Pd/Attapulgite Core–Shell Structured Catalytic Combustion Gas Sensor for Highly Sensitive Real-Time Methane Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensor Architecture
2.2. Fabrication of Detection/Compensation Elements
2.3. Sensor Bonding and Encapsulation
2.4. Characterization
2.5. Performance Testing
3. Results and Discussion
3.1. Structural and Elemental Analysis
3.2. Working Temperature
3.3. Ambient Temperature Effects
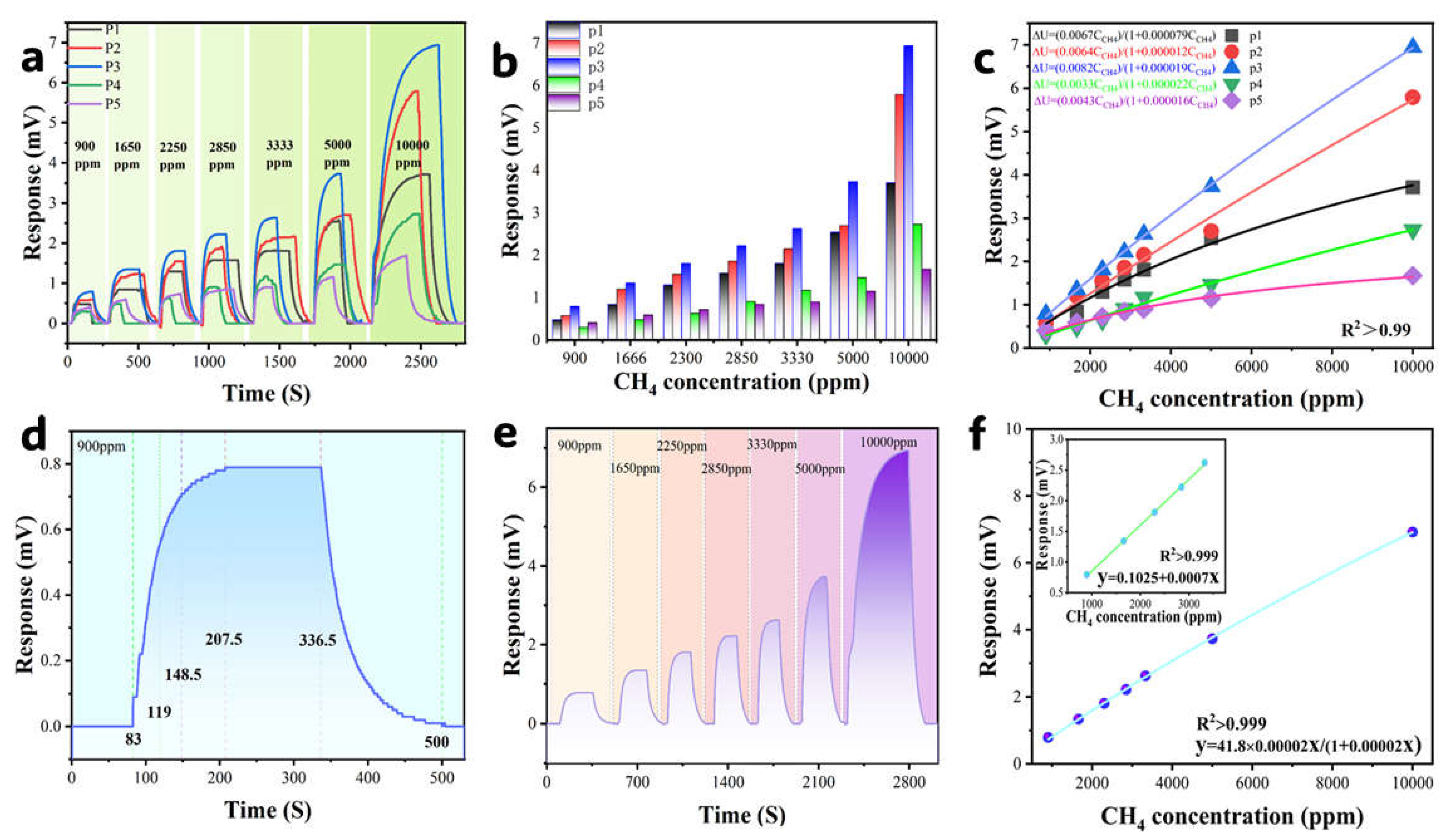
3.4. Electrical Signal Response
3.5. Response Rate
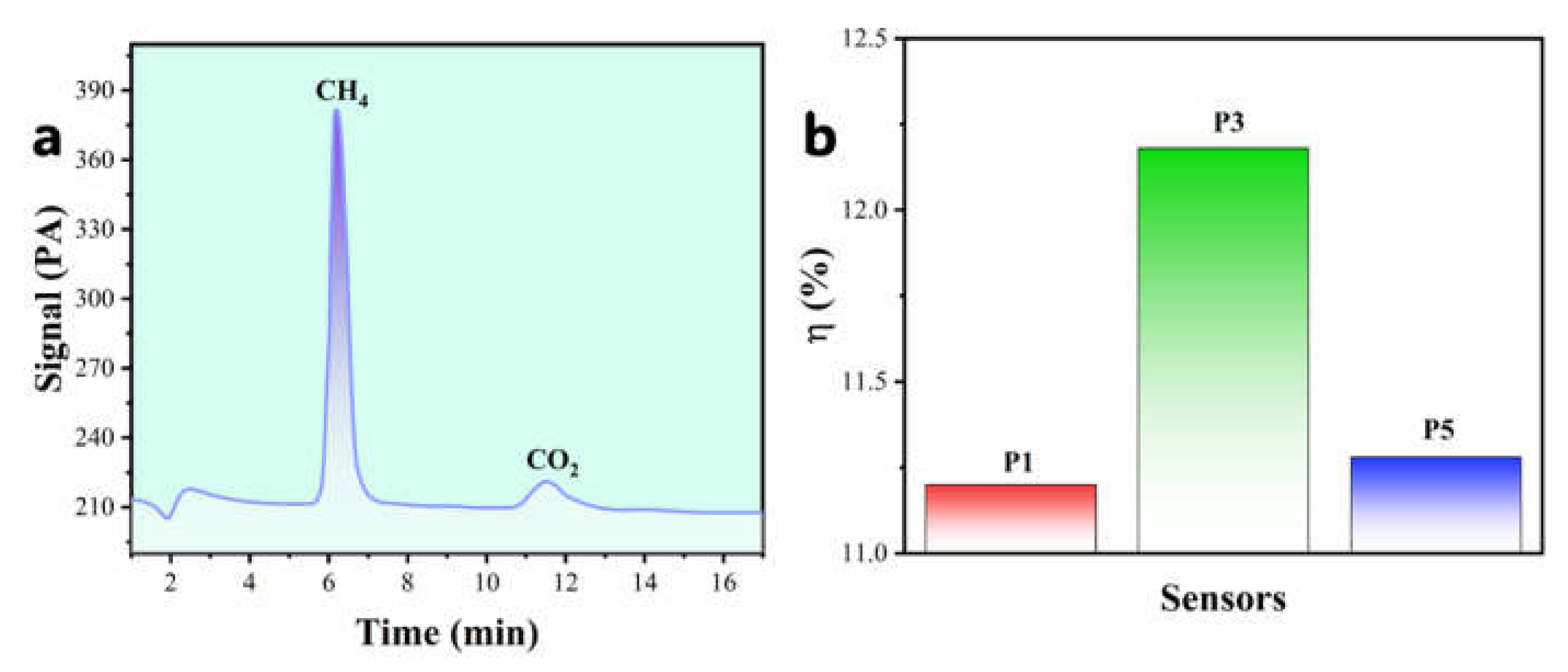
3.6. Catalytic Combustion Reaction Efficiency
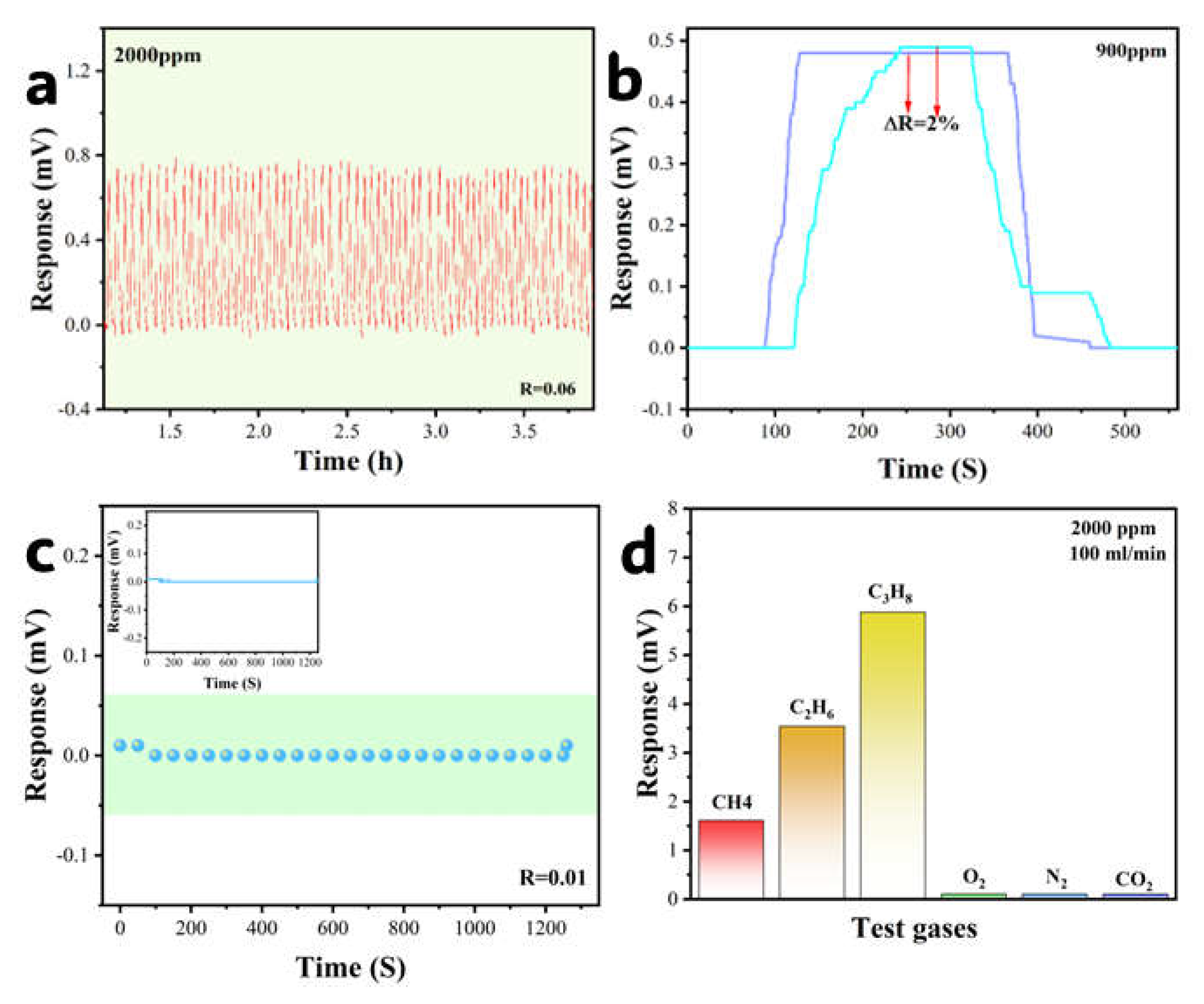
3.7. Repeatability and Specificity
3.8. Reaction Mechanism
3.9. Comparison of Detection Limits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Meng, X.; Zhu, Y.; Gao, W. Design of ultrahigh-response gas sensor based on Pd-WO3/WS2 ternary nanocomposites for ultrafast hydrogen detection. Sens. Actuators B Chem. 2024, 401, 134991. [Google Scholar] [CrossRef]
- Luo, Y.; Gao, J.; Fu, H.; Liu, S.; Hua, Z. A selective methane gas sensor based on SnO2 utilizing a reactive and porous substrate. Chin. J. Anal. Chem. 2025, 53, 100526. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Zheng, Z.; Zhang, Y.; Li, K.; Chen, T.; Guo, D.; Cao, H.; Zhan, R.; Lin, H. Comparative study on properties of Pd-Ce-Zr catalysts synthesized by flame spray pyrolysis and solution combustion: Application for methane catalytic oxidation in electric field. Appl. Surf. Sci. 2021, 566, 150536. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Wang, D.; He, R. Advances in the development of methane sensors with gas-sensing materials. Chin. Sci. Bull. 2019, 64, 1456–1470. [Google Scholar] [CrossRef]
- Kaur, L. Graphene based chemiresistor sensors for the detection of toxic air pollutants: A theoretical overview. J. Indian Chem. Soc. 2023, 100, 101019. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yang, Z.; Hu, N. Review of recent progress on graphene-based composite gas sensors. Ceram. Int. 2021, 47, 16367–16384. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Z.; Tian, C.; Yuan, W.; Hua, Z.; Fan, S.; Wu, Y.; Tian, X. Selective detection of methane by HZSM-5 zeolite/Pd-SnO2 gas sensors. Sens. Actuators B Chem. 2020, 321, 128567. [Google Scholar] [CrossRef]
- Shaposhnik, A.V.; Moskalev, P.V.; Arefieva, O.A.; Zvyagin, A.A.; Kul, O.V.; Vasiliev, A.A. Selective determination of hydrogen in a mixture with methane using a single metal oxide sensor. Int. J. Hydrogen Energy 2024, 82, 523–530. [Google Scholar] [CrossRef]
- Li, H.; Wu, R.; Tian, X.; Han, L.; Chen, T.; Yang, B.; Zhi, Z.; Hua, Z.; Fan, S. A catalytic filter based on Pt-CeO2 for selective methane detection with SnO2 sensors. Sens. Actuators B Chem. 2023, 389, 133872. [Google Scholar] [CrossRef]
- Wu, S. Preparation of attapulgite nanoparticles modified polypropylene adsorption membrane and its application in small molecular pollutant adsorption. J. Chromatogr. B 2024, 1247, 124338. [Google Scholar] [CrossRef]
- Sun, X.; Ke, C.; Lu, Q.; Liang, L.; Xie, G. Adsorption of fluoride ion on Al/La modified acidified attapulgite composite materials. Sep. Purif. Technol. 2025, 354, 128791. [Google Scholar] [CrossRef]
- Vereshchagina, E.; Tiggelaar, R.M.; Sanders, R.G.P.; Wolters, R.A.M.; Gardeniers, J.G.E. Low Power Micro-Calorimetric Sensors for Analysis of Gaseous Samples. Sens. Actuators B Chem. 2015, 206, 772–787. [Google Scholar] [CrossRef]
- Lee, S.M.; Dyer, D.C.; Gardner, J.W. Design and Optimisation of a High-Temperature Silicon Micro-Hotplate for Nanoporous Palladium Pellistors. Microelectron. J. 2003, 34, 115–126. [Google Scholar] [CrossRef]
- Nagai, D.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Thermal Balance Analysis of a Micro-Thermoelectric Gas Sensor Using Catalytic Combustion of Hydrogen. Sensors 2014, 14, 1822–1834. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Lan, C.; Mu, C.; Liu, J.; Wang, M.; Mu, B. Preparation of mesoporous carbon/clay composite adsorbent based on the mixed-dimension attapulgite: Dye adsorption, mechanism and molecular dynamics calculations. J. Environ. Chem. Eng. 2025, 13, 117528. [Google Scholar] [CrossRef]
- Boudriche, L.; Chamayou, A.; Calvet, R.; Hamdi, B.; Balard, H. Influence of different dry milling processes on the properties of an attapulgite clay, contribution of inverse gas chromatography. Powder Technol. 2014, 254, 352–363. [Google Scholar] [CrossRef]
- Moreroa-Monyelo, M.; Falayi, T.; Ntuli, F.; Magwa, N. Studies towards the adsorption of sulphate ions from acid mine drainage by modified attapulgite clays. S. Afr. J. Chem. Eng. 2022, 42, 241–254. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Yuan, Z.; Zhang, Y.; Li, X.; Wu, Y.; Jiang, Y.; Tai, H. Novel application of attapulgite on high performance and low-cost humidity sensors. Sens. Actuators B Chem. 2020, 305, 127534. [Google Scholar] [CrossRef]
- Duan, X.; Duan, Z.; Zhang, Y.; Liu, B.; Li, X.; Zhao, Q.; Yuan, Z.; Jiang, Y.; Tai, H. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Negm, H.H.; Allam, E.A.; Nabil, I.M.; Abdeltwab, E.; Mostafa, M.; El-Taher, A. Exploring the potential of attapulgite clay composites containing intercalated nano-cadmium oxide and nano-nickel oxide for efficient radiation shielding applications. Radiat. Phys. Chem. 2024, 225, 112149. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Zhao, M.; Xu, H.; Wang, J.; Chen, Y. Silicate-induced high-temperature-resistant small-crystallite ceria support enhancing palladium-catalyzed low-concentration methane combustion. Sep. Purif. Technol. 2025, 353, 128385. [Google Scholar] [CrossRef]
- Nurfarhana, M.M.; Asikin-Mijan, N.; Yusoff, S.F.M.; Abdulkareem-Alsultan, G.; Othman, M.A.R.; Kawi, S. Adsorption iso-therms and kinetic studies of nitrogen-doped porous carbon by sodium amide activation from natural rubber for CO2 capture. Mater. Chem. Phys. 2025, 343, 131058. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic combustion of methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Xu, G.; Liu, Z.; Shi, W.; Shi, X.; Yu, Y.; He, H. Palladium nanoparticles anchored on silanol nests of zeolite showed superior stability for methane combustion. Appl. Catal. B Environ. Energy 2024, 356, 124221. [Google Scholar] [CrossRef]
- An, B.; Zhou, H.; Cao, J.; Ming, P.; Xia, J.; Yao, J.; Persic, J. Simulation study on the effect of palladium layer thickness and temperature on the bonding properties of palladium coated copper wire. Microelectron. Reliab. 2024, 162, 115515. [Google Scholar] [CrossRef]
- Ballauri, S.; Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Fino, D.; Russo, N.; Bensaid, S. Wide range temperature stability of palladium on ceria-praseodymia catalysts for complete methane oxidation. Catal. Today 2022, 390–391, 185–197. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, B.; Li, Y.; Zhang, S.; Zhang, Y.; Hua, X.; Liu, G.; Li, B.; Zhou, J.; Xie, E.; et al. Methanol gas detection of elec-trospun CeO2 nanofibers by regulating Ce3+/Ce4+ mole ratio via Pd doping. Sens. Actuators B Chem. 2020, 307, 127638. [Google Scholar] [CrossRef]
- Qi, T.; Sun, J.; Yang, X. Research on Ethanol Sensing Characteristics of Pd/Neck-Connected-ZnO Based Chemiresistive Sensors. ChemistrySelect 2021, 6, 4797–4802. [Google Scholar] [CrossRef]
- Birajdar, S.N.; Adhyapak, P.V. Palladium-decorated vanadium pentoxide as NOx gas sensor. Ceram. Int. 2020, 46, 27381–27393. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, X.; Yang, M.; Ma, H.; Sun, Y.; Zhang, H.; Dong, T.; Liu, W. A high sensitivity and selectivity n-butanol sensor based on monodispersed Pd-doped SnO2 nanoparticles mediated by glucose carbonization. Semicond. Sci. Technol. 2020, 35, 095030. [Google Scholar] [CrossRef]
- Li, X.; He, H.; Tan, T.; Zou, Z.; Tian, Z.; Zhou, W.; Bao, Y.; Xia, X.; Gao, Y. Annealing effect on the methane sensing performance of Pt–SnO2/ZnO double layer sensor. Appl. Surf. Sci. 2023, 640, 158428. [Google Scholar] [CrossRef]
- GB 15322.1-2019; Combustible Gas Detectors—Part 1: Point-Type Combustible Gas Detectors for Industrial and Commercial use. National Standardization Administration: Beijing, China, 2019.
- EN 60079-29-1; Explosive Atmospheres—Part 29-1: Gas Detectors—Performance Requirements of Detectors for Flammable Gases. International Electrotechnical Commission: Geneva, Switzerland, 2020.
- JIS C 0901:2011; Mine Safety Law. Ministry of Economy, Trade and Industry (METI): Tokyo, Japan, 2022.
- Zhang, Y.; Chen, F.; Wang, D.; Wang, T.; Zhang, D. Vanadium dioxide/molybdenum telluride heterojunction gas sensor for methane detection. J. Alloys Compd. 2023, 969, 172023. [Google Scholar] [CrossRef]
- Parida, S.; Das, A.; Prasad, A.K.; Ghatak, J.; Dhara, S. Native defect-assisted enhanced response to CH4 near room temperature by Al0.07 Ga0.93 N nanowires. Phys. Chem. Chem. Phys. 2018, 20, 18391–18399. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lou, Q.; Wu, W.; Wang, K.; Xuan, C. NO2 Gas Sensing Performance of a VO2(B) Ultrathin Vertical Nanosheet Array: Experimental and DFT Investigation. ACS Appl. Mater. Interfaces 2021, 13, 31968–31977. [Google Scholar] [CrossRef]
- Mounasamy, V.; Mani, G.K.; Ponnusamy, D.; Tsuchiya, K.; Reshma, P.R.; Prasad, A.K.; Madanagurusamy, S. Investigation on CH4 sensing characteristics of hierarchical V2O5 nanoflowers operated at relatively low temperature using chemiresistive ap-proach. Anal. Chim. Acta 2020, 1106, 148–160. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Sun, Y.; Jiang, C.; Yao, Y.; Zhang, Y. Fabrication of platinum-loaded cobalt oxide/molybdenum disulfide nanocomposite toward methane gas sensing at low temperature. Sens. Actuators B Chem. 2017, 252, 624–632. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R. Characterization of nickel oxide decorated-reduced graphene oxide nanocomposite and its sensing properties toward methane gas detection. J. Mater. Sci. Mater. Electron. 2016, 27, 3723–3730. [Google Scholar] [CrossRef]
- Min, B.-K.; Choi, S.-D. Undoped and 0.1wt.% Ca-doped Pt-catalyzed SnO2 sensors for CH4 detection. Sens. Actuators B Chem. 2005, 108, 119–124. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Selective detection of methane and butane by temperature modulation in iron doped tin oxide sensors. Sens. Actuators B Chem. 2006, 115, 610–613. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a novel methane gas sensor based on SnO2 nano-rods-nanoporous graphene hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sens. Actuators B Chem. 2012, 166–167, 156–164. [Google Scholar] [CrossRef]
- Nikan, E.; Khodadadi, A.A.; Mortazavi, Y. Highly enhanced response and selectivity of electrospun ZnO-doped SnO2 sensors to ethanol and CO in presence of CH4. Sens. Actuators B Chem. 2013, 184, 196–204. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.-S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Karami Horastani, Z.; Sayedi, S.M.; Sheikhi, M.H.; Rahimi, E. Effect of silver additive on electrical conductivity and methane sensitivity of SnO2. Mater. Sci. Semicond. Process. 2015, 35, 38–44. [Google Scholar] [CrossRef]
- Nasresfahani, S.; Sheikhi, M.H.; Tohidi, M.; Zarifkar, A. Methane gas sensing properties of Pd-doped SnO2/reduced graphene oxide synthesized by a facile hydrothermal route. Mater. Res. Bull. 2017, 89, 161–169. [Google Scholar] [CrossRef]
- Lu, W.; Ding, D.; Xue, Q.; Du, Y.; Xiong, Y.; Zhang, J.; Pan, X.; Xing, W. Great enhancement of CH4 sensitivity of SnO2 based nanofibers by heterogeneous sensitization and catalytic effect. Sens. Actuators B Chem. 2018, 254, 393–401. [Google Scholar] [CrossRef]

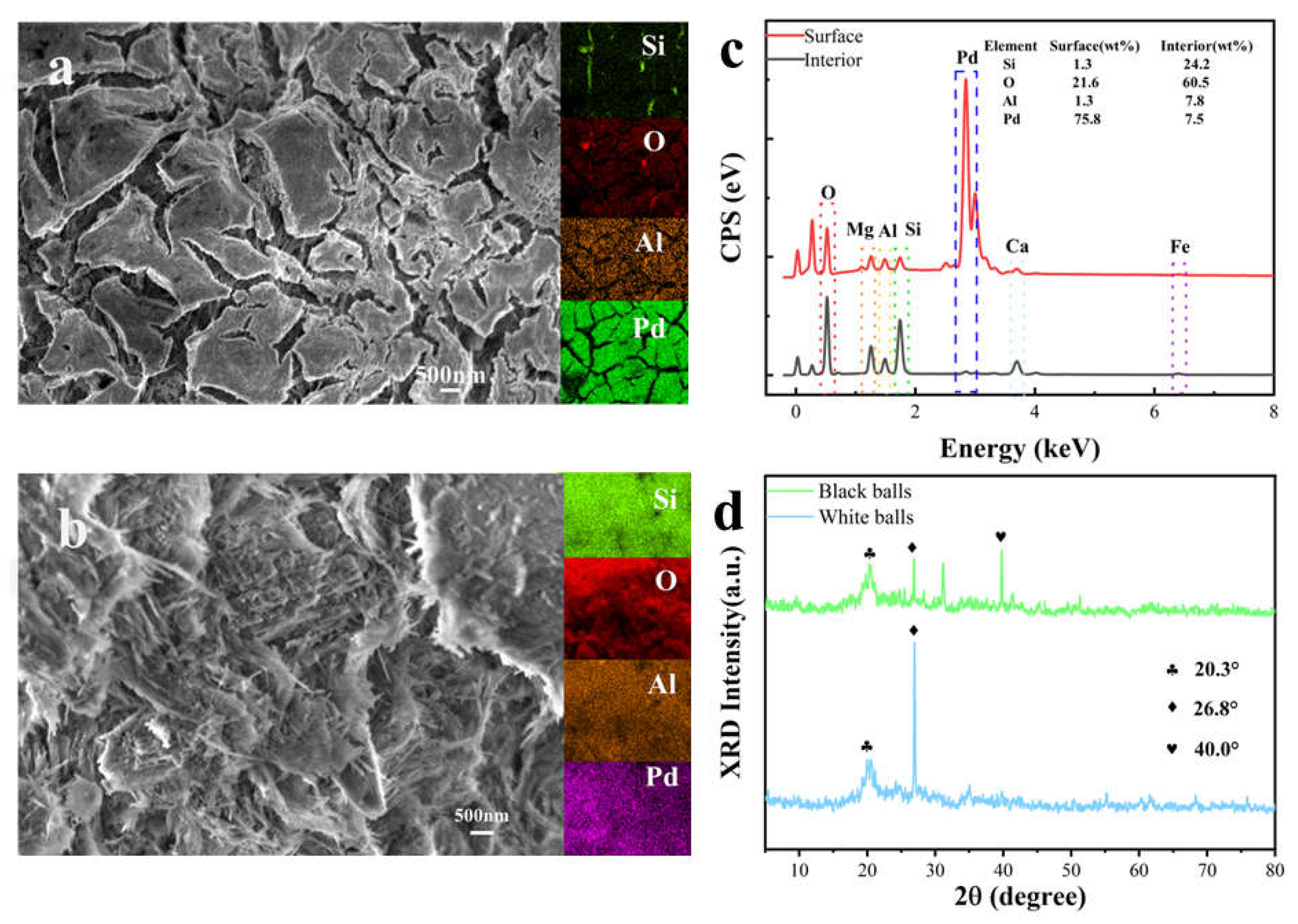

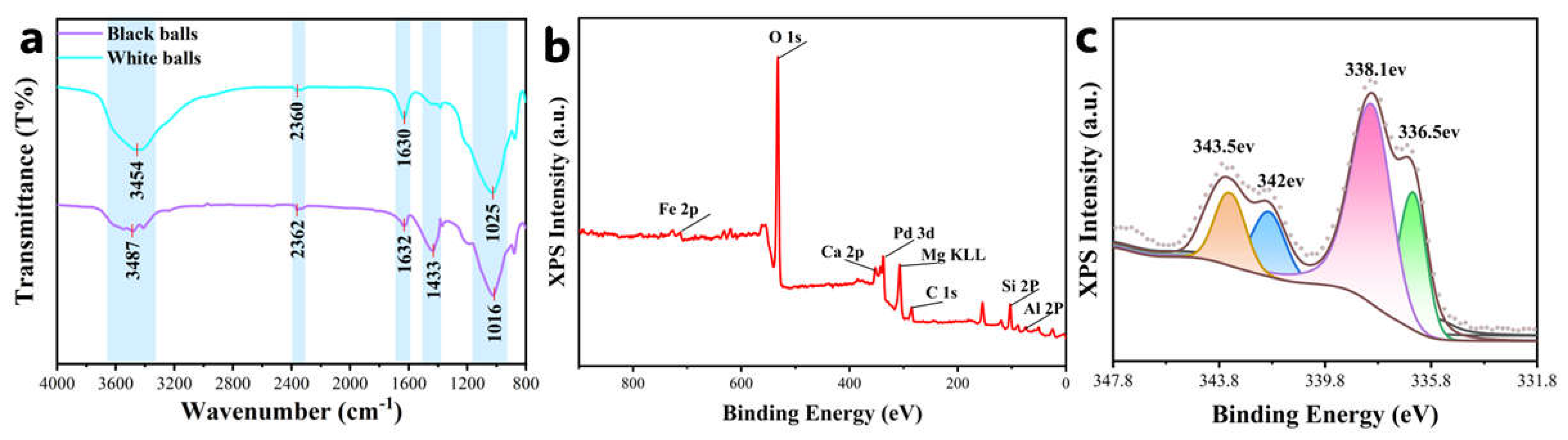
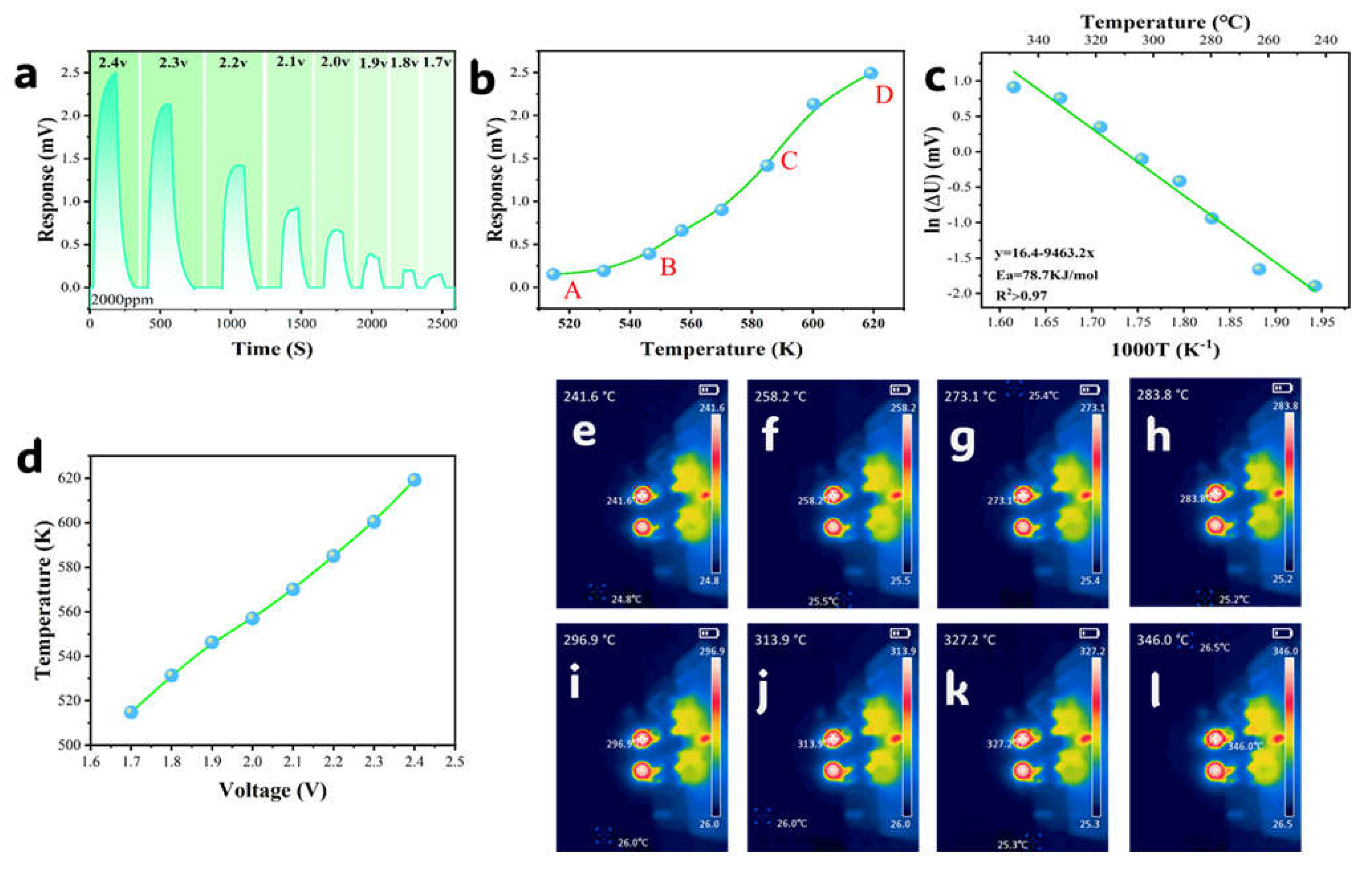
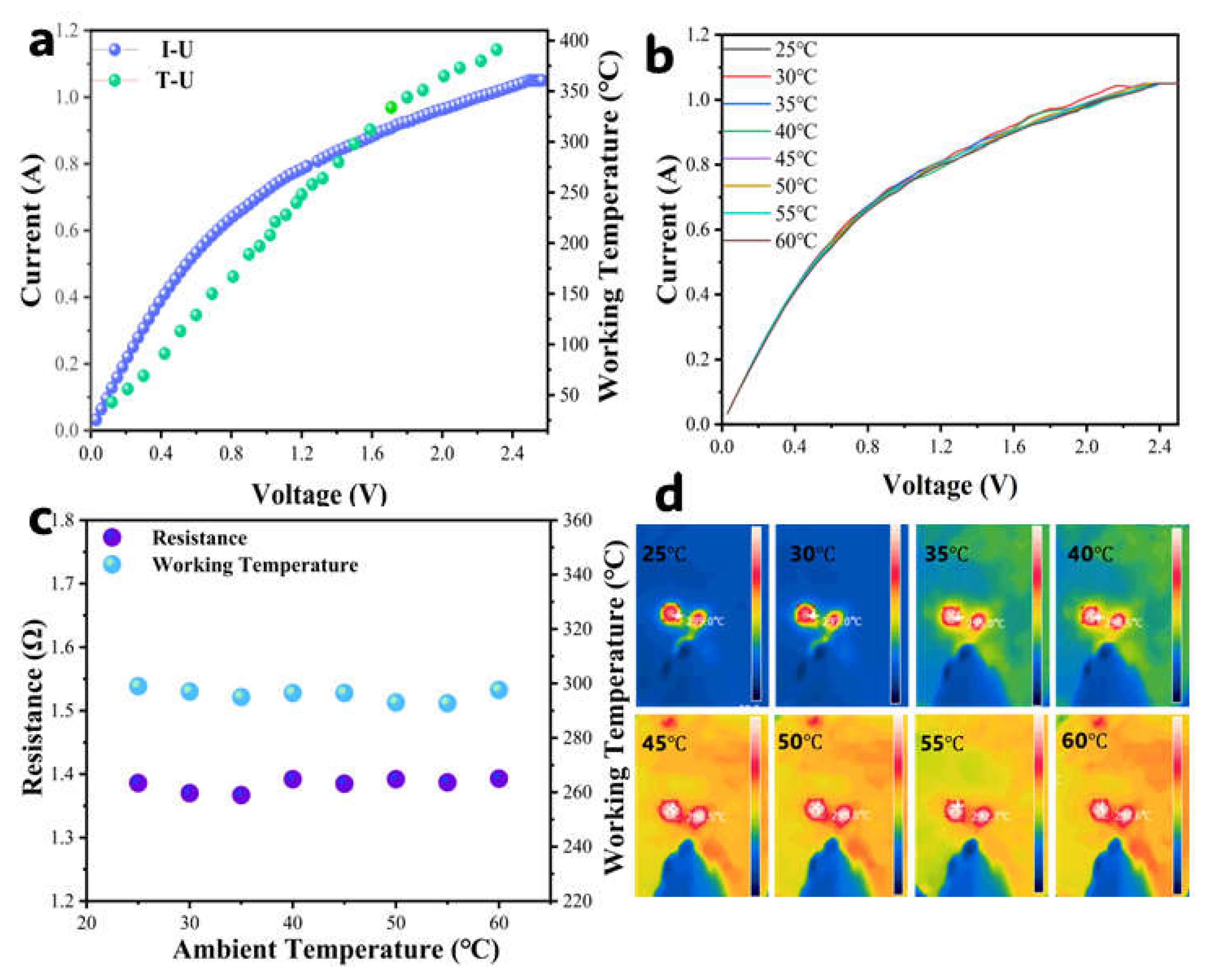
| Samples | SBET (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) | Main Pore Diameter (nm) |
|---|---|---|---|---|
| Raw materials | 138.98 | 0.3333 | 6.75 | 0.63 |
| Heat treatment | 91.39 | 0.3943 | 9.51 | 18.72 |
| Acid treatment | 139.98 | 0.4753 | 9.43 | 21.12 |
| 3454 cm−1 (-OH) | 2360 cm−1 (O=C=O) | 1630 cm−1 (H-O-H) | 1433 cm−1 (CO32−) | 1025 cm−1 (Si-O) | |
|---|---|---|---|---|---|
| Black | weaken | exist | exist | exist | exist |
| White | exist | exist | exist | absent | right shift |
| SAMPLE | U90 (mV) | T90 (S) | T (µV/S) | X (%) |
|---|---|---|---|---|
| P1 | 3.339 | 177.5 | 17.8 | 61.59 |
| P2 | 5.011 | 209.5 | 23.9 | 82.70 |
| P3 | 6.246 | 215.5 | 28.9 | 100 |
| P4 | 2.457 | 202.5 | 12.7 | 43.94 |
| P5 | 1.053 | 126 | 11.9 | 41.18 |
| Wt% | ||||
|---|---|---|---|---|
| P1 | 2 | 5937.95 | 748.62 | 11.20 |
| P3 | 3 | 5469.33 | 758.338 | 12.18 |
| P5 | 4 | 5268.34 | 670.27 | 11.28 |
| Sensing Material | Response (%) | τ Res/τ Recov (s) | LOD (ppm) | Working Temperature/°C | Ref. |
|---|---|---|---|---|---|
| VO2-MoTe2 | 13 (500 ppm) | 75/110 | 500 | RT | [35] |
| GaN | 4.5 (500 ppm) | 126/132 | 100 | 200 | [36] |
| ZnO | 62 (1000 ppm) | 190/204 | 100 | RT | [37] |
| V2O5 | 11.2 (500 ppm) | 230/187 | 50 | 100 | [38] |
| Pt-Co3O4/MoS2 | 21 (500 ppm) | 30/25 | 100 | 180 | [39] |
| NIO/rGO | 15.2 (1000 ppm) | 16/20 | 500 | 350 | [40] |
| Pt–Ca/SnO2 | 2.3 (5000 ppm) | _ | _ | 400 | [41] |
| Fe–SnO2 | 70 (1000 ppm) | _ | _ | 350 | [42] |
| SnO2 NRS-NPG | 24.9 (1000 ppm) | 369/_ | 1000 | 600 | [43] |
| Pd–SnO2 | 97.2 (200 ppm) | 26/70 | _ | 220 | [44] |
| ZnO–SnO2 | 80 (5000 ppm) | _ | _ | 350 | [45] |
| Ni2O3–SnO2 | 127 (200 ppm) | _ | _ | 400 | [46] |
| Ag–SnO2 | 75 (2000 ppm) | _ | _ | 430 | [47] |
| Pd/SnO2-rGO | 9.5 (12,000 ppm) | 300/420 | _ | RT | [48] |
| Pt–SnO2 | 4.5 (1000 ppm) | 25/141 | _ | 300 | [49] |
| Pd/attapulgite | 107 (900 ppm) | 65.5/163.5 | 36 | 296 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Pang, S.; Zhang, Z.; Feng, L.; Zhang, C.; Lin, J.; Liu, Z.; Sun, Y.; Wang, S.; Lou, Z. Pd/Attapulgite Core–Shell Structured Catalytic Combustion Gas Sensor for Highly Sensitive Real-Time Methane Detection. Sensors 2025, 25, 4950. https://doi.org/10.3390/s25164950
Cao S, Pang S, Zhang Z, Feng L, Zhang C, Lin J, Liu Z, Sun Y, Wang S, Lou Z. Pd/Attapulgite Core–Shell Structured Catalytic Combustion Gas Sensor for Highly Sensitive Real-Time Methane Detection. Sensors. 2025; 25(16):4950. https://doi.org/10.3390/s25164950
Chicago/Turabian StyleCao, Shuo, Shuang Pang, Zishuai Zhang, Lulu Feng, Chong Zhang, Jiahao Lin, Zhiyu Liu, Yifei Sun, Shiyu Wang, and Zhenning Lou. 2025. "Pd/Attapulgite Core–Shell Structured Catalytic Combustion Gas Sensor for Highly Sensitive Real-Time Methane Detection" Sensors 25, no. 16: 4950. https://doi.org/10.3390/s25164950
APA StyleCao, S., Pang, S., Zhang, Z., Feng, L., Zhang, C., Lin, J., Liu, Z., Sun, Y., Wang, S., & Lou, Z. (2025). Pd/Attapulgite Core–Shell Structured Catalytic Combustion Gas Sensor for Highly Sensitive Real-Time Methane Detection. Sensors, 25(16), 4950. https://doi.org/10.3390/s25164950






