Targeting MMP-9 in Diabetic Foot Ulcers
Abstract
1. Introduction
2. Effect of Treatments on MMPs in DFUs
2.1. Vacuum-Assisted Closure
2.2. Mesenchymal Stem Cells
2.3. N-Acetyl Cysteine
2.4. Wound Dressings
2.5. Growth Factors
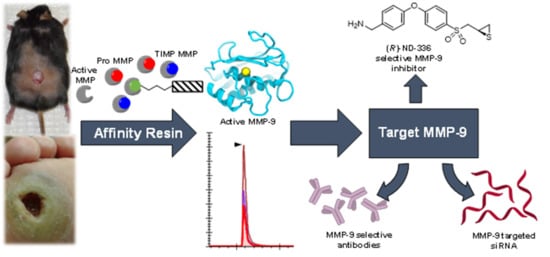
3. Affinity Enrichment Approaches to Identify MMPs
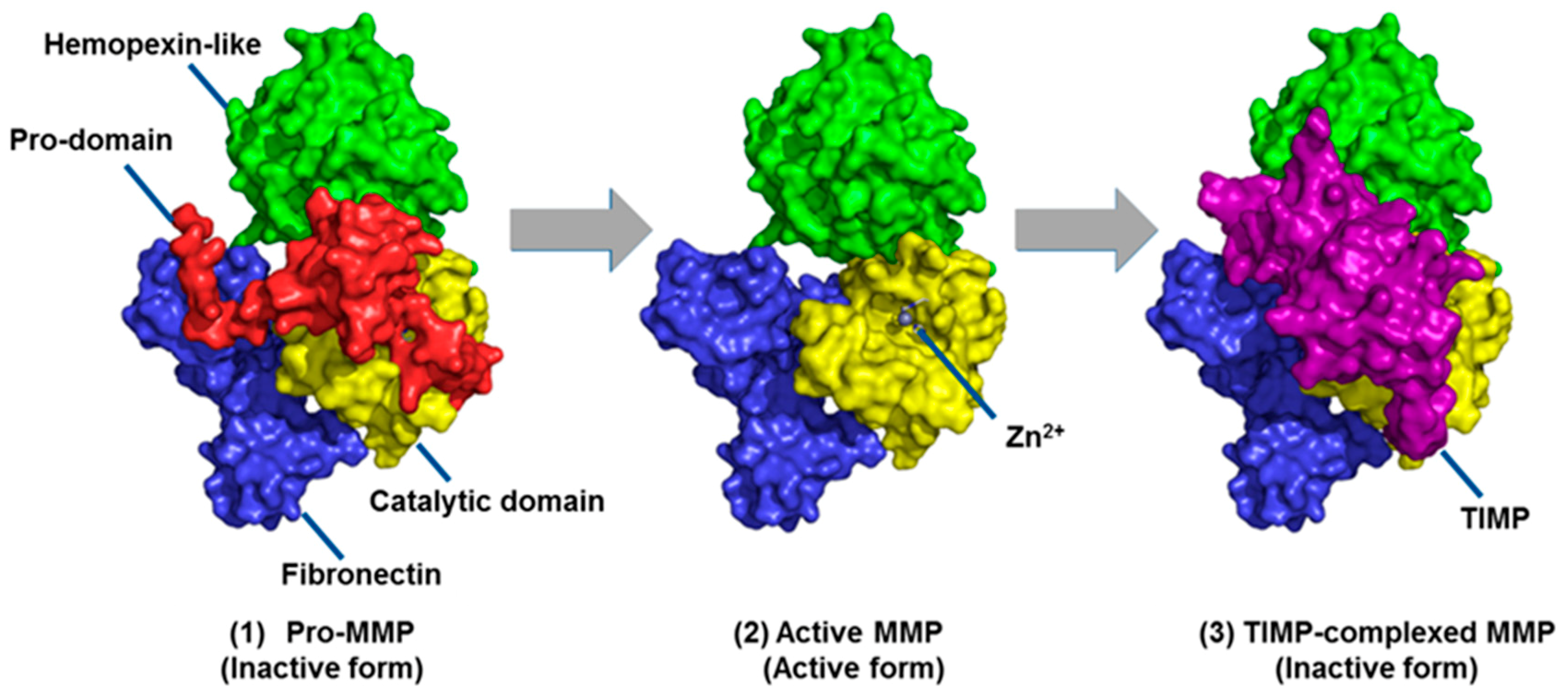
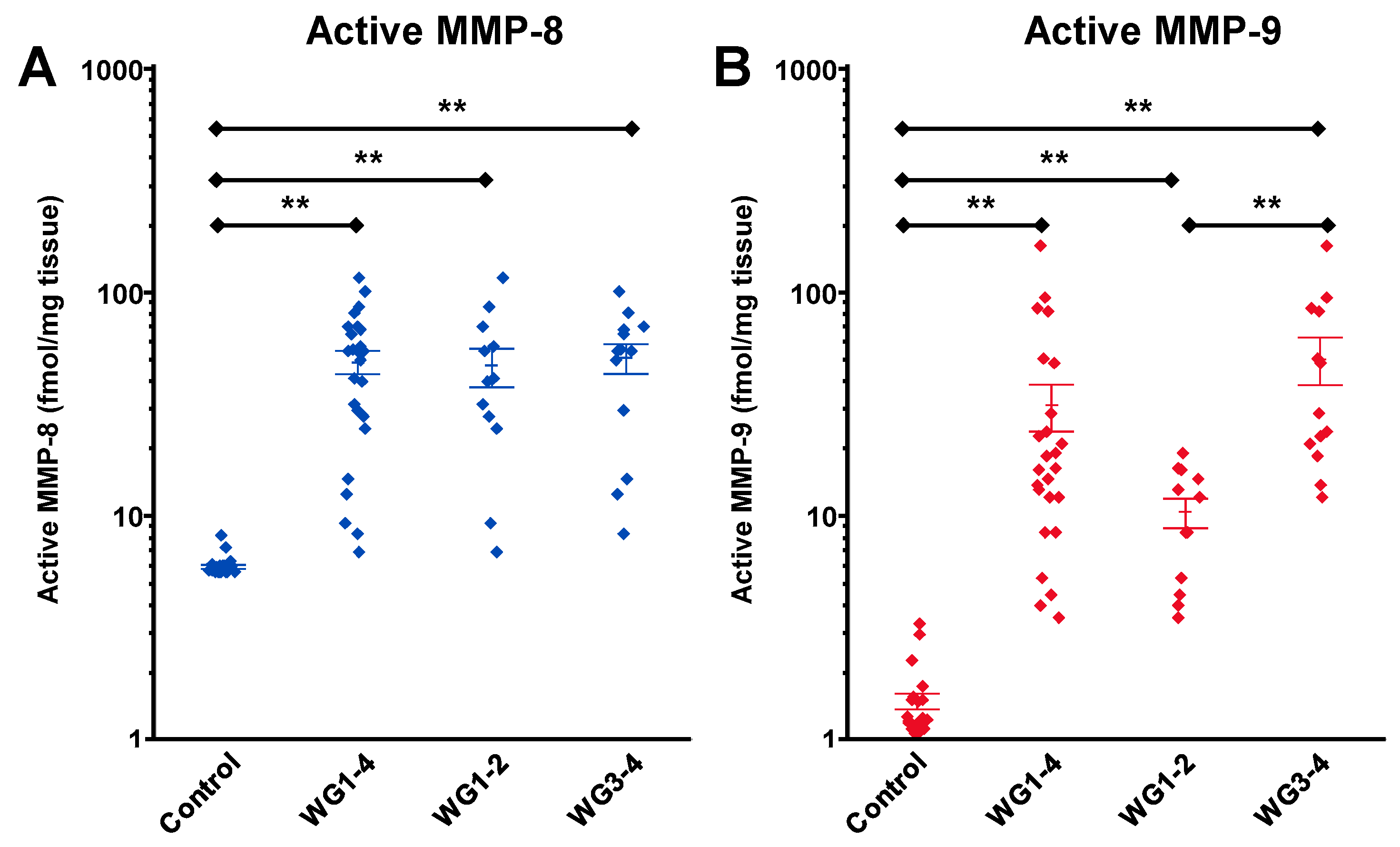
4. The Roles of MMPs in DFUs
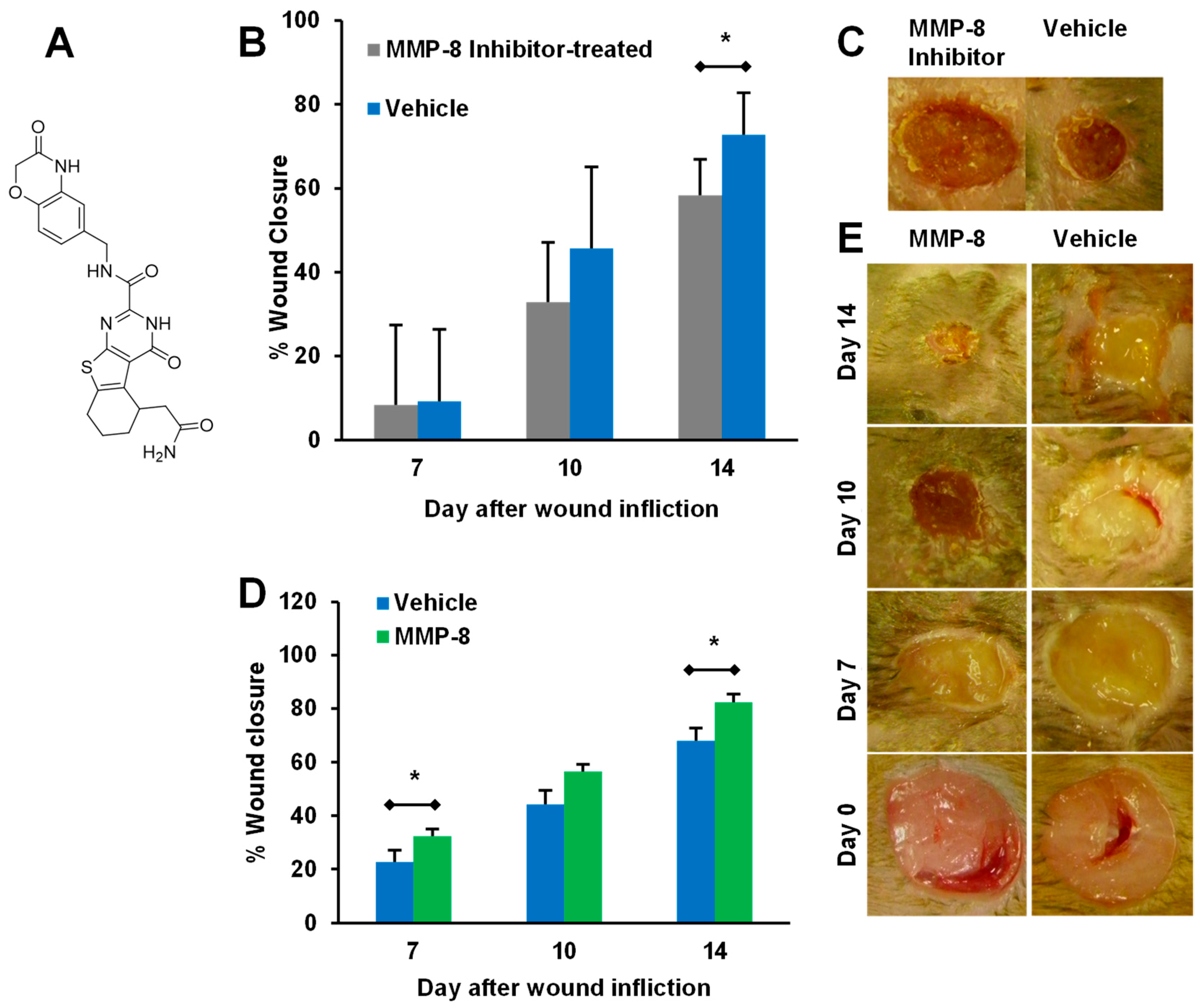
5. Targeting MMP-9 with Therapeutics
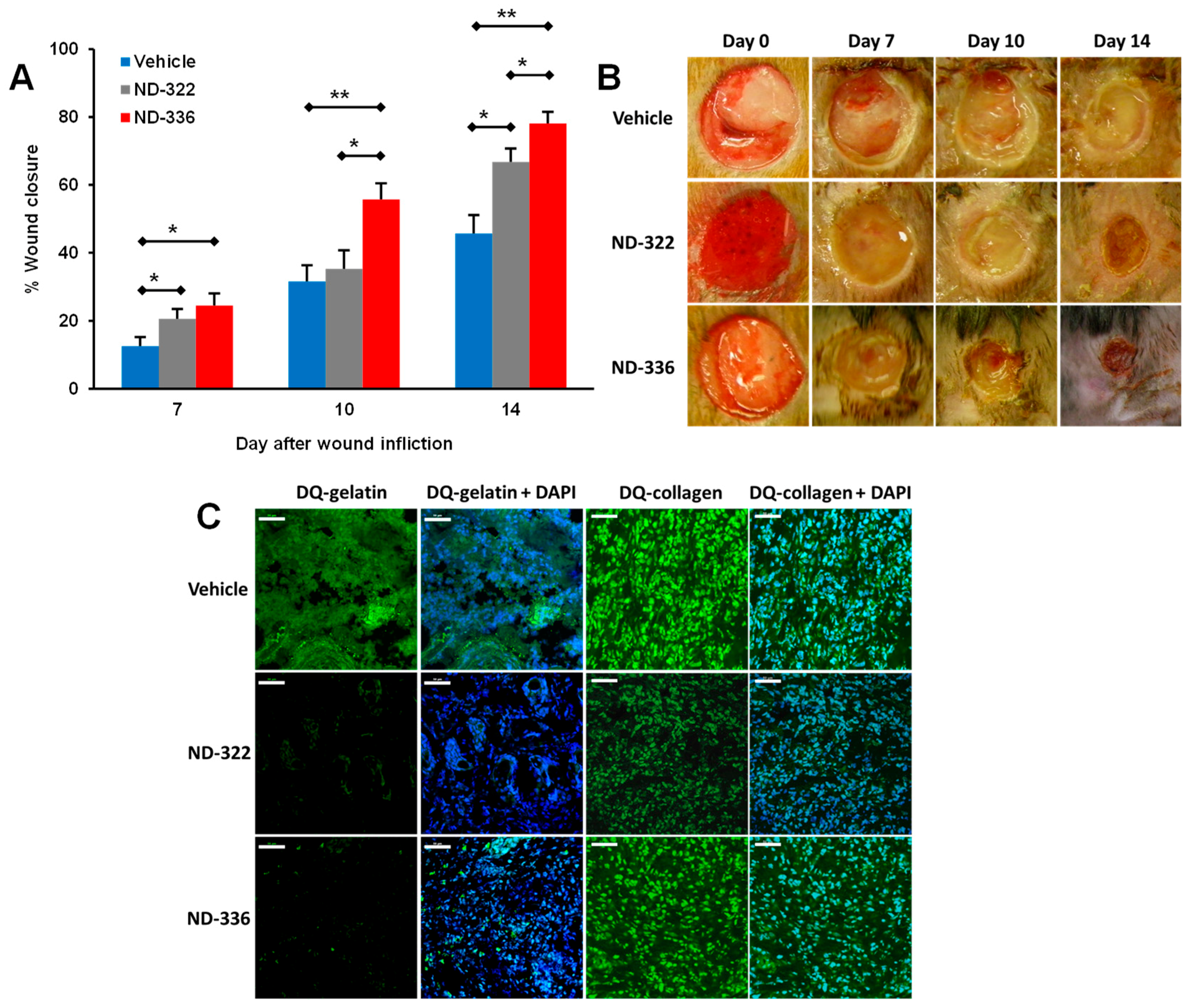
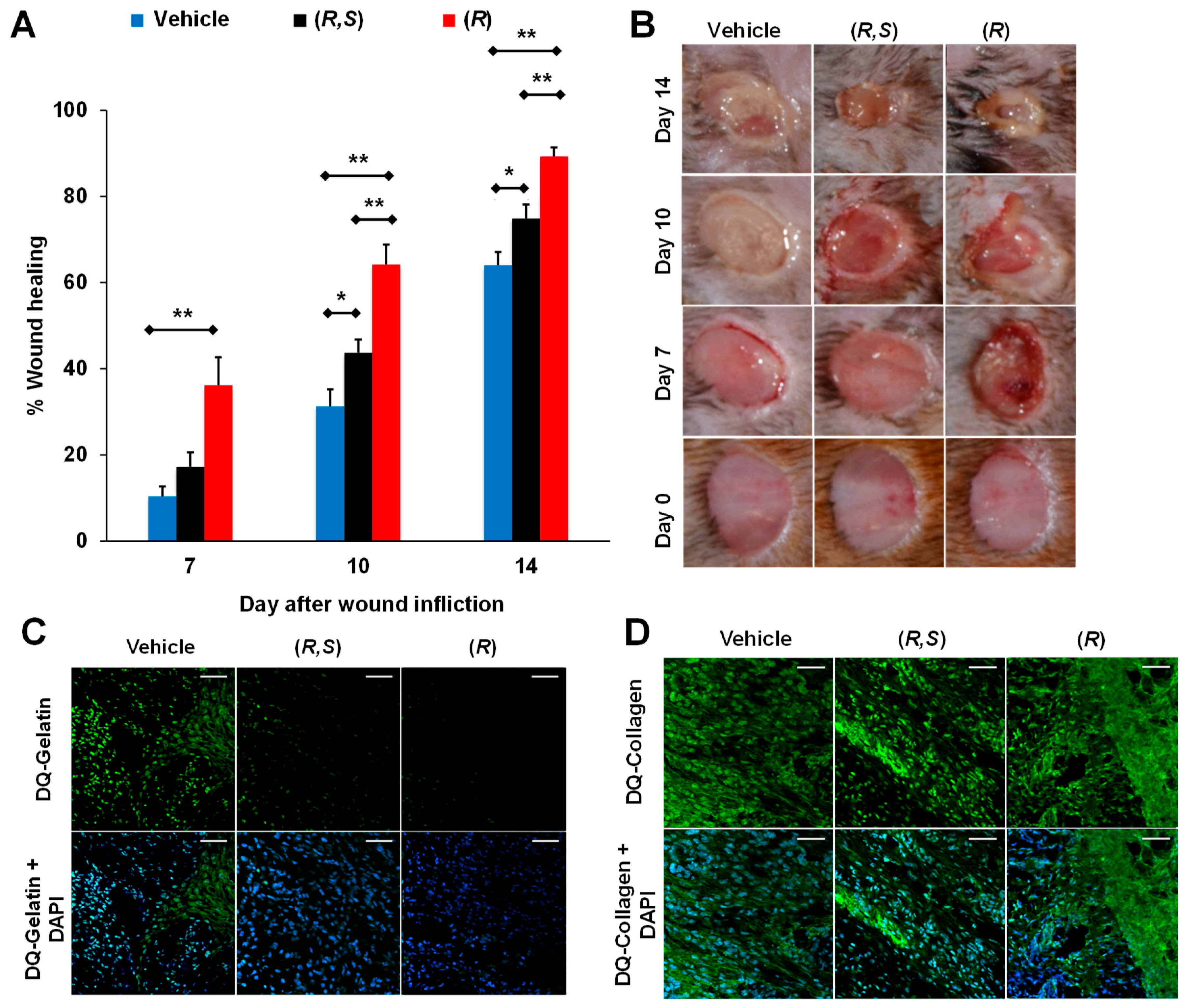
5.1. Small-Molecule Inhibitors
5.2. Antibody-Based Inhibitors
5.3. Advanced Wound Dressings
5.4. RNA-Based Therapies
6. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cooper, S. The biology of the skin. J. R. Soc. Med. 2002, 95, 109. [Google Scholar] [CrossRef]
- Dinh, T.; Tecilazich, F.; Kafanas, A.; Doupis, J.; Gnardellis, C.; Leal, E.; Tellechea, A.; Pradhan, L.; Lyons, T.E.; Giurini, J.M.; et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012, 61, 2937–2947. [Google Scholar] [CrossRef]
- Christian, L.M.; Graham, J.E.; Padgett, D.A.; Glaser, R.; Kiecolt-Glaser, J.K. Stress and Wound Healing. Neuroimmunomodulation 2006, 13, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nijm, J.; Kristenson, M.; Olsson, A.G.; Jonasson, L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J. Intern. Med. 2007, 262, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Ebrecht, M.; Hextall, J.; Kirtley, L.-G.; Taylor, A.; Dyson, M.; Weinman, J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology 2004, 29, 798–809. [Google Scholar] [CrossRef]
- Kometani, M.; Yoneda, T.; Demura, M.; Koide, H.; Nishimoto, K.; Mukai, K.; Gomez-Sanchez, C.E.; Akagi, T.; Yokota, T.; Horike, S.-I.; et al. Cortisol overproduction results from DNA methylation of CYP11B1 in hypercortisolemia. Sci. Rep. 2017, 7, 11205. [Google Scholar] [CrossRef] [PubMed]
- Vileikyte, L.; Shen, B.-j.; Pastar, I.; Boulton, A.J.; Kirsner, R.; Tomic-canic, M.; Hardman, M. Cortisol Synthesis Enzyme CYP11B1 as Tissue Biomarker for Diabetic Foot Ulcers. Diabetes 2018, 67, 641-P. [Google Scholar] [CrossRef]
- Szymanowski, A.; Nijm, J.; Kristenson, M.; Jonasson, L. Elevated levels of circulating matrix metalloproteinase-9 are associated with a dysregulated cortisol rhythm—A case-control study of coronary artery disease. Psychoneuroendocrinology 2011, 36, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.-H.; Kocieda, V.P.; Jing, H.; Ganea, D. Prostaglandin E2 Induces Matrix Metalloproteinase 9 Expression in Dendritic Cells through Two Independent Signaling Pathways Leading to Activator Protein 1 (AP-1) Activation. J. Biol. Chem. 2011, 286, 38913–38923. [Google Scholar] [CrossRef]
- Lazaro, J.L.; Izzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated levels of matrix metalloproteinases and chronic wound healing: An updated review of clinical evidence. J. Wound Care 2016, 25, 277–287. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Puente, X.S.; Sánchez, L.M.; Overall, C.M.; López-Otín, C. Human and mouse proteases: A comparative genomic approach. Nat. Rev. Genet. 2003, 4, 544. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mobashery, S.; Chang, M. Roles of matrix metalloproteinases in cutaneous wound healing. In Wound Healing—New Insights into Ancient Challenges; Alexandrescu, V.A., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Springman, E.B.; Angleton, E.L.; Birkedal-Hansen, H.; Van Wart, H.E. Multiple modes of activation of latent human fibroblast collagenase: Evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc. Natl. Acad. Sci. USA 1990, 87, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Bannikov, G.A.; Karelina, T.V.; Collier, I.E.; Marmer, B.L.; Goldberg, G.I. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J. Biol. Chem. 2002, 277, 16022–16027. [Google Scholar] [CrossRef]
- Okamoto, T.; Akaike, T.; Sawa, T.; Miyamoto, Y.; van der Vliet, A.; Maeda, H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar] [CrossRef]
- Sottrup-Jensen, L.; Birkedal-Hansen, H. Human fibroblast collagenase-alpha-macroglobulin interactions. Localization of cleavage sites in the bait regions of five mammalian alpha-macroglobulins. J. Biol. Chem. 1989, 264, 393–401. [Google Scholar] [PubMed]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: Multifunctional contributors to tumor progression. Mol. Med. Today 2000, 6, 149–156. [Google Scholar] [CrossRef]
- DeClerck, Y.A.; Imren, S. Protease inhibitors: Role and potential therapeutic use in human cancer. Eur. J. Cancer 1994, 30, 2170–2180. [Google Scholar] [CrossRef]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J.H. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, B. MMPs as therapeutic targets—Still a viable option? Semin. Cell Dev. Biol. 2008, 19, 61–68. [Google Scholar] [CrossRef]
- Martin, M.D.; Matrisian, L.M.J.C.; Reviews, M. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev. 2007, 26, 717. [Google Scholar] [CrossRef] [PubMed]
- Wynn, R.L. Latest FDA approvals for dentistry. Gen. Dent. 1999, 47, 19–22. [Google Scholar]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Sobrino, T.; Ramos-Cabrer, P.; Castillo, J. Reorganization of the cerebral vasculature following ischaemia. Rev. Neurol. 2009, 49, 0645–0654. [Google Scholar]
- Candelario-Jalil, E.; Yang, Y.; Rosenberg, G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009, 158, 983–994. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Rayment, E.A.; Upton, Z. Finding the culprit: A review of the influences of proteases on the chronic wound environment. Int. J. Low. Extrem. Wounds 2009, 8, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Trengove, N.J.; Stacey, M.C.; Macauley, S.; Bennett, N.; Gibson, J.; Burslem, F.; Murphy, G.; Schultz, G. Analysis of the acute and chronic wound environments: The role of proteases and their inhibitors. Wound Repair Regen. 1999, 7, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Rice, J.B.; Desai, U.; Cummings, A.K.G.; Birnbaum, H.G.; Skornicki, M.; Parsons, N.B. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2013, 37, 651–658. [Google Scholar] [CrossRef]
- Wagner, F.W. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle Int. 1981, 2, 64–122. [Google Scholar] [CrossRef]
- Fortington, L.V.; Geertzen, J.H.B.; van Netten, J.J.; Postema, K.; Rommers, G.M.; Dijkstra, P.U. Short and long term mortality rates after a lower limb amputation. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- McLennan, S.; Yue, D.; Twigg, S. Molecular aspects of wound healing in diabetes. Prim. Intent. 2006, 14, 8–13. [Google Scholar]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-kappaB. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Agren, M.S.; Mirastschijski, U.; Karlsmark, T.; Saarialho-Kere, U.K. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp. Dermatol. 2001, 10, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Hariono, M.; Yuliani, S.H.; Istyastono, E.P.; Riswanto, F.D.O.; Adhipandito, C.F. Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and structure-based drug design. Wound Med. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Greene, A.K.; Puder, M.; Roy, R.; Arsenault, D.; Kwei, S.; Moses, M.A.; Orgill, D.P. Microdeformational wound therapy: Effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann. Plast. Surg. 2006, 56, 418–422. [Google Scholar] [CrossRef]
- Mouës, C.M.; Van Toorenenbergen, A.W.; Heule, F.; Hop, W.C.; Hovius, S.E.R. The role of topical negative pressure in wound repair: Expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen. 2008, 16, 488–494. [Google Scholar] [CrossRef]
- Xu, J.; Zgheib, C.; Hodges, M.M.; Caskey, R.C.; Hu, J.; Liechty, K.W. Mesenchymal stem cells correct impaired diabetic wound healing by decreasing ECM proteolysis. Physiol. Genom. 2017, 49, 541–548. [Google Scholar] [CrossRef]
- Yang, C.; Meng, F.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.; Zhang, H.; Li, C. Inhibition of methylglyoxal-induced AGEs/RAGE expression contributes to dermal protection by N-acetyl-L-cysteine. Cell. Physiol. Biochem. 2017, 41, 742–754. [Google Scholar] [CrossRef]
- Tsang, K.-K.; Kwong, E.W.-Y.; To, T.S.-S.; Chung, J.W.-Y.; Wong, T.K.-S. A pilot randomized, controlled study of nanocrystalline silver, Manuka honey, and conventional dressing in healing diabetic foot ulcer. Evid.-Based Complement. Altern. Med. 2017, 2017, 15. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Perez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Champion, M.M.; Hesek, D.; Lee, M.; Pérez, R.L.; Schroeder, V.A.; Suckow, M.A.; Mobashery, S.; et al. Expression of active matrix metalloproteinase-9 as a likely contributor to the clinical failure of aclerastide in treatment of diabetic foot ulcers. Eur. J. Pharmacol. 2018, 834, 77–83. [Google Scholar] [CrossRef]
- Fleischmann, W.; Strecker, W.; Bombelli, M.; Kinzl, L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993, 96, 488–492. [Google Scholar]
- Armstrong, D.G.; Lavery, L.A. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet 2005, 366, 1704–1710. [Google Scholar] [CrossRef]
- Vuerstaek, J.D.D.; Vainas, T.; Wuite, J.; Nelemans, P.; Neumann, M.H.A.; Veraart, J.C.J.M. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J. Vasc. Surg. 2006, 44, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Badillo, A.T.; Redden, R.A.; Zhang, L.; Doolin, E.J.; Liechty, K.W.J.C.; Research, T. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007, 329, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, T.; Hu, M.S.; Marshall, C.D.; Barnes, L.A.; Longaker, M.T.; Lorenz, H.P. Stem cells and chronic wound healing: State of the art. Chronic Wound Care Manag. Res. 2016, 3, 7–27. [Google Scholar]
- Javazon, E.H.; Keswani, S.G.; Badillo, A.T.; Crombleholme, T.M.; Zoltick, P.W.; Radu, A.P.; Kozin, E.D.; Beggs, K.; Malik, A.A.; Flake, A.W. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 2007, 15, 350–359. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.; Zhang, M.; Chen, Y.; Wang, X.; Zhang, L.; Tian, Z.; Yan, Y.; Li, Q.; Zhong, W.; et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci. Rep. 2015, 5, 18104. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A review on various uses of N-acetyl cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [CrossRef]
- Khan, M.; Sekhon, B.; Jatana, M.; Giri, S.; Gilg, A.G.; Sekhon, C.; Singh, I.; Singh, A.K. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J. Neurosci. Res. 2004, 76, 519–527. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Aktunc, E.; Ozacmak, V.H.; Ozacmak, H.S.; Barut, F.; Buyukates, M.; Kandemir, O.; Demircan, N. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin. Exp. Dermatol. 2010, 35, 902–909. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L.J.D. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Gluud, C.; Nicola, S.; Simancas-Racines, D.; Reveiz, L.; Oliva, P.; Cedeño-Taborda, J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2015, CD005848. [Google Scholar] [CrossRef]
- Ziyadeh, N.; Fife, D.; Walker, A.M.; Wilkinson, G.S.; Seeger, J.D. A matched cohort study of the risk of cancer in users of becaplermin. Adv. Skin Wound Care 2011, 24, 31–39. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; Alexi, P.; Tihaa, L.; Haas, U.; Weiskirchen, R. Platelet-derived growth factor-D modulates extracellular matrix homeostasis and remodeling through TIMP-1 induction and attenuation of MMP-2 and MMP-9 gelatinase activities. Biochem. Biophys. Res. Commun. 2015, 457, 307–313. [Google Scholar] [CrossRef]
- Gooyit, M.; Peng, Z.; Wolter, W.R.; Ping, H.; Ding, D.; Hesek, D.; Lee, M.; Boggess, B.; Champion, M.M.; Suckow, M.A.; et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem. Biol. 2014, 9, 105–110. [Google Scholar] [CrossRef]
- Vukelic, S.; Griendling, K.K. Angiotensin II, from vasoconstrictor to growth factor. Circ. Res. 2014, 114, 754–757. [Google Scholar] [CrossRef]
- Yahata, Y.; Shirakata, Y.; Tokumaru, S.; Yang, L.; Dai, X.; Tohyama, M.; Tsuda, T.; Sayama, K.; Iwai, M.; Horiuchi, M.; et al. A novel function of Angiotensin II in skin wound healing: Induction of fibroblast and keratinocyte migration by angiotensin ii via heparin-binding epidermal growth factor (egf)-like growth factor-mediated egf receptor transactivation. J. Biol. Chem. 2006, 281, 13209–13216. [Google Scholar] [CrossRef]
- Chang, M. Restructuring of the extracellular matrix in diabetic wounds and healing: A perspective. Pharmacol. Res. 2016, 107, 243–248. [Google Scholar] [CrossRef]
- Rodgers, K.E.; Espinoza, T.; Felix, J.; Roda, N.; Maldonado, S.; diZerega, G. Acceleration of healing, reduction of fibrotic scar, and normalization of tissue architecture by an angiotensin analogue, NorLeu3-A(1-7). Plast. Reconstr. Surg. 2003, 111, 1195–1206. [Google Scholar] [CrossRef]
- Balingit, P.P.; Armstrong, D.G.; Reyzelman, A.M.; Bolton, L.; Verco, S.J.; Rodgers, K.E.; Nigh, K.A.; diZerega, G.S. NorLeu3-A(1–7) stimulation of diabetic foot ulcer healing: Results of a randomized, parallel-group, double-blind, placebo-controlled phase 2 clinical trial. Wound Repair Regen. 2012, 20, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.W.; Yang, L.X.; Wang, H.; Liu, B.; Wang, L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul. Pept. 2008, 147, 37–44. [Google Scholar] [CrossRef]
- Liu, Y.; Min, D.; Bolton, T.; Nubé, V.; Twigg, S.M.; Yue, D.K.; McLennan, S.V. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009, 32, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Rayment, E.A.; Upton, Z.; Shooter, G.K. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br. J. Dermatol. 2008, 158, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.F.; Mobashery, S. Mechanism-based profiling of MMPs. In Matrix Metalloproteinase Protocols; Clark, I.M., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 471–487. [Google Scholar]
- Meisel, J.E.; Chang, M. Selective small-molecule inhibitors as chemical tools to define the roles of matrix metalloproteinases in disease. Biochim. Biophys. Acta 2017, 1864, 2001–2014. [Google Scholar] [CrossRef]
- Vidova, V.; Spacil, Z. A review on mass spectrometry-based quantitative proteomics: Targeted and data independent acquisition. Anal. Chim. Acta 2017, 964, 7–23. [Google Scholar] [CrossRef]
- Saghatelian, A.; Jessani, N.; Joseph, A.; Humphrey, M.; Cravatt, B.F. Activity-based probes for the proteomic profiling of metalloproteases. Proc. Natl. Acad. Sci. USA 2004, 101, 10000–10005. [Google Scholar] [CrossRef]
- Freije, J.R.; Bischoff, R. Activity-based enrichment of matrix metalloproteinases using reversible inhibitors as affinity ligands. J. Chromatogr. 2003, 1009, 155–169. [Google Scholar] [CrossRef]
- Hesek, D.; Toth, M.; Krchňák, V.; Fridman, R.; Mobashery, S. Synthesis of an inhibitor-tethered resin for detection of active matrix metalloproteinases involved in disease. J. Org. Chem. 2006, 71, 5848–5854. [Google Scholar] [CrossRef]
- Brown, S.; Bernardo, M.M.; Li, Z.-H.; Kotra, L.P.; Tanaka, Y.; Fridman, R.; Mobashery, S. Potent and selective mechanism-based inhibition of gelatinases. J. Am. Chem. Soc. 2000, 122, 6799–6800. [Google Scholar] [CrossRef]
- Gooyit, M.D. Gelatinase Inhibition as a Therapeutic Approach for Treatment of Diseases of Matrix; University of Notre Dame: Notre Dame, IN, USA, 2013. [Google Scholar]
- Forbes, C.; Shi, Q.; Fisher, J.F.; Lee, M.; Hesek, D.; Llarrull, L.I.; Toth, M.; Gossing, M.; Fridman, R.; Mobashery, S. Active site ring-opening of a thiirane moiety and picomolar inhibition of gelatinases. Chem. Biol. Drug Des. 2009, 74, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Testero, S.A.; Lee, M.; Staran, R.T.; Espahbodi, M.; Llarrull, L.I.; Toth, M.; Mobashery, S.; Chang, M. Sulfonate-containing thiiranes as selective gelatinase inhibitors. ACS Med. Chem. Lett. 2011, 2, 177–181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, M.; Nguyen, T.T.; Suckow, M.A.; Wolter, W.R.; Gooyit, M.; Mobashery, S.; Chang, M. Acceleration of diabetic wound healing using a novel protease–anti-protease combination therapy. Proc. Natl. Acad. Sci. USA 2015, 112, 15226–15231. [Google Scholar] [CrossRef] [PubMed]
- Gooyit, M.; Lee, M.; Schroeder, V.A.; Ikejiri, M.; Suckow, M.A.; Mobashery, S.; Chang, M. Selective water-soluble gelatinase inhibitor prodrugs. J. Med. Chem. 2011, 54, 6676–6690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamarat, R.; Silvestre, J.S.; Durie, M.; Levy, B.I. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab. Investig. 2002, 82, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, A.; Inada, M.; Balbín, M.; Fueyo, A.; Pitiot, A.S.; Astudillo, A.; Hirose, K.; Hirata, M.; Shapiro, S.D.; Noël, A.; et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 2007, 21, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Smiell, J.M.; Wieman, T.J.; Steed, D.L.; Perry, B.H.; Sampson, A.R.; Schwab, B.H. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: A combined analysis of four randomized studies. Wound Repair Regen. 1999, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Kleifeld, O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br. J. Cancer 2006, 94, 941. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Minond, D.; Fields, G.B. Clinical implications of compounds designed to inhibit ECM-modifying metalloproteinases. Proteomics 2017, 17, 1600389. [Google Scholar] [CrossRef]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim. Biophys. Acta 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Appleby, T.C.; Greenstein, A.E.; Hung, M.; Liclican, A.; Velasquez, M.; Villaseñor, A.G.; Wang, R.; Wong, M.H.; Liu, X.; Papalia, G.A.; et al. Biochemical characterization and structure determination of a potent, selective antibody inhibitor of human MMP9. J. Biol. Chem. 2017, 292, 6810–6820. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Starodub, A.; Shah, M.A.; Sharma, S.; Wainberg, Z.A.; Thai, D.L. Phase I study of GS-5745 alone and in combination with chemotherapy in patients with advanced solid tumors. J. Clin. Oncol. 2015, 33, 4030. [Google Scholar] [CrossRef]
- Shah, M.A.; Starodub, A.; Sharma, S.; Berlin, J.; Patel, M.; Wainberg, Z.A.; Chaves, J.; Gordon, M.; Windsor, K.; Brachmann, C.B.; et al. Andecaliximab/GS-5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: Results from a phase I study. Clin. Cancer Res. 2018, 24, 3829–3837. [Google Scholar] [CrossRef] [PubMed]
- Sela-Passwell, N.; Kikkeri, R.; Dym, O.; Rozenberg, H.; Margalit, R.; Arad-Yellin, R.; Eisenstein, M.; Brenner, O.; Shoham, T.; Danon, T.; et al. Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat. Med. 2011, 18, 143–147. [Google Scholar] [CrossRef]
- Hu, J.; Van den Steen, P.E.; Houde, M.; Ilenchuk, T.T.; Opdenakker, G. Inhibitors of gelatinase B/matrix metalloproteinase-9 activity: Comparison of a peptidomimetic and polyhistidine with single-chain derivatives of a neutralizing monoclonal antibody. Biochem. Pharmacol. 2004, 67, 1001–1009. [Google Scholar] [CrossRef]
- Paemen, L.; Martens, E.; Masure, S.; Opdenakker, G. Monoclonal antibodies specific for natural human neutrophil gelatinase B used for affinity purification, quantitation by two-Site ELISA and inhibition of enzymatic activity. Eur. J. Biochem. 1995, 234, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2014, 32, 462–467. [Google Scholar] [CrossRef]
- Rayment, E.A.; Dargaville, T.R.; Shooter, G.K.; George, G.A.; Upton, Z. Attenuation of protease activity in chronic wound fluid with bisphosphonate-functionalised hydrogels. Biomaterials 2008, 29, 1785–1795. [Google Scholar] [CrossRef]
- Tronci, G.; Yin, J.; Holmes, R.A.; Liang, H.; Russell, S.J.; Wood, D.J. Protease-sensitive atelocollagen hydrogels promote healing in a diabetic wound model. J. Mater. Chem. B 2016, 4, 7249–7258. [Google Scholar] [CrossRef]
- Jeong, E.H.; Kim, H.; Jang, B.; Cho, H.; Ryu, J.; Kim, B.; Park, Y.; Kim, J.; Lee, J.B.; Lee, H. Technological development of structural DNA/RNA-based RNAi systems and their applications. Adv. Drug Deliv. Rev. 2016, 104, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L.-S. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Georgiou, T.K.; Vamvakaki, M.; Patrickios, C.S.; Yamasaki, E.N.; Phylactou, L.A. Nanoscopic cationic methacrylate star homopolymers: synthesis by group transfer polymerization, characterization and evaluation as transfection reagents. Biomacromolecules 2004, 5, 2221–2229. [Google Scholar] [CrossRef]
- Srinivasachari, S.; Fichter, K.M.; Reineke, T.M. Polycationic β-cyclodextrin “Click Clusters”: Monodisperse and versatile scaffolds for nucleic acid delivery. J. Am. Chem. Soc. 2008, 130, 4618–4627. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhang, Z.X.; Ping, Y.; Li, J.; Kang, E.T.; Neoh, K.G. Star-shaped cationic polymers by atom transfer radical polymerization from β-cyclodextrin cores for nonviral gene delivery. Biomacromolecules 2009, 10, 285–293. [Google Scholar] [CrossRef]
- Cryan, S.-A.; Holohan, A.; Donohue, R.; Darcy, R.; O’Driscoll, C.M. Cell transfection with polycationic cyclodextrin vectors. Eur. J. Pharm. Sci. 2004, 21, 625–633. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, P.; Yan, L.; Chen, L.; Meng, R.; Lao, G. Dynamic changes in matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 levels during wound healing in diabetic rats. J. Am. Podiatr. Med. Assoc. 2009, 99, 489–496. [Google Scholar] [CrossRef]
- Li, N.; Luo, H.-C.; Yang, C.; Deng, J.-J.; Ren, M.; Xie, X.-Y.; Lin, D.-Z.; Yan, L.; Zhang, L.-M. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int. J. Nanomed. 2014, 9, 3377–3387. [Google Scholar] [CrossRef]
- Li, N.; Luo, H.-C.; Ren, M.; Zhang, L.-M.; Wang, W.; Pan, C.-L.; Yang, L.-Q.; Lao, G.-J.; Deng, J.-J.; Mai, K.-j.; et al. Efficiency and safety of β-CD-(D3)7 as siRNA carrier for decreasing matrix metalloproteinase-9 expression and improving wound healing in diabetic rats. ACS Appl. Mater. Interfaces 2017, 9, 17417–17426. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, X.y.; Zhou, L.y.; Lao, G.j.; Liu, D.; Wang, C.; Hu, M.d.; Zeng, T.t.; Yan, L.; et al. MicroRNA-129 and -335 promote diabetic wound healing by inhibiting Sp1-Mediated MMP-9 expression. Diabetes 2018, 67, 1627–1638. [Google Scholar] [CrossRef]




| Treatment | Study Material | Method of MMP Measuring | Effect on MMPs | Reference |
|---|---|---|---|---|
| Vacuum-assisted closure (VAC) | Human chronic wound fluid | Gelatin zymography | Reduced MMP-9 and MMP-2 | [43] |
| VAC | Human chronic wound fluid | ELISA | Reduced MMP-9/TIMP-1 ratio, no change in MMP-9 | [44] |
| Mesenchymal stem cells (MSC) | Mouse model of diabetic wounds | Gelatin zymography, quantitative PCR | Reduced MMP-9 activity and expression | [45] |
| N-acetyl cysteine | HaCat cells treated with MGO | Western blot | Reduced MMP-9 expression | [46] |
| Manuka honey wound dressing | Human DFU patient wound fluid | ELISA | Increased MMP-9 expression (no improvement on ulcer healing) | [47] |
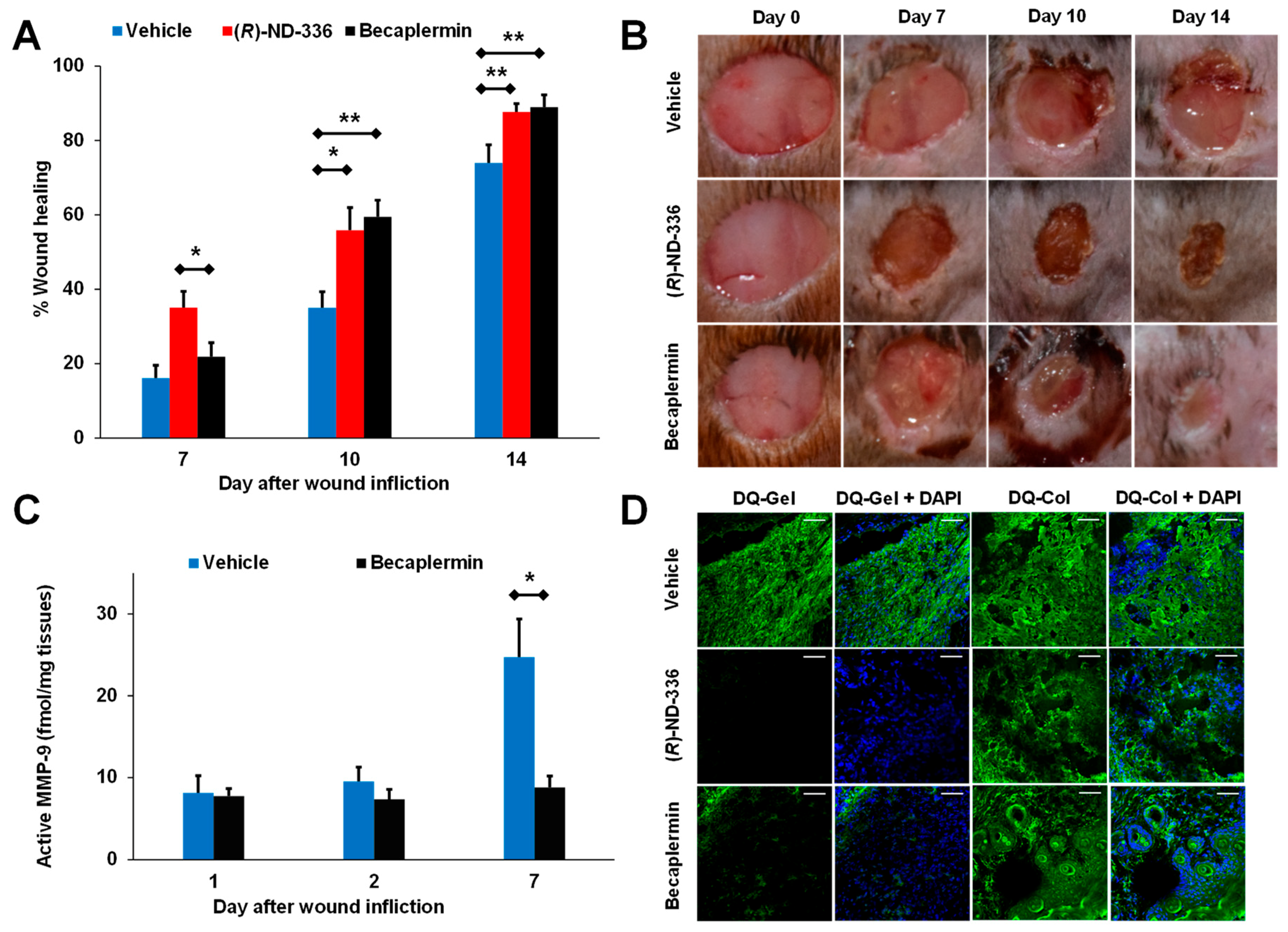
| Becaplermin | Mouse model of diabetic wounds | In-situ zymography and batimastat affinity resin coupled proteomics | Decreased gelatinase activity, no effect on collagenase activity; decreased active MMP-9, no effect on active MMP-8 | [48] |
| Aclerastide | Mouse model of diabetic wounds | Batimastat affinity resin coupled proteomics | Increased active MMP-9, no effect on active MMP-8 | [49] |
| Ki (nM) |  |  |  |  |
| Name | SB-3CT | (R,S)-ND-322 | (R,S)-ND-336 | (R)-ND-336 |
| MMP-9 | 400 ± 15 | 870 ± 110 | 150 ± 10 | 19 ± 3 |
| MMP-8 | 2100 ± 400 | 2600 ± 400 | 7700 ± 100 | 8590 ± 230 |
| Selectivity (Ki MMP-8/Ki MMP-9) | 3.5 | 3.0 | 51 | 450 |
| MMP-9 residence time (min) | 13.4 | 31.4 | 47.4 ± 4.4 | 300 ± 1 |
| Reference | [82,83] | [67] | [86] | [48] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, J.I.; Nguyen, T.T.; Peng, Z.; Chang, M. Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals 2019, 12, 79. https://doi.org/10.3390/ph12020079
Jones JI, Nguyen TT, Peng Z, Chang M. Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals. 2019; 12(2):79. https://doi.org/10.3390/ph12020079
Chicago/Turabian StyleJones, Jeffrey I., Trung T. Nguyen, Zhihong Peng, and Mayland Chang. 2019. "Targeting MMP-9 in Diabetic Foot Ulcers" Pharmaceuticals 12, no. 2: 79. https://doi.org/10.3390/ph12020079
APA StyleJones, J. I., Nguyen, T. T., Peng, Z., & Chang, M. (2019). Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals, 12(2), 79. https://doi.org/10.3390/ph12020079