Abstract
Industrial development has led to the widespread production of toxic materials, including carcinogenic, mutagenic, and toxic chemicals. Even with strict management and control measures, such materials still pose threats to human health. Therefore, convenient chemical sensors are required for toxic chemical monitoring, such as optical, electrochemical, nanomaterial-based, and biological-system-based sensors. Many existing and new chemical sensors have been developed, as well as new methods based on novel technologies for detecting toxic materials. The emergence of material sciences and advanced technologies for fabrication and signal-transducing processes has led to substantial improvements in the sensing elements for target recognition and signal-transducing elements for reporting interactions between targets and sensing elements. Many excellent reviews have effectively summarized the general principles and applications of different types of chemical sensors. Therefore, this review focuses on chemical sensor advancements in terms of the sensing and signal-transducing elements, as well as more recent achievements in chemical sensors for toxic material detection. We also discuss recent trends in biosensors for the detection of toxic materials.
1. Introduction
Anthropogenic activities, including industrial development and manufacturing, are responsible for various types of environmental pollution caused by the release of toxic chemicals [1]. These chemicals include airborne toxic chemicals, medicines, heavy metals, and the byproducts of anthropogenic activities. Although often generated for the good of humankind, failure to effectively manage or control such materials threatens both the environment and human health [2]. The release of harmful chemicals (such as phenols, polycyclic aromatic hydrocarbons, herbicides, insecticides, and nitroxides) into the environment can disrupt the balance between ecosystems and human well-being [3,4]. Therefore, tools and methods for detecting and monitoring toxic materials are crucial for protecting human health. As we live in an era of abundant material development, it is also important to characterize and assess the risks of newly developed materials. Many countries, including South Korea, have implemented laws such as “The Act on Registration and Evaluation of Chemicals” to document the chemical and physical properties of these materials [5]. Although these systems are strictly controlled by national authorities, toxic materials must also be thoroughly monitored to avoid potential threats to human health.
Traditionally, environmental monitoring of toxic chemicals has been performed using instrument-based analyses, such as spectrophotometry, high-performance liquid chromatography, gas chromatography, and mass spectrometry [6,7,8]. However, despite the superior sensitivity and precision of these tools, demand has increased for other methods that can compensate for the disadvantages of instrument-based analyses, such as high instrument and time costs. Chemical sensors represent a rapid and simple alternative method of target detection. As such, multiple types of chemical sensors with various applications have been developed using the novel techniques of different research fields.
Chemical sensors transform chemical information, ranging from the concentration of a specific sample component to the total composition, into an analytically useful signals [9]. In other words, chemical sensors systems can recognize and report signals originating from the chemical reaction of an analyte or changes in its chemical and physical properties. The IUPAC Commission defines chemical sensors as follows: “analytical chemical sensors are miniaturized transducers that selectively and reversibly respond to chemical compounds or ions and yield electrical signals which depend on the concentration” [10]. Janata et al. documented the definitions and types of existing chemical sensors [11]. Chemical sensing involves a data collection process that identifies the chemical components in a system by measuring the chemical and physical property changes induced by the interaction between targets and sensing platforms. Based on this unique interaction, diverse data acquisition techniques have been applied to develop many types of chemical sensor, including thermal, electrochemical, potentiometric, and optical techniques [12,13,14]. Although traditionally classified according to the method of data transduction, such as electrochemical responses, optical responses, electrical signals, optical signals, changes in electrical properties, and mass changes, chemical sensors can also be classified according to the materials used for the sensing devices; for example, nanostructure-based sensors, carbon nanotube-based sensors, biological system-based sensors, and graphene-based sensors [15,16,17,18,19]. Since the chemical sensors were designed using a combination of sensing and signal transducing elements, the types of chemical sensors could be diversified enormously. In this regard, it is not meaningful to classify chemical sensors simply by their signal transducing/translating type or by their sensing elements.
Many existing review articles have effectively summarized the principles and mechanisms of various types of existing chemical sensors, including nanostructured material-based sensors, carbon nanotube sensors, graphene-based sensors, nanomaterial-based sensors, and biological system-based sensors, which are also categorized as electrochemical and optical chemical sensors [12,15,20,21,22,23]. Biosensors that use biomolecules such as enzymes, proteins, and living cells as composites for sensing and transducing signals represent a subclass of chemical sensors [10,24]. As a review of all chemical sensors would be impractical because of their substantial diversity and the continual development of new biosensor techniques, this review focuses on recent trends in chemical sensors used to detect toxic chemicals and discusses recent achievements in novel chemical sensor technologies. First, we discuss the applications and prospects of chemical sensors and introduce different types of chemical sensors for detecting toxic chemicals. We then discuss recent findings and applications of chemical sensors for toxic chemical monitoring, including transcription factor (TF)-based biosensors.
2. Overview of Chemical Sensors for Detecting Toxic Materials
Chemical sensors are divided into various subclasses based on the materials used for the sensing elements and the types of output signal. Nonetheless, the basic principles and working mechanisms of chemical sensors are similar in terms of the major components, i.e., sensing elements and signal-transducing elements. The basic components of chemical sensors are shown in Figure 1. Chemical sensors comprise target sensing and signal-transducing elements and vary according to the sensing element source and type of signal-transducing element. Output type is also a significant factor in the classification of chemical sensors. Although chemical sensors are classically divided into optical, electrochemical, and thermal sensors based on the type of output signal, classification based on the sensing element has become more complicated. Chemical sensors that employ biomolecules as components are now categorized as biosensors. Owing to advances in materials science and fabrication technologies, new types of sensing element have recently been developed. Several types of chemical sensors detect the same target, and some chemical sensors can detect several targets via the sensing elements. For example, Balaji et al. reported that an optical sensor employing nanostructured cages as the sensing element can detect various metal ions including Sb, Hg, Pb, and Cd by modifying the cage structures [25]. In addition, methyl parathion, a component of pesticides, can be detected using electrochemical sensors that employ nanostructure-based sensing elements [26,27]. Therefore, the performance of chemical sensors can be determined using the target selectivity of the sensing elements and the sensitivity of the signal-transducing elements. From this perspective, new chemical sensors can be developed by combining sensing and signal-transducing elements.
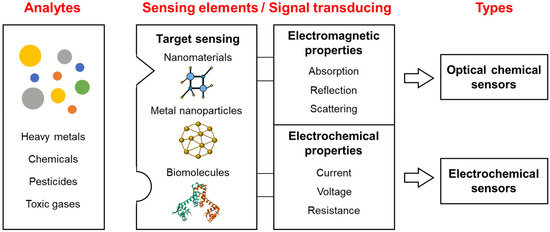
Figure 1.
Types of chemical sensors based on sensing and transducing elements.
The purpose of chemical sensors is to detect targets, including gases, chemicals, biomolecules, and cells, for diagnosis, monitoring, or determining production efficiency. However, considering the abundance of toxic materials released into the environment, the major application of chemical sensors is the detection of harmful materials that adversely affect human health. Among the many types of chemical sensors reported in different research fields, we focus on three types: optical, electrochemical, and biological system-based sensors.
3. Optical Chemical Sensors
Optical sensors are a group of chemical sensors that employ electromagnetic radiation as the energy source to induce transduction signals in the presence of targets. As described above, optical chemical sensors comprise target sensing (recognition) and signal-transducing elements; however, signal transduction is based on various optical principles, such as absorbance, emission, reflectance, and fluorescence. Therefore, direct spectroscopic methods such as UV spectroscopy and Raman spectroscopy are also considered optical sensors. In this review, according to the definition of chemical sensors, we include only sensors that employ changes in electromagnetic radiation as signal-transducing elements. Because the principles and working mechanisms of optical sensors have been discussed in many excellent reviews, we focus here on their applications for detecting toxic materials and recent progress in optical chemical sensors [14,28,29,30,31,32].
3.1. Fiber Optic Chemical Sensors
Optical fibers are the most widely investigated platform for optical chemical biosensors. Optical fibers are used as sensing elements to measure physical properties such as fluorescence, absorption, and reflectance. Although the different subclasses of fiber-optic chemical sensors have the same working principle, the target specificity and selectivity are determined by the fabrication of optical fibers. Fiber-optic chemical sensors (FOCS) represent an area of active research that has led to several new chemical sensors [14,33,34,35]. The optical fiber technology was coupled with surface plasmon resonance (SPR) to generate different novel fiber-optic sensors. Since the characteristics of SPR were varied by optic structures and metal fabrication on fiber optics, the novel SPR-based, fiber-optic sensors have been developed. Boruah et al. reported a SPR-based, fiber-optic sensor for monitoring lead in water samples [36]. They used U-shaped optic fiber fabrication with chitosan and glutathione as sensing elements and detected ppb ranges of lead ions employing SPR as a transducing element. In this regard, it was speculated that the new chemical sensors would be obtained by coupling new sensing elements and signal transducing elements if the appropriate sensing elements were developed. Additionally, advanced techniques such as microfluidic fabrication, lossy mode resonance, and nanoparticle fabrication have emerged to enhance the sensitivity and selectivity of FOCS [37,38,39]. However, new types of optical chemical sensors continue to emerge that combine various types of sensing and transducing elements. Table 1 lists existing types of optical chemical sensors according to their sensing element and transducer type.

Table 1.
List of optical chemical sensors classified by type of sensing element and transducer.
3.2. Microfluidic System-Based Optical Chemical Sensors
Among the new chemical sensors, biochip- and microfluidic-based optical chemical sensors can rapidly and conveniently detect and monitor various environmentally toxic materials [52,53]. Since microfluidic devices handle micron-scale samples using microfluidic channels, it has the advantage of requiring fewer samples and having faster analytic processes. Although the structure of fiber-optic microfluidic sensors differs from that of typical FOCS, they can also target toxic materials such as antibiotics, bisphenol A, and various pharmaceuticals with comparable detection limits. Similar to other chemical sensors, microfluidic devices would act as sensing platforms by integrating sensing elements into microfluidic devices. Recently, Li et al. fabricated a microfluidic chip-based, fiber-optic sensor for detecting the antibiotic minocycline [42], which comprised an optical fiber with a microstructured polymer, fabricated using polymethyl methacrylate and polystyrene, to generate an in-fiber optofluidic chemiluminescence device for minocycline detection. A luminol solution was added to report changes in chemiluminescence signals against minocycline concentrations. The sensor exhibited a minocycline detection limit of 100 ppb, which is comparable to that of high-performance liquid chromatography-based analysis [54]. Wang et al. has reported heavy-metal detecting sensors based on a paper-based microfluidic device [43]. They generated heavy metal monitoring microfluidic-based sensors by fabricating metal selective chromogenic reagents on paper-based microfluidic devices. And, it showed 0.29 ppm, 0.33 ppm, and 0.35 ppm detection limits for Cu(II), Ni(II), and Cr(VI), respectively. Consequently, it was emphasized that the fusion of scientific technologies from different research fields makes chemical sensors more advanced. Besides the microfluidic sensors based on the optic fiber mentioned here, many FOCS have been coupled with microfluidic systems for the detection of toxic materials [47,55,56].
FOCS are classified via their target sensing elements, sensing materials, and detection principles [55,56]. As shown in Figure 1, the target sensing element is used to identify chemical compounds, functionalized nanomaterials, aptamers, antibodies, proteins, and even cells. The target sensing elements are coupled with sensing materials such as nanoparticles, luminol, graphene oxide, carbon nanotubes (CNTs), and microfluidic devices, which transduce changes to the signals [57]. Therefore, the targets of chemical sensors are determined by the specificity and selectivity of the sensing elements. As such, it is critical to develop and investigate new sensing elements to expand the range of sensor targets. Once target-specific sensing elements are prepared, they can be coupled with different techniques to generate various types of chemical sensors.
3.3. Nanoparticle-Based Optical Chemical Sensors
Optical chemical sensors are based on changes in electromagnetic radiation. Among the many detection principles related to optical properties, colorimetric and fluorometric changes are suitable for the detection of toxic materials. Chemical sensors based on these principles must be equipped with a source for excitation and a detector [58,59]. However, samples must include species that exhibit intrinsic fluorescence. Thus, it is critical to endow samples with fluorescent properties for colorimetric and fluorometric measurements. Gold nanoparticles (AuNPs) are suitable sensor materials because they exhibit size-dependent colorimetric and fluorescent properties [60,61].
He et al. reported that rhodamine B functionalized AuNPs can be used in microfluidic devices to detect Hg ions [46]. Because AuNPs show different colorimetric changes according to their functionalization, size variation, and dispersion rate, AuNP-based chemical sensors have been developed to detect various targets, such as ribonucleotides, ascorbic acid, Cr, Pb, and glucose [62]. Although AuNPs are employed as signal-producing elements in chemical sensors, the target selectivity is determined using their functionalization/fabrication. For example, cysteine-capped AuNPs have been used for ethyl parathion and malathion detection [63,64], and DNA hybridization on AuNPs has been used for Hg ion detection [48]. Consequently, new functionalization (fabrication) technologies for AuNPs have accelerated the development of AuNP-based chemical sensors and expanded the range of targets, not only heavy metal ions but also various other chemicals [65,66,67]. The type of transducer used in these chemical sensors is related to the target sensitivity. For example, the sensitivity of AuNP-based chemical sensors toward Hg ions was enhanced from 5 μM to 0.002 μM of the detection limit by employing a surface-enhanced Raman as the transducer [68]. Developing chemical sensor based on metal nanoparticles including AuNPs and AgNPs is a state-of-the-art multidisciplinary science; thus, it is not reasonable to classify them just by the type of transducers. Rather, it would be better to put them into nanomaterial-based sensors, discussed in Section 3.4.
3.4. Nanomaterial-Based Optical Chemical Sensors
The rapid development of new materials and technologies in different research fields has diversified the range of materials and fabrication methods available for sensing elements. Notably, techniques for the fabrication of nanomaterials and nanostructures have opened the door to new sensing elements that can recognize various targets [69,70]. For example, metal nanoparticles, CNTs, metal oxides (quantum dots), and metal organic frameworks are current areas of active research in nanomaterials [70,71,72]. Sensor applications and target selectivity are determined by the type of fabrication and conjugation of nanomaterials. For example, carbon nanomaterials (CNMs) have been used to construct carbon dots, CNTs, graphene, and carbon black, all of which have different properties and are employed in various chemical sensors as sensing elements [73,74]. Carbon dots have been used to develop sensors for detecting cations and anions as well as small molecules and drugs with surface modifications [75,76]. CNTs have also been used as sensing elements with various modifications to detect gaseous analytes such as NH3, NO2, and CO, as well as biomolecules such as boronic acid and glucose [77,78].
Recently, optical sensors based on nanostructured cage material were developed by Balaji et al. for detecting toxic metal ions [25]. Heavy metal ions are typically determined using atomic absorption and emission spectroscopy. However, methods with greater sensitivity and simplicity are required because traditional methods are expensive and complicated. Nanomaterial-cage-based chemical sensors comprise optical-sensor-based cubic Fm3m cage monoliths with different fabrications as sensing elements that use colorimetric changes for signal transduction. Briefly, cubic Fm3m cage monoliths were designed as platforms, and dithizone, TMPyP (α, β, γ, and δ-tetrakis(1-methylpyridinium-4-yl) porphinep-toluenesulfonate), pyrogallol red, and tetraphenyl porphine tetrasulfonic acid were used as cages for detecting Pb, Cd, Sb, and Hg, respectively. The greatest advantage of the nanomaterial-cage-based sensor is that it enables target detection with a high quantification limit by the naked eye.
In addition to the studies mentioned here, many review papers have focused not only on specific nanomaterial-based chemical sensors but also on their working mechanisms, target selectivity upon fabrication, and applications [69,70,79]. Although some aspects of nanomaterial-based chemical sensors are discussed in this review, it should be noted that all chemical sensors share similarities in their basic principles, and the potential of chemical sensors continues to expand along with technological advances.
4. Electrochemical Sensors
Electrochemical sensors are an important subclass of chemical sensors that use electrodes as signal-transducing elements [12,80]. Electrochemical sensors can be further divided into potentiometric sensors, voltametric sensors, and conductimetric sensors. These sensors measure the changes in a potential signal caused by an ion-recognition event, whereby the potential between two electrodes causes the oxidation (or reduction) of an electroactive species, with the resistance representing the signal-transducing element [81,82]. Electrochemical sensors measure changes in electrochemical properties induced by the interactions between sensing elements and targets and are further classified based on the type of sensing element, which includes nanomaterials, biomolecules, CNTs, graphene, and optical chemical sensors. Advances in the fields of materials sciences, electrical engineering, device fabrication, and physical chemistry have continually improved the signal transducers and sensing elements of electrochemical sensors. Table 2 lists the types of transducers, sensing elements, and targets of electrochemical sensors.

Table 2.
List of electrochemical sensors classified by type of sensing element and transducer.
As many toxic materials are released from anthropogenic activities, heavy metal(loid)s (such as mercury, lead, cadmium, arsenic), chemicals (such as phenolic compounds, nitroaromatics, organophosphorus),and pesticides are increasingly found in environmental systems. Therefore, electrochemical sensors play a pivotal role in avoiding the impact of toxic materials by enabling constant monitoring via simple and fast analytical tools.
4.1. CNM-Based Electrochemical Sensors
Electrochemical sensors are defined as chemical sensors that employ potentiometric, amperometric, and conductometric changes as transduced signals, affording them superior sensitivity to optical chemical sensors. Nonetheless, electrochemical sensors are similar to optical chemical sensors in terms of sensing element advances and diversity. Recent advances in nanomaterial science have provided various materials for sensing elements, including metal nanoparticles (NPs) and CNMs such as carbon dots, CNTs, and graphene [26,93,94]. The integration of fabrication technologies has also enabled the use of nanostructured materials as sensing elements in chemical sensors [95,96].
As for optical chemical sensors, CNMs are widely used as sensing elements in electrochemical sensors [97,98]. Carbon nanostructures used for electrochemical sensors include fullerenes, CNTs, graphene, nanocones, and carbon dots, which are classified in terms of their size and shape. In general, nanostructured carbon is applied to electrodes via a fabrication process to detect targets such as pesticides, toxic chemicals, and heavy metal ions. Recently, Wu et al. reported CNT-based electrochemical sensors for measuring the cytotoxicity of 2,4,6-trichlorophenol, bisphenol AF, and polystyrene nanoplastics [83]. The electrodes were modified with tungsten disulfide nanosheets/hydroxylated multi-walled CNTs (WS2/MWCNTs-OH) for enhanced sensitivity. A recent study reported the development of laser-induced graphene printed on polyimide films for 4-nitrophenol detection in water [84]. The presence of 4-nitrophenol induced a cyclic voltammetry response that corresponded to the concentration of 4-nitrophenol. In addition, several studies and review papers have been written on CNM-based sensors [97,99,100]. Owing to their diverse intrinsic electronic and optical properties, chemical versatility, and stability, CNMs are attracting increasing research attention, with new findings on CNM-based chemical sensors also reported.
4.2. NP-Based Electrochemical Sensors
Recently, the use of NPs has increased in a variety of fields, including environmental, pharmaceutical, medical, and material sciences, as well as the cosmetics industry [101,102,103,104]. The wide application of NPs is related to their novel properties, which depend on the shape and size of the particles [105]. Thus, methods of synthesizing NPs represent a key research area [106,107]. Typically, NPs are divided into metal-based, metal-oxide-based, and carbon-based NPs [108]. Because the application of CNMs to electrochemical sensors was addressed in the previous section, only electrochemical sensors employing metal- and metal-oxide-based NPs are discussed in this section.
Among the metal-NP-based electrochemical sensors, AuNPs have received the most attention. Castañeda et al. reported AuNP-based electrochemical sensing of DNA [88], and Zhao et al. reported hydrazine detection using an AuNPs/CNTs-ErGO electrochemically reduced graphene oxide composite film [89]. In both cases, the AuNPs were employed as sensing elements to recognize targets. Silver nanoparticles (AgNPs) have also been used in electrochemical sensors [108]. Similar to AuNPs, AgNPs were fabricated on the electrodes of electrochemical sensors to serve as target sensors. AgNP-based electrochemical sensors targeting DNA, chemicals, and pesticides have been developed with variations in electrode types and other modifications [90,109].
Similar to NPs, metal oxides have been applied in various fields because of their novel characteristics, which are determined by their shape and size [110,111]. Among the metal oxides, the use of zinc oxide (ZnO) for sensing toxic materials has been extensively discussed in review articles [70,112,113,114]. ZnO is inexpensive and can be used to fabricate various nanostructures in a relatively simple manner. The shapes of ZnO nanostructures range from 1-D to 3-D, with each shape exhibiting distinct properties. For example, a thin layer of ZnO on the sensor surface enhances the electrochemical response to Hg by facilitating the migration of electrons between the redox-active analytes [115]. Moreover, Ibrahim et al. synthesized cauliflower-shaped ZnO and used it to modify electrodes for the detection of picric acid [91]. Many other types of ZnO have also been reported and employed for electrode modification. Unlike other nanostructured materials, ZnO plays a role in both target selectivity and transduced-signal amplification.
The versatility of nanomaterials, including metal NPs and metal oxides, has been accepted and proven in different research fields, which has accelerated research into their development and application. Advances in nanomaterials science have also provided significant benefits to many different research fields and industries, with their potential as components of chemical sensors being particularly important. Although metal NPs act to enhance the transducing signals through their integration with electrodes, they can also function as sensing elements with improvements to the chemical modification and surface fabrication of NPs. Although it is difficult to endow NPs with target selectivity, studies have achieved target sensitivity by conjugating NPs with organic ligands as well as biological molecules, such as chitosan, DNA, and proteins [116,117,118]. If NPs were conjugated with biomolecules and employed as sensing elements in chemical sensors, they could be classified as biosensors. In this regard, we discussed the application of biomolecule conjugated NPs further in Section 5 with biosensors.
5. Biosensors
Biosensors targeting biomolecules of interest are usually considered a subclass of chemical sensors because they use the same target sensing and transduction methods [119]. As shown in Figure 2, biosensors are divided into two categories based on their ability to transduce signals into digitized values. One category includes biosensors that integrate sensing and transducing elements with analytical devices, whereas the other requires additional instruments to read the transduced signals. The former type employs biomolecules and biochemical or biological mechanisms as sensing elements and recognition systems and are, therefore, considered to be conventional biosensors [120]. The latter are biosensing systems based on transcription factors (TFs), such as whole-cell biosensors, which require equipment to read the transduced signals. In this regard, various chemical sensors that use biomolecules, such as DNA, enzymes, and antibodies, for target recognition are considered biosensors [121,122,123]. In contrast, TF-based biosensors are not fully accepted as chemical sensors, despite target recognition occurring through the transduction of enzymatic activities and fluorescence. Nonetheless, TF-based biosensors are included in this review because the sensing systems can be chemical sensors equipped with sensing and signal-transducing elements.
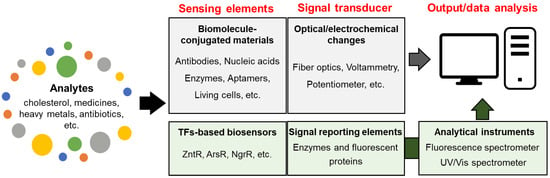
Figure 2.
The types of biosensors based on ability to transduce signals into digitized values.
5.1. Optical Biosensors and Electrochemical Biosensors
As discussed above, optical and electrochemical sensors are classified according to the method of transducing signals indicating the interaction between targets and sensing elements. In general, optical biosensors are based on technology that detects evanescent-wave changes caused by the interaction between targets and sensing elements incorporated with biological molecules [124,125]. Surface plasmon resonance, optical waveguides, optical resonators, and optical fibers have all been used as transducers to measure changes in the reflective index, fluorescence, Raman scattering, and optical absorption upon target recognition [126]. Electrochemical biosensors also employ biomolecules as sensing elements and electrodes to measure electrochemical properties such as voltametric, potentiometric, and electrometric changes as transduced signals [127,128,129]. Although different technologies have been employed for biosensors, the range of targets is determined by the sensing elements coupled with various biomolecules, such as antibodies, enzymes, DNA, aptamers, and cells. As biomolecules are more specific and selective to targets than other materials, biomolecule-coupled chemical sensors are a key area of research. Since the targets of biomolecules are diverse such as chemicals, heavy metals, chemicals, other proteins, and cells, the detecting ranges of targets were expanded. Therefore, the type and integration method of biomolecules used in chemical sensors are critical considerations. Table 3 lists the different types of biosensors according to their sensing and transducing elements.

Table 3.
List of biosensors classified by type of sensing element and transducer.
5.2. Enzyme-Based Biosensors
Because enzymes have specific targets, they are attractive for use as sensing elements in chemical sensors. The role of enzymes in chemical sensors is to sense targets and transduce signals based on their activities [14,145], with enzymes such as hydrolases, oxidoreductases, and transferases all used as bioreceptors for sensing targets. Enzyme activities induce optical or electrochemical changes and can be used to detect heavy metals, pharmaceuticals, and phenolics [146].
For example, cholinesterase is widely used as a sensing element to detect toxic materials such as pesticides, heavy metals, and toxins because its activity is inhibited by toxic materials [147]. Recently, Loewenthal et al. used acetylcholinesterase (AChE) coupled with a near-infrared, fluorescent, single-walled carbon nanotube optical sensor. AChE releases thiocholines from acetylthiocholine, increasing near-infrared fluorescence and, thereby, detecting AChE inhibitors by measuring the decrease in signals [130,148]. Another example of an enzyme used in chemical sensors is toluene monooxygenase for toluene detection [131]. This enzyme recognizes and degrades toluene, resulting in the consumption of oxygen, then induces changes in the phosphorescence intensity, which act as transduced signals.
The greatest advantage of enzymes is their target selectivity and enzymatic activity. Enzymes are integrated into chemical sensors to detect targets and produce output signals induced by catalytic activity [149]. However, the stability of enzymes would be an obstacle to enlarging their application because the enzymatic activity is dependent on the stable structure of enzymes. Thus, it would be necessary to consider the stability of enzymes during the integration on the components of sensors. Nonetheless, the advantageous aspects of enzymes accelerate the application of enzyme-based biosensors not only for the detection of toxic materials but also for biomedical analysis [150,151]. As biomolecules such as specific proteins and metabolites indicate certain diseases, enzymes that recognize these molecules have been employed as sensing elements in chemical sensors for biomedical analysis.
5.3. Biomolecule-Based Biosensors
Biomolecules such as DNA, peptides, antibodies, and aptamers have been employed as target-sensing elements in chemical sensors [121,152,153]. Target-specific biomolecules are integrated into sensing devices as sensing elements. Changes in the chemical properties induced by the interaction with targets are then transduced by various transducing elements. The basic components are the same as those of other chemical sensors; however, the biomolecules are used for sensing the targets. This type of sensor can also be classed as optical or electrochemical sensors according to the type of signal transducer.
Unlike enzymes, the biomolecules such as DNA, peptides, antibodies, and aptamers possess more versatile natures to modulate target selectivity and sensitivity. In this regard, those biomolecules were actively investigated as sensing elements for chemical sensors. With advances in nanomaterial fabrication technologies, various materials have been conjugated onto nanomaterials. In addition to chemicals and functional groups, biomolecules, such as DNA, antibodies, and aptamers, have been conjugated as ligands onto nanomaterials [123,154]. For example, Liu et al. developed mercury-sensing electrochemical sensors by integrating DNA strands onto cuprous oxide/nanochitosan composites [133]. Interfacial changes on the surface of the electrode caused by DNA–Hg interactions were measured using electrochemical impedance spectroscopy, which showed a nanomolar range of sensitivity. Aptamers, also known as chemical antibodies, are versatile materials used as sensing elements in chemical sensors. Aptamers are single-stranded DNA/RNA oligonucleotides with the advantages of low production costs, versatile applications, and easy modification and operation [155]. Wang et al. and Hu et al. have reported aptamer-based biosensors for monitoring Pseudomonas aeruginosa with 10 CFU/mL of detection limit using CDs and biotin integrated aptamers, respectively [135,136]. To facilitate the use of aptamers in chemical sensors, techniques for conjugating aptamers with nanomaterials have rapidly improved. Consequently, aptamer-conjugated NPs, CNMs, and quantum dots have been employed as elements in various chemical sensors [156,157,158]. For example, aptamer-based chemical sensors were applied to detect heavy metals, cancer cells, and various proteins according to the specificity of the aptamers. In addition to the biomolecules mentioned here, many other chemical sensors employ biomolecules as components, such as antibodies, proteins, chitosan, and carbohydrates. By integrating these biomolecules into sensing elements, biosensors can be used to detect a wide range of targets, including toxic gases, heavy metals, phenolic compounds, proteins, and even cancer cells.
Researchers have also reviewed chemical sensors with antibody-, aptamer-, and DNA-based sensors, emphasizing the huge potential of integrating biomolecules with nanomaterials, including CNTs, various NPs, quantum dots, and different types of electrodes for detecting toxic materials [121,159,160].
5.4. TF-Based Biosensors
Unlike the chemical sensors discussed above, TF-based biosensors do not transduce signals to digitized values but still comprise sensing and signal transduction elements [161,162]. The sensing elements are TFs, and targets interacting with the TFs are turned on or off via the transcription of genes. The target–TF interaction is indicated by gene expression; therefore, the gene expression level is the signal output [163,164]. For this reason, TF-based biosensors are often called bioreporters and are coupled with additional instruments such as UV/Vis spectroscopy and fluorescence spectroscopy.
Among the TF-based biosensors, whole-cell-based biosensors have been intensively investigated. In these biosensors, the cells are used as sensor platforms to serve as both sensing and signal-transducing elements [165]. Typically, the TFs are target-sensing elements and genes that encode enzymes or fluorescent proteins as signal-transducing elements. As TFs are involved in external stimuli, cells possessing genetically engineered TFs and reporter genes are responsible for specific stimuli. Thus, specific toxic material-sensing biosensors have been developed by employing TFs that respond to toxic materials as sensing elements. TF-based biosensors target a wide variety of materials, including biomolecules and cellular metabolites, but the targets are restricted to toxic materials in this review. The TFs and their corresponding toxic materials are listed in Table 3. For example, Escherichia coli contains operons responsive to arsenic and manganese, which are regulated by ArsR and MntR, respectively. When reporter genes such as egfp are inserted under the promoter regions of operons, E. coli cells serve as sensors to detect arsenic and manganese based on changes in fluorescent signals [142,166]. In addition, the applications of whole-cell biosensors can be increased by integrating cells with electrochemical devices. Because the cells produce electrochemically active materials upon exposure to toxic materials, the signal changes indicate the presence of toxic materials in samples. However, as the target recognition processes occur inside cells, targets impermeable to cells are not detected by whole-cell-based biosensors. To overcome this disadvantage, cell-free sensing systems that use a mixture of components are required for transcription and translation [167,168]. Alam et al. and colleagues have reported a cell-free, TFs-based sensor system named ROSALIND (RNA output sensors activated by ligand induction) [143]. The same TFs used as sensing elements for whole cell-based biosensors are employed as sensing elements. The interaction between target TFs induces the production of RNA reporting signals by forming a complex with fluorescent chemicals. Thus, it avoids the issue of cell permeability of the target. Nonetheless, the cell free system has disadvantages such as the purification of TFs and preparation of components for transcription and translation.
TF-based biosensors are a subclass of chemical sensors that include both sensing and signal-transducing elements. Therefore, similar to the chemical sensors discussed above, diverse targets and improved applications and performance can be achieved by enhancing the sensing elements and signal-transducing technologies. In addition, the target selectivity and specificity of TF-based biosensors could be modulated via genetic engineering of TFs. Although it is challenging to modulate the target interaction of TFs via genetic engineering, the advance in protein modelling and computational analysis makes the process more accurate and efficient. In this way, the performance of TF-based biosensor can be enhanced, and the targets can also be diversified from existing genetic systems. Because biosensors have many advantages over other chemical sensors, especially in terms of their target specificity and selectivity, efforts should be made to further improve biosensors. Moreover, advances in diverse scientific fields can be used to enhance the performance and application of both chemical sensors and biosensors because both sensors share sensing and signal-transducing elements. Consequently, the rapid development of scientific technologies related to chemical sensors will help protect humans from exposure to toxic materials in the environment.
6. Conclusions and Future Prospects
Advances in various industrial fields have increased the production of toxic materials. Although systems for the control and monitoring of toxic materials have been established in many countries, attempts to reduce the threat to human health have been hindered by the rapid increase in toxic materials. It is impossible to identify all toxic materials, but fast and convenient detection methods are vital for effective monitoring. Moreover, technologies must be developed to respond to and monitor newly generated toxic materials. Chemical sensors are commonly used to detect toxic materials and can, therefore, address these concerns. As described in this review, chemical sensors have undergone rapid improvements, and their applications have increased substantially following advances in different scientific fields. The basic structure of all types of chemical sensors is the same, comprising sensing and signal-transducing elements; however, these elements vary widely between sensor types. Although the chemical sensors discussed here were classified just by types of sensing elements and signal transducing elements, it would not be meaningful these days. To enhance the performance of chemical sensors, it is critical to integrate the interdisciplinary sciences as well as to upgrade sensing and signal transducing elements. In addition, the application fields of chemical sensors have been expended rapidly along with the advances in sensing and signal-transducing elements. Recently, various chemical sensors have been applied to medical sciences to diagnose diseases by monitoring biological markers and pathogenic markers [169,170]. Meanwhile, it has been also reported that whole-cell-based biosensors were used to monitor the toxicity and genotoxicity of harmful materials [171,172]. In this way, the chemical sensors could contribute to secure and improve the human health.
Consequently, the future prospects of chemical sensors not only depend on improving sensing elements and integrating them with signal-transducing elements but also integrating advanced technologies. Although this review only discusses a small number of chemical sensors, we focus on the similarity of chemical sensors in terms of their basic principles and future goals. As such, this review provides a unique perspective on chemical sensors and contributes to their continued development.
Author Contributions
Conceptualization, Y.K., Y.J. and Y.Y.; writing—original draft preparation, Y.K., Y.J., M.N. and S.-J.H.; writing—review and editing, Y.K., S.-J.H. and Y.Y.; visualization, Y.K. and Y.J.; supervision, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by Konkuk University Researcher Fund in 2023 (to S.-J.H.) and the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (2021R1F1A1056635 to Y.Y.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Ajibade, F.O.; Adelodun, B.; Lasisi, K.H.; Fadare, O.O.; Ajibade, T.F.; Nwogwu, N.A.; Sulaymon, I.D.; Ugya, A.Y.; Wang, H.C.; Wang, A. Environmental pollution and their socioeconomic impacts. In Microbe Mediated Remediation of Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–354. [Google Scholar]
- Young, S.; Balluz, L.; Malilay, J. Natural and technologic hazardous material releases during and after natural disasters: A review. Sci. Total Environ. 2004, 322, 3–20. [Google Scholar] [CrossRef]
- Corn, M. Handbook of Hazardous Materials; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Ha, S.; Seidle, T.; Lim, K.-M. Act on the Registration and Evaluation of Chemicals (K-REACH) and replacement, reduction or refinement best practices. Environ. Health Toxicol. 2016, 31, e2016026. [Google Scholar] [CrossRef]
- Rouessac, F.; Rouessac, A. Chemical Analysis: Modern Instrumentation Methods and Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Chen, H.; Zheng, J.; Zhang, X.; Luo, M.; Wang, Z.; Qiao, X. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J. Mass Spectrom. 2007, 42, 1045–1056. [Google Scholar] [CrossRef]
- Leary, P.E.; Kammrath, B.W.; Lattman, K.J.; Beals, G.L. Deploying portable gas chromatography–mass spectrometry (GC-MS) to military users for the identification of toxic chemical agents in theater. Appl. Spectrosc. 2019, 73, 841–858. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Cammann, K.; Lemke, U.; Rohen, A.; Sander, J.; Wilken, H.; Winter, B. Chemical sensors and biosensors—Principles and applications. Angew. Chem. Int. Ed. Engl. 1991, 30, 516–539. [Google Scholar] [CrossRef]
- Janata, J. Principles of Chemical Sensors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Karker, N.; Dharmalingam, G.; Carpenter, M.A. Thermal energy harvesting plasmonic based chemical sensors. ACS Nano 2014, 8, 10953–10962. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2006, 78, 3859–3874. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Choi, Y.-K. Chemical sensors based on nanostructured materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. Carbon nanotube-based chemical sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Faddah, L.M. Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 2010, 3, 65–76. [Google Scholar] [CrossRef]
- Ispas, C.R.; Crivat, G.; Andreescu, S. Recent developments in enzyme-based biosensors for biomedical analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Balaji, T.; El-Safty, S.A.; Matsunaga, H.; Hanaoka, T.; Mizukami, F. Optical sensors based on nanostructured cage materials for the detection of toxic metal ions. Angew. Chem. 2006, 118, 7360–7366. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Baghayeri, M.; Karimi, F.; Fu, L.; Rouhi, J.; Niculina, D.E.; Gündüz, E.S.; Dragoi, E. Recent developments in carbon nanomaterials-based electrochemical sensors for methyl parathion detection. J. Food Meas. Charact. 2023, 17, 5371–5389. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly sensitive electrochemical stripping analysis of methyl parathion at MWCNTs–CeO2–Au nanocomposite modified electrode. Sens. Actuators B Chem. 2013, 186, 774–780. [Google Scholar] [CrossRef]
- Qazi, H.H.; Mohammad, A.B.b.; Akram, M. Recent progress in optical chemical sensors. Sensors 2012, 12, 16522–16556. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F. Fluorescent chemical sensors: Applications in analytical, environmental, forensic, pharmaceutical, biological, and biomedical sample measurement, and clinical diagnosis. Appl. Spectrosc. Rev. 2023, 59, 1–89. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Lee, B.; Roh, S.; Park, J. Current status of micro-and nano-structured optical fiber sensors. Opt. Fiber Technol. 2009, 15, 209–221. [Google Scholar] [CrossRef]
- Lin, J. Recent development and applications of optical and fiber-optic pH sensors. TrAC Trends Anal. Chem. 2000, 19, 541–552. [Google Scholar] [CrossRef]
- Kim, J.A.; Hwang, T.; Dugasani, S.R.; Amin, R.; Kulkarni, A.; Park, S.H.; Kim, T. Graphene based fiber optic surface plasmon resonance for bio-chemical sensor applications. Sens. Actuators B Chem. 2013, 187, 426–433. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. An optical fiber based surface plasmon resonance technique for sensing of lead ions: A toxic water pollutant. Opt. Fiber Technol. 2018, 46, 152–156. [Google Scholar] [CrossRef]
- Ozcariz, A.; Ruiz-Zamarreno, C.; Arregui, F.J. A comprehensive review: Materials for the fabrication of optical fiber refractometers based on lossy mode resonance. Sensors 2020, 20, 1972. [Google Scholar] [CrossRef]
- Ju, S.; Nguyen, V.L.; Watekar, P.R.; Kim, B.H.; Jeong, C.; Boo, S.; Kim, C.J.; Han, W.-T. Fabrication and optical characteristics of a novel optical fiber doped with the Au nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 3555–3558. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.M.; Nevill, J.T.; Pettigrew, K.I.; Votaw, G.; Kung, P.-J.; Crenshaw, H.C. A low-cost, manufacturable method for fabricating capillary and optical fiber interconnects for microfluidic devices. Lab A Chip 2008, 8, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Usha, S.P.; Gupta, B.D. A lossy mode resonance-based fiber optic hydrogen gas sensor for room temperature using coatings of ITO thin film and nanoparticles. Meas. Sci. Technol. 2016, 27, 045103. [Google Scholar] [CrossRef]
- Usha, S.P.; Gupta, B.D. Performance analysis of zinc oxide-implemented lossy mode resonance-based optical fiber refractive index sensor utilizing thin film/nanostructure. Appl. Opt. 2017, 56, 5716–5725. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Teng, P.; Kong, D.; Gao, S.; Liu, Z.; Yang, J.; Gao, D.; Luo, M.; Wen, X. Determination of the antibiotic minocycline by integrated optofluidic microstructured polymer optical fiber chemiluminescence. Instrum. Sci. Technol. 2021, 49, 571–584. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.-j.; Wei, J.-f.; Xu, J.-r.; Wang, Y.-h.; Zheng, G.-x. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, K.-B.; Shin, K.-S.; Park, H.-D.; Kim, M.-C.; Kim, J.-R.; Park, S.-J.; Song, Y.-H. Design, fabrication and characterization of an integrated micro ammonia analysis system (IMAAS) with microreactor and in-plane type optical detector based on the Berthelot reaction. Sens. Actuators B Chem. 2006, 117, 516–522. [Google Scholar] [CrossRef]
- He, S.; Li, D.; Zhu, C.; Song, S.; Wang, L.; Long, Y.; Fan, C. Design of a gold nanoprobe for rapid and portable mercury detection with the naked eye. Chem. Commun. 2008, 40, 4885–4887. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, C.-J.; Tao, T.; Duan, M.; Fang, S.-W.; Zheng, M. A miniaturized fiber-optic colorimetric sensor for nitrite determination by coupling with a microfluidic capillary waveguide. Anal. Bioanal. Chem. 2016, 408, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, N.; Takarada, T.; Maeda, M. Rapid naked-eye detection of mercury ions based on non-crosslinking aggregation of double-stranded DNA-carrying gold nanoparticles. Chem. Commun. 2011, 47, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jin, J.; Chen, Y.; Shao, N.; Kang, H.; Xiao, Z.; Tang, Z.; Wu, Y.; Zhu, Z.; Tan, W. Carbon nanotube-quenched fluorescent oligonucleotides: Probes that fluoresce upon hybridization. J. Am. Chem. Soc. 2008, 130, 8351–8358. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Yan, Y.; Hou, Y.; Li, G.; Hao, C. Novel carbon quantum dots for fluorescent detection of phenol and insights into the mechanism. New J. Chem. 2018, 42, 11485–11492. [Google Scholar] [CrossRef]
- Babar, D.G.; Garje, S.S. Nitrogen and phosphorus co-doped carbon dots for selective detection of nitro explosives. ACS Omega 2020, 5, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jia, H.; Xu, D.; Wang, J. Novel method in emerging environmental contaminants detection: Fiber optic sensors based on microfluidic chips. Sci. Total Environ. 2023, 857, 159563. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, G.; Lee, L.M. Optical imaging techniques in microfluidics and their applications. Lab A Chip 2012, 12, 3566–3575. [Google Scholar] [CrossRef]
- Orti, V.; Audran, M.; Gibert, P.; Bougard, G.; Bressolle, F. High-performance liquid chromatographic assay for minocycline in human plasma and parotid saliva. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 357–365. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Arif, K.M. A comprehensive review of microfluidic water quality monitoring sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef]
- Yin, M.-j.; Gu, B.; An, Q.-F.; Yang, C.; Guan, Y.L.; Yong, K.-T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Kuswandi, B.; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Chemchem, M.; Chemchem, A.; Aydıner, B.; Seferoğlu, Z. Recent advances in colorimetric and fluorometric sensing of neurotransmitters by organic scaffolds. Eur. J. Med. Chem. 2022, 244, 114820. [Google Scholar] [CrossRef] [PubMed]
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samori, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Cho, H.H.; Jung, D.H.; Heo, J.H.; Lee, C.Y.; Jeong, S.Y.; Lee, J.H. Gold Nanoparticles as Exquisite Colorimetric Transducers for Water Pollutant Detection. ACS Appl. Mater. Interfaces 2023, 15, 19785–19806. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Sharma, R.K.; Wangoo, N. Highly sensitive colorimetric detection of ethyl parathion using gold nanoprobes. Sens. Actuators B Chem. 2015, 210, 425–430. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Wang, L.; Zhang, H.; Hu, J. A simple colorimetric probe based on anti-aggregation of AuNPs for rapid and sensitive detection of malathion in environmental samples. Anal. Bioanal. Chem. 2019, 411, 2645–2652. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S. Advances in gold nanoparticles for optical detection of nerve agents. In Sensing of Deadly Toxic Chemical Warfare Agents, Nerve Agent Simulants, and Their Toxicological Aspects; Elsevier: Amsterdam, The Netherlands, 2023; pp. 111–131. [Google Scholar]
- Sahu, B.; Kurrey, R.; Deb, M.K.; Khalkho, B.R.; Manikpuri, S. Recognition of malathion pesticides in agricultural samples by using α-CD functionalized gold nanoparticles as a colorimetric sensor. Talanta 2023, 259, 124526. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.-Y.; Liang, F.; Yang, Y.-W. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Ding, X.; Kong, L.; Wang, J.; Fang, F.; Li, D.; Liu, J. Highly sensitive SERS detection of Hg2+ ions in aqueous media using gold nanoparticles/graphene heterojunctions. ACS Appl. Mater. Interfaces 2013, 5, 7072–7078. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.; Baskoutas, S. Chemical sensing applications of ZnO nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Qamar, A.Z.; Slaughter, G. Application of nanomaterials for chemical and biological sensors: A review. IEEE Sens. J. 2020, 21, 12407–12425. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Amirfazli, M.; Massah, F.; Bagheri, Z. Application of functionalized carbon dots in detection, diagnostic, disease treatment, and desalination: A review. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025017. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.A.; Yuce, M.R. Recent progress in nanomaterial enabled chemical sensors for wearable environmental monitoring applications. Adv. Funct. Mater. 2020, 30, 2005703. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, H.; Xing, Y.; Wang, C.; Yuan, X.; Zhu, X. A sensitive electrochemical sensor for environmental toxicity monitoring based on tungsten disulfide nanosheets/hydroxylated carbon nanotubes nanocomposite. Chemosphere 2022, 286, 131602. [Google Scholar] [CrossRef]
- Wanjari, V.P.; Duttagupta, S.P.; Singh, S.P. Dual Linear Range Laser-Induced Graphene-Based Sensor for 4-Nitrophenol Detection in Water. ACS Appl. Nano Mater. 2023, 6, 11351–11360. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Guo, Y.; Ren, H.; Gao, C. Nitrogen dioxide sensing based on multiple-morphology cuprous oxide mixed structures anchored on reduced graphene oxide nanosheets at room temperature. Nanotechnology 2019, 30, 455502. [Google Scholar] [CrossRef]
- Yoon, H.J.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Reddy, T.M.; Palakollu, V.; Karpoormath, R.; Rao, Y.S.; Venkataprasad, G.; Gopal, T.V.; Gopal, P. Multi walled carbon nanotubes supported CuO-Au hybrid nanocomposite for the effective application towards the electrochemical determination of acetaminophen and 4-aminophenol. Synth. Met. 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Castañeda, M.e.T.; Alegret, S.; Merkoci, A. Electrochemical sensing of DNA using gold nanoparticles. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 743–753. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Hu, J.; Chen, Y. Preparation and characterization of AuNPs/CNTs-ErGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 2016, 158, 283–291. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.A.; Santana, E.R.; Piovesan, J.V.; Spinelli, A. Silver nanoparticle-modified electrode for the determination of nitro compound-containing pesticides. Anal. Bioanal. Chem. 2016, 408, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Kumar, R.; Umar, A.; Kim, S.; Bumajdad, A.; Ansari, Z.; Baskoutas, S. Cauliflower-shaped ZnO nanomaterials for electrochemical sensing and photocatalytic applications. Electrochim. Acta 2016, 222, 463–472. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Stelitano, S.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Antonucci, P.; Neri, G.; Neri, F.; Santangelo, S. Characterisation and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater. Chem. Phys. 2016, 170, 129–137. [Google Scholar] [CrossRef]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.; Saidur, R.; Singh, P. A review on CNTs-based electrochemical sensors and biosensors: Unique properties and potential applications. Crit. Rev. Anal. Chem. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Li, X.; Liu, X.; Lin, C.-T.; Karimi-Maleh, H. Strategies and Applications of Graphene and Its Derivatives-Based Electrochemical Sensors in Cancer Diagnosis. Molecules 2023, 28, 6719. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kailasa, S.K.; Kumar, V.; Tsang, Y.F.; Gobi, K.V.; Kim, K.-H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Deji, R.; Rahul; Choudhary, B.; Sharma, R.K. Role of Graphene-Based Materials in Gas Sensing Applications: From Synthesis to Device Fabrication. In Handbook of Porous Carbon Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 493–518. [Google Scholar]
- Krishna Perumal, P.; Chen, C.-w.; Giri, B.S.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Graphene-based functional electrochemical sensors for the detection of chlorpyrifos in water and food samples: A review. J. Food Sci. Technol. 2023, 1–11. [Google Scholar] [CrossRef]
- Das, P.K.; Mohanty, C.; Purohit, G.K.; Mishra, S.; Palo, S. Nanoparticle assisted environmental remediation: Applications, toxicological implications and recommendations for a sustainable environment. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100679. [Google Scholar]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Murphy, M.; Ting, K.; Zhang, X.; Soo, C.; Zheng, Z. Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. J. Nanomater. 2015, 2015, 696918. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Sakharwade, S. Silver nanoparticles in cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 48–53. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.-H.; Azzem, M.A. Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: A review. Mater. Adv. 2021, 2, 7350–7365. [Google Scholar] [CrossRef]
- Yari, A.; Saidikhah, M. Trithiane silver-nanoparticles-decorated polyaniline nanofibers as sensing element for electrochemical determination of Adenine and Guanine in DNA. J. Electroanal. Chem. 2016, 783, 288–294. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal-and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Zhao, R.; Du, J.; Li, J. nanostructural ZnO-based electrochemical sensor for environmental application. J. Electrochem. Soc. 2022, 169, 020573. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Nejad, F.G.; Safaei, M. Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Kumar, S. Zinc oxide quantum dots as efficient electron mediator for ultrasensitive and selective electrochemical sensing of mercury. Electrochim. Acta 2015, 178, 361–367. [Google Scholar] [CrossRef]
- Hu, P.; Chen, L.; Kang, X.; Chen, S. Surface functionalization of metal nanoparticles by conjugated metal–ligand interfacial bonds: Impacts on intraparticle charge transfer. Acc. Chem. Res. 2016, 49, 2251–2260. [Google Scholar] [CrossRef]
- Tran, P.H.; Duan, W.; Tran, T.T. Fucoidan-based nanostructures: A focus on its combination with chitosan and the surface functionalization of metallic nanoparticles for drug delivery. Int. J. Pharm. 2020, 575, 118956. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, chemical sensors, electrochemical sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Holford, T.R.; Davis, F.; Higson, S.P. Recent trends in antibody based sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, J.; Deng, H.; Zhang, L. Progress of mimetic enzymes and their applications in chemical sensors. Crit. Rev. Anal. Chem. 2016, 46, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Willner, B. Biomolecule-based nanomaterials and nanostructures. Nano Lett. 2010, 10, 3805–3815. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent advances in optical biosensors for sensing applications: A review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: Recent progress and future prospects. Adv. Colloid Interface Sci. 2022, 309, 102795. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In vivo electrochemical biosensors: Recent advances in molecular design, electrode materials, and electrochemical devices. Anal. Chem. 2023, 95, 388–406. [Google Scholar] [CrossRef]
- Loewenthal, D.; Kamber, D.; Bisker, G. Monitoring the activity and inhibition of cholinesterase enzymes using single-walled carbon nanotube fluorescent sensors. Anal. Chem. 2022, 94, 14223–14231. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Fritzsche, M.; Pieper, S.B.; Wood, T.K.; Lear, K.L.; Dandy, D.S.; Reardon, K.F. Fiber optic monooxygenase biosensor for toluene concentration measurement in aqueous samples. Biosens. Bioelectron. 2011, 26, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Atailia, S.; Baraket, A.; Rabai, S.; Benounis, M.; Jaffrezic, N.; Araar, H.; Naït-Bouda, A.; Boumaza, A.; Errachid, A.; Houhamdi, M. Electrochemical urea biosensor based on Proteus mirabilis urease immobilized over polyaniline PANi-Glassy carbon electrode. Electroanalysis 2023, 35, e202200502. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Yan, F.; Peng, D.; Yang, Y.; He, L.; Wang, M.; Fang, S.; Zhang, Z. Electrochemical DNA biosensor based on microspheres of cuprous oxide and nano-chitosan for Hg (II) detection. Electrochim. Acta 2015, 160, 64–73. [Google Scholar] [CrossRef]
- Mehto, N.K.; Sharma, P.; Kumar, S.; Khanuja, M.; Rawal, R.; Narang, J. Towards papertronics based electrode decorated with zinc oxide nanoparticles for the detection of the yellow fever virus consensus sequence. Process Biochem. 2022, 123, 36–43. [Google Scholar] [CrossRef]
- Wang, H.; Chi, Z.; Cong, Y.; Wang, Z.; Jiang, F.; Geng, J.; Zhang, P.; Ju, P.; Dong, Q.; Liu, C. Development of a fluorescence assay for highly sensitive detection of Pseudomonas aeruginosa based on an aptamer-carbon dots/graphene oxide system. RSC Adv. 2018, 8, 32454–32460. [Google Scholar] [CrossRef]
- Hu, J.; Fu, K.; Bohn, P.W. Whole-cell Pseudomonas aeruginosa localized surface plasmon resonance aptasensor. Anal. Chem. 2018, 90, 2326–2332. [Google Scholar] [CrossRef]
- Stocker, J.; Balluch, D.; Gsell, M.; Harms, H.; Feliciano, J.; Daunert, S.; Malik, K.A.; Van der Meer, J.R. Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ. Sci. Technol. 2003, 37, 4743–4750. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, S.; Chae, Y.; Jeong, S.-W.; An, Y.-J. Evaluation of bioavailable arsenic and remediation performance using a whole-cell bioreporter. Sci. Total Environ. 2016, 547, 125–131. [Google Scholar] [CrossRef]
- Riether, K.; Dollard, M.-A.; Billard, P. Assessment of heavy metal bioavailability using Escherichia coli zntAp::lux and copAp::lux-based biosensors. Appl. Microbiol. Biotechnol. 2001, 57, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, S.; Chae, Y.; Kang, Y.; Lee, Y.; Jeong, S.-W.; An, Y.-J. Use of tunable whole-cell bioreporters to assess bioavailable cadmium and remediation performance in soils. PLoS ONE 2016, 11, e0154506. [Google Scholar] [CrossRef] [PubMed]
- Tecon, R.; Beggah, S.; Czechowska, K.; Sentchilo, V.; Chronopoulou, P.-M.; McGenity, T.J.; van der Meer, J.R. Development of a multistrain bacterial bioreporter platform for the monitoring of hydrocarbon contaminants in marine environments. Environ. Sci. Technol. 2010, 44, 1049–1055. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, Y.; Kim, Y.; Park, C.; Choi, H.; Jang, G.; Yoon, Y. Development of novel Escherichia coli cell-based biosensors to monitor Mn (II) in environmental systems. Front. Microbiol. 2022, 13, 1051926. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.K.; Jung, J.K.; Verosloff, M.S.; Clauer, P.R.; Lee, J.W.; Capdevila, D.A.; Pastén, P.A.; Giedroc, D.P.; Collins, J.J.; Lucks, J.B. Rapid, low-cost detection of water contaminants using regulated in vitro transcription. BioRxiv 2019, 619296. [Google Scholar]
- Zhang, P.; Feng, H.; Yang, J.; Jiang, H.; Zhou, H.; Lu, Y. Detection of inorganic ions and organic molecules with cell-free biosensing systems. J. Biotechnol. 2019, 300, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nigam, V.K.; Shukla, P. Enzyme based biosensors for detection of environmental pollutants-a review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef]
- Coronado-Apodaca, K.G.; González-Meza, G.M.; Aguayo-Acosta, A.; Araújo, R.G.; Gonzalez-Gonzalez, R.B.; Oyervides-Muñoz, M.A.; Martínez-Ruiz, M.; Melchor-Martínez, E.M.; Barceló, D.; Parra-Saldívar, R.; et al. Immobilized Enzyme-based Novel Biosensing System for Recognition of Toxic Elements in the Aqueous Environment. Top. Catal. 2023, 66, 606–624. [Google Scholar] [CrossRef]
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.-L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef]
- Economou, A.; Karapetis, S.K.; Nikoleli, G.P.; Nikolelis, D.P.; Bratakou, S.; Varzakas, T.H. Enzyme-Based Sensors. Adv. Food Diagn. 2017, 231–250. [Google Scholar] [CrossRef]
- Wilson, G.S.; Hu, Y. Enzyme-based biosensors for in vivo measurements. Chem. Rev. 2000, 100, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yu, X.; Zheng, Y.; Yu, J. DNA based chemical sensor for the detection of nitrogen dioxide enabled by organic field-effect transistor. Sens. Actuators B Chem. 2016, 222, 1003–1011. [Google Scholar] [CrossRef]
- Schoukroun-Barnes, L.R.; Macazo, F.C.; Gutierrez, B.; Lottermoser, J.; Liu, J.; White, R.J. Reagentless, structure-switching, electrochemical aptamer-based sensors. Annu. Rev. Anal. Chem. 2016, 9, 163–181. [Google Scholar] [CrossRef]
- Xu, K.; Purahmad, M.; Brenneman, K.; Meshik, X.; Farid, S.; Poduri, S.; Pratap, P.; Abell, J.; Zhao, Y.; Nichols, B. Design and applications of nanomaterial-based and biomolecule-based nanodevices and nanosensors. Des. Appl. Nanomater. Sens. 2014, 16, 61–97. [Google Scholar]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, M.; Zhao, F.; Han, S. Application of nanomaterial modified aptamer-based electrochemical sensor in detection of heavy metal ions. Foods 2022, 11, 1404. [Google Scholar] [CrossRef]
- Sargazi, S.; Simge, E.; Mobashar, A.; Gelen, S.S.; Rahdar, A.; Ebrahimi, N.; Hosseinikhah, S.M.; Bilal, M.; Kyzas, G.Z. Aptamer-conjugated carbon-based nanomaterials for cancer and bacteria theranostics: A review. Chem. Biol. Interact. 2022, 361, 109964. [Google Scholar] [CrossRef]
- Lou, B.; Liu, Y.; Shi, M.; Chen, J.; Li, K.; Tan, Y.; Chen, L.; Wu, Y.; Wang, T.; Liu, X. Aptamer-based biosensors for virus protein detection. TrAC Trends Anal. Chem. 2022, 157, 116738. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Recent advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Rahman, M.M.; Li, X.-B.; Lopa, N.S.; Ahn, S.J.; Lee, J.-J. Electrochemical DNA hybridization sensors based on conducting polymers. Sensors 2015, 15, 3801–3829. [Google Scholar] [CrossRef]
- Pham, C.; Stogios, P.J.; Savchenko, A.; Mahadevan, R. Advances in engineering and optimization of transcription factor-based biosensors for plug-and-play small molecule detection. Curr. Opin. Biotechnol. 2022, 76, 102753. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Zhang, B.; Liu, S.; Liu, C.-g.; Li, F.; Song, H. Engineering whole-cell microbial biosensors: Design principles and applications in monitoring and treatment of heavy metals and organic pollutants. Biotechnol. Adv. 2022, 60, 108019. [Google Scholar] [CrossRef]
- Mahr, R.; Frunzke, J. Transcription factor-based biosensors in biotechnology: Current state and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.A.; Liu, D.; Zhang, F.; Oyarzún, D.A. Fundamental design principles for transcription-factor-based metabolite biosensors. ACS Synth. Biol. 2017, 6, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Wells, M.C.; Van der Meer, J.R. Whole-cell living biosensors—Are they ready for environmental application? Appl. Microbiol. Biotechnol. 2006, 70, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Elcin, E.; Öktem, H. Whole-cell fluorescent bacterial bioreporter for arsenic detection in water. Int. J. Environ. Sci. Technol. 2019, 16, 5489–5500. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lu, Y. Advances in cell-free biosensors: Principle, mechanism, and applications. Biotechnol. J. 2020, 15, 2000187. [Google Scholar] [CrossRef]
- Jung, J.K.; Alam, K.K.; Verosloff, M.S.; Capdevila, D.A.; Desmau, M.; Clauer, P.R.; Lee, J.W.; Nguyen, P.Q.; Pastén, P.A.; Matiasek, S.J. Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 2020, 38, 1451–1459. [Google Scholar] [CrossRef]
- Tricoli, A.; Neri, G. Miniaturized bio-and chemical-sensors for point-of-care monitoring of chronic kidney diseases. Sensors 2018, 18, 942. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Davis, J.J. Point of care sensors for infectious pathogens. Anal. Chem. 2020, 93, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.J.; Burmølle, M.; Hansen, L.H. Making bio-sense of toxicity: New developments in whole-cell biosensors. Curr. Opin. Biotechnol. 2006, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, G.; Xing, Y.; Zhang, D.; Jia, J.; Cui, Z.; Luan, X.; Tang, H. A whole-cell bioreporter assay for quantitative genotoxicity evaluation of environmental samples. Chemosphere 2017, 184, 384–392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).