Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats
Abstract
1. Introduction
2. Results
2.1. Microdialysis
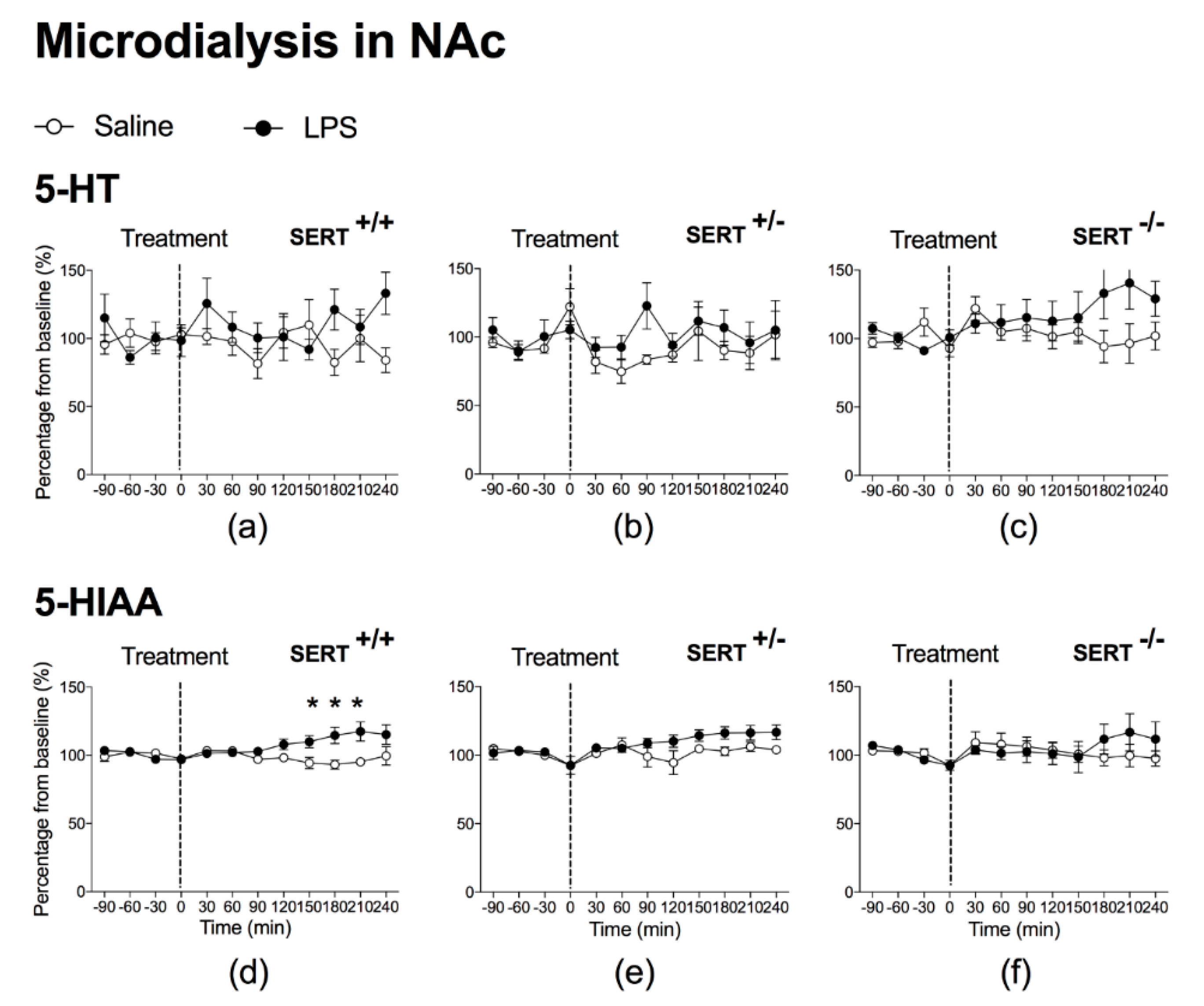
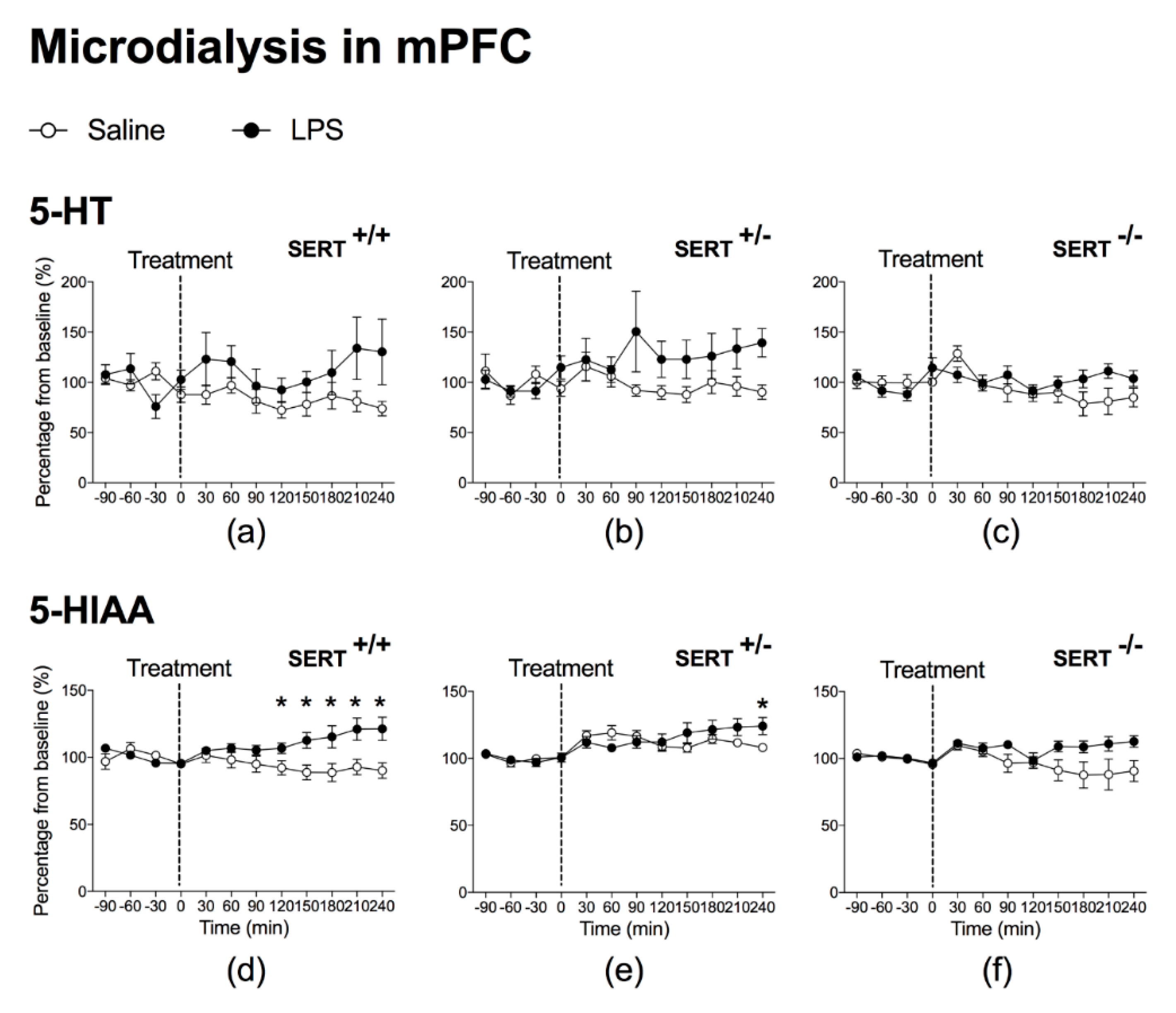
2.1.1. The Effect of Peripheral LPS on 5-HT and 5-HIAA Levels in the NAc and mPFC of Wild Type Rats and SERT (Partial and Total) Knockout Rats
2.1.2. Baseline 5-HT and 5-HIAA Levels in the NAc and mPFC of Wild Type Rats and SERT (Partial and Total) Knockout Rats
3. Discussion
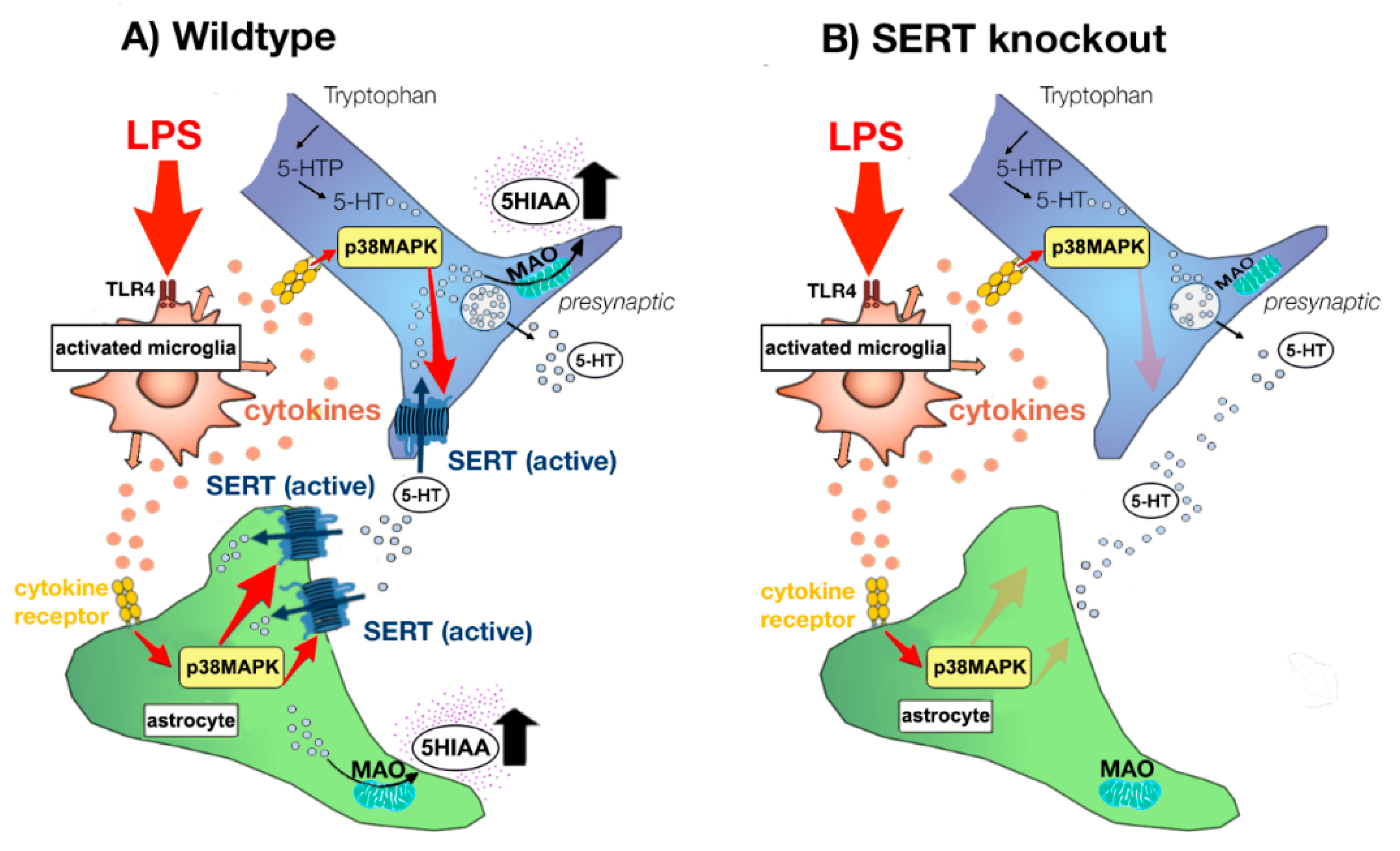
3.1. LPS Increases SERT Activity in Both NAc and mPFC
3.2. Underlying Mechanisms of Increased SERT Activity
3.3. Baseline Differences in 5-HT and 5-HIAA Levels
3.4. Consequences of LPS- and Cytokine-Induced Increases in SERT Activity
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Microdialysis
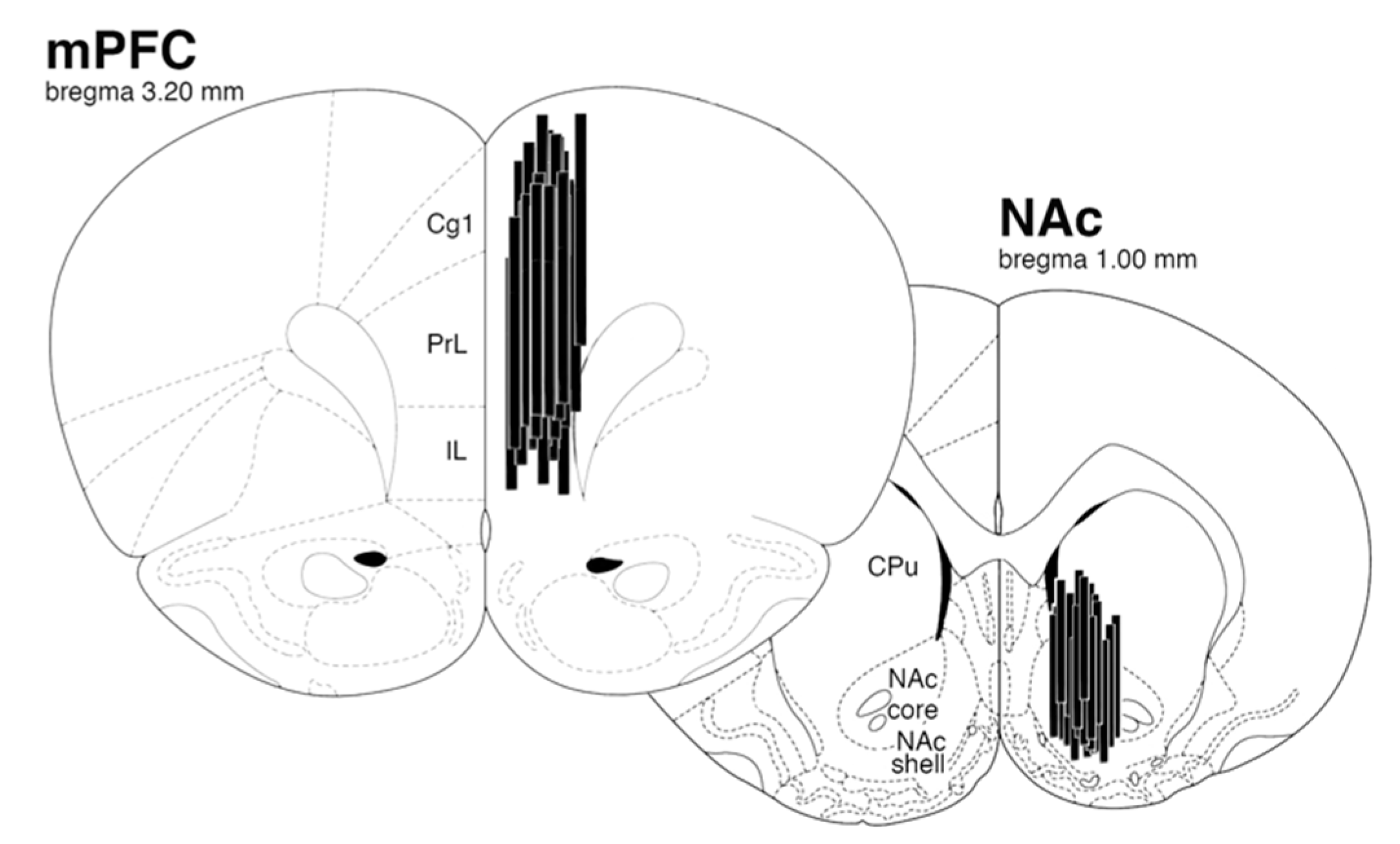
4.3.1. Microdialysis Surgery
4.3.2. Microdialysis Experiment
4.3.3. HPLC
4.3.4. Histology
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Konsman, J.P.; Parnet, P.; Dantzer, R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 2002, 25, 154–159. [Google Scholar] [CrossRef]
- Pollak, Y.; Yirmiya, R. Cytokine-induced changes in mood and behaviour: Implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int. J. Neuropsychopharmacol. 2002, 5, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Akay, A.; Pekcanlar, A.; Bozdag, K.E.; Altintas, L.; Karaman, A. Assessment of depression in subjects with psoriasis vulgaris and lichen planus. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.; Janke, K.H.; Klump, B.; Hinz, A. Anxiety and depression in patients with inflammatory bowel disease: Comparisons with chronic liver disease patients and the general population. Inflamm. Bowel. Dis. 2011, 17, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Isik, A.; Koca, S.S.; Ozturk, A.; Mermi, O. Anxiety and depression in patients with rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V.; Guérin, A.; Yu, A.P.; Wu, E.Q.; Yang, M.; Chao, J.; Mulani, P.M. Increased risks of developing anxiety and depression in young patients with crohn’s disease. Am. J. Gastroenterol. 2011, 106, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.J.; Polis, I.; Koob, G.F.; Markou, A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur. J. Neurosci. 2003, 17, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Borowski, T.; Kokkinidis, L.; Merali, Z.; Anisman, H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport 1998, 9, 3797–3802. [Google Scholar] [CrossRef] [PubMed]
- Van Heesch, F.; Prins, J.; Konsman, J.P.; Westphal, K.G.; Olivier, B.; Kraneveld, A.D.; Korte, S.M. Lipopolysaccharide-induced anhedonia is abolished in male serotonin transporter knockout rats: An intracranial self-stimulation study. Brain Behav. Immun. 2013, 29, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, M.J.; Page, T.H.; Urbaniak, A.M.; Mutch, B.E.; Horwood, N.J. Hck tyrosine kinase regulates TLR4-induced tnf and IL-6 production via AP-1. J. Immunol. 2011, 187, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R.; Olivier, J.D.; Smits, B.M.; Mul, J.D.; Mudde, J.; Verheul, M.; Nieuwenhuizen, O.F.; Cools, A.R.; Ronken, E.; Cremers, T.; et al. Characterization of the serotonin transporter knockout rat: A selective change in the functioning of the serotonergic system. Neuroscience 2007, 146, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- Siesser, W.B.; Sachs, B.D.; Ramsey, A.J.; Sotnikova, T.D.; Beaulieu, J.M.; Zhang, X.; Caron, M.G.; Gainetdinov, R.R. Chronic SSRI treatment exacerbates serotonin deficiency in humanized TPH2 mutant mice. ACS Chem. Neurosci. 2013, 4, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Mössner, R.; Heils, A.; Stöber, G.; Okladnova, O.; Daniel, S.; Lesch, K.P. Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem. Int. 1998, 33, 251–254. [Google Scholar] [CrossRef]
- Zhu, C.B.; Blakely, R.D.; Hewlett, W.A. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006, 31, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.J.; Song, C.; Leonard, B.E.; Anisman, H.; Merali, Z. Stressor-induced alterations in serotonergic activity in an animal model of depression. Neuroreport 1999, 10, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Van Heesch, F.; Prins, J.; Konsman, J.P.; Korte-Bouws, G.A.H.; Westphal, K.G.C.; Rybka, J.; Olivier, B.; Kraneveld, A.D.; Korte, S.M. Lipopolysaccharide increases degradation of central monoamines: An in vivo microdialysis study in the nucleus accumbens and medial prefrontal cortex of mice. Eur. J. Pharmacol. 2014, 725, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Lacosta, S.; Anisman, H. Effects of interleukin-1beta and mild stress on alterations of norepinephrine, dopamine and serotonin neurotransmission: A regional microdialysis study. Brain Res. 1997, 761, 225–235. [Google Scholar] [CrossRef]
- Van Heesch, F.; Prins, J.; Korte-Bouws, G.A.; Westphal, K.G.; Lemstra, S.; Olivier, B.; Kraneveld, A.D.; Korte, S.M. Systemic tumor necrosis factor-alpha decreases brain stimulation reward and increases metabolites of serotonin and dopamine in the nucleus accumbens of mice. Behav. Brain Res. 2013, 253, 191–195. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated byindoleamine 2,3 dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Tolliver, T.J.; Huang, S.J.; Martin, B.J.; Andrews, A.M.; Wichems, C.; Holmes, A.; Lesch, K.P.; Murphy, D.L. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 2005, 49, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Schwamborn, R.; Brown, E.; Haase, J. Elevation of cortical serotonin transporter activity upon peripheral immune challenge is regulated independently of p38 mitogen-activated protein kinase activation and transporter phosphorylation. J. Neurochem. 2016, 137, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Molliver, M.E. Serotonergic neuronal systems: What their anatomic organization tells us about function. J. Clin. Psychopharmacol. 1987, 7, 3S–23S. [Google Scholar] [CrossRef] [PubMed]
- Linthorst, A.C.; Flachskamm, C.; Holsboer, F.; Reul, J.M. Activation of serotonergic and noradrenergic neurotransmission in the rat hippocampus after peripheral administration of bacterial endotoxin: involvement of the cyclo-oxygenase pathway. Neuroscience 1996, 72, 989–997. [Google Scholar] [CrossRef]
- Linthorst, A.C.; Flachskamm, C.; Müller-Preuss, P.; Holsboer, F.; Reul, J.M. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J. Neurosci. 1995, 15, 2920–2934. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J. Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 2006, 6, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Choi, H.B.; Hines, R.M.; Phillips, A.G.; MacVicar, B.A. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS ONE 2013, 8, e60388. [Google Scholar] [CrossRef] [PubMed]
- Gorina, R.; Font-Nieves, M.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between myd88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, S.; Huang, Z.; Zhang, L.; Yang, X.; Bai, X.; Zhou, D.; Qin, Z.; Du, G. Lipopolysaccharide-induced serotonin transporter up-regulation involves pkg-i and p38mapk activation partially through a3 adenosine receptor. Biosci. Trends 2015, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lin, Y.S.; Cheng, J.T.; Lin, C.F.; Wu, H.T.; Wu, S.R.; Tsai, W.H. Interferon-alpha-induced serotonin uptake in Jurkat T cells via mitogen-activated protein kinase and transcriptional regulation of the serotonin transporter. J. Psychopharmacol. 2008, 22, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.; Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—A central role for the serotonin transporter? Pharmacol. Ther. 2015, 147, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Hewlett, W.A.; Feoktistov, I.; Biaggioni, I.; Blakely, R.D. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 2004, 65, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Carneiro, A.M.; Dostmann, W.R.; Hewlett, W.A.; Blakely, R.D. P38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2a-dependent process. J. Biol. Chem. 2005, 280, 15649–15658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Steiner, J.A.; Munn, J.L.; Daws, L.C.; Hewlett, W.A.; Blakely, R.D. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J. Pharmacol. Exp. Ther. 2007, 322, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Morón, J.A.; Zakharova, I.; Ferrer, J.V.; Merrill, G.A.; Hope, B.; Lafer, E.M.; Lin, Z.C.; Wang, J.B.; Javitch, J.A.; Galli, A.; et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 2003, 23, 8480–8488. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, D.P.; Blakely, R.D. Kinase-dependent regulation of monoamine neurotransmitter transporters. Pharmacol. Rev. 2016, 68, 888–953. [Google Scholar] [CrossRef] [PubMed]
- Mathews, T.A.; Fedele, D.E.; Coppelli, F.M.; Avila, A.M.; Murphy, D.L.; Andrews, A.M. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J. Neurosci. Methods 2004, 140, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.D.; Van Der Hart, M.G.; Van Swelm, R.P.; Dederen, P.J.; Homberg, J.R.; Cremers, T.; Deen, P.M.; Cuppen, E.; Cools, A.R.; Ellenbroek, B.A. A study in male and female 5-ht transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience 2008, 152, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.C.; Lesch, K.P.; Murphy, D.L. Serotonin uptake into dopamine neurons via dopamine transporters: A compensatory alternative. Brain Res. 2002, 942, 109–119. [Google Scholar] [CrossRef]
- Olivier, J.D.; Jans, L.A.; Korte-Bouws, G.A.; Korte, S.M.; Deen, P.M.; Cools, A.R.; Ellenbroek, B.A.; Blokland, A. Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology 2008, 200, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Bengel, D.; Murphy, D.L.; Andrews, A.M.; Wichems, C.H.; Feltner, D.; Heils, A.; Mössner, R.; Westphal, H.; Lesch, K.P. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 1998, 53, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Ortiz, N.; Pittman, B.; Bhagwagar, Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav. Immun. 2011, 25, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Galvao-de Almeida, A.; Guindalini, C.; Batista-Neves, S.; de Oliveira, I.R.; Miranda-Scippa, A.; Quarantini, L.C. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen. Hosp. Psychiatry 2010, 32, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Van Heesch, F.; Prins, J.; Westphal, K.G.C.; Korte-Bouws, G.A.H.; Hoevenaar, W.H.M.; Olivier, B.; Kraneveld, A.D.; Korte, S.M. Pro-inflammatory cytokines affect monoamine (metabolite) levels in the nucleus accumbens and induce anhedonia in mice. In Proceedings of the SfN Neuroscience 42nd Annual Meeting of SFN Neuroscience, New Orleans, LA, USA, 13–17 October 2012. [Google Scholar]
- Cunningham, E.T.; Wada, E.; Carter, D.B.; Tracey, D.E.; Battey, J.F.; De Souza, E.B. In situ histochemical localization of type I interleukin-1 receptor messenger rna in the central nervous system, pituitary, and adrenal gland of the mouse. J. Neurosci. 1992, 12, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Baganz, N.L.; Lindler, K.M.; Zhu, C.B.; Smith, J.T.; Robson, M.J.; Iwamoto, H.; Deneris, E.S.; Hewlett, W.A.; Blakely, R.D. A requirement of serotonergic p38α mitogen-activated protein kinase for peripheral immune system activation of cns serotonin uptake and serotonin-linked behaviors. Transl. Psychiatry 2015, 5, e671. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial activation, increased tnf and sert expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Smits, B.M.; Mudde, J.B.; van de Belt, J.; Verheul, M.; Olivier, J.; Homberg, J.; Guryev, V.; Cools, A.R.; Ellenbroek, B.A.; Plasterk, R.H.; et al. Generation of gene knockouts and mutant models in the laboratory rat by enu-driven target-selected mutagenesis. Pharmacogenet. Genom. 2006, 16, 159–169. [Google Scholar]
- Paxinos, G.; Franklin, K.B. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
| Brain Area | Genotype | 5-HT (nM) | 5-HIAA (nM) |
|---|---|---|---|
| NAc | SERT+/+ | 0.11 | 154.25 |
| SERT+/− | 0.15 | 122.51 * | |
| SERT−/− | 0.64 ** | 86.375 ** | |
| mPFC | SERT+/+ | 0.16 | 65.67 |
| SERT+/− | 0.22 | 51.34 ** | |
| SERT−/− | 0.71 ** | 34.91 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korte-Bouws, G.A.H.; Van Heesch, F.; Westphal, K.G.C.; Ankersmit, L.M.J.; Van Oosten, E.M.; Güntürkün, O.; Korte, S.M. Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats. Pharmaceuticals 2018, 11, 66. https://doi.org/10.3390/ph11030066
Korte-Bouws GAH, Van Heesch F, Westphal KGC, Ankersmit LMJ, Van Oosten EM, Güntürkün O, Korte SM. Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats. Pharmaceuticals. 2018; 11(3):66. https://doi.org/10.3390/ph11030066
Chicago/Turabian StyleKorte-Bouws, Gerdien A. H., Floor Van Heesch, Koen G. C. Westphal, Lisa M. J. Ankersmit, Edwin M. Van Oosten, Onur Güntürkün, and S. Mechiel Korte. 2018. "Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats" Pharmaceuticals 11, no. 3: 66. https://doi.org/10.3390/ph11030066
APA StyleKorte-Bouws, G. A. H., Van Heesch, F., Westphal, K. G. C., Ankersmit, L. M. J., Van Oosten, E. M., Güntürkün, O., & Korte, S. M. (2018). Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats. Pharmaceuticals, 11(3), 66. https://doi.org/10.3390/ph11030066





