Nano-Tailored Triple Gas Sensor for Real-Time Monitoring of Dough Preparation in Kitchen Machines
Abstract
1. Introduction
2. Materials and Methods
2.1. Dough Preparation and Experimental Setup
2.2. S3+ Electronic Nose and Gas Sensor Technology
2.3. Sensor Integration with the Planetary Mixer
2.4. Sensor Calibration and Data Acquisition
Signal Preprocessing
2.5. Chromatography–Mass Spectrometry (GC-MS) Analysis
2.6. Data Analysis and Statistical Processing
3. Results and Discussion
3.1. Leavening Process Evaluation Through Volatile Profiles of Different Doughs
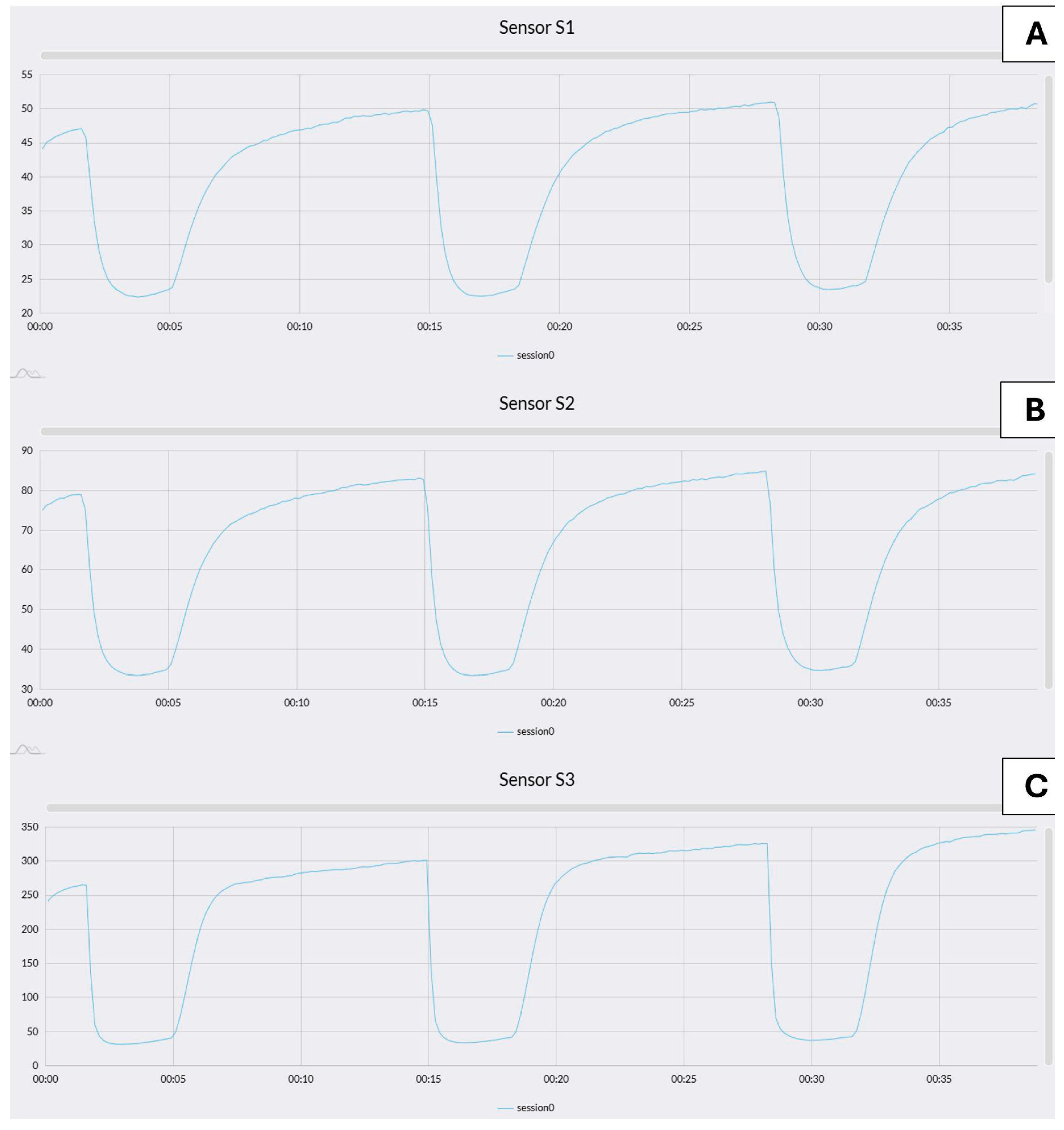
3.1.1. VOCs Analysis by MOS-Sensors (e-Nose)
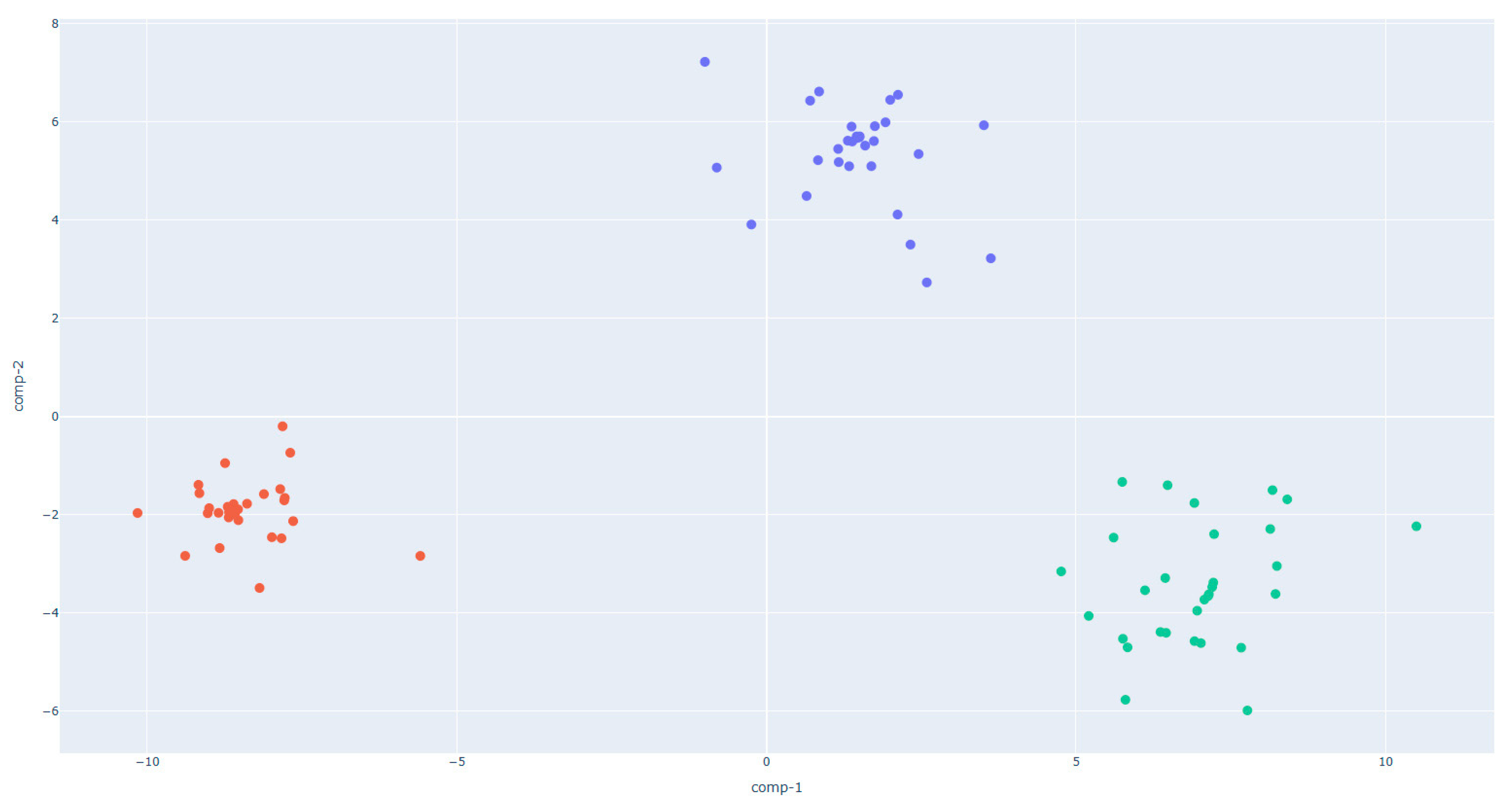
- In the W390 sample (Figure 5), the separation between PRE_W390 and POST_W390 is particularly evident along the first and second discriminant components. The two phases form compact and distinct clusters, indicating significant differences in VOC composition before and after fermentation. This reflects the high gas-retention capacity and slower fermentation kinetics of stronger flours, which lead to more distinct metabolic profiles as fermentation progresses.
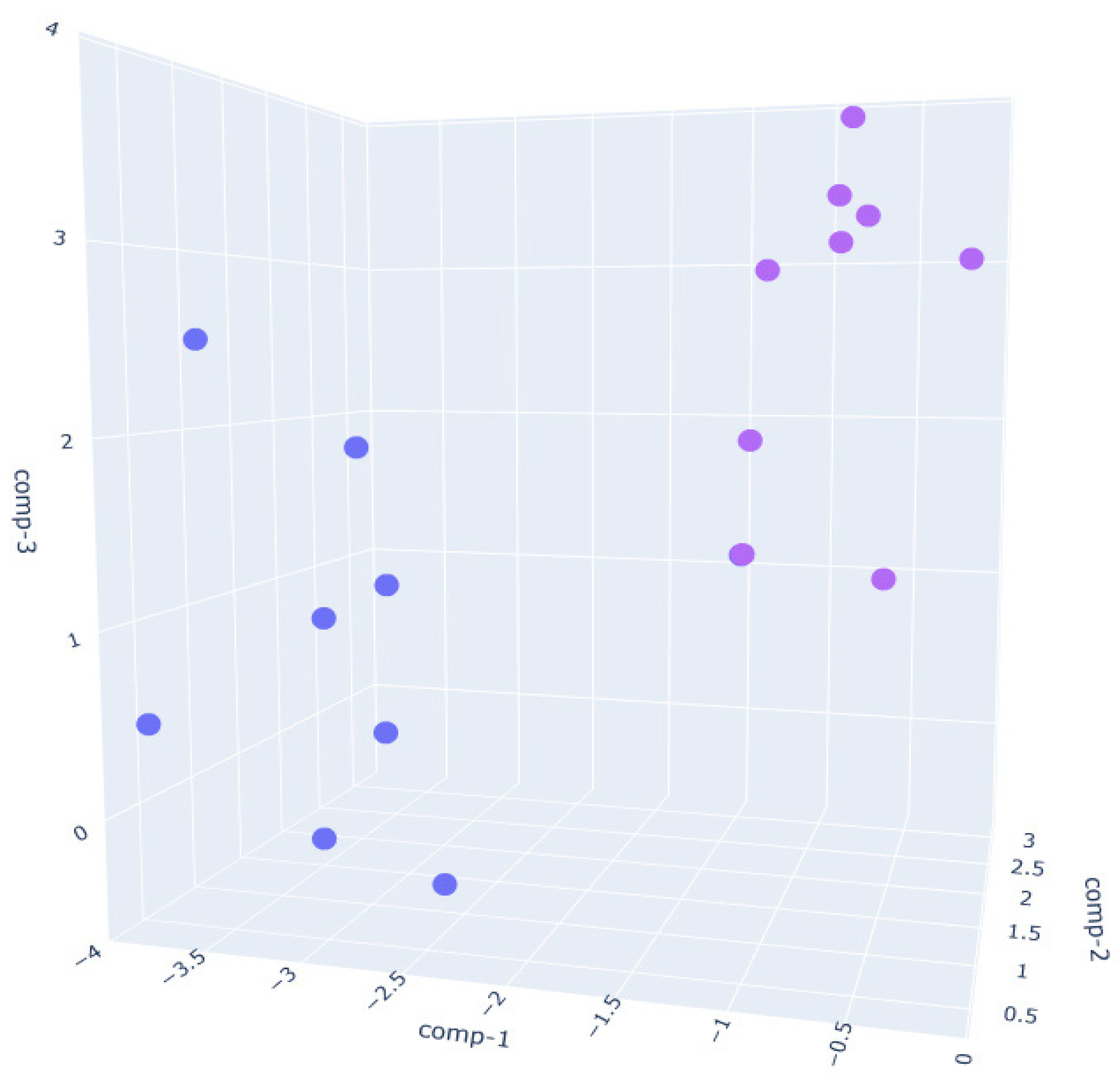
- In the W250 sample (Figure 6), the separation is also clear, though slightly more dispersed than in the W390 case. The clusters remain non-overlapping, suggesting that the VOC fingerprint evolves noticeably during the fermentation phase, albeit with more heterogeneity than W390. This aligns with the intermediate gluten strength of W250, which allows for moderate elasticity and extensibility, leading to a well-structured yet less tightly controlled VOC development.
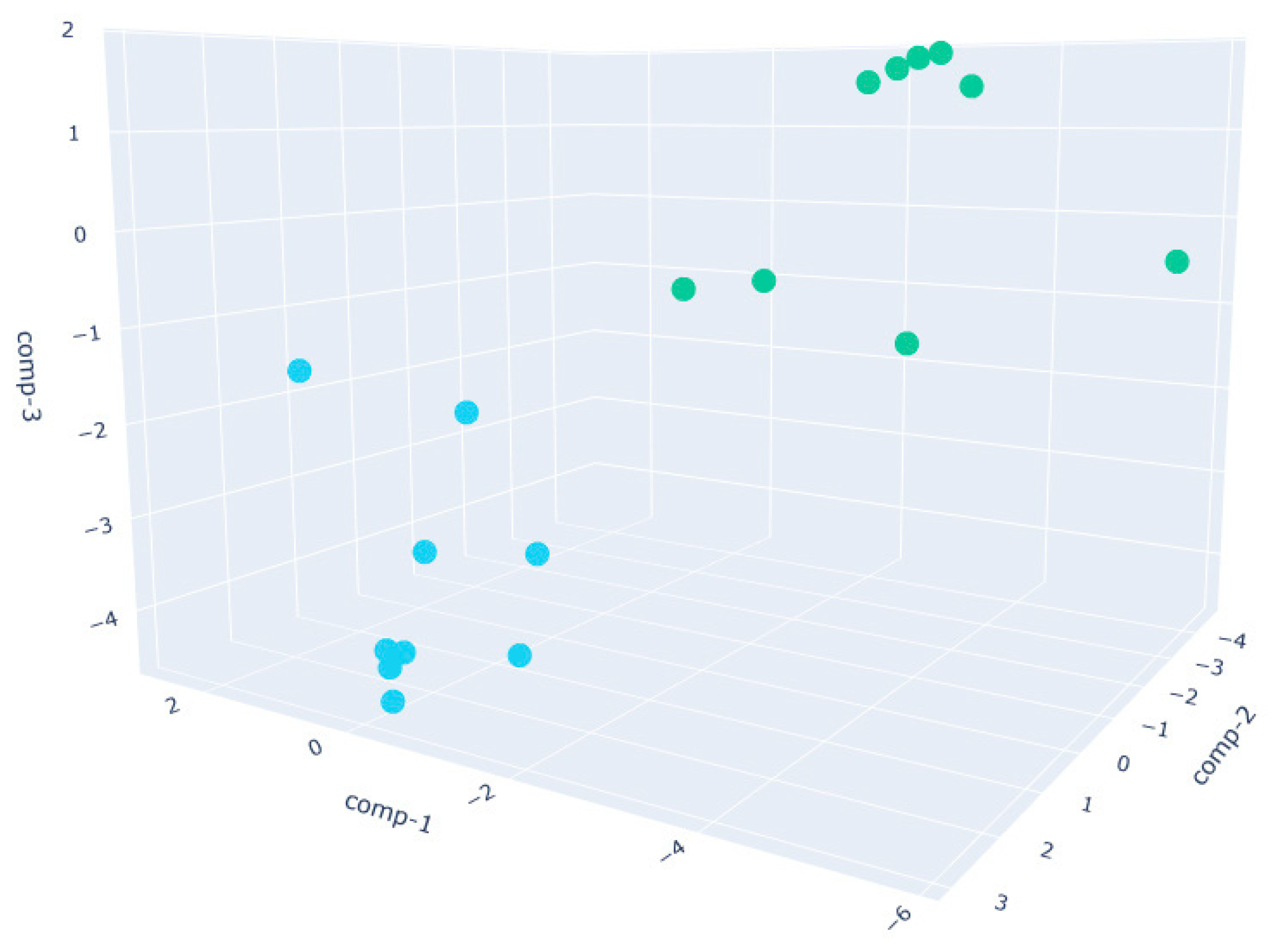
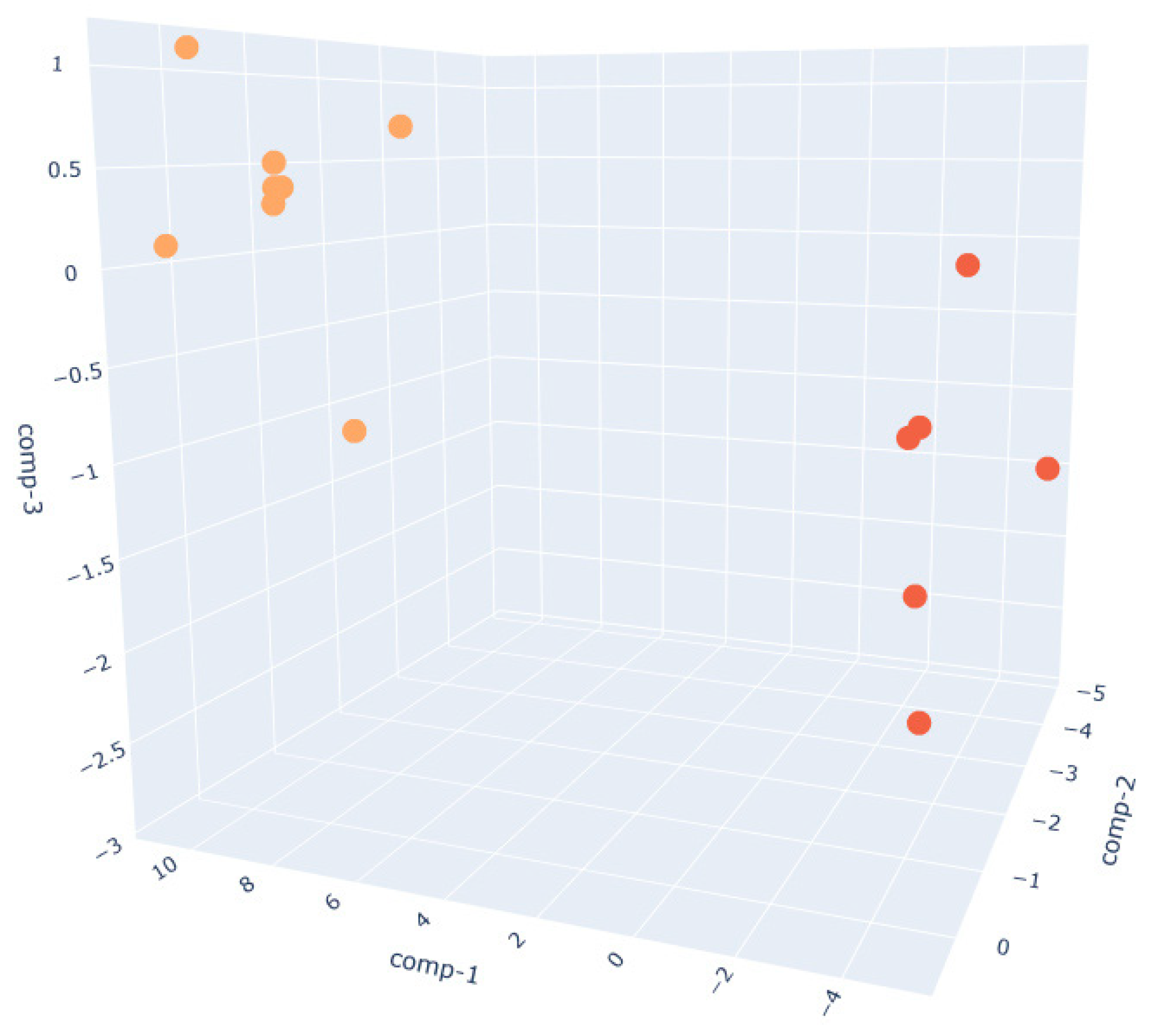
- The W200 sample (Figure 7) exhibits the greatest intra-class variance among the three flour types, as shown by the broader spread of both PRE and POST clusters.This higher signal dispersion observed in W200 samples may be attributed to the intrinsic variability of weaker flours during fermentation. However, sensor-related variability was minimized through baseline normalization, and environmental parameters were kept as constant as possible throughout the trials. Nevertheless, a clear distinction between the two phases is still observed. This broader dispersion may be attributed to the lower gluten content and reduced gas-holding capacity of weaker flours, which can result in less consistent fermentation dynamics and a more variable VOC output. These observations are supported by literature indicating that weaker flours exhibit faster fermentation onset but less stable leavening behavior, with VOC production being highly sensitive to environmental parameters such as humidity and temperature [60].
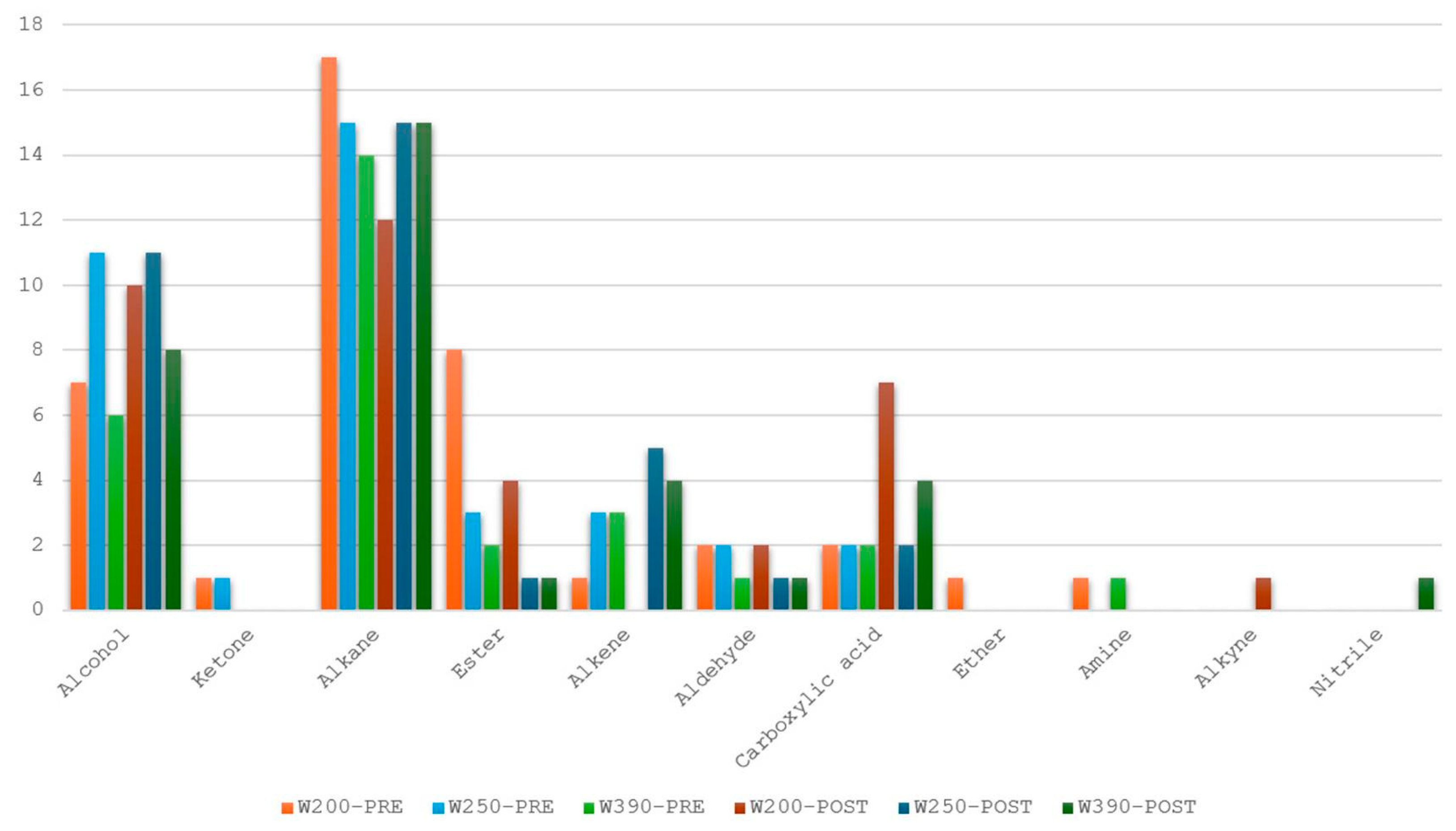
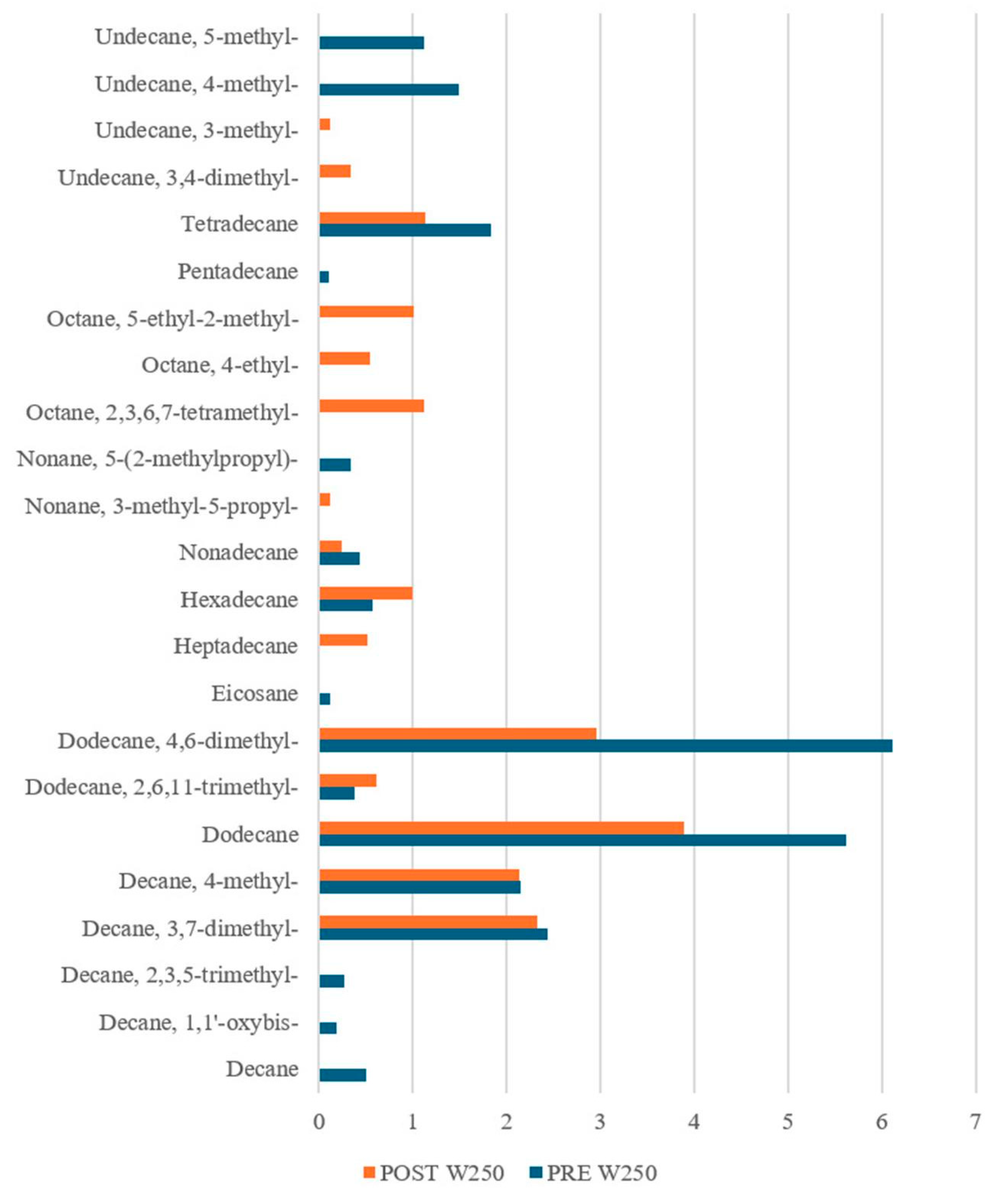
3.1.2. VOC Detection by SPME-GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| SPME-GC-MS | Solid-phase micro-extraction coupled with gas chromatography-mass spectometry |
| MOS | Metal oxide semiconductors |
| IoT | Internet of Things |
References
- Hassoun, A.; Jagtap, S.; Trollman, H.; Garcia-Garcia, G.; Abdullah, N.A.; Goksen, G.; Bader, F.; Ozogul, F.; Barba, F.J.; Cropotova, J.; et al. Food processing 4.0: Current and future developments spurred by the fourth industrial revolution. Food Control 2023, 145, 109507. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, M.; Mujumdar, A.S.; Li, C. AI-based processing of future prepared foods: Progress and prospects. Int. Food Res. 2025, 201, 115675. [Google Scholar] [CrossRef] [PubMed]
- da Costa, T.P.; Gillespie, J.; Cama-Moncunill, X.; Ward, S.; Condell, J.; Ramanathan, R.; Murphy, F. A Systematic Review of Real-Time Monitoring Technologies and Its Potential Application to Reduce Food Loss and Waste: Key Elements of Food Supply Chains and IoT Technologies. Sustainability 2023, 15, 614. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Significance of sensors for industry 4.0: Roles, capabilities, and applications. Sens. Int. 2021, 2, 100110. [Google Scholar] [CrossRef]
- Saeed, R.; Glamuzina, B.; Thi Tuyet Nga, M.; Zhao, F.; Zhang, X. Supervised Learning-Based Artificial Senses for Non-Destructive Fish Quality Classification. Biosensors 2025, 13, 116770. [Google Scholar] [CrossRef]
- Alvarado, V.L.S.; Diaz, F.J.; Parra, L.; Lloret, J.; Aldana, C.; Saavedra, Y. Proposal for a Gas Sensor Device to Classify Hydrobiological Species and Estimate Non-Refrigeration Time. IEEE Sens. J. 2025, 23. in press. [Google Scholar] [CrossRef]
- Poeta, E.; Liboà, A.; Mistrali, S.; Núñez-Carmona, E.; Sberveglieri, V. Nanotechnology and E-Sensing for Food Chain Quality and Safety. Sensors 2023, 23, 8429. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Adamek, M.; Zvonkova, M.; Buresova, I.; Buran, M.; Sevcikova, V.; Sebestikova, R.; Adamkova, A.; Skowronkova, N.; Mlcek, J. Use of a Thermodynamic Sensor in Monitoring Fermentation Processes in Gluten-Free Dough Proofing. Sensors 2023, 23, 534. [Google Scholar] [CrossRef]
- Toska, D.; Pulla, A.; Robustelli, S.; Fiamma, G. Leavening control system based on machine learning techniques. In Proceedings of the 7th World Forum on Internet of Things, New Orleans, LA, USA, 14 June–31 July 2021. [Google Scholar]
- Skaf, A.; Nassar, G.; Lefebvre, F.; Nongaillard, B. A new acoustic technique to monitor bread dough during the fermentation phase. J. Food Eng. 2009, 93, 365–378. [Google Scholar] [CrossRef]
- Romano, A.; Toraldo, G.; Cavella, S.; Masi, P. Description of leavening of bread dough with mathematical modelling. J. Food Eng. 2007, 83, 142–148. [Google Scholar] [CrossRef]
- Rathnayake, H.A.; Navaratne, S.B.; Navaratne, C.M. Porous Crumb Structure of Leavened Baked Products. Int. J. Food Sci. 2018, 1, 8187318. [Google Scholar] [CrossRef] [PubMed]
- Neeharikam, B.; Suneetha, W.J.; Anila Kumari, A.B.; Tejashree, M. Leavening Agents for Food Industry. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1812–1817. [Google Scholar] [CrossRef]
- Kamel, B.; Stauffer, C.E. Advances in Baking Technology; Springer Science + Business Media: Dordrecht, The Netherlands, 1993; pp. 88–106. [Google Scholar]
- de Wijk, R.A.; Smeets, P.A.M.; Polet, I.A.; Holthuysen, N.T.E.; Zoon, J.; Vingerhoeds, M.H. Aroma effects on food choice task behavior and brain responses to bakery food product cues. Food Qual. Prefer. 2018, 68, 304–314. [Google Scholar] [CrossRef]
- Akbari, G.A.; Shehzad, A.; Khan, M.R.; Shabbir, M.A.; Amjid, M.R. Yeast, its types and role in fermentation during bread making process—A Review. Pak. J. Food Sci. 2012, 22, 171–179. [Google Scholar]
- Mesta-Corral, M.; Gomez-Garcia, R.; Balagurusamy, N.; Torres-Leon, C.; Hernández-Almanza, A.Y. Technological and Nutritional Aspects of Bread Production: An Overview of Current Status and Future Challenges. Foods 2024, 13, 2062. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Wang, R. Research progress on the formation mechanism and detection technology of bread flavor. J. Food Sci. 2022, 87, 3724–3736. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast Fermentation at Low Temperatures: Adaptation to Changing Environmental Conditions and Formation of Volatile Compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Petel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Lai, H.; Lin, T. Bakery products: Science and technology. Bak. Prod. Sci. Technol. 2006, 3, 65. [Google Scholar]
- Chen, J.; Pu, D.; Cao, B.; Sun, B.; Zhang, Y. Effect of Temperature and Water Addition Ratio on the Aroma Release of Yeast Proteins. Foods 2025, 14, 1037. [Google Scholar] [CrossRef] [PubMed]
- Aplevicz, K.S.; Ogliari, P.J.; Sant’Anna, E.S. Influence of fermentation time on characteristics of sourdough bread. Braz. J. Pharm. Sci. 2013, 49, 233–239. [Google Scholar] [CrossRef]
- Khromeenkov, V.M. Technology of Long-Term Fermentation of Dough; Confectionery and Bakery Production: Mumbai, India, 2017; Volume 3, pp. 30–32. [Google Scholar]
- Temieva, K.A.; Sheveleva, G.I.; Grigorieva, R.Z. The influence of baking methods on the quality of bakery products. In Food Innovations in Biotechnology: Materials of the VI International Scientific Conference of Students, Postgraduates and Young Scientists; Under the general editorship of A.Y. Prosekov; Kemerovo State University (Kemerovo): Kemerovo, Russia, 2018; Volume 2, pp. 274–275. [Google Scholar]
- Makhoul, S.; Romano, A.; Cappellin, L.; Spano, G.; Capozzi, V.; Aprea, E.; Benozzi, E.; Märk, T.D.; Gasperi, F.; El Nakat, H.; et al. Volatile compound production during the bread-making process: Effect of flour, yeast and their interaction. Food Bioprocess Technol. 2015, 8, 1925–1937. [Google Scholar] [CrossRef]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Sberveglieri, V.; Bhandari, M.P.; Núñez Carmona, E.; Betto, G.; Sberveglieri, G. A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese. Biosensors 2016, 6, 60. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V. Novel equipment for food quality control: An IoT nanowire gas sensors array. Chem. Eng. Trans. 2019, 75, 25–30. [Google Scholar]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors 2021, 21, 4266. [Google Scholar] [CrossRef]
- Sevcikova, V.; Martin Adamek, M.; Sebestikova, R.; Iva Buresova, I.; Buran, M.; Adamkova, A.; Zvonkova, M.; Skowronkova, N.; Matyas, J.; Mlcek, J. New Insights into the Comprehensive System of Thermodynamic Sensors and Electronic Nose and Its Practical Applications in Dough Fermentation Monitoring. Sensors 2024, 24, 352. [Google Scholar] [CrossRef]
- Bax, C.; di Diodoro, B.; Ticozzi, A.; Dellarosa, N.; Corazza, F.; Langfelder, G.; Capelli, L. Smart Odour Sensing for Automated Monitoring of Bread Products. Proceedings 2024, 97, 172. [Google Scholar] [CrossRef]
- di Diodoro, B.; Bax, C.; Dellarosa, N.; Corazza, F.; Langfelder, G.; Capelli, L. Bread baking monitoring by smart sensory system: A feasibility study. In Proceedings of the IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Aveiro, Portugal, 29 May–1 June 2022; pp. 1–4. [Google Scholar]
- Gancarz, M.; Malaga-Toboła, U.; Oniszczuk, A.; Tabor, S.; Oniszczuk, T.; Gawrysiak-Witulska, M.; Rusinek, R. Detection and measurement of aroma compounds with the electronic nose and a novel method for MOS sensor signal analysis during the wheat bread making process. Food Bioprod. Process. 2021, 127, 90–98. [Google Scholar] [CrossRef]
- Ponzoni, A.; Depari, A.; Falasconi, M.; Comini, E.; Flammini, A.; Marioli, D.; Taroni, A.; Sberveglieri, G. Bread baking aromas detection by low-cost electronic nose. Sens. Actuators B Chem. 2008, 130, 100–104. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. On-line monitoring of food fermentation processes using electronic noses and electronic tongues: A review. Anal. Chim. Acta 2013, 804, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, V.; Genzardi, D.; Greco, G.; Nunez-Carmona, E.; Pezzottini, S.; Sberveglieri, G. Discrimination of the Olfactive Fraction of Different Renewable Organic Sources and Their By-products. A New Generation of Mox Sensor Tailor Made Device to Classify the Volatile Fingerprint. Chem. Eng. Trans. 2022, 95, 181–186. [Google Scholar]
- Zhang, S.; Xie, C.; Li, H.; Bai, Z.; Xia, X.; Zeng, D. A reaction model of metal oxide gas sensors and a recognition method by pattern matching. Sens. Actuators B Chem. 2009, 135, 552–559. [Google Scholar] [CrossRef]
- Rossi, A.; Spagnoli, E.; Visonà, A.; Ahmed, D.; Marzocchi, M.; Guidi, V.; Fabbri, B. SO2 Detection over a Wide Range of Concentrations: An Exploration on MOX-Based Gas Sensors. Chemosensors 2024, 12, 111. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. An Array of MOX Sensors and ANNs to Assess Grated Parmigiano Reggiano Cheese Packs’ Compliance with CFPR Guidelines. Biosensors 2020, 10, 47. [Google Scholar] [CrossRef]
- Mariotti, R.; Núñez-Carmona, E.; Genzardi, D.; Pandolfi, S.; Sberveglieri, V.; Mousavi, S. Volatile Olfactory Profiles of Umbrian Extra Virgin Olive Oils and Their Discrimination through MOX Chemical Sensors. Sensors 2022, 22, 7164. [Google Scholar] [CrossRef]
- Genzardi, D.; Núñez Carmona, E.; Poeta, E.; Gai, F.; Caruso, I.; Fiorilla, E.; Schiavone, A.; Sberveglieri, V. Unraveling the Chicken Meat Volatilome with Nanostructured Sensors: Impact of Live and Dehydrated Insect Larvae Feeding. Sensors 2024, 24, 4921. [Google Scholar] [CrossRef]
- Sahner, K.; Schonauer, D.; Matam, M.; Post, M.; Moos, R. Selectivity enhancement of p-type semiconducting hydrocarbon sensors—The use of sol-precipitated nano-powders. Sens. Actuators B Chem. 2008, 130, 470–476. [Google Scholar] [CrossRef]
- Xanthopoulos, P.; Pardalos, P.M.; Trafalis, T.B. Linear Discriminant Analysis. Robust Data Mining, SpringerBriefs in Optimization; Springer: New York, NY, USA, 2013. [Google Scholar]
- Guiné, R.P.F. Textural Properties of Bakery Products: A Review of Instrumental and Sensory Evaluation Studies. Appl. Sci. 2022, 12, 8628. [Google Scholar] [CrossRef]
- Niçin, R.T.; Özdemir, N.; Şimşek, Ö; Çon, A.H. Production of volatiles relation to bread aroma in flour-based fermentation with yeast. Food Chem. 2022, 378, 132125. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Bernal, J.; Gómez, M. Wheat Bread Aroma Compounds in Crumb and Crust: A Review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G. Rapid Quantification of Major Volatile Metabolites in Fermented Food and Beverages Using Gas Chromatography-Mass Spectrometry. Metabolites 2017, 7, 37. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, L.; Chai, D.; Jiang, Y.; Wang, M.; Xu, X.; Li, C.; Dong, L. Metabolite analysis of wheat dough fermentation incorporated with buckwheat. Food Sci. Nutr. 2020, 8, 4242–4251. [Google Scholar] [CrossRef]
- Galoburda, R.; Straumite, E.; Sabovics, M.; Kruma, Z. Dynamics of Volatile Compounds in Triticale Bread with Sourdough: From Flour to Bread. Foods 2020, 9, 1837. [Google Scholar] [CrossRef]
- Sabovics, M.; Straumite, E.; Galoburda, R. Detection of volatile compounds during wheat dough fermentation. Food Chem. 2017, 1, 93–99. [Google Scholar]
- Wang, H.; Han, P.; Zhang, P.; Li, Y. Influence of yeast concentrations and fermentation durations on the physical properties of white bread. LWT-Food Sci. Technol. 2024, 19, 116063. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Yang, Z.; Zhang, M.; Guo, P.; Li, H. Influence of the fermentation time and potato pulp addition on the technological properties and volatile compounds of wheat dough and steamed bread. LWT-Food Sci. Technol. 2020, 128, 10937. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.; Sadiq, F.A.; Sakandar, H.A.; He, G. Evaluation of the effect of Saccharomyces cerevisiae on fermentation characteristics and volatile compounds of sourdough. J. Food Sci. Technol. 2018, 55, 2079–2086. [Google Scholar] [CrossRef]
- Troadec, R.; Regnault, S.; Nestora, S.; Jacolot, P.; Niquet Léridon, C.; Anton, P.M.; Jouquand, C. Effect of fermentation conditions of bread dough on the sensory and nutritional properties of French bread. Eur. Food Res. Technol. 2023, 249, 2749–2762. [Google Scholar] [CrossRef]
- Annett, L.E.; Spaner, D.; Wismer, W.V. Sensory profiles of bread made from paired samples of organic and conventionally grown wheat grain. J. Food Sci. 2007, 72, S254–S260. [Google Scholar] [CrossRef] [PubMed]
- Heiniö, R.L. Sensory Attributes of Bakery Products. In Bakery Products Science and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Salovaara, H.; Valjakka, T. The effect of fermentation temperature, flour type, and starter on the properties of sour wheat bread. Int. J. Food Sci. Technol. 1987, 22, 591–597. [Google Scholar] [CrossRef]
- Starr, G.; Petersen, M.A.; Jespersen, B.M.; Hansen, Å.S. Variation of volatile compounds among wheat varieties and landraces. Food Chem. 2015, 174, 527–537. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.; Gebruers, K.; Delcour, J. Wheat flour constituents: How they impact bread quality and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Lutter, L.; Jõudu, I.; Andreson, H. Volatile organic compounds and their generation in sourdough. Agron. Res. 2023, 21, 504–536. [Google Scholar]
- Mohammadi, N.; Ostovar, N. Chemical composition, fatty acid composition, volatile compounds of a traditional Kurdish fermented cereal food: Tarkhineh. Food Chem. Adv. 2023, 2, 100187. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, A.; Zhao, W.; Liu, Y.; Zhang, Y. Volatile profiles of 13 Foxtail Millet Commercial Cultivars (Setaria italica Beauv.) from China. Cereal Chem. 2017, 94, 170–176. [Google Scholar] [CrossRef]
- Pizarro, F.; Franco, F. Volatile organic compounds at early stages of sourdough preparation via static headspace and GC/MS analysis. Curr. Res. Nutr. 2017, 5, 89–99. [Google Scholar] [CrossRef]
- Fan, H.; Zheng, X.; Ai, Z.; Liu, C.; Li, R.; Bian, K. Analysis of volatile aroma components from Mantou fermented by different starters. Food Process. Preserv. 2018, 42, 13627. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Zou, Y.; He, R.; Ju, X.; Yuan, J. Effect of static-state fermentation on volatile composition in rapeseed meal. J. Sci. Food Agric. 2020, 100, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cao, M.; Cao, A.; Zhang, W. The effect of magnetic nanoparticles plus microwave thawing on the volatile flavor characteristics of Largemouth Bass (Micropterus salmoides) Fillets. Food Bioprocess Technol. 2019, 12, 1340–1351. [Google Scholar] [CrossRef]
- Kieronczyk, A.; Skeie, S.; Olsen, K.; Langsrud, T. Metabolism of amino acids by resting cells of non-starter lactobacilli in relation to flavour development in cheese. Int. Dairy J. 2001, 11, 217–224. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. 2017, 1, 128. [Google Scholar] [CrossRef]
- Hansen, A.S.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Sousa de Almeida, B.; Waszczynskyj, N.; Spier, M.R. Influence of Temperature and of Starter Culture on Biochemical Characteristics and the Aromatic Compounds Evolution on Type II Sourdough and Wheat Bread. LWT 2019, 108, 199–206. [Google Scholar] [CrossRef]
- Özdemir, N.; Şimşek, Ö.; Temiz, H.; Çon, A.H. The effect of fermentation time on the volatile aromatic profile of tarhana dough. Int. J. Food Sci. Technol. 2019, 25, 212–222. [Google Scholar] [CrossRef]
- Frasse, P.; Lambert, S.; Richard-Molard, D.; Chiron, H. The Influence of Fermentation on Volatile Compounds in French Bread Dough. LWT 1993, 26, 126–132. [Google Scholar] [CrossRef]
- Nor Qhairul Izzreen, M.N.; Petersen, M.A.; Hansen, Å.S. Volatile compounds in crumb of whole-meal wheat bread fermented with different yeast levels and fermentation temperatures. Cereal Chem. 2016, 93, 209–216. [Google Scholar] [CrossRef]
- Tasan, M. Fatty acid composition of traditional fermented and unfermented Turkish corn bread with the emphasis on trans fatty acids. Eur. Food Res. Technol. 2003, 217, 125–127. [Google Scholar]




| Ingredients | Amount | Unit |
|---|---|---|
| Flour | 500 | g |
| Water | 300 | mL |
| Dry yeast | 7 | g |
| Salt | 10 | g |
| Sugar | 3 | g |
| Type of Sensor | Doping | Working Temperature (°C) |
|---|---|---|
| MOX sensor | SnO2 | 500 |
| MOX sensor | SnO2 + Pd | 500 |
| MOX sensor | SnO2 + Au | 500 |
| Features | Description |
|---|---|
| Sharpe Forward 25% | Variability index equivalent to the ratio between the mean and the standard deviation, calculated from the beginning of the signal to 25% of it. |
| Sharpe Back 25% | Variability index equivalent to the ratio between the mean and the standard deviation, calculated from the end of the signal to 25% of it. |
| Sharpe Forward 50% | Variability index equivalent to the ratio between the mean and the standard deviation, calculated from the beginning of the signal to 50% of it. |
| Sharpe Back 50% | Variability index equivalent to the ratio between the mean and the standard deviation, calculated from the end of the signal to 50% of it. |
| Minimum derivative | Calculation of the minimum derivative of the function in the selected interval. |
| Maximum derivative | Calculation of the maximum derivative of the function in the selected interval. |
| Integral | Calculation of the integral of the function in the selected interval. |
| ΔR | Often called excursion range, this feature represents the difference between the maximum and minimum values observed in the time series. |
| Logarithm of sum | The sum of the natural logarithm of the signal. |
| Minimum | The minimum value observed in the time series. |
| Maximum | The maximum value observed in the time series. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genzardi, D.; Caruso, I.; Poeta, E.; Sberveglieri, V.; Núñez Carmona, E. Nano-Tailored Triple Gas Sensor for Real-Time Monitoring of Dough Preparation in Kitchen Machines. Sensors 2025, 25, 2951. https://doi.org/10.3390/s25092951
Genzardi D, Caruso I, Poeta E, Sberveglieri V, Núñez Carmona E. Nano-Tailored Triple Gas Sensor for Real-Time Monitoring of Dough Preparation in Kitchen Machines. Sensors. 2025; 25(9):2951. https://doi.org/10.3390/s25092951
Chicago/Turabian StyleGenzardi, Dario, Immacolata Caruso, Elisabetta Poeta, Veronica Sberveglieri, and Estefanía Núñez Carmona. 2025. "Nano-Tailored Triple Gas Sensor for Real-Time Monitoring of Dough Preparation in Kitchen Machines" Sensors 25, no. 9: 2951. https://doi.org/10.3390/s25092951
APA StyleGenzardi, D., Caruso, I., Poeta, E., Sberveglieri, V., & Núñez Carmona, E. (2025). Nano-Tailored Triple Gas Sensor for Real-Time Monitoring of Dough Preparation in Kitchen Machines. Sensors, 25(9), 2951. https://doi.org/10.3390/s25092951








