Black Phosphorous-Based Surface Plasmon Resonance Biosensor for Malaria Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Framework and Performance Metrics
2.2. Biosensor Configuration and Initial Parameters
3. Results and Discussion
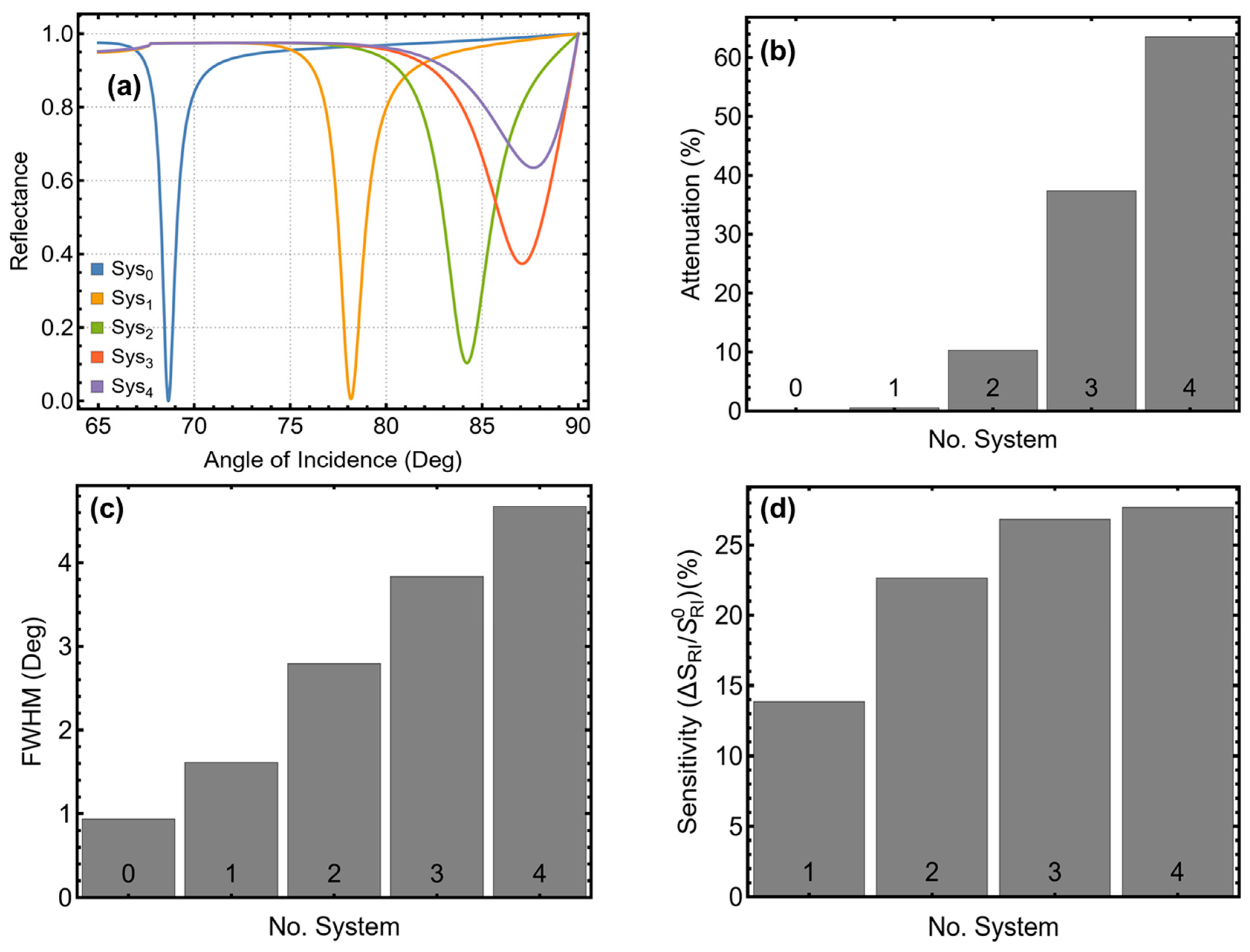
3.1. Selection of the Best Configurations
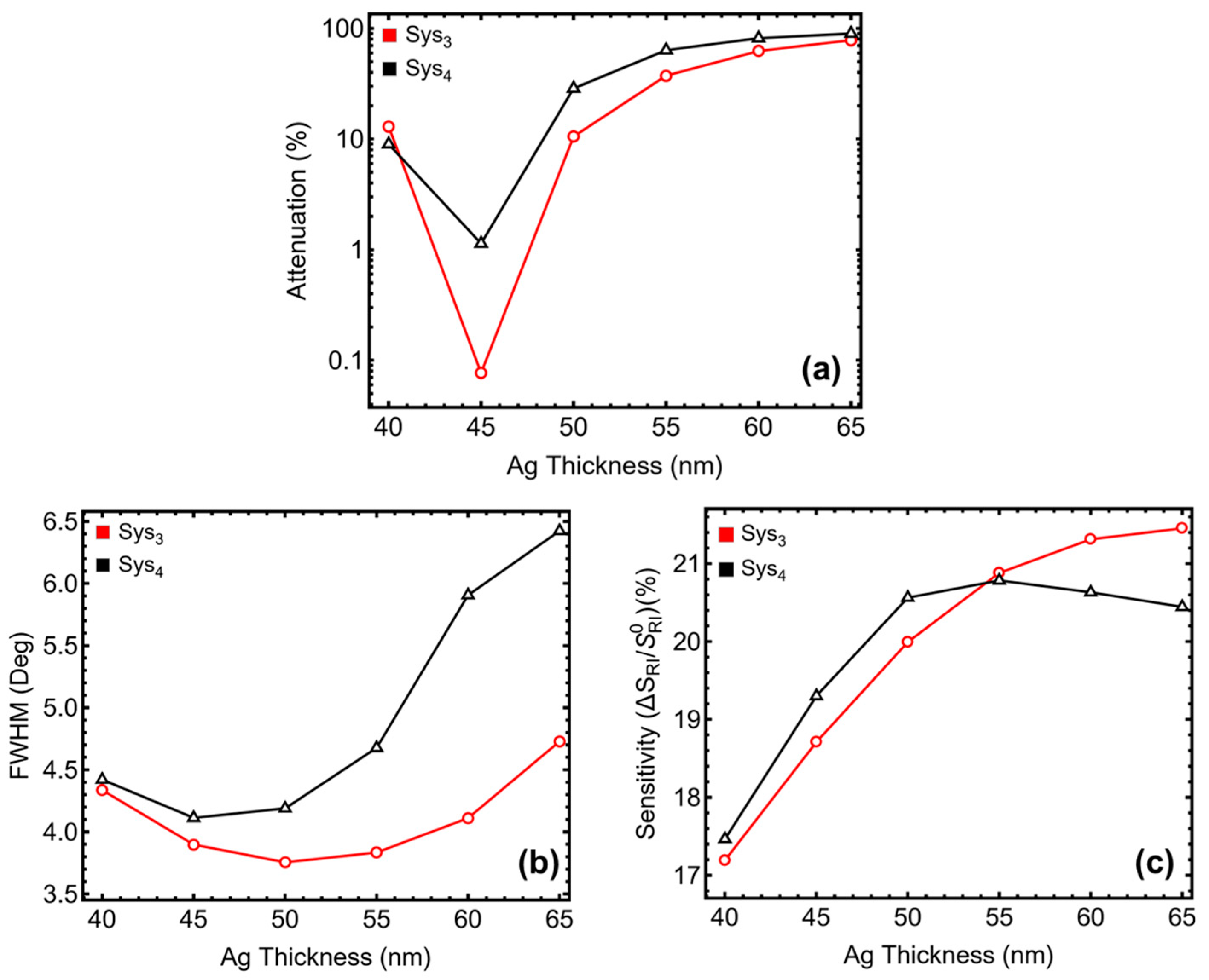
3.2. Metal Thickness Optimization
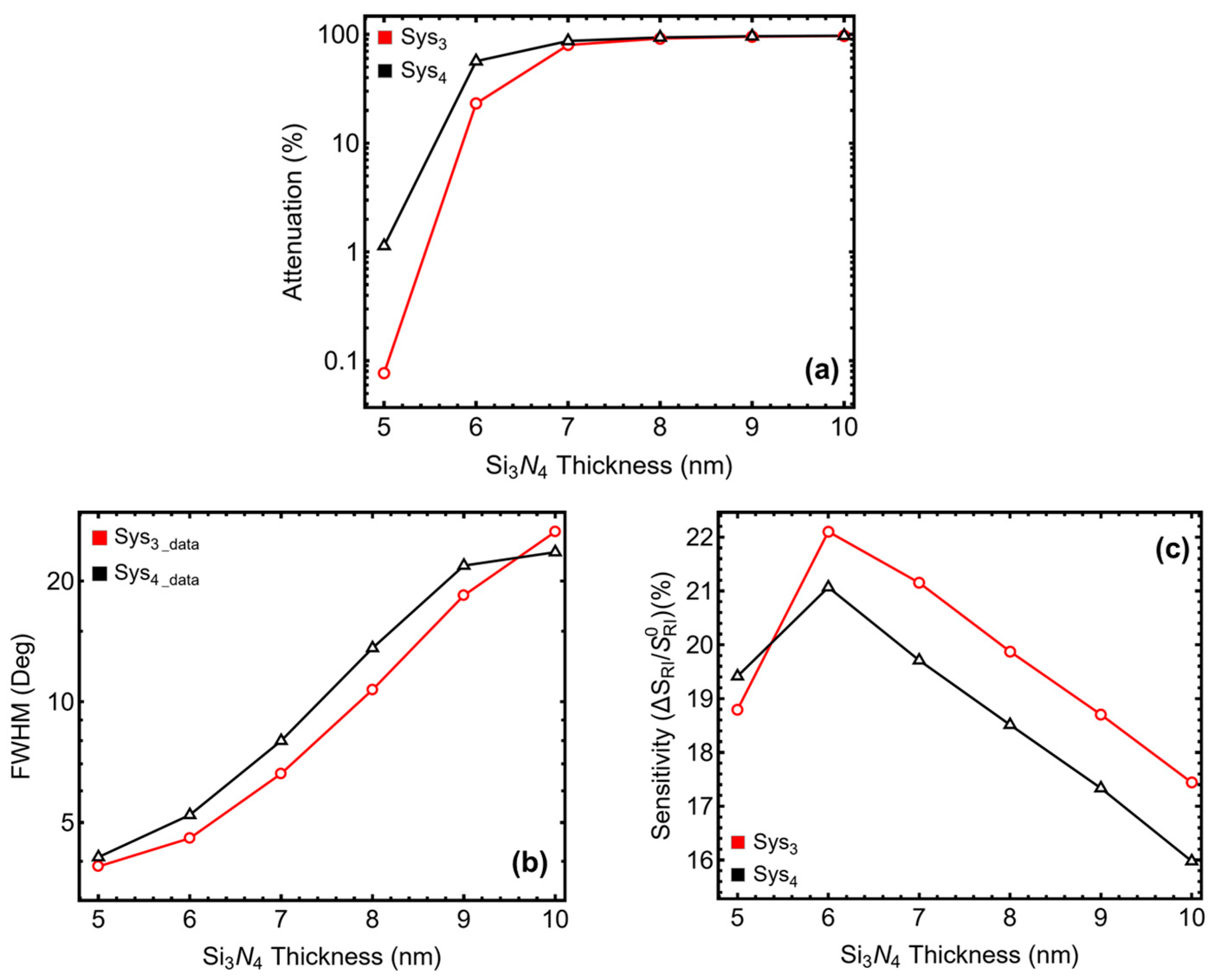
3.3. Silicon Nitride Thickness Optimization
3.4. Two-Dimensional Nanolayers Optimization
- The BK7 prism serves as the optical coupling element.
- A 55 nm silver (Ag) layer could be deposited via thermal evaporation or sputtering.
- A 5 nm Si3N4 layer could be deposited using plasma-enhanced chemical vapor deposition (PECVD) [35].
- Red phosphorus or phosphine gas (PH3) could be heated under an inert atmosphere (Ar/N2).
- The BP monolayer can be directly grown on Si3N4/Au using a low-pressure CVD system at 400–600 °C.
- Controlled growth time and carrier gas flow regulate monolayer thickness.
- Immediately after growth, the BP monolayer can be encapsulated with hexagonal boron nitride (hBN) or atomic-layer-deposited (ALD) Al2O3 to prevent degradation [36]. While encapsulation enhances BP stability, it may slightly modify the plasmonic response of the sensor, which should be considered in experimental evaluations.
- The SPR chip could be cleaned under inert conditions to avoid oxidation.
3.5. ssDNA Thickness Optimization
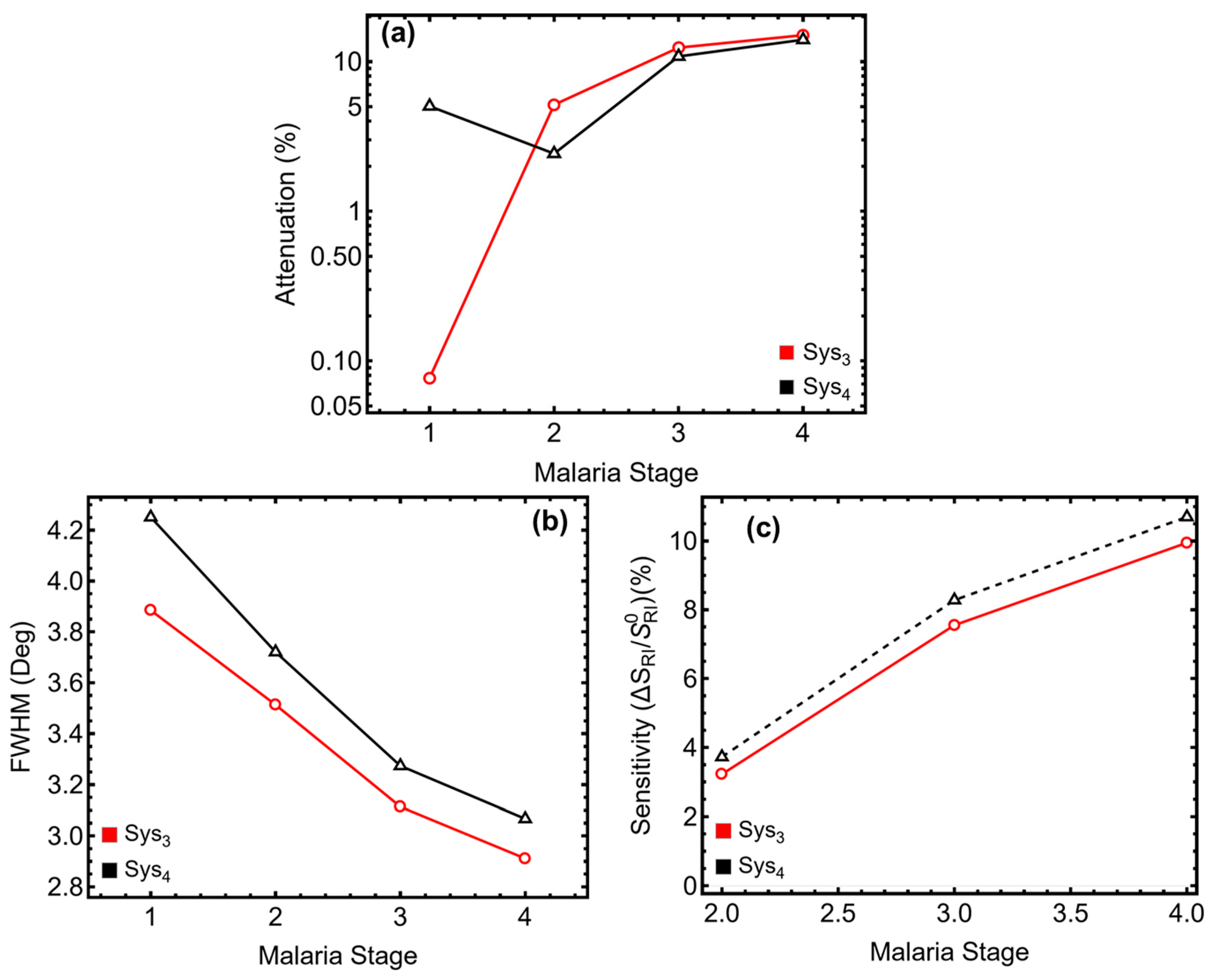
3.6. Malaria Detection
3.7. Performance Sensing Metrics
3.8. State-of-the-Art Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kar, N.P.; Kumar, A.; Singh, O.P.; Carlton, J.M.; Nanda, N. A review of malaria transmission dynamics in forest ecosystems. Parasites Vectors 2014, 7, 265. [Google Scholar] [CrossRef]
- Boyle, M.J.; Engwerda, C.R.; Jagannathan, P. The impact of Plasmodium-driven immunoregulatory networks on immunity to malaria. Nat. Rev. Immunol. 2024, 24, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Ogbaunu, I.U.; Otieno, K.; Varo, R.; Sow, S.O.; Ojulong, J.; Duduyemi, B.; Kowour, D.; Cain, C.J.; Rogena, E.A.; Onyango, D.; et al. Burden of child mortality from malaria in high endemic areas: Results from the CHAMPS network using minimally invasive tissue sampling. J. Infect. 2024, 88, 106107. [Google Scholar] [CrossRef]
- Onyinyechi, O.M.; Ismail, S.; Nashriq Mohd Nazan, A.I. Prevention of malaria in pregnancy through health education intervention programs on insecticide-treated nets use: A systematic review. BMC Public Health 2024, 24, 755. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E.; Gorres, J.P.; Healy, S.A.; Fried, M. Malaria vaccines: A new era of prevention and control. Nat. Rev. Microbiol. 2024, 22, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Littmann, J.; Achu, D.; Laufer, M.K.; Karema, C.; Schellenberg, D. Making the most of malaria chemoprevention. Malar. J. 2024, 23, 51. [Google Scholar] [CrossRef]
- Hamid, M.M.A.; Mohamed, A.O.; Mohammed, F.O.; Elaagip, A.; Mustafa, S.A.; Elfaki, T.; Jebreel, W.M.A.; Albsheer, M.M.; Dittrich, S.; Owusu, E.D.A.; et al. Diagnostic accuracy of an automated microscope solution (miLab®) in detecting malaria parasites in symptomatic patients at point-of-care in Sudan: A case–control study. Malar. J. 2024, 23, 200. [Google Scholar] [CrossRef]
- Tegegn, G.; Gnanasekaren, N.; Gadisa, E.; Getie, M.; Molla, A.; Meharie, T.; Debash, H.; Berhan, A.; Almaw, A.; Hussien, A.; et al. Comparative assessment of microscopy, malaria rapid diagnostic test and polymerase chain reaction as malaria diagnostic tools in Adama Woreda, East Shoa Zone of Ethiopia: A cross-sectional study. BMC Infect. Dis. 2024, 24, 1363. [Google Scholar] [CrossRef]
- Adebusuyi, S.A.; Olorunfemi, A.B.; Fagbemi, K.A.; Nderu, D.; Amoo, A.O.J.; Thomas, B.N.; Velavan, T.P.; Ojurongbe, O. Performance of rapid diagnostic test, light microscopy, and polymerase chain reaction in pregnant women with asymptomatic malaria in Nigeria. IJID Reg. 2024, 12, 100416. [Google Scholar] [CrossRef]
- Shrikrishna, N.S.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the landscape of optical biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhang, G.-J. State-of-the-art strategies of surface plasmon resonance biosensors in clinical analysis: A comprehensive review. Coord. Chem. Rev. 2024, 520, 216149. [Google Scholar] [CrossRef]
- Aftab, M.; Mansha, M.S.; Iqbal, T.; Farooq, M. Surface plasmon excitation: Theory, configurations, and applications. Plasmonics 2024, 19, 1701–1719. [Google Scholar] [CrossRef]
- Sindona, A.; Vacacela Gomez, C.; Pisarra, M. Dielectric screening versus geometry deformation in two-dimensional allotropes of silicon and germanium. Sci. Rep. 2022, 12, 15107. [Google Scholar] [CrossRef]
- Pisarra, M.; Vacacela Gomez, C.; Sindona, A. Massive and massless plasmons in germanene nanosheets. Sci. Rep. 2022, 12, 18624. [Google Scholar] [CrossRef]
- Pandey, P.S.; Raghuvanshi, S.K.; Kumar, S. Recent advances in two-dimensional materials-based Kretschmann configuration for SPR sensors: A review. IEEE Sens. J. 2022, 22, 1069–1080. [Google Scholar] [CrossRef]
- Karki, B.; Pal, A.; Sarkar, P.; Uniyal, A.; Yadav, R.B. Gold, MXene, and graphene nanofilm-based surface plasmon resonance sensor for malaria detection. J. Opt. 2024, 53, 4805–4816. [Google Scholar] [CrossRef]
- Chithravel, A.; Murugan, D.; Shekhawat, A.S.; Diwan, A.; Srivastava, T.; Munjal, S.; Lakkakula, S.; Saxena, S.K.; Shrivastav, A.M. 2D material assisted prism-based surface plasmon resonance sensors: A comprehensive survey. Opt. Laser Eng. 2024, 182, 108452. [Google Scholar] [CrossRef]
- Sindona, A.; Pisarra, M.; Bellucci, S.; Tene, T.; Guevara, M.; Vacacela Gomez, C. Plasmon oscillations in two-dimensional arrays of ultranarrow graphene nanoribbons. Phys. Rev. B 2019, 100, 235422. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Svozilík, J.; Coello-Fiallos, D.; Briceño, J.; Vacacela Gomez, C. Proving Surface Plasmons in Graphene Nanoribbons Organized as 2D Periodic Arrays and Potential Applications in Biosensors. Chemosensors 2022, 10, 514. [Google Scholar] [CrossRef]
- Tene, T.; Coello-Fiallos, D.; Palacios Robalino, M.d.L.; Londo, F.; Vacacela Gomez, C. The Effect of MoS2 and Si3N4 in Surface Plasmon Resonance Biosensors for HIV DNA Hybridization Detection: A Numerical Study. Micromachines 2025, 16, 295. [Google Scholar] [CrossRef]
- Srivastava, A.; Verma, A.; Das, R.; Prajapati, Y.K. A theoretical approach to improve the performance of SPR biosensor using MXene and black phosphorus. Optik 2020, 203, 163430. [Google Scholar] [CrossRef]
- Singh, N.P.; Mishra, A.C.; Yadav, S.; Lohia, P.; Dwivedi, D.K. Numerical analysis of SPR biosensor using zinc telluride, ferric oxide and black phosphorous for malaria detection. J. Opt. 2025, 54, 141–151. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Srivastava, S.K. Silicon nitride-BP-based surface plasmon resonance highly sensitive biosensor for virus SARS-CoV-2 detection. Plasmonics 2022, 17, 1065–1077. [Google Scholar] [CrossRef]
- Tene, T.; Coello-Fiallos, D.; Borja, M.; Sánchez, N.; Londo, F.; Vacacela Gomez, C.; Bellucci, S. Surface plasmon resonance biosensors for SARS-CoV-2 sensing: The role of silicon nitride and graphene. Biosens. Bioelectron. X 2025, 23, 100586. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef]
- Tene, T.; Svozilík, J.; Colcha, D.; Cevallos, Y.; Vinueza-Naranjo, P.G.; Vacacela Gomez, C.; Bellucci, S. The Tunable Parameters of Graphene-Based Biosensors. Sensors 2024, 24, 5049. [Google Scholar] [CrossRef]
- Akib, T.B.A.; Rana, M.M.; Mehedi, I.M. Multi-layer SPR biosensor for in-situ amplified monitoring of the SARS-CoV-2 omicron (B.1.1.529) variant. Biosens. Bioelectron. X 2024, 16, 100434. [Google Scholar] [CrossRef]
- Bendib, S.; Bendib, C. Photonic crystals for malaria detection. J. Biosens. Bioelectron. 2018, 9, 3. [Google Scholar] [CrossRef]
- Agnero, M.A.; Konan, K.; Tokou, Z.G.C.S.; Kossonou, Y.T.A.; Dion, B.S.; Kaduki, K.A.; Zoueu, J.T. Malaria-Infected Red Blood Cell Analysis through Optical and Biochemical Parameters Using the Transport of Intensity Equation and the Microscope’s Optical Properties. Sensors 2019, 19, 3045. [Google Scholar] [CrossRef]
- Lee, S.A.; Link, S. Chemical interface damping of surface plasmon resonances. Acc. Chem. Res. 2021, 54, 1950–1960. [Google Scholar] [CrossRef]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z.; et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef]
- Mu, Y.; Si, M.S. The mechanical exfoliation mechanism of black phosphorus to phosphorene: A first-principles study. EPL 2015, 112, 37003. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J.; Yuan, S.; Lin, S.; Yau, H.M.; Daj, J.; Lau, S.P. Field-effect transistors based on amorphous black phosphorus ultrathin films by pulsed laser deposition. Adv. Mater. 2015, 27, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; Hagaman, D.; Ji, H.-F. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.J.; Kim, K.S.; Kim, K.H.; Byun, J.Y.; Yeom, G.Y. A brief review of plasma enhanced atomic layer deposition of Si3N4. Appl. Sci. Converg. Technol. 2019, 28, 142–147. [Google Scholar] [CrossRef]
- Avsar, A.; Vera-Marun, I.J.; Tan, J.Y.; Watanabe, K.; Taniguchi, T.; Castro Neto, A.H.; Özyilmaz, B. Air-stable transport in graphene-contacted, fully encapsulated ultrathin black phosphorus-based field-effect transistors. ACS Nano 2015, 9, 4138–4145. [Google Scholar] [CrossRef]
- Moznuzzaman, M.; Khan, I.; Islam, M.R. Nano-layered surface plasmon resonance-based highly sensitive biosensor for virus detection: A theoretical approach to detect SARS-CoV-2. AIP Adv. 2021, 11, 065023. [Google Scholar] [CrossRef]
- Mostufa, S.; Paul, A.K.; Chakrabarti, K. Detection of hemoglobin in blood and urine glucose level samples using a graphene-coated SPR-based biosensor. OSA Contin. 2021, 4, 2164–2176. [Google Scholar] [CrossRef]
- Guo, S.; Wu, X.; Li, Z.; Tong, K. High-sensitivity biosensor-based enhanced SPR by ZnO/MoS2 nanowires array layer with graphene oxide nanosheet. Int. J. Opt. 2020, 2020, 7342737. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, S.K. Gas sensing in Kretschmann configuration utilizing bi-metallic layer of rhodium-silver in visible region. Sens. Actuators B Chem. 2016, 237, 969–973. [Google Scholar] [CrossRef]
- Park, Y.-L.; Chen, B.-R.; Wood, R.J. Design and fabrication of soft artificial skin using embedded microchannels and liquid conductors. IEEE Sens. J. 2012, 12, 2711–2718. [Google Scholar] [CrossRef]

| Malaria Stage | S () | DA | QF (RIU−1) | |
|---|---|---|---|---|
| Opt-Sys3 | ||||
| Ring (II) | 2.758 | 394.000 | 0.785 | 112.148 |
| Trophozoite (III) | 6.457 | 307.460 | 2.073 | 98.751 |
| Schizont (IV) | 8.507 | 274.430 | 2.922 | 94.265 |
| Opt-Sys4 | ||||
| Ring (II) | 3.251 | 464.381 | 0.873 | 124.818 |
| Trophozoite (III) | 7.222 | 343.937 | 2.206 | 105.061 |
| Schizont (IV) | 9.333 | 301.075 | 3.043 | 98.172 |
| Malaria Stage | FoM (RIU−1) | LoD (10−5) | CSF |
|---|---|---|---|
| Opt-Sys3 | |||
| Ring (II) | 106.375 | 1.269 | 103.822 |
| Trophozoite (III) | 86.519 | 1.626 | 84.279 |
| Schizont (IV) | 80.111 | 1.822 | 77.843 |
| Opt-Sys4 | |||
| Ring (II) | 121.791 | 1.076 | 118.939 |
| Trophozoite (III) | 93.689 | 1.454 | 91.316 |
| Schizont (IV) | 84.434 | 1.660 | 82.135 |
| Configuration | S (°/RIU) | Ref. |
|---|---|---|
| TiO2/Ag/MoSe/Graphene | 194.0 | [37] |
| Au/PtSe2/Graphene | 200.0 | [38] |
| TiO2/ZnO/Au/MoS2/GO | 210.8 | [39] |
| Rh/Ag/Si/Graphene | 220.0 | [40] |
| Au/MXene/Au/Graphene (Trophozoite (III)) | 258.3 | [16] |
| Ag (Sys1) | 190.95 (II) | This work |
| 175.95 (III) | ||
| 167.63 (IV) | ||
| Ag/Si3N4 (Sys2) | 302.62 (II) | This work |
| 254.48 (III) | ||
| 233.20 (IV) | ||
| Ag/Si3N4/Black Phosphorus (Sys3) | 394.0 (II) | This work |
| 307.5 (III) | ||
| 274.4 (IV) | ||
| Ag/Si3N4/Black Phosphorus/ssDNA (Sys4) | 464.4 (II) | This work |
| 343.9 (III) | ||
| 301.1 (IV) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Cevallos, Y.; Vinueza-Naranjo, P.G.; Inca, D.; Vacacela Gomez, C. Black Phosphorous-Based Surface Plasmon Resonance Biosensor for Malaria Diagnosis. Sensors 2025, 25, 2068. https://doi.org/10.3390/s25072068
Tene T, Cevallos Y, Vinueza-Naranjo PG, Inca D, Vacacela Gomez C. Black Phosphorous-Based Surface Plasmon Resonance Biosensor for Malaria Diagnosis. Sensors. 2025; 25(7):2068. https://doi.org/10.3390/s25072068
Chicago/Turabian StyleTene, Talia, Yesenia Cevallos, Paola Gabriela Vinueza-Naranjo, Deysi Inca, and Cristian Vacacela Gomez. 2025. "Black Phosphorous-Based Surface Plasmon Resonance Biosensor for Malaria Diagnosis" Sensors 25, no. 7: 2068. https://doi.org/10.3390/s25072068
APA StyleTene, T., Cevallos, Y., Vinueza-Naranjo, P. G., Inca, D., & Vacacela Gomez, C. (2025). Black Phosphorous-Based Surface Plasmon Resonance Biosensor for Malaria Diagnosis. Sensors, 25(7), 2068. https://doi.org/10.3390/s25072068








