Genes Cancer 2013, 4, 135–147. [Google Scholar] [CrossRef]
- Hutadilok-Towatana, N.; Chaiyamutti, P.; Panthong, K.; Mahabusarakam, W.; Rukachaisirikul, V. Antioxidative and Free Radical Scavenging Activities of Some Plants Used in Thai Folk Medicine. Pharm. Biol. 2006, 44, 221–228. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarami, G.; Siddhuraju, P. The medicinal and nutritional role of underutilized citrus fruit Citrus hystrix (Kaffir lime): A review. Drug Invent. Today 2014, 6, 1–5. [Google Scholar]
- Irawaty, W.; Ayucitra, A. Assessment on antioxidant and in vitro antidiabetes activities of different fractions of Citrus hystrix peel. Int. Food Res. J. 2018, 6, 2467–2477. [Google Scholar]
- Abirami, A.; Nagarami, G.; Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci. Hum. Wellness 2014, 3, 16–25. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarami, G.; Siddhuraju, P. Hepatoprotective of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rat. Food Sci. Hum. Wellness 2015, 4, 35–41. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Aromdee, C.; Khunkitti, W. Antibacterial activity of citronella oil solid lipid particles in oleogel against Propionibacterium acnes and itschemical stability. Int. J. Essent. Oil Ther. 2008, 2, 167–171. [Google Scholar]
- Pattnaik, S.; Vemulpad, S.; Kole, C. Antibacterial and Antifungal activity of ten essential oils in vitro. Microbios 1996, 86, 237–246. [Google Scholar] [PubMed]
- Jantamas, S.; Matan, N.; Aewsiri, T. Improvement of antifungal activity of Citronella oil against Aspergillus flavus on rubberwood (Hevea Brasiliensis) using heat curing. J. Trop. For. Sci. 2016, 28, 39–47. [Google Scholar]
- Kandimalla, R.; Kalita, S.; Choudhury, B.; Dash, S.; Kalita, K.; Kotoky, J. Chemical Composition and Anti-Candidiasis Mediated Wound Healing Property of Cymbopogon nardus Essential Oil on Chronic Diabetic Wounds. Front. Pharmacol. 2016, 7, 1–8. [Google Scholar]
- Batubara, I.; Suparto, I.H.; Sa’diah, S.; Matsuoka, R.; Mitsunaga, T. Effects of Inhaled Citronella oil and Related Compounds on Rat Body Weight and Brown Adipose Tissue Sympathetic Nerve. Nutrients 2015, 7, 1859–1870. [Google Scholar] [CrossRef]
- Wasito, W.; Noorhamdani, N.; Sukardi, S.; Suratmo, S. Assessment of antioxidant activity of citronellal extract and fractions of essential oils of Citrus hystrix DC. Trop. J. Pharm. Res. 2018, 17, 1119–1125. [Google Scholar] [CrossRef]
- Santos, P.L.; Matos, J.P.S.C.F.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J. Citronellol, a monoterpene alcohol with promising pharmacological activities—A systematic review. Food Chem. Toxicol. 2018, 123, 459–469. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Goncalves, J.C.R.; Quintans-Junior, L.; Cruz, J.S.; Araujo, D.A.M.; de Almeida, R.N. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci. Lett. 2006, 401, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.F.; Moreira, I.J.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; de Sousa, D.P.; Santos, M.R. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 2009, 106, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Matyas, C.; Erdelyi, K.; Cinar, R.; Nieri, D.; Chicca, A.; Nemeth, B.T.; Paloczi, J.; Lajtos, T.; Corey, L.; et al. β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2018, 175, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. Beta-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.N.; de Almeida, A.; da Silva Oliveira, J.; dos Santos, P.; de Sousa, D.; de Freitas, R. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef]
- Hada, T.; Shiraishi, A.; Furuse, S.; Inoue, Y.; Hamashima, H.; Matsumoto, Y.; Masuda, K.; Shiojima, K.; Shimada, J. Inhibitory effects of terpenes on the growth of Staphylococcus aureus. Nat. Med. 2003, 57, 64–67. [Google Scholar]
- Lee, K.; Lee, J.H.; Kim, S.I.; Cho, M.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef]
- Curvelo, J.A.R.; Marques, A.M.; Barreto, A.L.S.; Romanos, M.T.V.; Portela, M.B.; Kaplan, M.A.C.; Soares, R.M.A. A novel nerolidol-rich essential oil from Piper claussenianum modulates Candida albicans biofilm. J. Med. Microbiol. 2014, 63, 697–702. [Google Scholar] [CrossRef]
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choi, I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Renan, O.S.; Sousa, F.B.M.; Damasceno, S.R.B.; Carvalho, N.S.; Silva, V.G.; Oliviera, F.R.; Sousa, D.P.; Aragao, K.S.; Barbosa, A.L.R.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 4, 455–464. [Google Scholar]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- Plat, J.; Mensink, R.P. Effects of plant sterols and stanols on lipid metabolism and cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 31–40. [Google Scholar]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, W.; Zhang, J.J.; Qu, S.S.; Wang, L.Y. Determination of β-Sitosterol and Total Sterols Content and Antioxidant Activity of Oil in Acai (Euterpe Oleracea). China J. Chin. Mater. Med. 2014, 39, 4620–4624. [Google Scholar]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Brimson, J.M.; Brimson, S.J.; Brimson, C.A.; Rakkhitawatthana, V.; Tencomnao, T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-β neurotoxicity in HT-22 mouse hippocampal cells: Possible active compounds include lupeol, stigmasterol and β-sitosterol. Int J. Mol. Sci. 2012, 13, 5074–5097. [Google Scholar] [CrossRef]
- Sabino, C.K.; Ferreria-Filho, E.S.; Mendes, M.B.; Da SilvaFilho, J.C. Cardiovascular effects induced by α-terpineol in hypertensive rats. Flavour Frag. J. 2013, 28, 333–339. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An update review. Flavour Frag. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar]
- De Sousa, G.M.; Cazarin, C.B.B.; Junior, M.R.M.; Lamas, C.A.; Quitete, V.H.A.C.; Pastore, G.M.; Bicas, J.L. The effect of α-terpineol enantiomers on biomarkers of rats fed a high-fat diet. Heliyon 2020, 6, e03752. [Google Scholar] [CrossRef]
- Srisukh, V.; Tribuddharat, C.; Nukoolkarn, V.; Bunyapraphatsara, N.; Chokephaibulkit, K.; Phoomniyom, S.; Chaunphung, S.; Srifuengfung, S. Antibacterial activity of essential oils from Citrus hystrix (makrut lime) against respiratory tract pathogens. Science 2012, 38, 212–217. [Google Scholar]
- Chaikul, P.; Khat-Udomkiri, N.; Iangthanarat, K.; Manosroi, J.; Manosroi, A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB noisome. Eur. J. Pharm. Sci. 2019, 131, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblasts and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [PubMed]
- Li, L.; Ng, T.B.; Gao, W.; Li, W.; Fu, M.; Niu, S.M.; Zhao, L.; Chen, R.R.; Liu, F. Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice. Life Sci. 2005, 77, 230–240. [Google Scholar]
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Effect of gallic acid on dementia type of Alzheimer disease in rats: Electrophysiological and Histological studies. Basic Clin. Neurosci. 2015, 7, 97–106. [Google Scholar]
- Patel, S.S.; Goyal, R.K. Cardioprotective effects of gallic acid indiabetes-induced myocardial dysfunction in rats. Pharmacogn. Res. 2011, 3, 239–245. [Google Scholar]
- Elahi, E.; Ashfaq, A.; Mustafa, S. Impact of gallic acid on oxidative stress in diabetes. PJMHS 2018, 12, 80–82. [Google Scholar]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar]
- Saeki, Y.; Shiohara, M. Physiological effects of inhaling fragnances. Int. J. Aromather. 2001, 11, 118–125. [Google Scholar] [CrossRef]
- Sayowan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The Harmonizing Effects of Citronella Oil on Mood States and Brain Activities. J. Health Res. 2012, 26, 69–75. [Google Scholar]
- Brito, R.G.; Guimaraes, A.G.; Quintans, J.S.; Santos, M.R.V.; De Sousa, D.P.; Badaue-Passos, D., Jr.; de Lucca, W., Jr.; Brito, F.A.; Barreto, E.O.; Oliviera, A.P.; et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activites in rodents. J. Nat. Med. 2012, 66, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, J.; Rossano, M.; Touraud, D.; Obermayr, U.; Geier, M.; Rose, A.; Kunz, W. Green synthesis of para-Menthan-3,8-diol from Eucalyptus citriodora: Application for repellent products. C. R. Chim. 2011, 14, 629–635. [Google Scholar] [CrossRef]
- Carroll, S.P.; Loye, J. PMD, a Registered Botanical Mosquito Repellent with Deet-Like Efficacy. J. Am. Mosq. Control Assoc. 2006, 22, 507–514. [Google Scholar] [CrossRef]
- Chang, H.J.; Kim, J.M.; Lee, J.C.; Kim, W.K.; Chun, H.S. Protective effect of beta-caryophyllene, a natural bicyclic sesquiterpene, against cerebral ischemic injury. J. Med. Food 2013, 16, 471–480. [Google Scholar] [CrossRef]
- Viveros-Paredes, J.M.; Gonzalez-Castaneda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; Lopez-Roa, R.I.; Vazquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of beta-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. Beta-Caryophyllene protects the C6 glioma cells against glutamate-induced excitotoxicity through the Nrf2 pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-gamma receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.; Vieira, J.L.; Raposo, N.R.; Dutra, R.C. (-)-beta-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.A.; Jeong, T.S.; Park, D.S.; Xu, M.Z.; Oh, H.W.; Song, K.B.; Lee, W.S.; Park, H.Y. Antioxidant effects of quinolone alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol. Pharm. Bull. 2006, 29, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.K.; Kim, H.K.; Harris, K.; Kim, C.J.; Park, G.G.; Park, C.S.; Shin, D.H. 2,4-Di-tert-butylphenol from potato protects against oxidative stress in PC12 cells and mice. J. Med. Food 2013, 16, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.V.R.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-inflammatory and anticancer activities of erythrodiol-3-acetate and 2,4-di-tert-butylphenol isolated from Humboldtia unijuga. Nat. Prod. Res. 2018, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butyphenol and Its Analogs. Toxins 2019, 12, 35. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, S.Y.; Chen, C.T. Increasing antioxidant activity and reducing decay of blueberries by essential oils. J. Agric. Food Chem. 2008, 56, 3587–3592. [Google Scholar] [CrossRef]
- Pinheiro, B.G.; Silva, A.S.B.; Souza, G.E.P.; Figueiredo, J.G.; Cunha, F.Q.; Lahlou, S.; da Silva, J.K.R.; Maia, J.G.S.; Sousa, P.J.C. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (SW.) Loud. J. Ethnopharmacol. 2011, 138, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Fonsêca, D.V.; Salgado, P.R.R.; de Carvalho, F.L.; Salvadori, M.G.S.S.; Penha, A.R.S.; Leite, F.C.; Borges, C.J.S.; Piuvezam, M.R.; Pordeus, L.C.d.M.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2015, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Arbale, V.A.; Kamble, G.S.; Khatiwora, E.; Ghayal, N. Antioxidant capacity of leaves and stem of Ehretia laevis. Int. J. Pharm. 2011, 3, 149–151. [Google Scholar]
- Tyagi, T.; Agarwal, M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (mart.) solms. J. Pharmacogn. Phytochem. 2017, 6, 195–206. [Google Scholar]
- Syad, N.A.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective Effect of the Marine Macroalga Gelidiella Acerosa: Identification of Active Compounds Through Bioactivity-Guided Fractionation. Pharm Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef]
- García-Argáez, A.N.; Apan, T.O.R.; Delgado, H.P.; Velázquez, G.; Martínez-Vázquez, M. Anti-inflammatory Activity of Coumarins from Decatropis Bicolor on TPA Ear Mice Model. Planta Med. 2000, 66, 279–281. [Google Scholar] [CrossRef]
- Zahri, S.; Razavi, S.M.; Moatamed, Z. Antioxidant activity and cytotoxic effect of aviprin and aviprin-3”-O-D-glucopyranoside on LNCaP and HeLa cell lines. Nat. Prod. Res. 2012, 26, 540–547. [Google Scholar] [CrossRef]
- Kang, T.J.; Lee, S.Y.; Singh, R.P.; Agarwal, R.; Yim, D.S. Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells. Acta Oncol. 2009, 48, 895–900. [Google Scholar] [CrossRef]
- Altameme, H.J.; Hameed, I.H.; Idan, S.A. Artemisia annua: Biochemical products analysis of methanolic aerial parts extract and anti-microbial capacity. RJPBCS 2016, 7, 1843–1868. [Google Scholar]
- Costa, E.V.; Dutra, L.M.; De Jesus, H.C.R.; Nogueira, P.C.D.L.; Moraes, V.R.D.S.; Salvador, M.J.; Cavalcanti, S.C.D.H.; Santos, R.L.C.D.; Prata, A.P.D.N. Chemical composition and antioxidant, antimicrobial, and larvicidal activities of the essential oils of Annona salzmannii and A. pickelii (Annonaceae). Nat. Prod. Commun. 2011, 6, 907–912. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Tatar, A.; Geyikoglu, F. Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biologia 2014, 69, 936–942. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Ahluwalia, V.; Kaur, C. Antioxidant potential of essential oil and cadinene sesquiterpenes of Eupatorium adenophorum. Toxicol. Environ. Chem. 2013, 95, 127–137. [Google Scholar] [CrossRef]
- Ramesh, B.; Pugalendi, K.V. Antihyperlipidemic and antidiabetic effects of umbelliferone in streptozotocin diabetic rats. Yale J. Biol. Med. 2005, 78, 189–196. [Google Scholar]
- Ramu, R.; Shirahatti, P.S.; Nanjunda, S.S.; Zameer, F.; Dhananjaya, B.L.; Nagendra, P.M.N. Correction: Assessment of In vivo Antidiabetic Properties of Umbelliferone and Lupeol Constituents of Banana (Musa sp. Var. Nanjangud Rasa Bale) Flower in Hyperglycaemic Rodent Model. PLoS ONE 2016, 11, e0151135. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.O.; Lee, H.I.; Ham, R.; Seo, K.I.; Kim, M.J.; Lee, M.K. Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats. Nutr. Res. Pract. 2015, 9, 364–369. [Google Scholar] [CrossRef]
- Sigh, R.; Sigh, B.; Sigh, S.; Kumar, N. Umbelliferone—An antioxidant isolated from Acacia nilotica (L.) Willd. Ex. Del. Food Chem. 2009, 120, 825–830. [Google Scholar]
- Seong, S.H.; Ali, M.Y.; Jung, H.A.; Choi, J.S. Umbelliferone derivatives exert neuroprotective effects by inhibiting monoamine oxidase A, self-amyloidβ aggregation, and lipid peroxidation. Bioorganic Chem. 2019, 92, 103293. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Ellis, E.M. Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 453–461. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Z.; Li, H.; Cang, Z.; Yin, K.; Wu, M.; Luo, S. Neuroprotective effect of Umbelliferone against Cerebral ischemia/Reperfusion induced neurological deficits: In-vivo and in-silico studies. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Ҁakmak, Y.S.; Aktumsek, A.; Duran, A. Studies on antioxidant activity, volatile compound and fatty acid composition of different parts of Glycyrrhiza echinata L. EXCLI J. 2012, 11, 178–187. [Google Scholar]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative Activity of Natural Products from Plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [PubMed]
- He, W.; Chen, W.; Zhou, Y.; Tian, Y.; Liao, F. Xanthotoxol exerts neuroprotective effect via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Cell Mol. Neurobiol. 2013, 33, 715–722. [Google Scholar] [PubMed]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary In Vitro and In Vivo Evaluation of Antidiabetic Activity of Ducrosia anethifolia boiss. and Its Linear Furanocoumarins. BioMed Res. Int. 2014. [Google Scholar] [CrossRef]
- Doungsaard, P.; Chansakaow, S.; Sirithunyalug, J.; Shang-Chian, L.; Wei-Chao, L.; Chia-Hua, L.; Kuan-Ha, L.; Leelapornpisid, P. In vitro Biological Activities of the Anti-aging Potential of Dimocarpus longan Leaf Extracts. CMU J. Nat. Sci. 2020, 19, 235–251. [Google Scholar]
- Cascale, H.S.; Mullers, E.; Lindqvist, A. How the cell cycle enforce senescence. Aging 2017, 9, 2022–2023. [Google Scholar]
- Mombach, J.C.M.; Bug, C.A.; Chaouiya, C. Modelling the onset of senescence at the G1/S cell cycle checkpoint. BMC Genom. 2014, 15, 1–11. [Google Scholar]
- Gire, V.; Dulic’, V. Senecence from G2 arrest, revisited. Cell Cycle 2014, 14, 297–304. [Google Scholar]
- Zhu, Y.; Armstrong, J.L.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr. Opin. Clin. 2014, 17, 324–328. [Google Scholar]
- Campisi, J. Cancer, Aging and Cellular Senescence. In Vivo 2000, 14, 183–188. [Google Scholar] [PubMed]
- Kumar, M.; Seeger, W.; Voswinckel, R. Senescence-associated Secretory Phenotype and Its Possicle Role in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2014, 51, 323–333. [Google Scholar] [PubMed]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Tateno, K.; Kunieda, T.; Komuro, I. Vascular Cell Senescence and Vascular Aging. J. Mol. Cell Cardiol. 2004, 36, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Umran, N.S.S.; Mohamed, S.; Lau, S.F.; Ishak, N.I.M. Citrus hystrix leaf extract attenuated diabetic-cataract in STZ-rats. J. Food Biochem. 2020, 44, e13258. [Google Scholar] [PubMed]
- Santiago, L.A.; Mayor, A.B. Prooxidant Effect of the Crude Ethanolic Leaf Extract of Ficus odorata Blanco Merr. In Vitro: Its Medical Significance. Int. J. Biotechnol. Bioeng. 2014, 8, 53–60. [Google Scholar]
- Nurse, P. Checkpoint Pathways Come of Age. Cell 1997, 91, 865–867. [Google Scholar] [CrossRef]
- Ezhevsky, S.A.; Ho, A.; Becker-Hapak, M.; Davis, P.K.; Dowdy, S.F. Differential Regulation of Retinoblastoma Tumor Suppressor Protein by G1 Cyclin-Dependent Kinae Complexes in Vivo. Mol. Cell. Biol. 2001, 21, 4773–4784. [Google Scholar]
- Ezhevsky, S.A.; Nagahara, H.; Vocero-Akbani, A.M.; Gius, D.R.; Wei, M.C.; Dowdy, S.F. Hypo-phophorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc. Natl. Acad. Sci. USA 1997, 94, 10699–10704. [Google Scholar]
- Harbour, J.W.; Dean, D.C. The Rb/E2F pathway: Expanding roles and emerging paradigms. Gene Dev. 2000, 14, 2393–2409. [Google Scholar] [CrossRef]
- He, G.; Thuillier, P.; Fischer, S.M. Troglitazone Inhibits Cyclin D1 Expression and Cell Cycling Independently of PPARγ in Normal Mouse Skin Keratinocytes. J. Investig. Dermatol. 2004, 123, 1110–1119. [Google Scholar] [CrossRef]
- Hosooka, T.; Ogawa, W. A novel role for the cell cycle regulatory complex cyclin D1-CDK4 in gluconeogenesis. J. Diabetes Investig. 2015, 7, 27–28. [Google Scholar] [CrossRef]
- Chang, C.; Su, H.; Zhang, D.; Wang, Y.; Shen, Q.; Liu, B.; Huang, R.; Zhou, T.; Peng, C.; Wong, C.C.; et al. AMPK-Dependent Phosphorylation of GAPDH Triggers Sirt1 Activation and Is Necessary for Autophagy upon Glucose Starvation. Mol. Cell 2015, 60, 930–940. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Gao, W.; Huang, S.; Liu, Z.; Li, W.; Jia, L. SIRT1 is downregulated in gastric cancer and leads to G1-phase arrest via NF-kB/Cyclin D1 signaling. Mol. Cancer Res. 2013, 11, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, M.T.; Jia, Y.Y.; Liu, J.J.; Wang, Q.; Tian, Z.; Liu, Y.T. Regulation of Cell Cycle Regulators by SIRT1 Contributes to Resveratrol-Mediated Prevention of Pulmonary Arterial Hypertension. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, E.S.; Osagie, A.U. Effect of Jatropha tanjoresis leaves supplement on activities of some antioxidant enzymes, vitamins and lipid peroxidation in rat. J. Food Biochem. 2011, 35, 409–424. [Google Scholar] [CrossRef]
- Omoregie, E.S.; Osagie, A.U. Phytochemical screening and anti-anaemia effect of Jatropha tanjoresis leaf in protein malnourished rats. Plant. Arch. 2007, 7, 509–516. [Google Scholar]
- Shafaquat, N.; Syed, T.; Showkat, A.G. Glutathione-S-transferase, Superoxide Dismutase (GST, SOD) levels, Protein content and lipid Perioxidation in Schizothorax plagiostomus under the infection of pomphorhynchus in Nallah Sukhnag of Kashmir Valley. Pakistan. J. Biol. Sci. 2017, 20, 442–446. [Google Scholar]
- AshokKumar, T. Antioxidants: New-generation therapeutic base for treatment of polygenic disorders. Curr. Sci. 2004, 86, 496–504. [Google Scholar]
- Jonas, C.R.; Puckett, A.B.; Jones, D.P.; Griffith, D.P.; Szeszycki, E.E.; Bergman, G.F.; Furr, C.E.; Tyre, C.; Carlson, J.L.; Galloway, J.R.; et al. Plasma antioxidant status after high-dose chemotherapy a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am. J. Clin. Nutr. 2000, 72, 181–189. [Google Scholar] [CrossRef]
- Bahrami, S.; Jalali, M.H.; Jafari, A. Evaluation of hepatic antioxidant changes in ovine discrocoliosis. J. Parasit. Dis. 2015, 39, 766–769. [Google Scholar]
- Deger, S.; Deger, Y.; Ertekin, A.; Gul, K.; Ozdal, N. Determination of the status of lipid perioxidation and antioxidant in Cattle infected with Dictyocaulus viviparous. Turk. Parasitol. 2008, 32, 234–237. [Google Scholar]
- Siwela, A.H.; Motsi, L.R.; Dube, S. Alternation of some hepatic enzyme activities by gastrointestinal helminth parasite in domesticated ostrishes. Adv. Biores. 2013, 4, 145–150. [Google Scholar]
- Pranitha, A.; Lakshmi, P.K. Effect of pH on weakly acidic and basic model drugs and determination of their ex vivo transdermal permeation routes. Braz. J. Pharm. Sci. 2018, 54, e00070. [Google Scholar]
- Persson, L.C.; Porter, C.J.H.; Charman, W.N.; Bergström, C.A.S. Computational Prediction of Drug Solubility in Lipid Based Formulation Excipients. Pharm. Res. 2013, 30, 3225–3237. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Donovan, L.; Welford, S.M.; Haaga, J.; LaManna, J.; Strohl, K.P. Hypoxia-implications for pharmaceutical developments. Sleep Breath. 2010, 14, 291–298. [Google Scholar] [CrossRef]
- Hussain, K.; Ismail, Z.; Sadikun, A.; Ibrahim, P. Bioactive Markers Based Pharmacokinetic Evaluation of Extracts of a Traditional Medicinal Plant, Piper sarmentosum. Evid.-Based Complement. Altern. Med. 2011, 980760, 1–7. [Google Scholar] [CrossRef]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Volume of Distribution in Drug Design. J. Med. Chem. 2015, 58, 5691–5698. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, G.; Tang, Y.; Cao, D.; Qi, T.; Qi, Y.; Fan, G. Physicochemical characterization and pharmacokinetics evaluation of β-caryophyllene/β-cyclodextrin inclusion complex. Int. J. Pharm. 2013, 450, 304–310. [Google Scholar] [CrossRef]

 ); Low expression of proteins (
); Low expression of proteins ( ); Hyperphosphorylation of proteins (
); Hyperphosphorylation of proteins (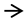 ); Hypophosphorylation of proteins (
); Hypophosphorylation of proteins ( ).
).
 ); Low expression of proteins (
); Low expression of proteins ( ); Hyperphosphorylation of proteins (
); Hyperphosphorylation of proteins (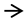 ); Hypophosphorylation of proteins (
); Hypophosphorylation of proteins ( ).
).
| Sample | % Radical Scavenging Activity (of 1 mg/mL Extract) | mg VCEAC/g Dry Weight Sample |
|---|---|---|
| CHP | 14.98 ± 5.25 | 210.06 ± 11.95 |
| CHL | 15.36 ± 6.79 | 238.89 ± 12.25 |
| Sample | % Radical Scavenging Activity (of 1 mg/mL extract) | mg VCEAC/g Dry Weight Sample |
|---|---|---|
| CHP | 90.38 ± 0.11 | 3063.67 ± 3.71 |
| CHL | 65.18 ± 0.33 | 2219.99 ± 11.12 |
| Sample | Total Phenolic (mg(GA)/g of Dry Weight) | Total Flavonoid (mg(QE)/g of Dry Weight) |
|---|---|---|
| CHP | 1796.55 ± 1.38 | 1521.54 ± 3.54 |
| CHL | 2134.48 ± 1.06 | 2856.15 ± 1.24 |
| Peak No. | RT | Area (%) | MF | MW | Name of Compound |
|---|---|---|---|---|---|
| 12 | 12.635 | 0.9 | C10H18O | 154 | Citronellal |
| 15 | 13.735 | 0.87 | C10H18O | 154 | α-Terpineol |
| 18 | 14.765 | 1.01 | C10H20O | 156 | Citronellol |
| 23 | 16.061 | 1.08 | C8H14O3 | 158 | Methyl 6-oxoheptanoate |
| 30 | 18.872 | 0.83 | C15H24 | 204 | α-Copaene |
| 36 | 20.018 | 0.58 | C15H24 | 204 | Caryophyllene |
| 40 | 21.559 | 1.13 | C15H24 | 204 | β-Cubebene |
| 46 | 22.558 | 3.54 | C15H24 | 204 | Cadinene |
| 44 | 22.2 | 0.24 | C14H22O | 206 | Phenol, 2,4-bis(1,1-dimethylethyl)- |
| 61 | 29.504 | 0.7 | C9H6O3 | 162 | 7-Hydroxycoumarin |
| 62 | 31.821 | 1.23 | C16H32O2 | 256 | n-Hexadecanoic acid |
| 63 | 32.495 | 0.39 | C18H36O2 | 284 | Hexadecanoic acid, ethyl ester |
| 68 | 34.698 | 0.41 | C20H40O | 296 | Phytol |
| 76 | 36.32 | 6.86 | C11H6O4 | 202 | 7H-Furo(3,2-g)(1)benzopyran-7-one, 9-hydroxy- |
| 77 | 40.871 | 14.86 | C16H14O5 | 286 | 7H-Furo(3,2-g)(1)benzopyran-7-one, 4-(2,3-epoxy-3-methylbutoxy)-, (S)-(−)- |
| 78 | 41.405 | 1.37 | C16H14O5 | 286 | 4-(3-Methyl-2-oxobutoxy)-7H-furo(3,2-g)(1)benzopyran-7-one |
| 82 | 44.565 | 12.4 | C16H16O6 | 304 | 4-(2,3-Dihydroxy-3-methylbutoxy)furo(3,2-g)chromen-7-one |
| 88 | 52.412 | 0.81 | C29H50O | 414 | Sitosterol |
| Peak No. | RT | Area (%) | MF | MW | Name of Compound |
|---|---|---|---|---|---|
| 9 | 12.631 | 2.35 | C10H18O | 154 | Citronellal |
| 13 | 14.758 | 3.53 | C10H20O | 156 | Citronellol |
| 17 | 17.664 | 4.24 | C10H20O2 | 172 | Cyclohexanol, 2-(2-hydroxy-2-propyl)-5-methyl- |
| 28 | 20.014 | 3.51 | C15H24 | 204 | Caryophyllene |
| 36 | 22.193 | 0.78 | C14H22O | 206 | Phenol, 2,4-bis(1,1-dimethylethyl)- |
| 40 | 23.447 | 2.39 | C15H26O | 222 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- |
| 55 | 31.918 | 0.19 | C20H30O4 | 334 | 1,2-Benzenedicarboxylic acid, butyl octyl ester |
| 57 | 32.494 | 2.52 | C18H36O2 | 284 | Hexadecanoic acid, ethyl ester |
| 61 | 34.701 | 17.3 | C20H40O | 296 | Phytol |
| 65 | 40.803 | 1.53 | C16H14O5 | 286 | 7H-Furo(3,2-g)(1)benzopyran-7-one, 4-(2,3-epoxy-3-methylbutoxy)-, (S)-(−)- |
| 68 | 44.484 | 0.73 | C16H16O6 | 304 | 4-(2,3-Dihydroxy-3-methylbutoxy)furo(3,2-g)chromen-7-one |
| 71 | 52.408 | 4.91 | C29H50O | 414 | Sitosterol |
| Name of Compound | Compound Nature | Bioactivity |
|---|---|---|
| Citronellal | Monoterpenoid | Antibacterial and antifungal activities [24,40] |
| Wound healing property on chronic diabetic wounds [27] | ||
| Relaxing effects [61,62] | ||
| Citronellol | Monoterpene alcohol | Anti-inflammatory and analgesic activities [30,63] |
| Cyclohexanol, 2-(2-hydroxy-2-propyl)-5-methyl- | Monoterpenoid | Insect repellents [64,65] |
| Caryophyllene | Monoterpenes | Anti-inflammatory pathologies, atherosclerosis and tumors [35,66,67] |
| Antioxidant activity [68,69] | ||
| Analgesic activity [70,71] | ||
| 2,4-bis(1,1-dimethylethyl)Phenol | Phenol | Antioxidant activity [72,73,74] |
| Anti-inflammatory activity [74,75] | ||
| 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- or (Nerolidol) | Sesquiterpene alcohol | Antioxidant activity [76,77,78,79,80] Anti-inflammatory and analgesic activities [81,82] Neuroprotective effect [36] |
| 1,2-Benzenedicarboxylic acid, butyloctyl ester | Ester | Antioxidant activity [83] |
| Hexadecanoic acid, ethyl ester or (Ethyl palmitate) | Palmitic acid ester (Fatty acid ethyl ester) | Antioxidant, hypocholesterolemic, anti-androgenic [84] Anti-inflammatory activities [42] |
| Phytol | Diterpene alcohol | Antioxidant and neuroprotective effects [41,85] |
| 7H-Furo(3,2-g)(1)benzopyran-7-one, 4-(2,3-epoxy-3-methylbutoxy)-, (S)-(−)- or (Heraclenin) | Furanocoumarin | Anti-inflammatory activity [86] |
| 4-(2,3-Dihydroxy-3-methylbutoxy) furo(3,2-g)chromen-7-one or (Oxypeucedanin hydrate oraviprin) | Furanocoumarin | Antioxidant activity Anticancer activity [87,88] |
| Sitosterol | Phytosterol | Prevention of the coronary heart disease [43,44] Anti-Alzheimer’s activity [45] |
| Antioxidant activity [46,47] Prevention of glutamate and β-amyloid toxicity [48] | ||
| α-Terpineol | Monoterpene alcohol | Antioxidant activity, antiulcer activity |
| Cardiovascular and antihypertensive effects | ||
| Anticonvulsant and sedative activity Re-establish insulin sensitivity Antibacterial activity Anti-nociceptive activity [49,50,51,52,53] | ||
| Methyl 6-oxoheptanoate | Methyl ester | Anticancer activity [89] |
| α-Copaene | Sesquiterpene | Antioxidant and anticancer activities [90,91] |
| Cadinene | Sesquiterpene | Antioxidant activity [92] |
| 7-Hydroxycoumarin or Umbelliferone | Coumarin | Antihyperlipidemic and antidiabetic effects [93,94] Anti-inflammatory and antioxidant activities [95,96] Neuroprotective effect [97,98,99] |
| n-Hexadecanoic acid | Palmitic acid | Anti-inflammatory and antioxidant activities [84,100] |
| 7H-Furo(3,2-g)(1)benzopyran-7-one, 9-hydroxy- or (Xanthotoxol) | Furanocoumarin | Antioxidant activity and neuroprotective effect [101,102] |
| 4-(3-Methyl-2-oxobutoxy)-7H-furo(3,2-g)(1)benzopyran-7-one or (Isooxypeucedanin) | Furanocoumarin | Antidiabetic effects [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarachotanant, N.; Tencomnao, T. Citrus hystrix Extracts Protect Human Neuronal Cells against High Glucose-Induced Senescence. Pharmaceuticals 2020, 13, 283. https://doi.org/10.3390/ph13100283
Pattarachotanant N, Tencomnao T. Citrus hystrix Extracts Protect Human Neuronal Cells against High Glucose-Induced Senescence. Pharmaceuticals. 2020; 13(10):283. https://doi.org/10.3390/ph13100283
Chicago/Turabian StylePattarachotanant, Nattaporn, and Tewin Tencomnao. 2020. "Citrus hystrix Extracts Protect Human Neuronal Cells against High Glucose-Induced Senescence" Pharmaceuticals 13, no. 10: 283. https://doi.org/10.3390/ph13100283
APA StylePattarachotanant, N., & Tencomnao, T. (2020). Citrus hystrix Extracts Protect Human Neuronal Cells against High Glucose-Induced Senescence. Pharmaceuticals, 13(10), 283. https://doi.org/10.3390/ph13100283






