Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches
Abstract
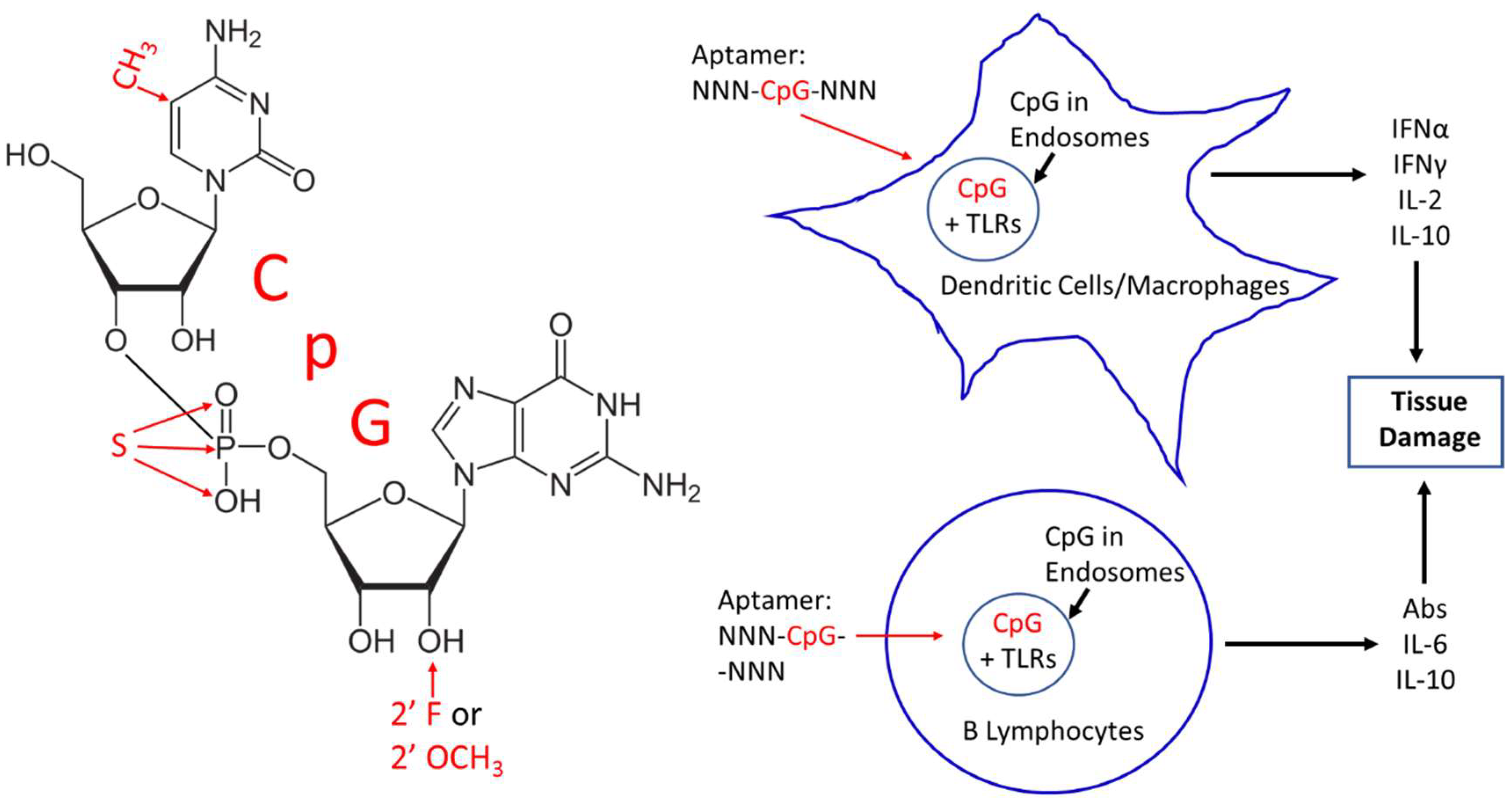
1. Introduction and Background
2. CpG Toxicity Based on Route of Administration and Molecular Context
3. Corrective Strategies
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Mechanisms and applications of immune stimulatory CpG oligodeoxynucleotides. Biochim. Biophys. Acta 1999, 1489, 107–116. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Ann. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Nejad, P.; Häcker, H.; Rutz, M.; Bauer, S.; Vabulas, R.M.; Wagner, H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002, 32, 1958–1968. [Google Scholar] [CrossRef]
- Stacey, K.J.; Young, G.R.; Clark, F.; Sester, D.P.; Roberts, T.L.; Naik, S.; Sweet, M.J.; Hume, D.A. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J. Immunol. 2003, 170, 3614–3620. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, B.D.; Rabadan, R.; Levine, A.J. Patterns of oligonucleotide sequences in viral and host cell RNA identify mediators of the host innate immune system. PLoS ONE 2009, 4, e5969. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Chikh, G.; Luu, R.; Patel, S.; Davis, H.L.; Weeratna, R.D. Effects of KLK peptide on adjuvanticity of different ODN sequences. Vaccines (Basel) 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kuo, C.C. Pivotal role of ADP-ribosylation factor 6 in Toll-like receptor 9-mediated immune signaling. J. Biol. Chem. 2012, 287, 4323–4334. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kuo, C.C. TLR9-mediated ARF6 activation is involved in advancing CpG ODN cellular uptake. Commun. Integr. Biol. 2012, 5, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Kandimalla, E.R. Antisense and/or immunostimulatory oligonucleotide therapeutics. Curr. Cancer Drug Targets 2001, 1, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Lundin, K.E.; Gissberg, O.; Smith, C.I. Oligonucleotide therapies: The past and the present. Hum. Gene Ther. 2015, 26, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, Z.; Tang, X. Chemical modifications of nucleic acid drugs and their delivery systems for gene-based therapy. Med. Res. Rev. 2018, 38, 829–869. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Hann, L.; Michel, T.; Steinle, H.; Stoppelkamp, S.; Stang, K.; Narita, M.; Schlensak, C.; Wendel, H.P. In vitro test system for evaluation of immune activation potential of new single-stranded DNA-based therapeutics. Drug Test Anal. 2015, 7, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Steinle, H.; Michel, T.; Schlensak, C.; Wendel, H.P. Potential capacity of aptamers to trigger immune activation in human blood. PLoS ONE 2013, 8, e68810. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Namperumalsamy, P.; Goldbaum, M.; Cunningham, E.T. Pegaptanib sodium for ocular vascular disease. Indian J. Ophthalmol. 2007, 55, 427–430. Available online: http://www.ijo.in/temp/IndianJOphthalmol556427-4865626_133056.pdf (accessed on 22 May 2018). [PubMed]
- Lee, Y.; Urban, J.H.; Xu, L.; Sullenger, B.A.; Lee, J. 2′ Fluoro modification differentially modulates the ability of RNAs to activate pattern recognition receptors. Nucleic Acid Ther. 2016, 26, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, B.M. The unsolved “solved-problem” of protein folding. J. Biomol. Struct. Dyn. 2013, 31, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Hanson, D.; Bohmann, J.A. DNA aptamer beacon assay for C-telopeptide and handheld fluorometer to monitor bone resorption. J. Fluoresc. 2011, 21, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G. Do it yourself 3-dimensional aptamer-ligand molecular modeling. J. Bionanosci. 2017, 11, 183–186. [Google Scholar] [CrossRef]
- Gong, S.; Wang, Y.; Wang, Z.; Zhang, W. Computational methods for modeling aptamers and designing riboswitches. Int. J. Mol. Sci. 2017, 18, 2442. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, R.; Nahar, S.; Aggarwal, S.; Ramachandran, S.; Maiti, S.; Nahar, P. In silico selection of an aptamer to estrogen receptor alpha using computational docking employing estrogen response elements as aptamer-alike molecules. Sci. Rep. 2016, 6, 21285. [Google Scholar] [CrossRef] [PubMed]
- Albada, H.B.; Golub, E.; Willner, I. Computational docking simulations of a DNA-aptamer for argininamide and related ligands. J. Comput. Aided Mol. Des. 2015, 29, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Borsenberger, V.; Kukwikila, M.; Howorka, S. Synthesis and enzymatic incorporation of modified deoxyuridine triphosphates. Org. Biomol. Chem. 2009, 7, 3826–3835. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Zuniga, M.A.; Carrillo, M.P.; Phillips, T. Development of naturally selected and molecularly engineered intrachain and competitive FRET-aptamers and aptamer beacons. Comb. Chem. High Throughput Screen. 2011, 14, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Andrews, C.J. A novel screening method for competitive FRET-aptamers applied to E. coli assay development. J. Fluoresc. 2010, 20, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. The sweetness of the DNA backbone drives Toll-like receptor 9. Curr. Opin. Immunol. 2008, 20, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, J.W.; Zhang, Y.; Leong, K.W.; Pisetsky, D.; Sullenger, B.A. Nucleic acid-binding polymers as anti-inflammatory agents. Proc. Natl. Acad. Sci. USA 2011, 108, 14055–14060. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Ishii, K.J.; Klinman, D.M. Suppressive oligodeoxynucleotides inhibit CpG-induced inflammation of the mouse lung. Crit. Care Med. 2004, 32, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.J.; Gursel, I.; Gursel, M.; Klinman, D.M. Immunotherapeutic utility of stimulatory and suppressive oligodeoxynucleotides. Curr. Opin. Mol. Ther. 2004, 6, 166–174. [Google Scholar] [PubMed]
- Tluk, S.; Jurk, M.; Forsbach, A.; Weeratna, R.; Samulowitz, U.; Krieg, A.M.; Bauer, S.; Vollmer, J. Sequences derived from self-RNA containing certain natural modifications act as suppressors of RNA-mediated inflammatory immune responses. Int. Immunol. 2009, 21, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Klaschik, S.; Tross, D.; Klinman, D.M. Inductive and suppressive networks regulate TLR9-dependent gene expression in vivo. J. Leukoc. Biol. 2009, 85, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.C.; Shirey, K.A.; Pletneva, L.M.; Boukhvalova, M.S.; Garzino-Demo, A.; Vogel, S.N.; Blanco, J.C.G. Novel drugs targeting Toll-like receptors for antiviral therapy. Future Virol. 2014, 9, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to nano therapeutics. Front. Physiol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G. Predicting the uncertain future of aptamer-based diagnostics and therapeutics. Molecules 2015, 20, 6866–6887. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G. A review of therapeutic aptamer conjugates with emphasis on new approaches. Pharmaceuticals 2013, 6, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T. In vitro antibacterial effects of anti-lipopolysaccharide DNA aptamer-C1qrs complexes. Folia Microbiol. 2008, 53, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Crowell, R. Preliminary development of DNA aptamer-Fc conjugate opsonins. J. Biomed. Mat. Res. A 2009, 90, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Kristian, S.A.; Hwang, J.H.; Hall, B.; Leire, E.; Iacomini, J.; Old, R.; Galili, U.; Roberts, C.; Mullis, K.B.; Westby, M.; et al. Retargeting pre-existing human antibodies to a bacterial pathogen with an alpha-Gal conjugated aptamer. J. Mol. Med. (Berlin) 2015, 93, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Richarte, A.M.; Phillips, T.; Andrews, C.; Lee, J.S. Development, screening, and analysis of a small DNA aptamer library potentially useful for diagnosis and passive immunity of arboviruses. BMC Res. Notes 2012, 5, 633. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Dong, J.; Yao, L.; Chen, A.; Jia, R.; Huan, L.; Guo, J.; Shu, Y.; Zhang, Z. Potent inhibition of human influenza H5N1 virus by oligonucleotides derived by SELEX. Biochem. Biophys. Res. Commun. 2008, 366, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Binning, J.M.; Wang, T.; Luthra, P.; Shabman, R.S.; Borek, D.M.; Liu, G.; Xu, W.; Leung, D.W.; Basler, C.F.; Amarasinghe, G.K. Development of RNA aptamers targeting Ebola virus VP35. Biochemistry 2013, 52, 8406–8419. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.E.; Tanaka, T. Aptamer therapeutics in cancer: Current and future. Cancers (Basel) 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Stecker, J.R.; Savage, A.; Bruno, J.G.; Garcia, D.M.; Koke, J.R. Dynamics and visualization of MCF7 adenocarcinoma cell death by aptamer-C1q-mediated membrane attack. Nucleic Acid Ther. 2012, 22, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Phillips, T.; Montez, T. Preliminary development of DNA aptamers to inhibit phospholipase A2 activity of bee and cobra venoms. J. Bionanosci. 2015, 9, 270–275. [Google Scholar] [CrossRef]
- Lauridsen, L.H.; Veedu, R.N. Nucleic acid aptamers against biotoxins: A new paradigm toward the treatment and diagnostic approach. Nucleic Acid Ther. 2012, 22, 371–399. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.S.; Sullenger, B.A. Modulation of the coagulation cascade using aptamers. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Esposito, C.L.; Rienzo, A.; Catuogno, S.; Iaboni, M.; Condorelli, G.; de Franciscis, V.; Cerchia, L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRβ aptamer. Mol. Ther. 2014, 22, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, J.G. Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches. Pharmaceuticals 2018, 11, 62. https://doi.org/10.3390/ph11030062
Bruno JG. Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches. Pharmaceuticals. 2018; 11(3):62. https://doi.org/10.3390/ph11030062
Chicago/Turabian StyleBruno, John G. 2018. "Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches" Pharmaceuticals 11, no. 3: 62. https://doi.org/10.3390/ph11030062
APA StyleBruno, J. G. (2018). Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches. Pharmaceuticals, 11(3), 62. https://doi.org/10.3390/ph11030062



