Potential for Wearable Sensor-Based Field-Deployable Diagnosis and Monitoring of Mild Traumatic Brain Injury: A Scoping Review
Abstract
1. Introduction
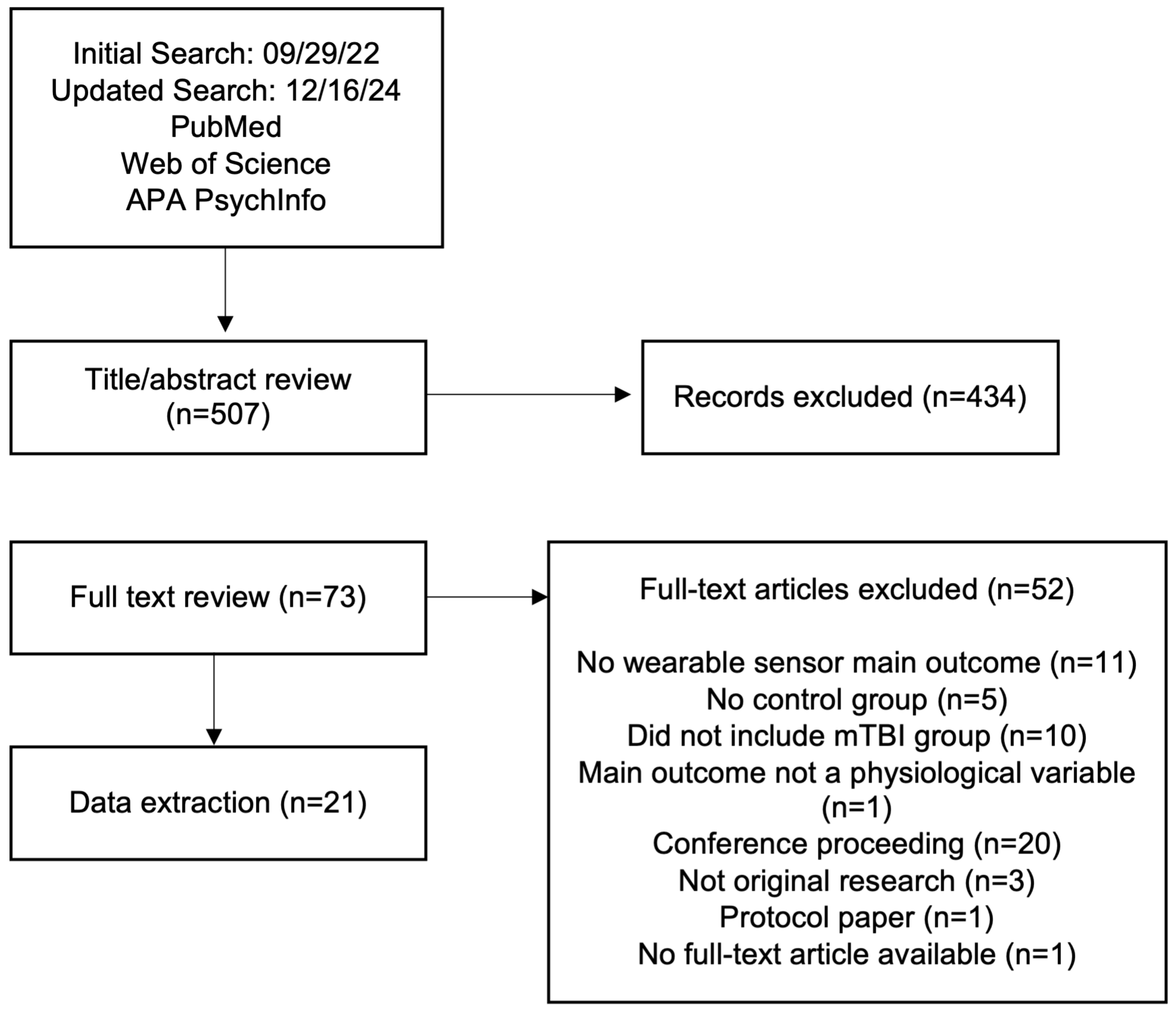
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Selected Studies
3.2. Static Balance
3.2.1. Study Participants
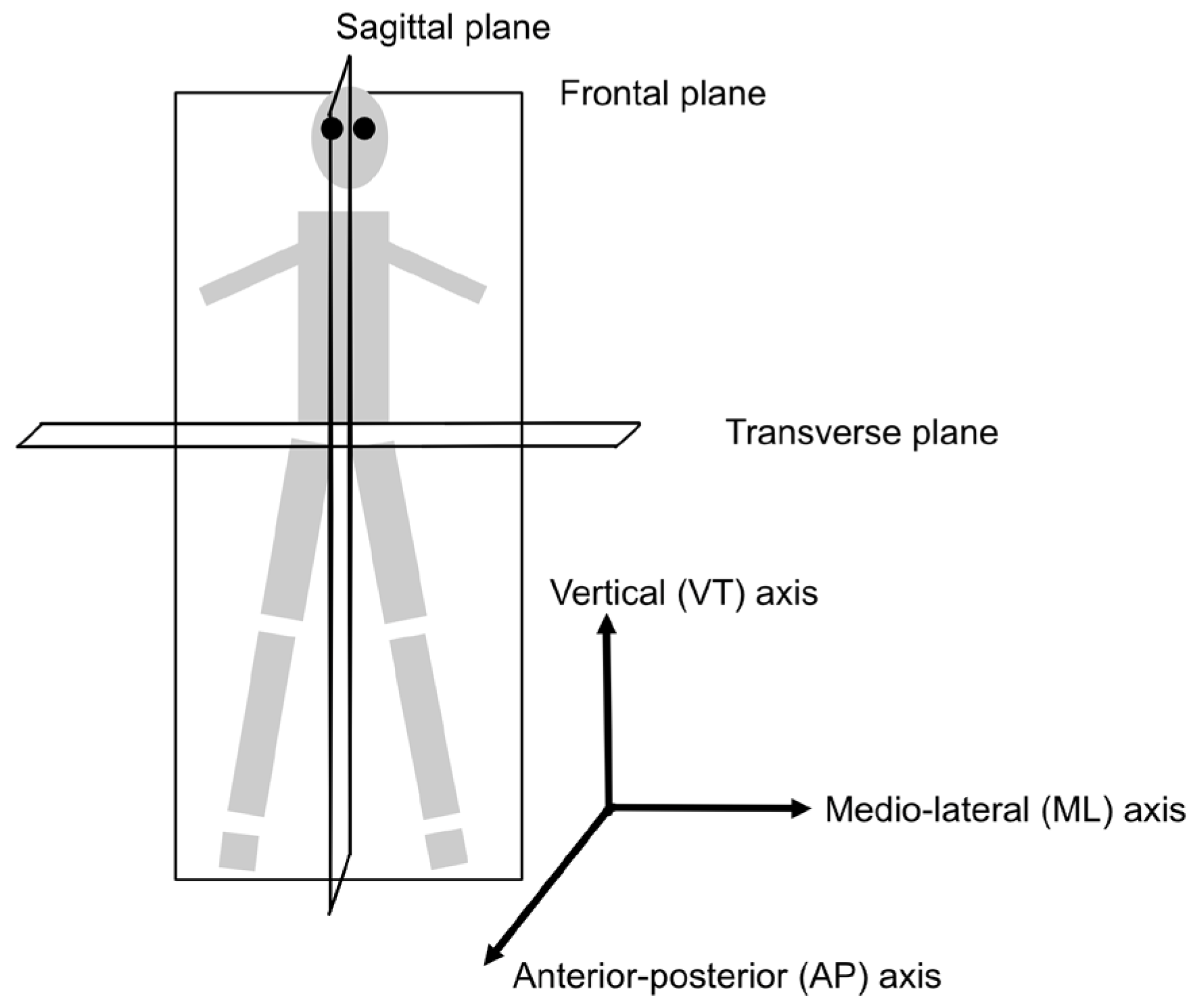
3.2.2. Wearable Sensors and Protocols
3.2.3. Measurement Outcomes
3.3. Gait Tests
3.3.1. Study Participants
3.3.2. Wearable Sensors and Protocols
3.3.3. Measurement Outcomes
3.4. Heart Rate Variability Tests
3.4.1. Study Participants
3.4.2. Wearable Sensors and Protocols
3.4.3. Measurement Outcomes
4. Discussion
4.1. General Observations
4.2. Static Balance
4.3. Gait and Dynamic Tasks
4.4. Heart Rate Variability
4.5. Methodological Limitations and Confounders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DePalma, R.G.; Hoffman, S.W. Combat blast related traumatic brain injury (TBI): Decade of recognition; promise of progress. Behav. Brain Res. 2018, 340, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.J.; Leddy, J.J.; Guskiewicz, K.M.; Seifert, T.; McCrea, M.; Silverberg, N.D.; Feddermann-Demont, N.; Iverson, G.L.; Hayden, A.; Makdissi, M. Rest and treatment/rehabilitation following sport-related concussion: A systematic review. Br. J. Sports Med. 2017, 51, 930–934. [Google Scholar] [CrossRef]
- Stewart, I.J.; Amuan, M.E.; Wang, C.P.; Kennedy, E.; Kenney, K.; Werner, J.K.; Carlson, K.F.; Tate, D.F.; Pogoda, T.K.; Dismuke-Greer, C.E.; et al. Association Between Traumatic Brain Injury and Subsequent Cardiovascular Disease Among Post-9/11-Era Veterans. JAMA Neurol. 2022, 79, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Pugh, M.J.; Orman, J.A.; Jaramillo, C.A.; Salinsky, M.C.; Eapen, B.C.; Towne, A.R.; Amuan, M.E.; Roman, G.; McNamee, S.D.; Kent, T.A.; et al. The prevalence of epilepsy and association with traumatic brain injury in veterans of the Afghanistan and Iraq wars. J. Head Trauma Rehabil. 2015, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.E.; Watrous, J.; Poltavskiy, E.; Howard, J.T.; Janak, J.C.; Pettey, W.B.P.; Zarzabal, L.A.; Sim, A.; Gundlapalli, A.; Stewart, I.J. Longitudinal mental health outcomes of combat-injured service members. Brain Behav. 2021, 11, e02088. [Google Scholar] [CrossRef]
- Peixoto, C.; Buchanan, D.M.; Nahas, R. Missed emergency department diagnosis of mild traumatic brain injury in patients with chronic pain after motor vehicle collision. Pain Physician 2023, 26, 101. [Google Scholar]
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M. The sport concussion assessment tool 5th edition (SCAT5): Background and rationale. Br. J. Sports Med. 2017, 51, 848–850. [Google Scholar] [CrossRef]
- Health.mil. Military Acute Concussion Evaluation (MACE-2). 2021. Available online: https://health.mil/Reference-Center/Publications/2020/07/30/Military-Acute-Concussion-Evaluation-MACE-2 (accessed on 2 April 2025).
- Silverberg, N.D.; Iaccarino, M.A.; Panenka, W.J.; Iverson, G.L.; McCulloch, K.L.; Dams-O’Connor, K.; Reed, N.; McCrea, M.; American Congress of Rehabilitation Medicine Brain Injury Interdisciplinary Special Interest Group Mild TBI Task Force. Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice Guidelines. Arch. Phys. Med. Rehabil. 2020, 101, 382–393. [Google Scholar] [CrossRef]
- Makdissi, M.; Davis, G.; McCrory, P. Clinical challenges in the diagnosis and assessment of sports-related concussion. Neurol. Clin. Pract. 2015, 5, 2–5. [Google Scholar] [CrossRef]
- Levin, H.S.; Diaz-Arrastia, R.R. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015, 14, 506–517. [Google Scholar] [CrossRef]
- Mac Donald, C.L.; Barber, J.; Andre, J.; Panks, C.; Zalewski, K.; Temkin, N. Longitudinal neuroimaging following combat concussion: Sub-acute, 1 year and 5 years post-injury. Brain Commun. 2019, 1, fcz031. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, C.L.; Barber, J.; Andre, J.; Evans, N.; Panks, C.; Sun, S.; Zalewski, K.; Sanders, R.E.; Temkin, N. 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage Clin. 2017, 14, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pacheco, V.; Vargas-Medrano, J.; Tran, E.; Nicolas, M.; Price, D.; Patel, R.; Tonarelli, S.; Gadad, B.S. Prognosis and Diagnostic Biomarkers of Mild Traumatic Brain Injury: Current Status and Future Prospects. J. Alzheimer’s Dis. 2022, 86, 943–959. [Google Scholar] [CrossRef]
- Korfias, S.; Stranjalis, G.; Papadimitriou, A.; Psachoulia, C.; Daskalakis, G.; Antsaklis, A.; Sakas, D. Serum S-100B protein as a biochemical marker of brain injury: A review of current concepts. Curr. Med. Chem. 2006, 13, 3719–3731. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Schmid, K.; Berger, R.P.; Kobeissy, F.; Italiano, D.; Jeromin, A.; Hayes, R.L.; Tortella, F.C.; Buki, A. The challenge of mild traumatic brain injury: Role of biochemical markers in diagnosis of brain damage. Med. Res. Rev. 2014, 34, 503–531. [Google Scholar] [CrossRef]
- King, L.A.; Mancini, M.; Fino, P.C.; Chesnutt, J.; Swanson, C.W.; Markwardt, S.; Chapman, J.C. Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion. Ann. Biomed. Eng. 2017, 45, 2135–2145. [Google Scholar] [CrossRef]
- King, L.A.; Horak, F.B.; Mancini, M.; Pierce, D.; Priest, K.C.; Chesnutt, J.; Sullivan, P.; Chapman, J.C. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2014, 95, 353–359. [Google Scholar] [CrossRef]
- Fino, P.C.; Wilhelm, J.; Parrington, L.; Stuart, S.; Chesnutt, J.C.; King, L.A. Inertial Sensors Reveal Subtle Motor Deficits When Walking With Horizontal Head Turns After Concussion. J. Head Trauma Rehabil. 2019, 34, E74–E81. [Google Scholar] [CrossRef]
- Howell, D.; Osternig, L.; Chou, L.-S. Monitoring recovery of gait balance control following concussion using an accelerometer. J. Biomech. 2015, 48, 3364–3368. [Google Scholar] [CrossRef]
- Russell, K.N.; Preble, E.A.; Hegarty-Craver, M.; Arrieux, J.P.; Cole, W.R.; Choi, Y.S.; Grego, S.; Rae Olmsted, K.; Gilchrist, K.H. Feasibility of Mild Traumatic Brain Injury Assessment Based on Cardiovascular Response to Postural Change. J. Head Trauma Rehabil. 2020, 35, E422–E428. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narvaez, E.; Guder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid.-Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Campbell, K.R.; Wilhelm, J.L.; Antonellis, P.; Scanlan, K.T.; Pettigrew, N.C.; Martini, D.N.; Chesnutt, J.C.; King, L.A. Assessing the Effects of Mild Traumatic Brain Injury on Vestibular Home Exercise Performance with Wearable Sensors. Sensors 2023, 23, 9860. [Google Scholar] [CrossRef]
- Delling, A.C.; Jakobsmeyer, R.; Coenen, J.; Christiansen, N.; Reinsberger, C. Home-Based Measurements of Nocturnal Cardiac Parasympathetic Activity in Athletes during Return to Sport after Sport-Related Concussion. Sensors 2023, 23, 4190. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Zhao, L.; Ryan, J.; Komaba, Y.; Inomata, A.; Caulfield, B. Quantification of postural control deficits in patients with recent concussion: An inertial-sensor based approach. Clin. Biomech. 2017, 42, 79–84. [Google Scholar] [CrossRef]
- Doherty, C.; Zhao, L.; Ryan, J.; Komaba, Y.; Inomata, A.; Caulfield, B. Concussion is associated with altered preparatory postural adjustments during gait initiation. Hum. Mov. Sci. 2017, 52, 160–169. [Google Scholar] [CrossRef]
- Favorov, O.; Kursun, O.; Challener, T.; Cecchini, A.; McCulloch, K.L. Wearable Sensors Detect Movement Differences in the Portable Warrior Test of Tactical Agility After mTBI in Service Members. Mil. Med. 2023, 188, e637–e645. [Google Scholar] [CrossRef]
- Gera, G.; Chesnutt, J.; Mancini, M.; Horak, F.B.; King, L.A. Inertial Sensor-Based Assessment of Central Sensory Integration for Balance After Mild Traumatic Brain Injury. Mil. Med. 2018, 183, 327–332. [Google Scholar] [CrossRef]
- Harrison, A.; Lane-Cordova, A.; La Fountaine, M.F.; Moore, R.D. Concussion History and Heart Rate Variability During Bouts of Acute Stress. J. Athl. Train. 2022, 57, 741–747. [Google Scholar] [CrossRef]
- Loyd, B.J.; Dibble, L.E.; Weightman, M.M.; Pelo, R.; Hoppes, C.W.; Lester, M.; King, L.A.; Fino, P.C. Volitional Head Movement Deficits and Alterations in Gait Speed Following Mild Traumatic Brain Injury. J. Head. Trauma. Rehabil. 2023, 38, E223–E232. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.N.; Gera, G.; Brumbach, B.H.; Campbell, K.R.; Parrington, L.; Chesnutt, J.; King, L.A. Symptoms and Central Sensory Integration in People With Chronic mTBI: Clinical Implications. Mil. Med. 2023, 188, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.; Chen, S.-H.; Chou, L.-S. Using IMU-based kinematic markers to monitor dual-task gait balance control recovery in acutely concussed individuals. Clin. Biomech. 2020, 80, 105145. [Google Scholar] [CrossRef]
- Powell, D.; Godfrey, A.; Parrington, L.; Campbell, K.R.; King, L.A.; Stuart, S. Free-living gait does not differentiate chronic mTBI patients compared to healthy controls. J. Neuroeng. Rehabil. 2022, 19, 49. [Google Scholar] [CrossRef]
- Ralston, J.D.; Raina, A.; Benson, B.W.; Peters, R.M.; Roper, J.M.; Ralston, A.B. Physiological Vibration Acceleration (Phybrata) Sensor Assessment of Multi-System Physiological Impairments and Sensory Reweighting Following Concussion. Med. Devices 2020, 13, 411–438. [Google Scholar] [CrossRef] [PubMed]
- Sas, A.R.; Popovich, M.J.; Gillenkirk, A.; Greer, C.; Grant, J.; Almeida, A.; Ichesco, I.K.; Lorincz, M.T.; Eckner, J.T. Orthostatic Vital Signs After Sport-Related Concussion: A Cohort Study. Am. J. Sports Med. 2024, 52, 2902–2910. [Google Scholar] [CrossRef]
- Sharma, B.; Obeid, J.; DeMatteo, C.; Noseworthy, M.D.; Timmons, B.W. New Insights Into Accelerometer-Measured Habitual Physical Activity and Sedentary Time During Early Recovery in Pediatric Concussion. Pediatr. Exerc. Sci. 2024, 36, 58–65. [Google Scholar] [CrossRef]
- Sinnott, A.M.; Kochick, V.L.; Eagle, S.R.; Trbovich, A.M.; Collins, M.W.; Sparto, P.J.; Flanagan, S.D.; Elbin, R.J.; Connaboy, C.; Kontos, A.P. Comparison of physiological outcomes after dynamic exertion between athletes at return to sport from concussion and controls: Preliminary findings. J. Sci. Med. Sport 2023, 26, 682–687. [Google Scholar] [CrossRef]
- Stuart, S.; Parrington, L.; Martini, D.N.; Kreter, N.; Chesnutt, J.C.; Fino, P.C.; King, L.A. Analysis of Free-Living Mobility in People with Mild Traumatic Brain Injury and Healthy Controls: Quality over Quantity. J. Neurotrauma 2020, 37, 139–145. [Google Scholar] [CrossRef]
- Riemann, B.L.; Guskiewicz, K.M. Effects of mild head injury on postural stability as measured through clinical balance testing. J. Athl. Train. 2000, 35, 19. [Google Scholar]
- Kristoffersson, A.; Linden, M. A Systematic Review of Wearable Sensors for Monitoring Physical Activity. Sensors 2022, 22, 573. [Google Scholar] [CrossRef]
- Degani, A.M.; Santos, M.M.; Leonard, C.T.; Rau, T.F.; Patel, S.A.; Mohapatra, S.; Danna-Dos-Santos, A. The effects of mild traumatic brain injury on postural control. Brain Inj. 2017, 31, 49–56. [Google Scholar] [CrossRef]
- Fino, P.C.; Parrington, L.; Pitt, W.; Martini, D.N.; Chesnutt, J.C.; Chou, L.S.; King, L.A. Detecting gait abnormalities after concussion or mild traumatic brain injury: A systematic review of single-task, dual-task, and complex gait. Gait Posture 2018, 62, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.J.; Batycky, J.; Campbell, C.; Schneider, K.; Smirl, J.; Debert, C.T. Autonomic dysfunction in adults following mild traumatic brain injury: A systematic review. NeuroRehabilitation 2022, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.G.; Allerberger, F. How to: Evaluate a diagnostic test. Clin. Microbiol. Infect. 2019, 25, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.D.; Terry, D.P.; Ko, J.; Newell, K.M.; Miller, L.S. Balance Regularity Among Former High School Football Players With or Without a History of Concussion. J. Athl. Train. 2018, 53, 109–114. [Google Scholar] [CrossRef]
- Merritt, E.D.; Brown, C.N.; Queen, R.M.; Simpson, K.J.; Schmidt, J.D. Concussion History and Time Since Concussion Do not Influence Static and Dynamic Balance in Collegiate Athletes. J. Sport Rehabil. 2017, 26, 518–523. [Google Scholar] [CrossRef]
- Hilz, M.J.; Liu, M.; Koehn, J.; Wang, R.; Ammon, F.; Flanagan, S.R.; Hosl, K.M. Valsalva maneuver unveils central baroreflex dysfunction with altered blood pressure control in persons with a history of mild traumatic brain injury. BMC Neurol. 2016, 16, 61. [Google Scholar] [CrossRef]
- Hilz, M.J.; DeFina, P.A.; Anders, S.; Koehn, J.; Lang, C.J.; Pauli, E.; Flanagan, S.R.; Schwab, S.; Marthol, H. Frequency analysis unveils cardiac autonomic dysfunction after mild traumatic brain injury. J. Neurotrauma 2011, 28, 1727–1738. [Google Scholar] [CrossRef]
- Franklin, J.M.; Howell, G.; Ledgerwood, S.; Griffith, J.L. Security Analysis of First Responder Mobile and Wearable Devices; US Department of Commerce, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [Google Scholar]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-term heart rate variability--influence of gender and age in healthy subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef]
- Røgind, H.; Lykkegaard, J.; Bliddal, H.; Danneskiold-Samsøe, B. Postural sway in normal subjects aged 20–70 years. Clin. Physiol. Funct. Imaging 2003, 23, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Schreiber, S.; Pick, C.; Been, E. Gait, balance and posture in major mental illnesses: Depression, anxiety and schizophrenia. Austin Med. Sci. 2020, 5, 1039. [Google Scholar]
| Author, Year | Paper Group |
Wearable Sensor (# of Sensors Used) | Participants with mTBI | Control Participants | |||||
|---|---|---|---|---|---|---|---|---|---|
|
Age (Years) Mean (SD) |
Time Since mTBI Mean (SD or Range) | N | % Female |
Age (Years) Mean (SD) | N | % Female | |||
| Campbell 2023 [25] | SBT, GT | IMU (2) | 37 (12) | 74 (32) days | 73 | na | 41 (12) | 50 | Not available |
| Deling 2023 [26] | HRT | PPG (1) | 23 (5) | 13 (21) days | 18 | 17 (n = 3) | 23 (5) | 18 | 17 (n = 3) |
| Doherty 2017 [27] | SBT | IMU (3) | 22 (4) | 9 (7) days | 15 | 27 (n = 4) | 22 (4) | 15 | 27 (n = 4) |
| Doherty 2017 [28] | SBT | IMU (3) | 22 (4) | 9 (7) days | 15 | 27 (n = 4) | 22 (4) | 15 | 27 (n = 4) |
| Favorov 2021 [29] | GT | IMU (2) | 29 (6) | Within 2 years | 42 | na | 30 (6.7) | 57 | na |
| Fino 2019 [19] | GT | IMU (4) | 20.3 (1.3) | 2 (0.6) days | 24 | 25 (n = 6) | 20.9 (1.4) | 25 | 24 (n = 6) |
| Gera 2018 [30] | SBT | IMU (1) | 20.6 (1.3) | 2–3 days | 38 | 34 (n = 13) | 21.0 (1.4) | 81 | 46 (n = 37) |
| Harrison 2022 [31] | HRT | ECG (1) | 16.06 (0.73) | 24.13 (17.7) months | 16 | 0 (n = 0) | 15.98 (0.62) | 18 | 0 (n = 0) |
| Howell 2015 [20] | GT | IMU (1) | 19.0 (5.5) | 2.0 (0.8) days | 10 | 30 (n = 3) | 20.0 (4.5) | 7 | 57 (n = 4) |
| King 2014 [18] | SBT | IMU (1) | 16.3 (2.0) | 5 (3.3) months | 13 | 77 (n = 10) | 16.7 (2.0) | 13 | 77 (n = 10) |
| King 2017 [17] | SBT | IMU (1) | 20.4 (1.3) | 2.2 (1.2) days | 52 | 33 (n = 17) | 20.6 (1.4) | 76 | 50 (n = 38) |
| Loyd 2023 [32] | GT | IMU (7) | 33.0 (9.5) | 244 (21–989) days | 45 | 80% (n = 36) | 31.5 (9.5) | 46 | 72 (n = 33) |
| Martini 2022 [33] | SBT | IMU (1) | 39.8 (11.5) | 2.3 (2.0) years | 41 | 71 (n = 29) | 36.5 (12.1) | 53 | 60 (n = 32) |
| Pitt 2020 [34] | GT | IMU (3) | 20.1 (1.3) | 1.8 (0.6) days | 11 | 64 (n = 7) | 20.6 (1.9) | 11 | 64 (n = 7) |
| Powell 2022 [35] | GT | IMU (1) | 40.9 (11.8) | 440.7 (700.6) days | 32 | 81 (n = 26) | 48.6 (22.6) | 23 | 74 (n = 17) |
| Ralston 2020 [36] | SBT | IMU (1) | 18.8 (13.2) | Within 30 days | 92 | 55 (n = 51) | 17.2 (7.7) | 83 | 52 (n = 43) |
| Russell 2020 [21] | HRT | ECG (1) | 23.8 (4.6) | Within 72 h | 31 | 10 (n = 3) | 24.0 (4.8) | 32 | 12.5 (n = 4) |
| Sas 2024 [37] | HRT | PPG (1) | 15.3 | Within 30 days | 133 | 45.9 | 15.7 | 100 | 54 |
| Sharma 2024 [38] | GT | ACC (1) | 12.7 (2.8) | Within 4 weeks | 60 | 52 (n = 31) | 12.4 (2.7) | 60 | 52 (n = 31) |
| Sinnott 2023 [39] | HRT | ECG (1) | 16.3 (2.3) | 18.5 (12.3) | 13 | 31 (n = 4) | 16.3 (2.3) | 13 | 31 (n = 4) |
| Stuart 2020 [40] | GT | IMU (1) | 40.2 (12.1) | 419 days | 29 | 79 (n = 23) | 48.6 (23.1) | 23 | 74 (n = 17) |
| Task | Condition | Postural Sway Outcome Stratified by Time Since mTBI | ||
|---|---|---|---|---|
| Days | Weeks | Months | ||
| Postural adjustment during gait initiation | Leading with dominant and non-dominant limb | Reduced COM acceleration and displacement [28] | ||
| BESS or Modified BESS | Bilateral stance | Greater COM acceleration, greater power, greater sway area [17] | Greater COM sway volume [27] | |
| Tandem stance | No change in COM sway volume [27] | |||
| Unilateral stance | No change in COM sway volume [27] | |||
| Average of three stances | Greater COM acceleration; BESS AUROC = 0.70 [0.50–0.91]; modified BESS AUROC = 0.81 (0.64–0.99) [18] | |||
| CTSIB | Eyes open, firm surface | Greater COM sway area [30] | Greater COM acceleration [33] | |
| Eyes closed firm surface | Greater COM sway area [30] | Greater COM sway area [33] | ||
| Eyes closed foam surface | ||||
| Eyes open foam surface | ||||
| Average of four conditions | Greater COM sway area; AUROC = 0.77 (0.60–0.85) [33] | |||
| Sensory reweighting firm surface | No change in COM sway area [30] | |||
| Sensory reweighting foam surface | Greater COM sway area [30] | |||
| Eyes closed + eyes open balance | Average of eyes closed and eyes open on firm surface | Greater average power, AUROC = 0.98 (0.96–0.99) [36] | ||
| Balance with head turns | Eyes open, firm surface | Slower forehead and sternum peak angular velocity, larger forehead and sternum range of motion [25] | ||
| Task | Condition | Gait Outcomes Stratified by Time Since mTBI | ||
|---|---|---|---|---|
| Days | Weeks | Months | ||
| Walking | Head rotation | Reduced angular velocity, AUROC = 0.73 [0.56–0.85] [19] | Reduced forehead peak angular velocity [25] Slower and smaller head rotations horizontally and slower head rotations vertically [32] Slower gait speed and greater percent reduction in gait speed during walking with horizontal head rotations [32] | |
| Dual task walking gait | Gait | Reduced speed [20] | Reduced speed [20,34] | NC [20] |
| Second half of gait cycle | Reduced peak frontal acceleration [20] | Reduced peak frontal acceleration [20] | ||
| Reduced peak ML acceleration, AUROC = 0.889 [20] | Reduced peak ML acceleration, AUROC = 0.810 [20] | |||
| Right heel strike | Reduced VT peak angular velocity [34] | Reduced VT peak angular velocity [34] | ||
| Free-living gait | Turning | Greater number of turns, turn angle and CV, turn duration and CV, peak velocity CV, average velocity CV [40] Reduced peak velocity and average velocity [40] | ||
| Free-living PA | Sedentary activity | More sedentary time [38] | ||
| Light PA | Less light PA time [38] | |||
| Moderate PA | Less moderate PA time [38] | |||
| Vigorous PA | Less vigorous PA time [38] | |||
| Tactical agility assessment | Lowering | Greater duration [29] | ||
| Rolling | ||||
| Rising/Running | ||||
| Lowering and rolling | Greater duration, AUROC = 0.83 [0.72–0.93] [29] | |||
| Task/ Condition | Outcome | Change Stratified by Time Since mTBI | ||
|---|---|---|---|---|
| Days | Weeks | Months | ||
| Sleep | HR, RMSDD, HR CV, RMSDD CV | NC [26] | NC [26] | NC [26] |
| Awake | Mean RR, RMSDD, SDNN | NC [31] | ||
| Post-exertion | RMSDD, SDNN | NC [39] | Greater [31] | |
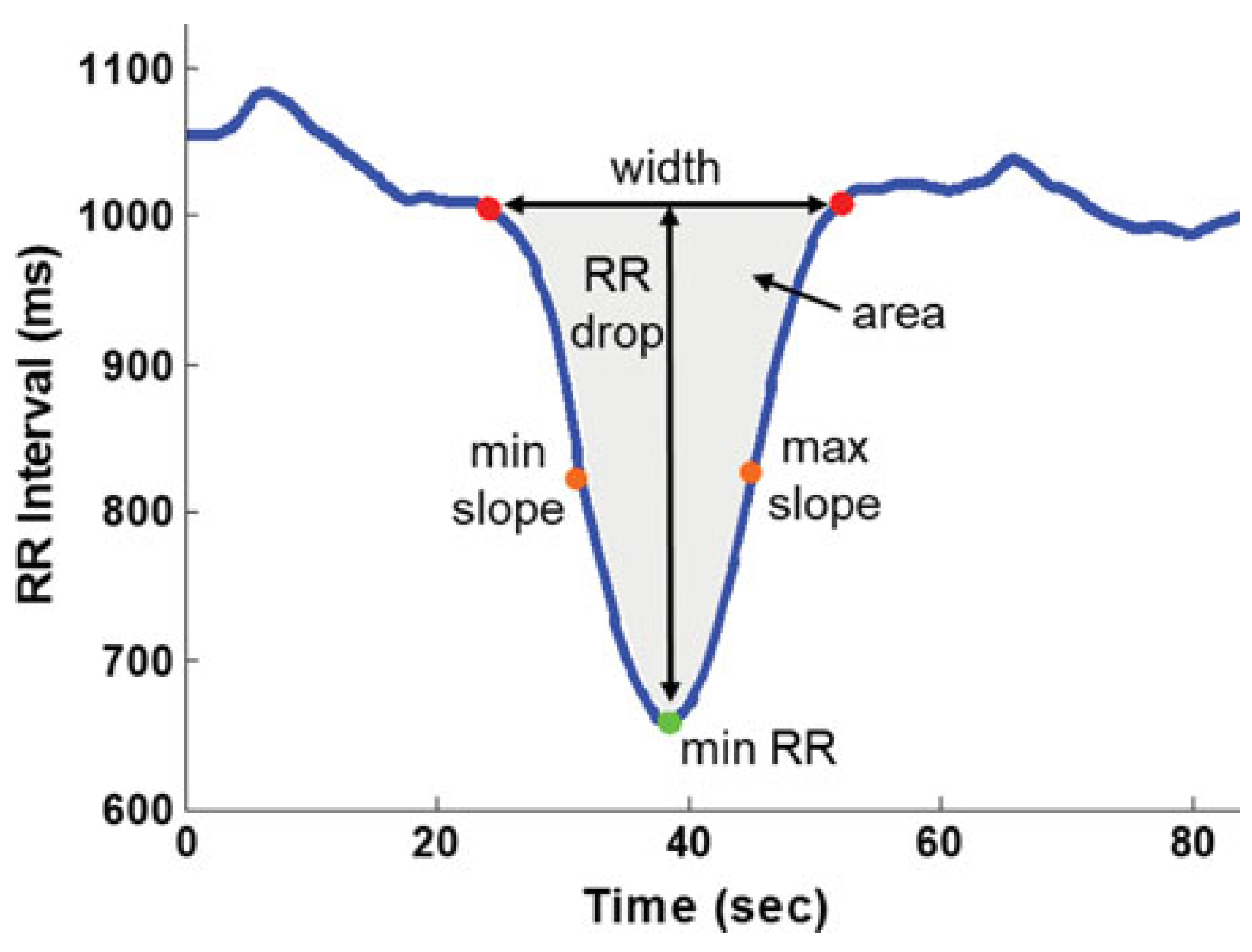
| Lie-to-Stand Transition | ΔHR | Reduced [37] | ||
| ΔHR, ΔRSA, ΔLF | NC [21] | |||
| RR drop, time of max slope, width and area of the valley | Greater [21] | |||
| Sit-to-Stand Transition | Time of min RR, RR drop, time of max slope, width and area of the valley | Greater [21] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis-Wilson, H.C.; Maldonado-Rosado, E.; Hegarty-Craver, M.; Temple, D.S. Potential for Wearable Sensor-Based Field-Deployable Diagnosis and Monitoring of Mild Traumatic Brain Injury: A Scoping Review. Sensors 2025, 25, 2803. https://doi.org/10.3390/s25092803
Davis-Wilson HC, Maldonado-Rosado E, Hegarty-Craver M, Temple DS. Potential for Wearable Sensor-Based Field-Deployable Diagnosis and Monitoring of Mild Traumatic Brain Injury: A Scoping Review. Sensors. 2025; 25(9):2803. https://doi.org/10.3390/s25092803
Chicago/Turabian StyleDavis-Wilson, Hope C., Erika Maldonado-Rosado, Meghan Hegarty-Craver, and Dorota S. Temple. 2025. "Potential for Wearable Sensor-Based Field-Deployable Diagnosis and Monitoring of Mild Traumatic Brain Injury: A Scoping Review" Sensors 25, no. 9: 2803. https://doi.org/10.3390/s25092803
APA StyleDavis-Wilson, H. C., Maldonado-Rosado, E., Hegarty-Craver, M., & Temple, D. S. (2025). Potential for Wearable Sensor-Based Field-Deployable Diagnosis and Monitoring of Mild Traumatic Brain Injury: A Scoping Review. Sensors, 25(9), 2803. https://doi.org/10.3390/s25092803









