Assessing the Sensitivity and Efficiency of Laser-Induced Breakdown Spectroscopy (LIBS) for High-Concentration Cadmium Detection in Cocoa Powder
Abstract
Highlights
- The LIBS technique was successfully applied for the first time to quantify cadmium in commercial cocoa powder across concentrations ranging from 70 ppm to 5000 ppm.
- A mechanical mixing and pelletization protocol for sample preparation, combined with a background correction algorithm, significantly improved the accuracy and robustness of LIBS for cadmium detection in complex cocoa matrices.
- LIBS demonstrates its potential as a rapid, field-deployable method for food safety monitoring and industrial contamination assessments.
- The methodology validates its applicability in high-contamination scenarios and expands the use of LIBS to other plant-based matrices and environmental monitoring.
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
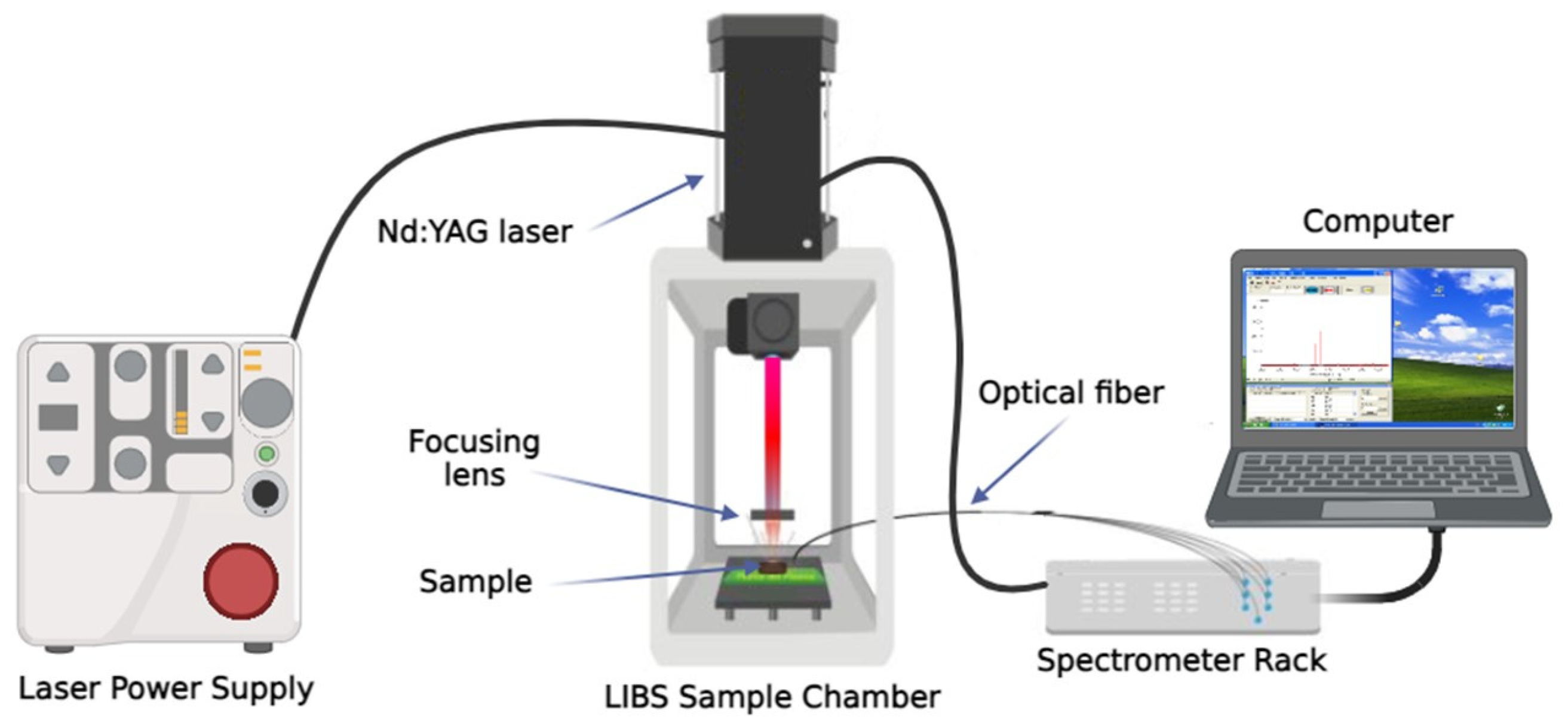
2.2. Experimental Setup
3. Results
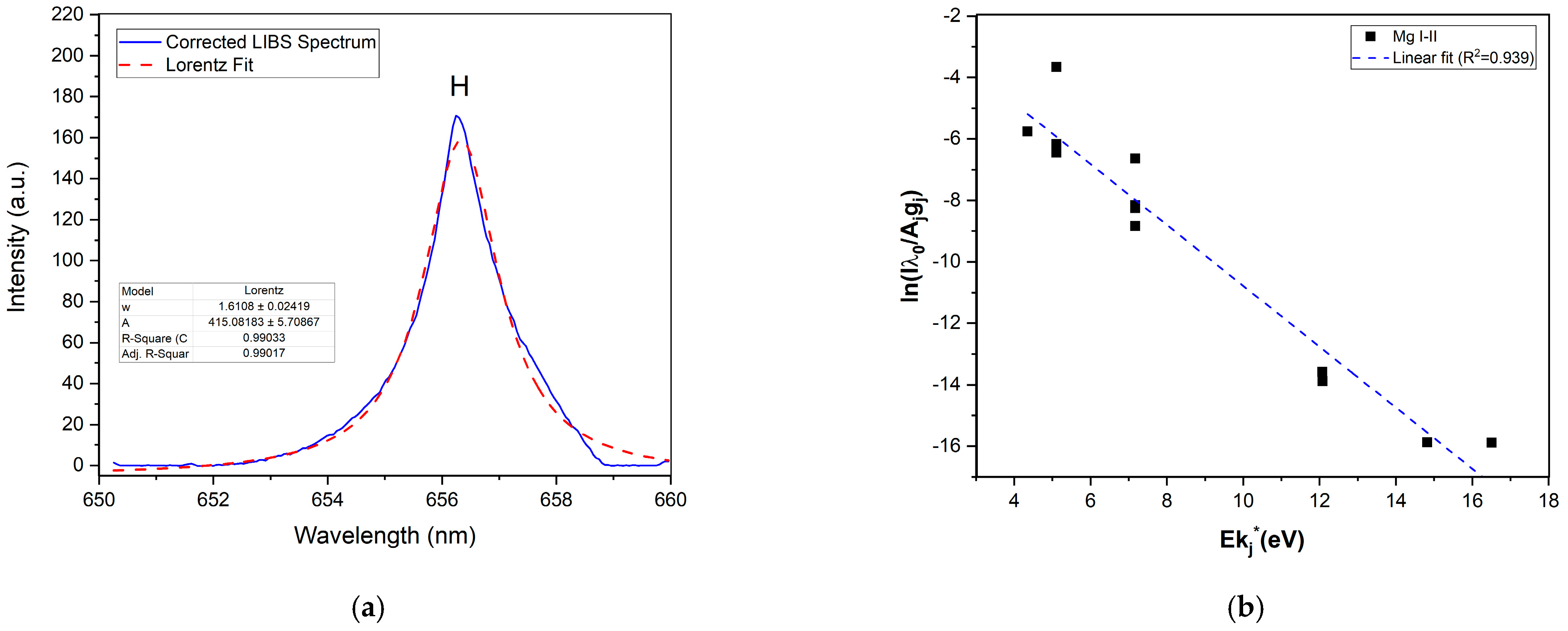
3.1. Spectral Treatment
Base Correction Algorithm
3.2. LIBS Analysis
3.2.1. Plasma Characterization
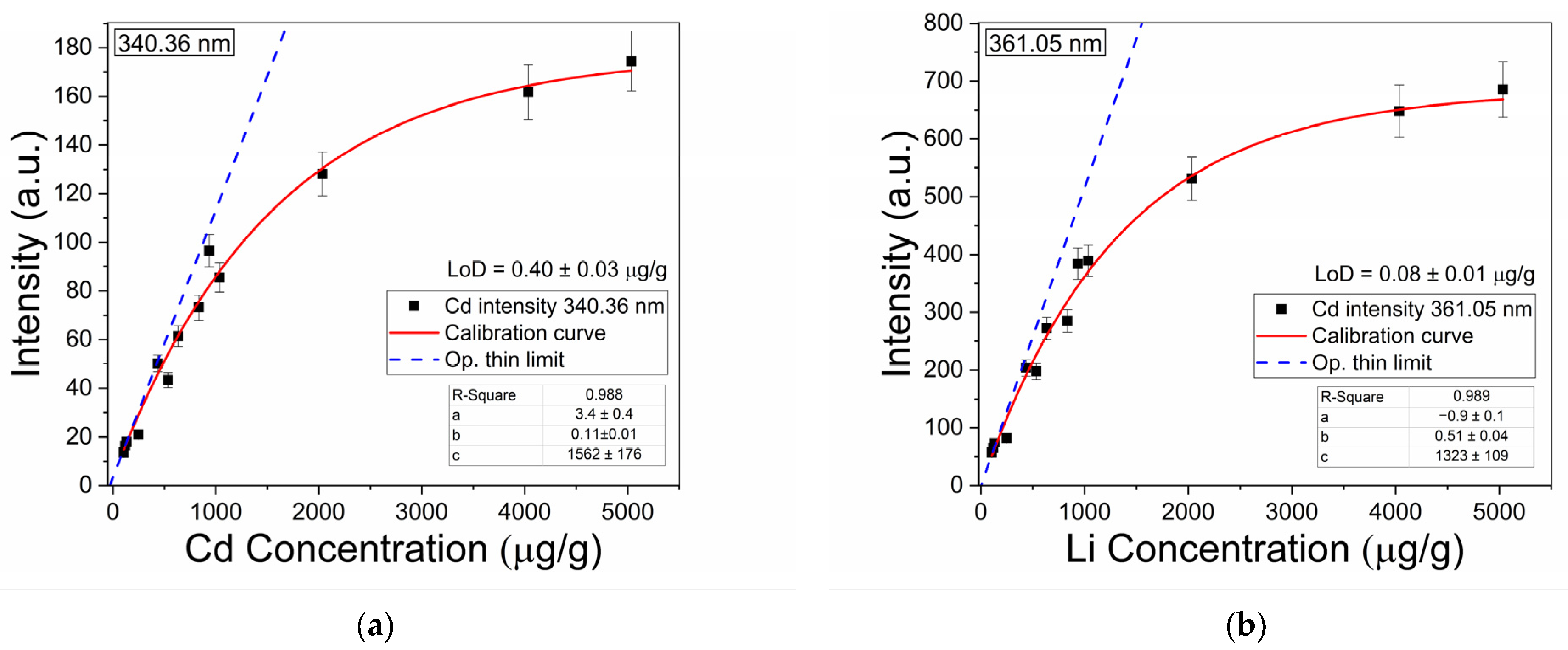
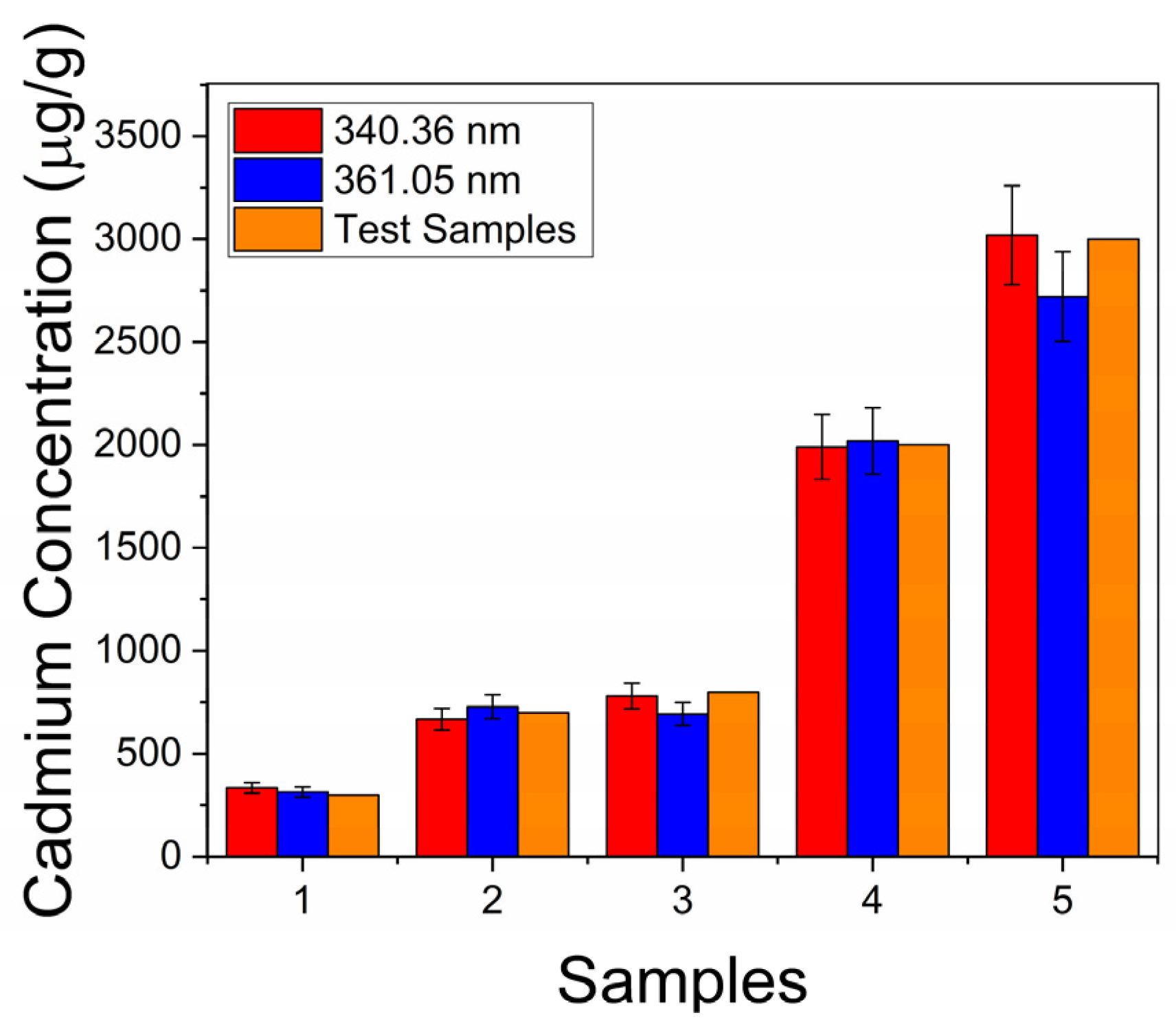
3.2.2. Calibration Curve and Detection Limits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The use and domestication of Theobroma cacao during the mid-Holocene in the upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Lanaud, C.; Vignes, H.; Utge, J.; Valette, G.; Rhoné, B.; Garcia Caputi, M.; Angarita Nieto, N.S.; Fouet, O.; Gaikwad, N.; Zarrillo, S.; et al. A revisited history of cacao domestication in pre-Columbian times revealed by archaeogenomic approaches. Sci. Rep. 2024, 14, 2972. [Google Scholar] [CrossRef] [PubMed]
- Lippi, D.; Vannozzi, G. Cacao bioactive compounds: Chemistry, analysis, and bioavailability. In Chocolate in Health and Nutrition; Humana: Cham, Switzerland, 2019; pp. 1–20. [Google Scholar]
- International Cocoa Organization (ICCO). Quarterly Bulletin of Cocoa Statistics, Volume XLVIII, No. 1–4. 2022. Available online: https://www.icco.org/categorie-produit/qbcs/ (accessed on 20 January 2025).
- CXS 193-1995; General Standard for Contaminants and Toxins in Food and Feed. FAO/WHO: Rome, Italy, 1995.
- European Commission. Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Smith, R.M.; Martell, A.E. Critical evaluation of selected atomic absorption techniques for the determination of metals in plants. Anal. Chem. 1976, 48, 45–51. [Google Scholar]
- Marguí, E.; Queralt, I.; Hidalgo, M. Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. TrAC Trends Anal. Chem. 2009, 28, 362–372. [Google Scholar] [CrossRef]
- Zhong, W.-S.; Ren, T.; Zhao, L.-J. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J. Food Drug Anal. 2016, 24, 46–55. [Google Scholar] [CrossRef]
- Gamela, R.R.; Costa, V.C.; Babos, D.V.; Araújo, A.S.; Pereira-Filho, E.R. Direct determination of Ca, K, and Mg in cocoa beans by laser-induced breakdown spectroscopy (LIBS): Evaluation of three univariate calibration strategies for matrix matching. Food Anal. Methods 2020, 13, 1017–1026. [Google Scholar] [CrossRef]
- Moros, J.; Laserna, J.J. Laser-induced breakdown spectroscopy (LIBS) of organic compounds: A review. Appl. Spectrosc. 2019, 73, 963–1011. [Google Scholar] [CrossRef]
- Douglas, A.S.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Jantzi, S.C.; Motto-Ros, V.; Trichard, F.; Markushin, Y.; Melikechi, N.; De Giacomo, A. Sample treatment and preparation for laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 115, 52–63. [Google Scholar] [CrossRef]
- Fu, X.; Li, G.; Tian, H.; Dong, D. Detection of cadmium in soils using laser-induced breakdown spectroscopy combined with spatial confinement and resin enrichment. RSC Adv. 2018, 8, 39635–39640. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, R.; Zhang, W.; Yi, R.; Tang, S.; Guo, L.; Hao, Z.; Li, X.; Lu, Y.; Zeng, X. High-sensitivity determination of cadmium and lead in rice using laser-induced breakdown spectroscopy. Food Chem. 2019, 272, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kong, W.; Shen, T.; Man, Z.; Zhu, W.; He, Y.; Liu, F.; Liu, Y. Application of laser-induced breakdown spectroscopy in detecting cadmium content in rice stems. Front. Plant Sci. 2020, 11, 599616. [Google Scholar] [CrossRef] [PubMed]
- Díaz Pace, D.M.; D’Angelo, C.A.; Bertuccelli, D.; Bertuccelli, G. Analysis of heavy metals in liquids using laser-induced breakdown spectroscopy by liquid-to-solid matrix conversion. Spectrochim. Acta Part B 2006, 61, 929–933. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Zhao, Z.; Jiang, J.; Zhou, F.; Liu, F.; Shen, T. Fast visualization of chromium in rice leaves using dual-pulse LIBS and chemometric methods. Environ. Pollut. 2019, 252, 1125–1132. [Google Scholar] [CrossRef]
- Gondal, M.A.; Hussain, T.; Yamani, Z.H.; Baig, M.A. Detection of heavy metals in Arabian crude oil residue using LIBS. Talanta 2006, 69, 1072–1078. [Google Scholar] [CrossRef]
- Tognoni, E.; Palleschi, V.; Corsi, M.; Cristoforetti, G.; Omenetto, N.; Gornushkin, I.; Smith, B.W.; Winefordner, J.D. From Sample to Signal in laser-Induced Breakdown Spectroscopy: A Complex Route to Quantitative Analysis. In Laser-Induced Breakdown Spectroscopy (LIBS) Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006; pp. 122–170. [Google Scholar] [CrossRef]
- El Haddad, J.; Canioni, L.; Bousquet, B. Good practices in LIBS analysis: Review and advice. Spectrochim. Acta Part B 2014, 101, 171–182. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (Ver. 5.10). Available online: https://physics.nist.gov/asd (accessed on 7 August 2023).
- Dyar, M.D.; Giguere, S.; Carey, C.J.; Boucher, T. Comparison of baseline removal methods for laser-induced breakdown spectroscopy of geological samples. Spectrochim. Acta Part B 2016, 126, 53–64. [Google Scholar] [CrossRef]
- Giguere, S.; Boucher, T.; Carey, C.J.; Mahadevan, S.; Dyar, M.D. A fully customized baseline removal framework for spectroscopic applications. Appl. Spectrosc. 2017, 71, 1457–1470. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Li, X.; Chen, J.; Liu, J.; Qiu, J.; Gao, X.; Cui, J.; Heshig, B. Continuous background correction using effective points selected in third-order minima segments in low-cost laser-induced breakdown spectroscopy without intensified CCD. Opt. Express 2018, 26, 16171–16186. [Google Scholar] [CrossRef]
- Palleschi, V. Chemometrics and Numerical Methods in LIBS; Wiley Online Library: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Noll, R. Laser-Induced Breakdown Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Singh, J.P.; Thakur, S.N. Laser-Induced Breakdown Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- National Institute of Standards and Technology (NIST). Atomic Spectra Database. Available online: http://physics.nist.gov/PhysRefData (accessed on 7 August 2023).
- Cristoforetti, G.; Lorenzetti, G.; Palleschi, V.; Capitelli, M. Local Thermodynamic Equilibrium in Laser-Induced Breakdown Spectroscopy: Beyond the McWhirter Criterion. Spectrochim. Acta Part B 2010, 65, 86–95. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A. Characterization of laser-induced plasmas by optical emission spectroscopy: A review of experiments and methods. Spectrochim. Acta Part B 2008, 63, 893–916. [Google Scholar] [CrossRef]
- Konjević, N.; Ivković, M.; Sakan, N. Hydrogen Balmer lines for low electron number density plasma diagnostics. Spectrochim. Acta Part B 2012, 76, 16. [Google Scholar] [CrossRef]
- Mansour, S.A.M. Self-Absorption Effects on Electron Temperature Measurements Utilizing Laser-Induced Breakdown Spectroscopy (LIBS) Techniques. Opt. Photonics J. 2015, 5, 79–90. [Google Scholar] [CrossRef]
- Diaz Pace, D.M.; Molina, M.J. τ-algorithm for gathering spectroscopic information by modeling emission line shapes: Application to laser-induced plasmas. J. Opt. Soc. Am. B 2023, 40, C1–C7. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A.; Pen, F.; Alba, Á. Improvements in Quantitative Analysis of Steel Composition by Laser-Induced Breakdown Spectroscopy at Atmospheric Pressure Using an Infrared Nd:YAG Laser. Appl. Spectrosc. 1999, 53, 1259. [Google Scholar] [CrossRef]
- Sabsabi, M.; Cielo, P. Quantitative Analysis of Aluminum Alloys by Laser-Induced Breakdown Spectroscopy and Plasma Characterization. Spectrochim. Acta Part B 1995, 50, 1841–1851. [Google Scholar] [CrossRef]
- Argüello, D.; Chávez, E.; Montalvo, D. Soil Properties and Agronomic Factors Affecting Cadmium Concentrations in Cacao Beans: A Nationwide Survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef]


| Element | Ionization State | Wavelength (nm) | |||||
|---|---|---|---|---|---|---|---|
| Cd | I | 340.36 | 7.07 × 107 | 3.73 | 7.36 | 0 | 1 |
| Cd | I | 361.05 | 1.30 × 108 | 3.94 | 7.38 | 2 | 3 |
| Species | Wavelength (Å) | |||||
|---|---|---|---|---|---|---|
| Mg I | 2776.47 | 1.32 | 2.712 | 7.175 | 3 | 5 |
| Mg I | 2778.45 | 1.82 | 2.709 | 7.170 | 1 | 3 |
| Mg I | 2779.65 | 1.36 | 2.712 | 7.170 | 3 | 3 |
| Mg I | 2781.00 | 5.43 | 2.717 | 7.173 | 5 | 3 |
| Mg I | 2782.78 | 2.14 | 2.717 | 7.170 | 5 | 3 |
| Mg I | 2852.14 | 4.91 | 0.000 | 4.345 | 1 | 3 |
| Mg I | 5167.09 | 1.13 | 2.709 | 5.107 | 1 | 3 |
| Mg I | 5172.59 | 3.37 | 2.712 | 5.108 | 3 | 3 |
| Mg I | 5183.53 | 5.61 | 2.717 | 5.108 | 5 | 3 |
| Mg II | 2790.78 | 4.01 | 4.422 | 8.864 | 2 | 4 |
| Mg II | 2795.23 | 2.60 | 0.000 | 4.434 | 2 | 4 |
| Mg II | 2798.25 | 4.79 | 4.434 | 8.863 | 4 | 6 |
| Mg II | 2802.50 | 2.57 | 0.000 | 4.422 | 2 | 2 |
| Test Samples Mixed-in Concentration (μg/g) | Calculated Concentration (μg/g) | |
|---|---|---|
| Cadmium Lines | ||
| 361.05 nm | 340.43 nm | |
| 300 | 314 ± 25 | 335 ± 26 |
| 700 | 728 ± 58 | 668 ± 53 |
| 800 | 728 ± 55 | 780 ± 62 |
| 2000 | 2019 ± 161 | 1990 ± 159 |
| 3000 | 2720 ± 217 | 3018 ± 241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina M., J.; Pincay, R.; Santos, V.; González, J.L.; Trujillo Guerrero, M.F.; Díaz Pace, D.; Costa-Vera, C. Assessing the Sensitivity and Efficiency of Laser-Induced Breakdown Spectroscopy (LIBS) for High-Concentration Cadmium Detection in Cocoa Powder. Sensors 2025, 25, 2434. https://doi.org/10.3390/s25082434
Molina M. J, Pincay R, Santos V, González JL, Trujillo Guerrero MF, Díaz Pace D, Costa-Vera C. Assessing the Sensitivity and Efficiency of Laser-Induced Breakdown Spectroscopy (LIBS) for High-Concentration Cadmium Detection in Cocoa Powder. Sensors. 2025; 25(8):2434. https://doi.org/10.3390/s25082434
Chicago/Turabian StyleMolina M., Juan, Raquel Pincay, Víctor Santos, José Luis González, María Fernanda Trujillo Guerrero, Diego Díaz Pace, and César Costa-Vera. 2025. "Assessing the Sensitivity and Efficiency of Laser-Induced Breakdown Spectroscopy (LIBS) for High-Concentration Cadmium Detection in Cocoa Powder" Sensors 25, no. 8: 2434. https://doi.org/10.3390/s25082434
APA StyleMolina M., J., Pincay, R., Santos, V., González, J. L., Trujillo Guerrero, M. F., Díaz Pace, D., & Costa-Vera, C. (2025). Assessing the Sensitivity and Efficiency of Laser-Induced Breakdown Spectroscopy (LIBS) for High-Concentration Cadmium Detection in Cocoa Powder. Sensors, 25(8), 2434. https://doi.org/10.3390/s25082434









