Use of Accelerometers to Monitor Motor Activity During HABIT-ILE for Chronic Stroke: An Exploratory Study
Highlights
- Individuals with stroke showed high inter-individual variability in their upper and lower extremities activity pattern throughout the intervention.
- Individuals with a moderate to high dexterity baseline showed a trend for similar evolution of their activity pattern on the upper extremities during a 2-week HABIT-ILE intervention.
- Defining common motor activity patterns in individuals with stroke with moderate to high upper extremity dexterity baseline seems possible.
- Developing and implementing such robust protocols in a large number of individuals with stroke would allow defining clear patterns for different profiles of individuals with stroke.
- Defining patterns of treatment based on the characteristics of the patients should help therapists to optimally adapt their treatment content and improve the specificity and efficiency of their interventions.
Abstract
1. Introduction
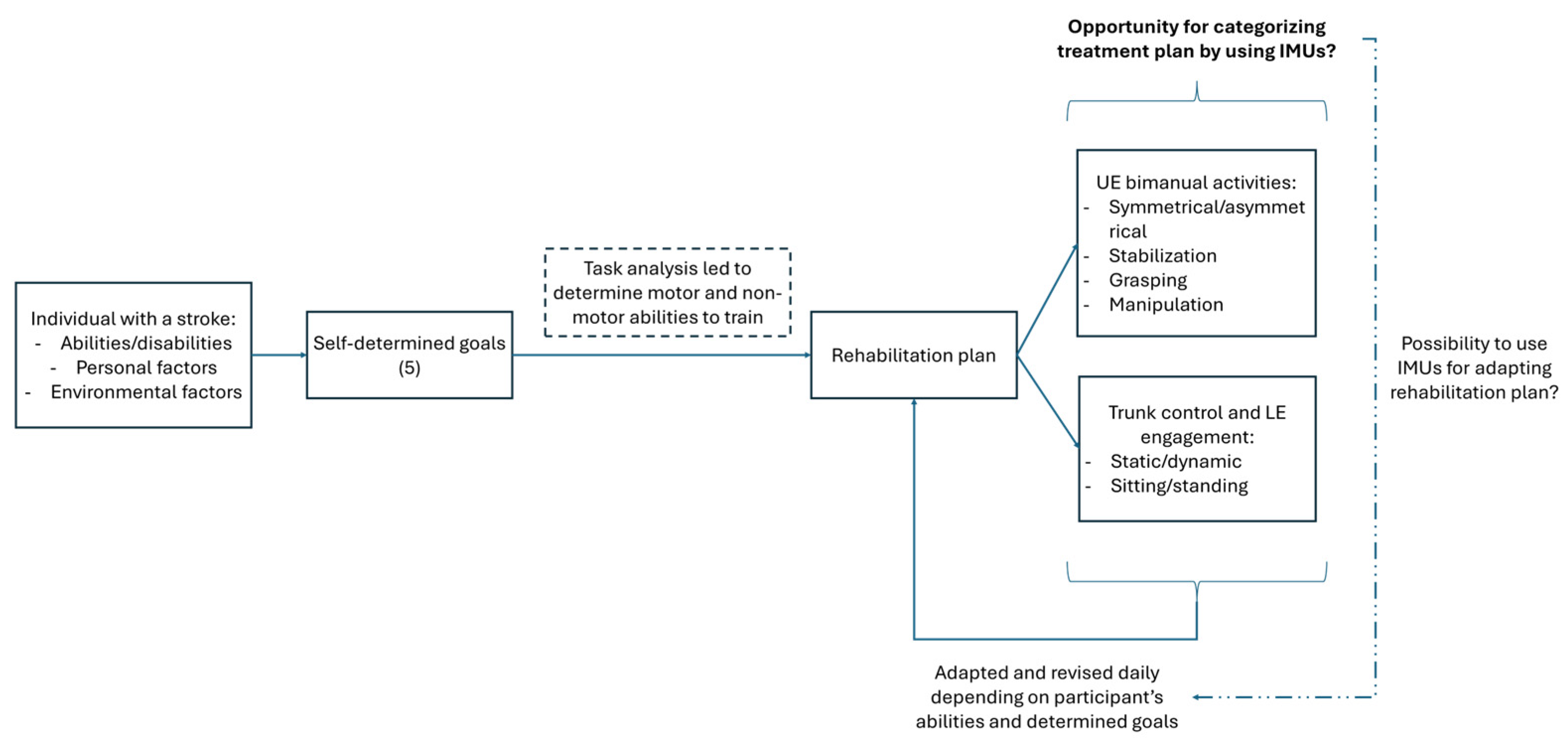
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Participants
2.4. Procedure
2.5. IMU Raw Data Treatment
2.6. Outcome Measures
- (1)
- The percentage of use for each of the UEs represents the percentage of time where the UE activity (wrists) is above the threshold to detect motor activity during the daily recorded total time.
- (2)
- Activity magnitude (AM) measures the intensity of the detected movements for each of the UEs, expressed in counts per second (counts/s).
- (3)
- The bimanual use (BU) represents the percentage of motor activity time, where a bimanual activity is recorded by the IMU in the daily recorded total active time.
- (4)
- Bilateral magnitude represents the intensity of detected movements in both of the UEs. It is calculated by summing the AM of both of the UEs for each second of activity, expressed in counts/s [17].
- (5)
- The use ratio is calculated by dividing the percentage of use of the affected UE with the one of the non-affected UE. A ratio > 1 expresses a higher percentage of time with movement recorded on the affected UE. A ratio < 1 expresses a higher percentage of time with movement recorded on the non-affected UE.
- (6)
- The magnitude ratio represents each UE’s contribution to the movement. Its calculation has been performed based on the methodology described by Bailey et al. (2014) [17]: one activity count is added to the smoothed vector magnitude of both UEs, the smoothed vector magnitude of the non-dominant UE is then divided by that of the dominant UE, and the resulting values are finally log-transformed by natural logarithm. Values range from −7 to 7, where 0 indicates an equal contribution of both UEs, with positive values indicating a higher contribution of the affected UE and negative values a higher contribution of the non-affected UE [17].
- (7)
- The percentage of use of the LE represents the percentage of time where a movement is recorded by the IMU worn on the right thigh during the daily recorded total time.
- (8)
- The activity magnitude measures the intensity of the movements detected at the thigh of the right LE, expressed in counts per second (counts/s).
- (9)
- The positioning of individuals with stroke, expressed in percent of the daily total recorded time, are calculated based on the orientation of the IMU worn at the thigh. Based on a simple vertical 45° threshold, the positioning was determined to be standing (>45°) or sitting/lying (<45°).
2.7. Baseline Functional Performance
2.8. Data Analysis
3. Results
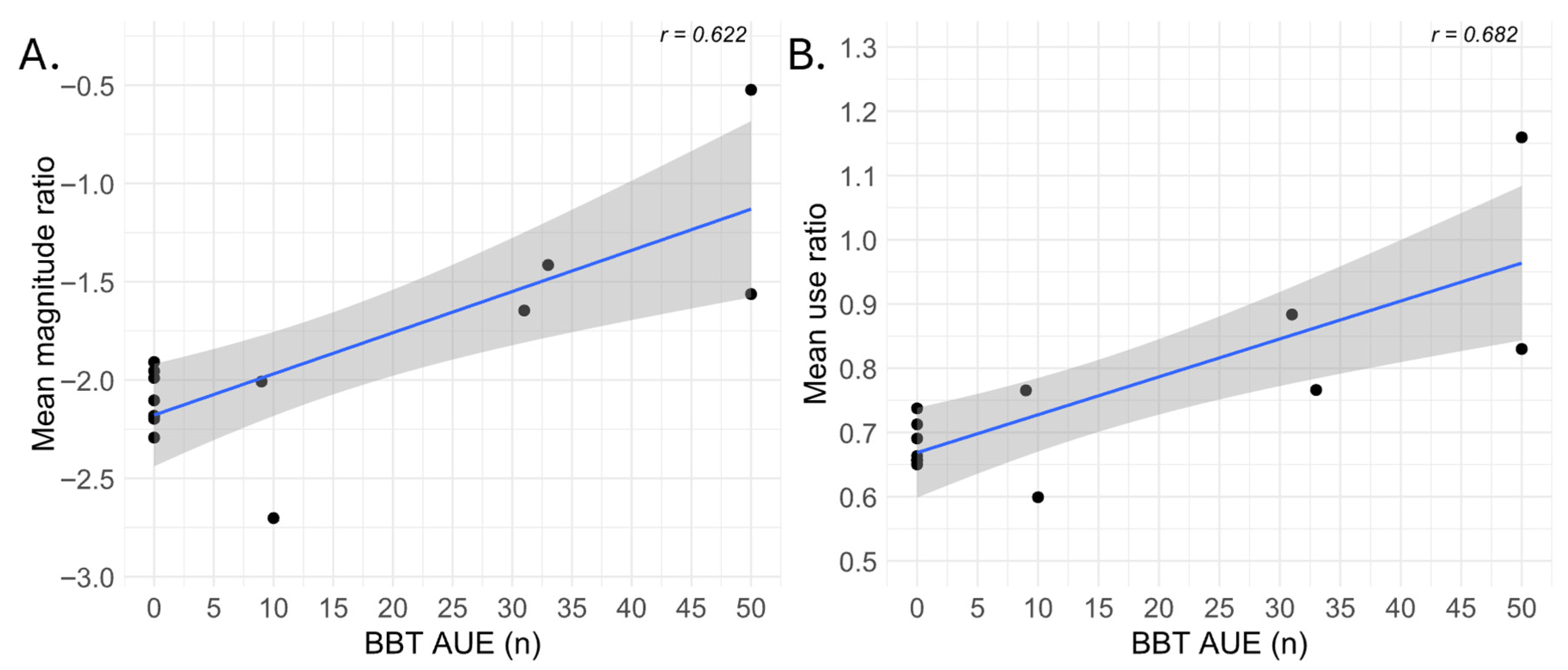
3.1. Correlations Between Baseline Abilities and IMUs-Derived Variables
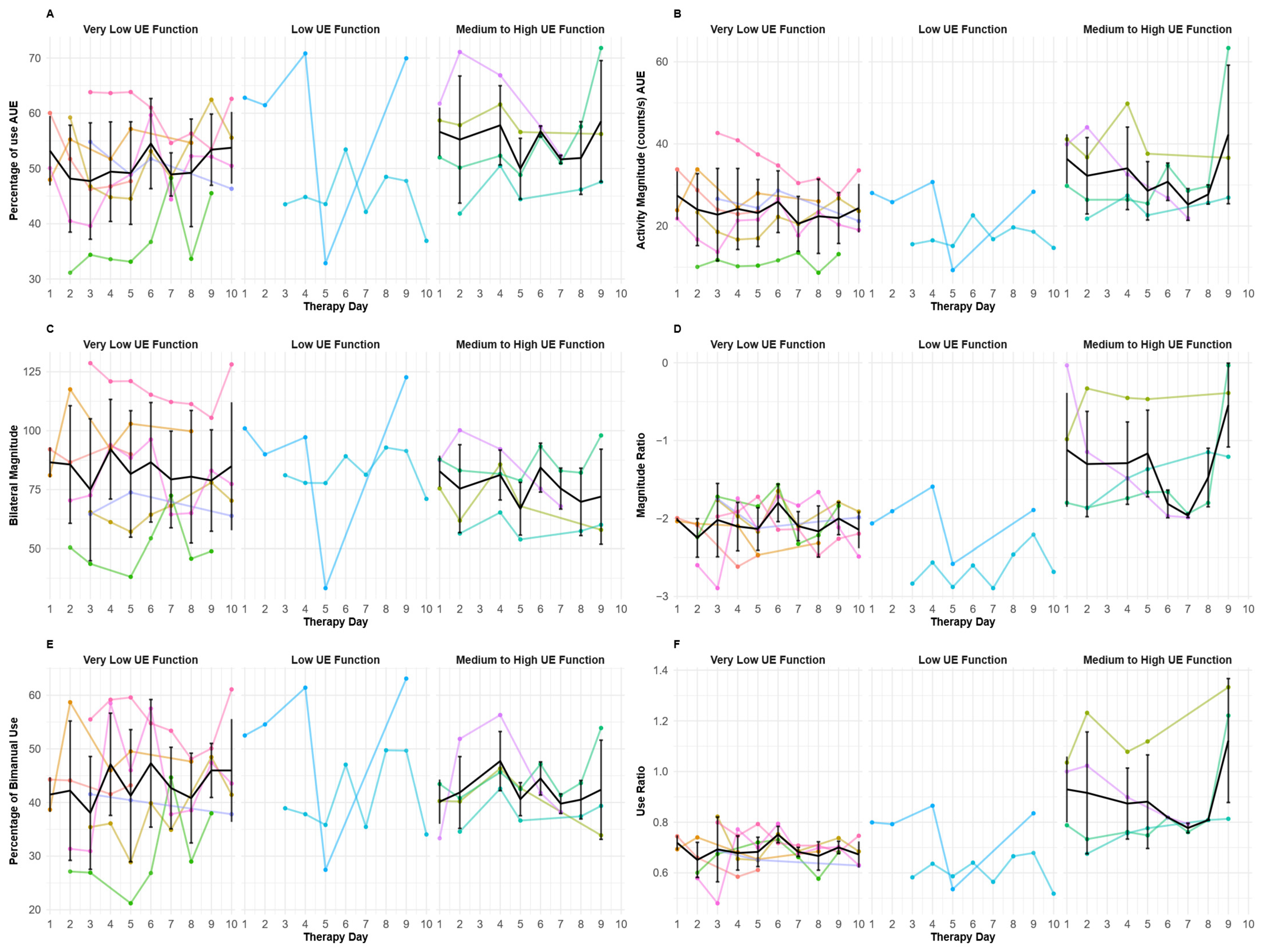
3.2. Day-to-Day Patterns for Upper Extremities’ IMU-Derived Variables
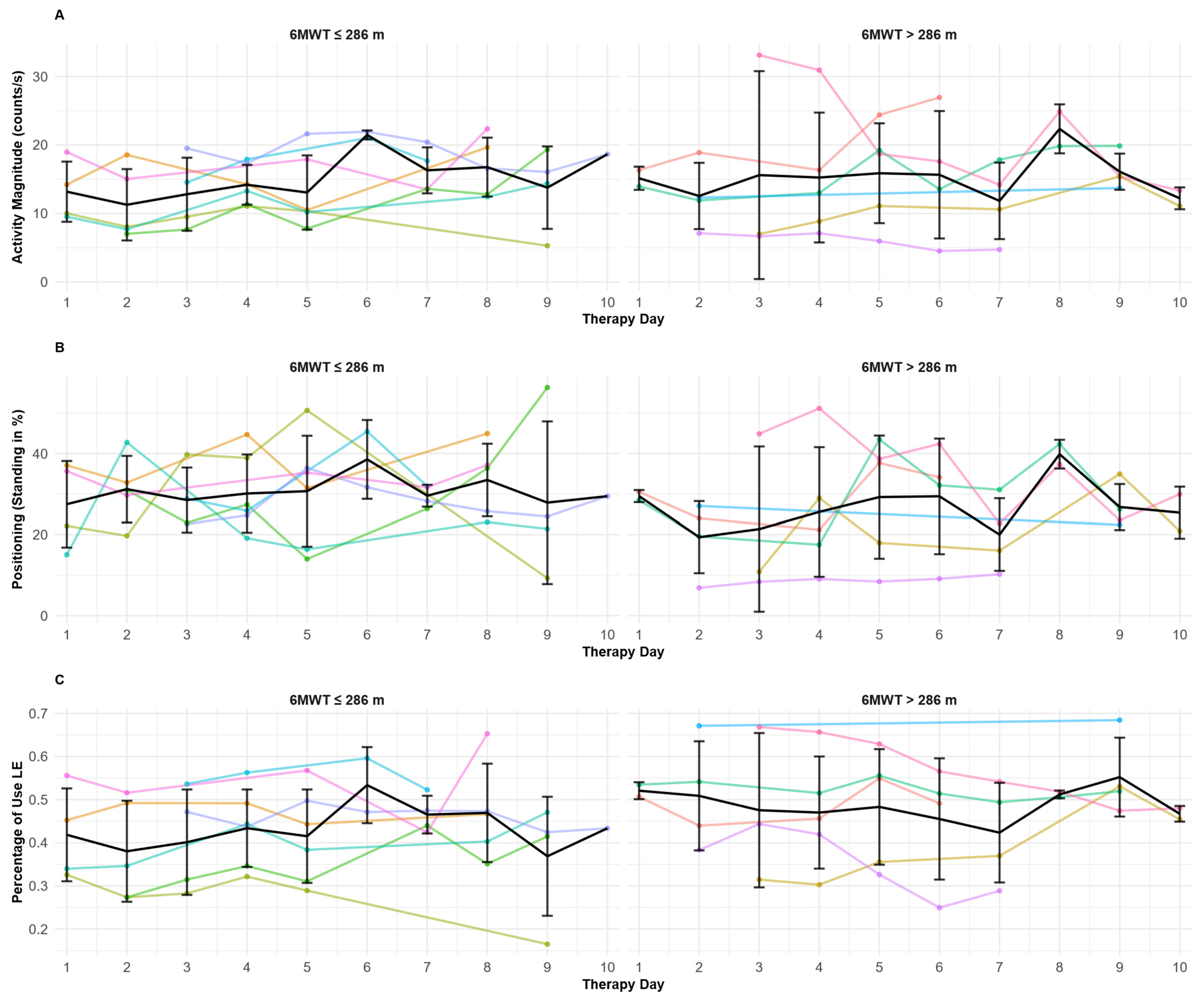
3.3. Determining Day-to-Day Patterns for Lower Extremities’ IMU-Derived Variables
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6-Minute Walk Test |
| AC | Activity Count (counts·s−1) |
| ADLs | Activities of Daily Living |
| AM | Activity Magnitude (counts·s−1) |
| AUE | Affected Upper Extremity |
| BBT | Box and Block Test |
| BM | Bilateral Magnitude |
| BU | Bimanual Use |
| CIMT | Constraint-induced Movement Therapy |
| CP | Cerebral Palsy |
| EMG | Electromyography |
| HABIT-ILE | Hand and Arm Bimanual Intensive Therapy Including Lower Extremities |
| Hz | Hertz |
| IMU | Inertial Measurement Unit (wearable sensor) |
| LE | Lower Extremity |
| MATLAB | Matrix Laboratory (MathWorks software (Build 8.6.0.267246 (R2015b) 64 bit).) |
| MR | Magnitude Ratio |
| mRS | Modified Rankin Scale |
| NAUE | Non-Affected Upper Extremity |
| ROC | Receiver Operating Characteristic (curve) |
| R2 | Coefficient of Determination (R-squared) |
| SD | Standard Deviation |
| TUG | Timed Up and Go |
| UE | Upper Extremity |
| UR | Use Ratio |
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Lai, S.M.; Studenski, S.; Duncan, P.W.; Perera, S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002, 33, 1840–1844. [Google Scholar] [CrossRef]
- Winterbottom, L.; Nilsen, D. Motor Learning Following Stroke. Phys. Med. Rehabil. Clin. N. Am. 2023, 35, 277–291. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Stevenson, T.; Thalman, L.; Christie, H.; Poluha, W. Constraint-Induced Movement Therapy Compared to Dose-Matched Interventions for Upper-Limb Dysfunction in Adult Survivors of Stroke: A Systematic Review with Meta-analysis. Physiother. Can. 2012, 64, 397–413. [Google Scholar] [CrossRef]
- Christie, L.J.; Rendell, R.; McCluskey, A.; Fearn, N.; Hunter, A.; Lovarini, M. Adult experiences of constraint-induced movement therapy programmes: A qualitative study using the Theoretical Domains Framework and Capability, Opportunity, Motivation—Behaviour system. Brain Impair. 2023, 24, 274–289. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, H.; Lin, J.; Zhai, H.; Shen, X. Influence of the constraint-induced method of constraint-induced movement therapy on improving lower limb outcomes after stroke: A meta-analysis review. Front. Neurol. 2023, 14, 2023. [Google Scholar] [CrossRef] [PubMed]
- Bleyenheuft, Y.; Gordon, A.M. Hand-arm bimanual intensive therapy including lower extremities (HABIT-ILE) for children with cerebral palsy. Phys. Occup. Ther. Pediatr. 2014, 34, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Bleyenheuft, Y.; Arnould, C.; Brandao, M.B.; Bleyenheuft, C.; Gordon, A.M. Hand and Arm Bimanual Intensive Therapy Including Lower Extremity (HABIT-ILE) in Children With Unilateral Spastic Cerebral Palsy: A Randomized Trial. Neurorehabilit. Neural Repair 2015, 29, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Ebner-Karestinos, D.; Gathy, E.; Carton de Tournai, A.; Herman, E.; Araneda, R.; Dricot, L.; Macq, B.; Vandermeeren, Y.; Bleyenheuft, Y. Hand-Arm Bimanual Intensive Therapy Including Lower Extremities (HABIT-ILE) in adults with chronic stroke: Protocol of a randomised controlled trial. BMJ Open 2023, 13, e070642. [Google Scholar] [CrossRef] [PubMed]
- Ducoffre, E.; Arnould, C.; Somville, M.; Rosselli, Z.; Saussez, G.; Bleyenheuft, Y. Feasibility of HABIT-ILE@home in children with cerebral palsy and adults with chronic stroke: A pilot study. PLoS Digit. Health 2025, 4, e0000850. [Google Scholar] [CrossRef]
- Hale, L.A.; Pal, J.; Becker, I. Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch. Phys. Med. Rehabil. 2008, 89, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Saussez, G.; Bailly, R.; Araneda, R.; Paradis, J.; Ebner-Karestinos, D.; Klocker, A.; Sogbossi, E.S.; Riquelme, I.; Brochard, S.; Bleyenheuft, Y. Efficacy of integrating a semi-immersive virtual device in the HABIT-ILE intervention for children with unilateral cerebral palsy: A non-inferiority randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Sakzewski, L.; Reedman, S.; McLeod, K.; Thorley, M.; Burgess, A.; Trost, S.; Ahmadi, M.; Rowell, D.; Chatfield, M.; Bleyenheuft, Y.; et al. Preschool HABIT-ILE: Study protocol for a randomised controlled trial to determine efficacy of intensive rehabilitation compared with usual care to improve motor skills of children, aged 2–5 years, with bilateral cerebral palsy. BMJ Open 2021, 11, e041542. [Google Scholar] [CrossRef]
- Hayward, K.; Eng, J.; Boyd, L.; Lakhani, B.; Bernhardt, J.; Lang, C. Exploring the Role of Accelerometers in the Measurement of Real World Upper-Limb Use After Stroke. Brain Impair. 2015, 17, 16–33. [Google Scholar] [CrossRef]
- Bailey, R.; Klaesner, J.; Lang, C. An Accelerometry-Based Methodology for Assessment of Real-World Bilateral Upper Extremity Activity. PLoS ONE 2014, 9, e103135. [Google Scholar] [CrossRef]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults With Chronic Stroke. Neurorehabilit. Neural Repair 2015, 29, 969–978. [Google Scholar] [CrossRef]
- Brønd, J.C.; Andersen, L.B.; Arvidsson, D. Generating ActiGraph Counts from Raw Acceleration Recorded by an Alternative Monitor. Med. Sci. Sports Exerc. 2017, 49, 2351–2360. [Google Scholar] [CrossRef]
- Poitras, I.; Clouâtre, J.; Campeau-Lecours, A.; Mercier, C. Accelerometry-Based Metrics to Evaluate the Relative Use of the More Affected Arm during Daily Activities in Adults Living with Cerebral Palsy. Sensors 2022, 22, 1022. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Butland, R.J.; Pang, J.; Gross, E.R.; Woodcock, A.A.; Geddes, D.M. Two-, six-, and 12-minute walking tests in respiratory disease. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 1607–1608. [Google Scholar] [CrossRef]
- Alvarenga, M.T.; Avelino, P.R.; KK, D.E.M.; Texeira-Salmela, L.F.; Faria, C.D.; Scianni, A.A. Deficits in dynamic balance were the motor impairments that best explained limitations in community ambulation after stroke. Eur. J. Phys. Rehabil. Med. 2023, 59, 145–151. [Google Scholar] [CrossRef]
- Mattlage, A.E.; Redlin, S.A.; Rippee, M.A.; Abraham, M.G.; Rymer, M.M.; Billinger, S.A. Use of Accelerometers to Examine Sedentary Time on an Acute Stroke Unit. J. Neurol. Phys. Ther. 2015, 39, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, K.; Bever, C.T.; Tian, J.; Zhan, M.; Conroy, S.S. Comparing Home Upper Extremity Activity with Clinical Evaluations of Arm Function in Chronic Stroke. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100048. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, M.E.; Selles, R.W.; Stam, H.J.; Ribbers, G.M.; Bussmann, J.B. Quantifying Nonuse in Chronic Stroke Patients: A Study Into Paretic, Nonparetic, and Bimanual Upper-Limb Use in Daily Life. Arch. Phys. Med. Rehabil. 2012, 93, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Flury, D.; Massé, F.; Paraschiv-Ionescu, A.; Aminian, K.; Luft, A.R.; Gonzenbach, R. Clinical value of assessing motor performance in postacute stroke patients. J. Neuroeng. Rehabil. 2021, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Y.; Ye, B.; Babineau, J.; Ding, Y.; Mihailidis, A. Technology-Based Compensation Assessment and Detection of Upper Extremity Activities of Stroke Survivors: Systematic Review. J. Med. Internet Res. 2022, 24, e34307. [Google Scholar] [CrossRef]
- Dahms, C.; Brodoehl, S.; Witte, O.W.; Klingner, C.M. The importance of different learning stages for motor sequence learning after stroke. Hum. Brain Mapp. 2020, 41, 270–286. [Google Scholar] [CrossRef]
- Kwakkel, G.; Veerbeek, J.M.; van Wegen, E.E.; Wolf, S.L. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef]
- Lee, S.I.; Liu, Y.; Vergara-Díaz, G.; Pugliese, B.L.; Black-Schaffer, R.; Stoykov, M.E.; Bonato, P. Wearable-Based Kinematic Analysis of Upper-Limb Movements During Daily Activities Could Provide Insights into Stroke Survivors’ Motor Ability. Neurorehabilit. Neural Repair 2024, 38, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The use of wearable sensors to assess and treat the upper extremity after stroke: A scoping review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef] [PubMed]
- Rajda, C.; Desabrais, K.; Levin, M. Relationships Between Cognitive Impairments and Motor Learning After Stroke: A Scoping Review. Neurorehabilit. Neural Repair 2024, 39, 15459683241300458. [Google Scholar] [CrossRef] [PubMed]
- Bernaldo de Quirós, M.; Douma, E.H.; van den Akker-Scheek, I.; Lamoth, C.J.C.; Maurits, N.M. Quantification of Movement in Stroke Patients under Free Living Conditions Using Wearable Sensors: A Systematic Review. Sensors 2022, 22, 1050. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]



| Age (years) | |
| mean ± SD | 57.1 ± 11.3 |
| Time since stroke (months) | |
| mean ± SD | 30.0 ± 20.7 |
| Gender, n (%) | |
| Male | 6 (46.2%) |
| Female | 7 (53.8%) |
| Affected side, n (%) | |
| Right | 8 (61.5%) |
| Left | 5 (38.5%) |
| Type of stroke, n (%) | |
| Ischemic | 10 (76.9%) |
| Hemorrhagic | 3 (23.1%) |
| mRS, n (%) | |
| 1 | 1 (7.7%) |
| 2 | 4 (30.8%) |
| 3 | 8 (61.5%) |
| 6MWT (m) | |
| mean ± SD | 311 ± 124.1 |
| BBT (n) | |
| mean ± SD | 14 ± 19.7 |
| Therapeutic Days with Available Data (n/130) | Mean ± SD | |
|---|---|---|
| Upper Extremities | ||
| Percentage of use (%) | ||
| Non-affected side | 79 | 67.3 ± 9.7 |
| Affected side | 84 | 51.5 ± 9.2 |
| Activity magnitude (counts/s) | ||
| Non-affected side | 79 | 57.3 ± 17.3 |
| Affected side | 84 | 25.3 ± 9.6 |
| Bimanual use (%) | 74 | 43.4 ± 8.9 |
| Bilateral magnitude (counts/s) | 74 | 81.3 ± 21.3 |
| Use ratio | 74 | 0.75 ± 0.16 |
| Magnitude ratio | 74 | –1.91 ± 0.59 |
| Lower Extremities | ||
| Percentage of use (%) | 90 | 47.6 ± 14.0 |
| Activity magnitude (counts/s) | 90 | 17.2 ± 12.0 |
| Standing time (%) | 90 | 27.7 ± 14.5 |
| Sitting time (%) | 90 | 72.3 ± 14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somville, M.; Rosselli, Z.; Ducoffre, E.; Penta, M.; Smeesters, T.; Bleyenheuft, Y.; Saussez, G. Use of Accelerometers to Monitor Motor Activity During HABIT-ILE for Chronic Stroke: An Exploratory Study. Sensors 2025, 25, 6656. https://doi.org/10.3390/s25216656
Somville M, Rosselli Z, Ducoffre E, Penta M, Smeesters T, Bleyenheuft Y, Saussez G. Use of Accelerometers to Monitor Motor Activity During HABIT-ILE for Chronic Stroke: An Exploratory Study. Sensors. 2025; 25(21):6656. https://doi.org/10.3390/s25216656
Chicago/Turabian StyleSomville, Merlin, Zélie Rosselli, Edouard Ducoffre, Massimo Penta, Tristan Smeesters, Yannick Bleyenheuft, and Geoffroy Saussez. 2025. "Use of Accelerometers to Monitor Motor Activity During HABIT-ILE for Chronic Stroke: An Exploratory Study" Sensors 25, no. 21: 6656. https://doi.org/10.3390/s25216656
APA StyleSomville, M., Rosselli, Z., Ducoffre, E., Penta, M., Smeesters, T., Bleyenheuft, Y., & Saussez, G. (2025). Use of Accelerometers to Monitor Motor Activity During HABIT-ILE for Chronic Stroke: An Exploratory Study. Sensors, 25(21), 6656. https://doi.org/10.3390/s25216656







