Measuring Daylight: A Review of Dosimetry in Daylight Photodynamic Therapy
Abstract
1. Introduction
2. Methodology and Literature Searches
2.1. Quantifying Dosimetry in Daylight PDT
- Irradiance (W m−2) is the radiant flux received by a surface per unit area, where radiant flux (W, commonly called ‘power’) is the electromagnetic energy received per unit time.
- Spectral irradiance (W m−2 nm−1) is the irradiance per unit wavelength and gives spectral information about a light source, which is important when considering the application of broadband light sources in the medical setting and weighting for functions such as PpIX absorption.
- Radiant exposure (J m−2) is the irradiance received by a surface integrated over a set duration. In DPDT radiant exposure is given in the units J cm−2 and, when the irradiance is weighted for PpIX absorption, may be referred to as either ‘PpIX-weighted dose’, ‘PpIX-effective dose’ or simply ‘PpIX dose’.
- Illuminance (lumens m−2; lx) is the photometric equivalent to irradiance after weighting to the luminosity function—the perception of the human eye to the light brightness. Defined as the luminous flux (lumens) received by a surface per unit area.
- Intensity and brightness should not be used for quantitative measurements so should be avoided where possible in the context of DPDT dosimetry.
2.2. Dosimetry Methods Reported in the Literature
2.2.1. Measurements of Spectral Irradiance
2.2.2. Broadband Measurements
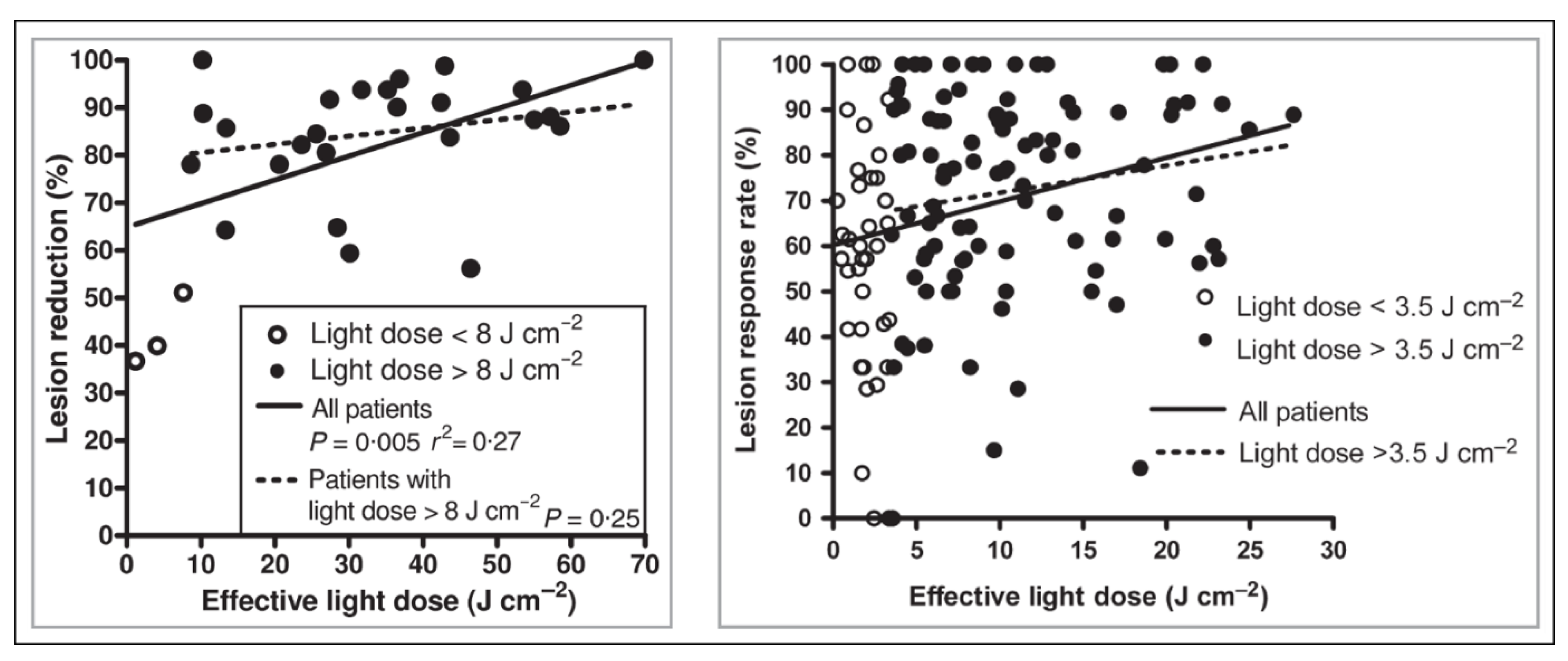
2.2.3. Clinical Relevance of Dosimetry in DPDT
2.3. Dose Measurements by Location
2.4. Sunscreens and PpIX-Effective Light Dose
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Wiegell, S.R.; Wulf, H.C.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Gerritsen, M.J.; Gilaberte, Y.; Calzavara-Pinton, P.; Morton, C.A.; Sidoroff, A.; et al. Daylight photodynamic therapy for actinic keratosis: An international consensus. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Wulf, H.C.; Szeimies, R.M.; Gilaberte, Y.; Basset-Seguin, N.; Sotiriou, E.; Piaserico, S.; Hunger, R.E.; Baharlou, S.; Sidoroff, A.; et al. Practical approach to the use of daylight photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: A European consensus. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Spelman, L.; Rubel, D.M.; Murrell, D.F.; See, J.; Hewitt, D.; Foley, P.; Salmon, R.; Kerob, D.; Pascual, T.; Shumack, S.; et al. Treatment of face and scalp solar (actinic) keratosis with daylight-mediated photodynamic therapy is possible throughout the year in Australia: Evidence from a clinical and meteorological study. Australas. J. Dermatol. 2015, 57, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lacour, J.-P.; Ulrich, C.; Gilaberte, Y.; Von Felbert, V.; Basset-Seguin, N.; Dreno, B.; Girard, C.; Redondo, P.; Serra-Guillen, C.; Synnerstad, I.; et al. Daylight photodynamic therapy with methyl aminolevulinate cream is effective and nearly painless in treating actinic keratoses: A randomised, investigator-blinded, controlled, phase III study throughout Europe. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- McLellan, L.J.; O’Mahoney, P.; Logan, S.; Yule, S.; Goodman, C.; Lesar, A.; Fullerton, L.; Ibbotson, S.; Eadie, E. Daylight PDT: Patient willingness to undertake home treatment. Br. J. Dermatol. 2019, 1–2. [Google Scholar]
- Braathen, L.R. Daylight photodynamic therapy in private practice in Switzerland: Gain without pain. Acta Derm. Venereol. 2012, 92, 652–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Nguyen, J.K.; Austin, E.; Mamalis, A.; Jagdeo, J. Updates on Treatment Approaches for Cutaneous Field Cancerization. Curr. Dermatol. Rep. 2019, 8, 122–132. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Haedersdal, M.; Eriksen, P.; Wulf, H.C. Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home-based daylight exposure: A double-blinded randomized clinical trial. Br. J. Dermatol. 2009, 160, 1308–1314. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Fabricius, S.; Gniadecka, M.; Stender, I.M.; Berne, B.; Kroon, S.; Andersen, B.L.; Mørk, C.; Sandberg, C.; Ibler, K.S.; et al. Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: A randomized multicentre study. Br. J. Dermatol. 2012, 166, 1327–1332. [Google Scholar] [CrossRef]
- Galderma (UK) Ltd. Metvix: Method of Administration. 2018. Available online: https://www.medicines.org.uk/emc/product/6777/smpc (accessed on 19 July 2019).
- Biofrontera Pharma GmbH. Ameluz: Method of Administration. 2019. Available online: https://www.medicines.org.uk/emc/product/3158/smpc (accessed on 19 July 2019).
- Wiegell, S.R.; Haedersdal, M.; Philipsen, P.A.; Eriksen, P.; Enk, C.D.; Wulf, H.C. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br. J. Dermatol. 2008, 158, 740–746. [Google Scholar] [CrossRef]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.-E.; Nesland, J.M. 5-aminolevulinic acid-based photodynamic therapy—Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Fabricius, S.; Stender, I.M.; Berne, B.; Kroon, S.; Andersen, B.L.; Mørk, C.; Sandberg, C.; Jemec, G.B.E.; Mogensen, M.; et al. A randomized, multicentre study of directed daylight exposure times of 1½ vs. 2½ h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br. J. Dermatol. 2011, 164, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Rubel, D.M.; Spelman, L.; Murrell, D.F.; See, J.; Hewitt, D.; Foley, P.; Bosc, C.; Kerob, D.; Kerrouche, N.; Wulf, H.C.; et al. Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: A randomized controlled trial. Br. J. Dermatol. 2014, 171, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Grinblat, B.M.; Festa Neto, C.; Sanches, J.A.; Szeimies, R.-M.; Oliveira, A.P.; Torezan, L.A.R. Daylight photodynamic therapy for actinic keratoses in São Paulo, Brazil. Photodermatol. Photoimmunol. Photomed. 2014, 31, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Togsverd-Bo, K.; Lei, U.; Erlendsson, A.M.; Taudorf, E.H.; Philipsen, P.A.; Wulf, H.C.; Skov, L.; Haedersdal, M. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—A randomized controlled trial. Br. J. Dermatol. 2015, 172, 467–474. [Google Scholar] [CrossRef]
- O’Gorman, S.; Clowry, J.; Manley, M.; McCavana, J.; Gray, L.; Kavanagh, A.; Lally, A.; Collins, P. Artificial White Light vs. Daylight Photodynamic Therapy for Actinic Keratoses. JAMA Dermatol. 2016, 152, 638–644. [Google Scholar] [CrossRef]
- Nissen, C.V.; Heerfordt, I.M.; Wiegell, S.R.; Mikkelsen, C.S.; Wulf, H.C. Pretreatment with 5-fluorouracil cream enhances the efficacy of daylight-mediated photodynamic therapy for actinic keratosis. Acta Derm. Venereol. 2017, 97, 617–621. [Google Scholar] [CrossRef]
- Räsänen, J.E.; Neittaanmäki, N.; Ylitalo, L.; Hagman, J.; Rissanen, P.; Ylianttila, L.; Salmivuori, M.; Snellman, E.; Grönroos, M. 5-Aminolaevulinic Acid Nanoemulsion Is More Effective Than Methyl-5-Aminolaevulinate in Daylight Photodynamic Therapy for Actinic Keratosis: A Nonsponsored Randomized Double-Blind Multicentre Trial. Br. J. Dermatol. 2019, 181, 265–274. [Google Scholar] [CrossRef]
- Heerfordt, I.M.; Wulf, H.C. Daylight photodynamic therapy of actinic keratosis without curettage is as effective as with curettage: A randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2019. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Fabricius, S.; Heydenreich, J.; Enk, C.D.; Rosso, S.; Bäumler, W.; Baldursson, B.T.; Wulf, H.C. Weather conditions and daylight-mediated photodynamic therapy: Protoporphyrin IX-weighted daylight doses measured in six geographical locations. Br. J. Dermatol. 2013, 168, 186–191. [Google Scholar] [CrossRef]
- Grinblat, B.; Galimberti, G.; Pantoja, G.; Sanclemente, G.; Lopez, M.; Alcala, D.; Torezan, L.; Kerob, D.; Pascual, T.; Chouela, E. Feasibility of daylight-mediated photodynamic therapy for actinic keratosis throughout the year in Central and South America: A meteorological study. Int. J. Dermatol. 2016, 55, 1–6. [Google Scholar] [CrossRef]
- O’Mahoney, P.; Khazova, M.; Higlett, M.; Lister, T.; Ibbotson, S.; Eadie, E. Use of illuminance as a guide to effective light delivery during daylight photodynamic therapy in the U.K. Br. J. Dermatol. 2017, 176, 1607–1616. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of absorption and fluorescence spectra of 300 common compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef]
- Marra, K.; LaRochelle, E.; Chapman, M.S.; Hoopes, P.J.; Lukovits, K.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparison of Blue and White Lamp Light with Sunlight for Daylight-Mediated, 5-ALA Photodynamic Therapy, in vivo. Photochem. Photobiol. 2018, 94, 1049–1057. [Google Scholar] [CrossRef]
- Campbell, C.L.; Wood, K.; Valentine, R.M.; Brown, C.T.; Moseley, H. Monte Carlo modelling of daylight activated photodynamic therapy. Phys. Med. Biol. 2015, 60, 4059–4073. [Google Scholar] [CrossRef][Green Version]
- Vignion-Dewalle, A.S.; Baert, G.; Thecua, E.; Lecomte, F.; Vicentini, C.; Abi-Rached, H.; Mortier, L.; Mordon, S. Comparison of 10 efficient protocols for photodynamic therapy of actinic keratosis: How relevant are effective light dose and local damage in predicting the complete response rate at 3 months? Lasers Surg. Med. 2018, 50, 576–589. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.M.; Sidoroff, A.; Braathen, L.R. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications—Actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef]
- Manley, M.; Collins, P.; Gray, L.; O’Gorman, S.; McCavana, J. Quantifying the radiant exposure and effective dose in patients treated for actinic keratoses with topical photodynamic therapy using daylight and LED white light. Phys. Med. Biol. 2018, 63, 35013. [Google Scholar] [CrossRef]
- CIE Commission Internationale de l’Éclairage Proceedings; Cambridge University Press: Cambridge, UK, 1924.
- Wiegell, S.R.; Skiveren, J.; Philipsen, P.A.; Wulf, H.C. Pain during photodynamic therapy is associated with protoporphyrin IX fluorescence and fluence rate. Br. J. Dermatol. 2008, 158, 727–733. [Google Scholar] [CrossRef]
- Timmins, K. A Dosimetry Review of Daylight Photodynamic Therapy; University of St. Andrews: St Andrews, UK, 2016. [Google Scholar]
- Wenande, E.; Phothong, W.; Bay, C.; Karmisholt, K.E.; Haedersdal, M.; Togsverd-Bo, K. Efficacy and safety of daylight photodynamic therapy after tailored pretreatment with ablative fractional laser or microdermabrasion: A randomized, side-by-side, single-blind trial in patients with actinic keratosis and large-area field cancerization. Br. J. Dermatol. 2018, 180, 756–764. [Google Scholar] [CrossRef]
- Cordey, H.; Valentine, R.M.; Lesar, A.; Moseley, H.; Eadie, E.; Ibbotson, S. Daylight photodynamic therapy in Scotland. Scott. Med. J. 2017, 62, 48–53. [Google Scholar] [CrossRef]
- Thieden, E.; Agren, M.S.; Wulf, H.C. The wrist is a reliable body site for personal dosimetry of ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2000, 16, 57–61. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Heydenreich, J.; Fabricius, S.; Wulf, H.C. Continuous ultra-low-intensity artificial daylight is not as effective as red LED light in photodynamic therapy of multiple actinic keratoses. Photodermatol. Photoimmunol. Photomed. 2011, 27, 280–285. [Google Scholar] [CrossRef]
- Olsen, E.A.; Lisa Abernethy, M.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef]
- Röwert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Erratum: Actinic keratosis is an early in situ squamous cell carcinoma: A proposal for reclassification (British Journal of Dermatology (2007) 156, SUPPL., (S13-S17)). Br. J. Dermatol. 2007, 157, 431. [Google Scholar]
- Dirschka, T.; Pellacani, G.; Micali, G.; Malvehy, J.; Stratigos, A.J.; Casari, A.; Schmitz, L.; Gupta, G.; Panagiotopoulos, A.; Hasapi, V.; et al. A proposed scoring system for assessing the severity of actinic keratosis on the head: Actinic keratosis area and severity index. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1295–1302. [Google Scholar] [CrossRef]
- Remund, J.; Müller, S.; Kunz, S.; Huguenin-Landl, B.; Studer, C.; Cattin, R. Meteonorm Handbook, Part II: Theory; Meteotest: Bern, Switzerland, 2018. [Google Scholar]
- Gansler, R.; Klein, S.A.; Beckman, W.A. Assessment of the Accuracy of Generated Meteorological Data for Use in Solar Energy Simulation Studies. Sol. Energy 1994, 53, 279–287. [Google Scholar] [CrossRef]
- Lerche, C.; Heerfordt, I.; Heydenreich, J.; Wulf, H.C. Alternatives to Outdoor Daylight Illumination for Photodynamic Therapy—Use of Greenhouses and Artificial Light Sources. Int. J. Mol. Sci. 2016, 17, 309. [Google Scholar] [CrossRef]
- McLellan, L.J.; O’Mahoney, P.; Khazova, M.; Higlett, M.; Ibbotson, S.H.; Eadie, E. Ultraviolet radiation exposure during daylight Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2019, 27, 19–23. [Google Scholar] [CrossRef]
- UK Air Information Resource. Available online: https://uk-air.defra.gov.uk/data/uv-index-graphs (accessed on 2 August 2019).
- Osman-Ponchet, H.; Sevin, K.; Gaborit, A.; Kouidhi, M.; Hanaizi, J.; Comby, P.; Ruty, B.; Bouvier, G. Lack of effect of selected sunscreens applied on ex vivo human skin for 5-methyl-aminolevulinic acid penetration and protoporphyrin IX photoactivation. Photodiagnosis Photodyn. Ther. 2017, 17, 75–81. [Google Scholar] [CrossRef]
- O’Mahoney, P.; Khazova, M.; Ibbotson, S.; Eadie, E. The effects of sunscreen use and window glass on daylight photodynamic therapy dosimetry. Br. J. Dermatol. 2019, 181, 220–221. [Google Scholar] [CrossRef]

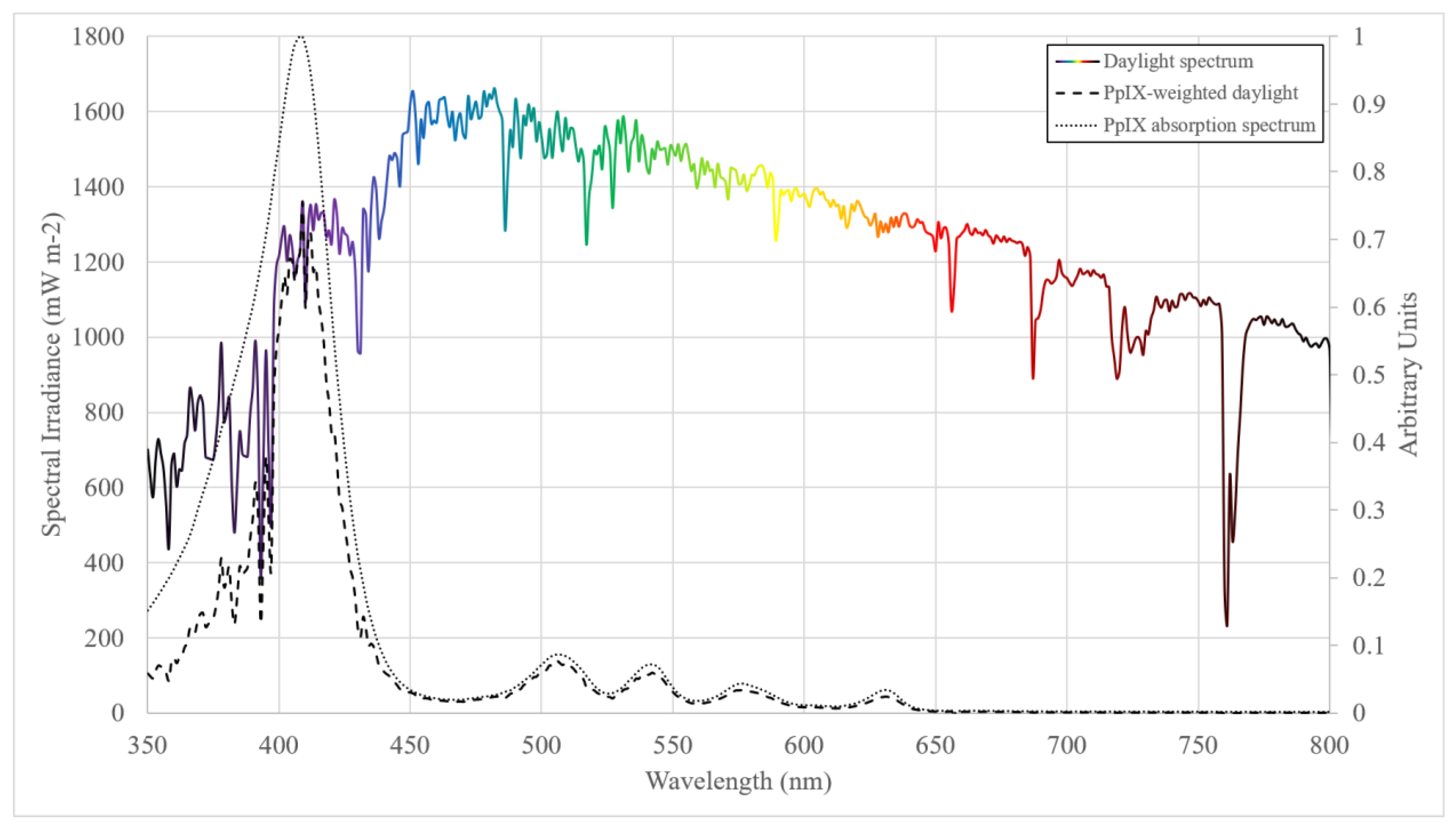
| Author (et al.) | Year | Study Type | Dosimetry Method | PpIX Spectrum Source | Sunscreen | Time (hours) | Light Dose (J cm−2) | Range |
|---|---|---|---|---|---|---|---|---|
| Wiegell [12] | 2008 | RCT | Luxmeter informs radiative transfer model | [13] | 2.5 | 43.2 | 11.7–65.9 | |
| Wiegell [8] | 2009 | RCT | Wristwatch luxmeter informs radiative transfer model | [13] | P20 | 4 | 30.1 | 1.2–69.8 |
| Wiegell [14] | 2011 | RCT | Wristwatch (PpIX-weighted) | [13] | P20 | 2 or 3 | 9.4 | 0.2–28.3 |
| Wiegell [9] | 2012 | RCT | Wristwatch (PpIX-weighted) | [13] | P20 | 1.5 or 2.5 | 6.5 and 9.6 | |
| Rubel [15] | 2014 | RCT | Spectroradiometer | N/A | ‘High SPF’ | 2 | 22.8 | 3–46 |
| Grinblat [16] | 2014 | Non-RCT | Pyranometer (Irradiance) | N/A | ‘SPF30’ | 1–1.5 | 28.25 | 7.9–45.3 |
| Lacour [4] | 2015 | RCT | Spectroradiometer | N/A | Actinica | 2 | 267 (W m−2) | 44–601 (W m−2) |
| Togsverd-Bo [17] | 2015 | RCT | Illuminance | N/A | P20 | 2 | 48773 (lx) | 10600–86357 (lx) |
| O’Gorman [18] | 2016 | RCT | Irradiance, illuminance and spectral irradiance. | N/A | P20 | 2 | 21.38 | 3.2–43 |
| Nissen [19] | 2017 | RCT | Illuminance | N/A | P20 | 2 | 36477 (lx) | 1322–94234 (lx) |
| Räsänen [20] | 2018 | RCT | Irradiance | N/A | P20 | 2 | 12.89 | 5.96–17.93 |
| Heerfordt [21] | 2019 | RCT | Illuminance | N/A | N/A | 2 | 38640 (lx) | 7200–85200 (lx) |
| Wiegell [22] | 2013 | Dose measurement | Wristwatch (PpIX-weighted) | [13] | 2 | |||
| Morton [2] | 2015 | Dose measurement | Meteonorm (Radiative transfer outputs irradiance) | N/A | ||||
| Spelman [3] | 2015 | Dose measurement | Meteonorm (Radiative transfer outputs irradiance) | N/A | ||||
| Grinblat [23] | 2016 | Dose measurement | Meteonorm (Radiative transfer outputs irradiance) | N/A | ||||
| O’Mahoney [24] | 2017 | Dose measurement | Spectroradiometer and illuminance | [25] | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Mahoney, P.; Khazova, M.; Eadie, E.; Ibbotson, S. Measuring Daylight: A Review of Dosimetry in Daylight Photodynamic Therapy. Pharmaceuticals 2019, 12, 143. https://doi.org/10.3390/ph12040143
O’Mahoney P, Khazova M, Eadie E, Ibbotson S. Measuring Daylight: A Review of Dosimetry in Daylight Photodynamic Therapy. Pharmaceuticals. 2019; 12(4):143. https://doi.org/10.3390/ph12040143
Chicago/Turabian StyleO’Mahoney, Paul, Marina Khazova, Ewan Eadie, and Sally Ibbotson. 2019. "Measuring Daylight: A Review of Dosimetry in Daylight Photodynamic Therapy" Pharmaceuticals 12, no. 4: 143. https://doi.org/10.3390/ph12040143
APA StyleO’Mahoney, P., Khazova, M., Eadie, E., & Ibbotson, S. (2019). Measuring Daylight: A Review of Dosimetry in Daylight Photodynamic Therapy. Pharmaceuticals, 12(4), 143. https://doi.org/10.3390/ph12040143





