Ratiometric Sensing of Glyphosate in Water Using Dual Fluorescent Carbon Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the CDs
2.3. Fluorescence Spectroscopy
2.4. UV–Visible Absorbance Spectroscopy (UV–Vis)
2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. Fluorescence Lifetimes
2.8. Transmission Electron Spectroscopy (TEM)
3. Results and Discussion
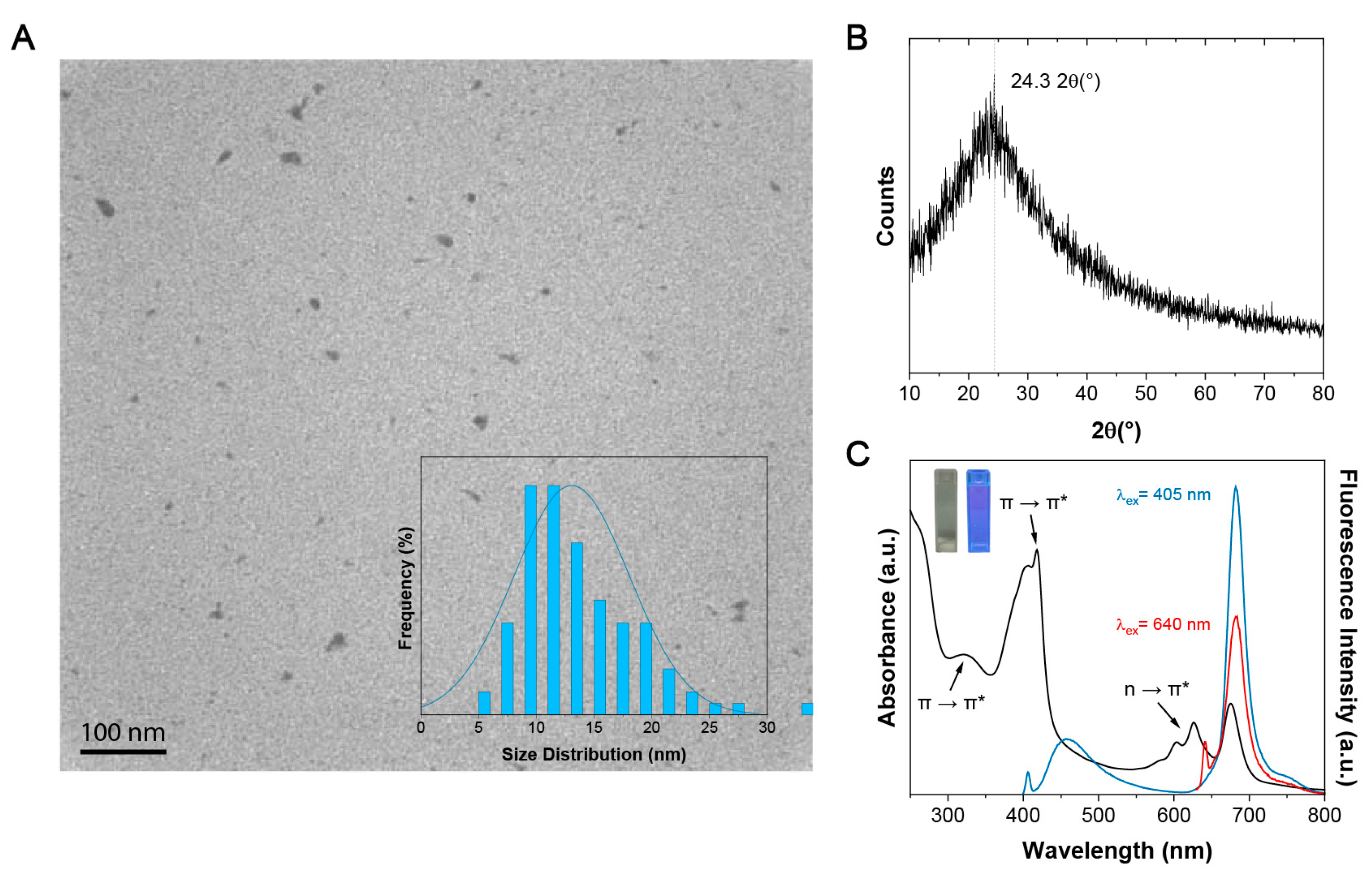
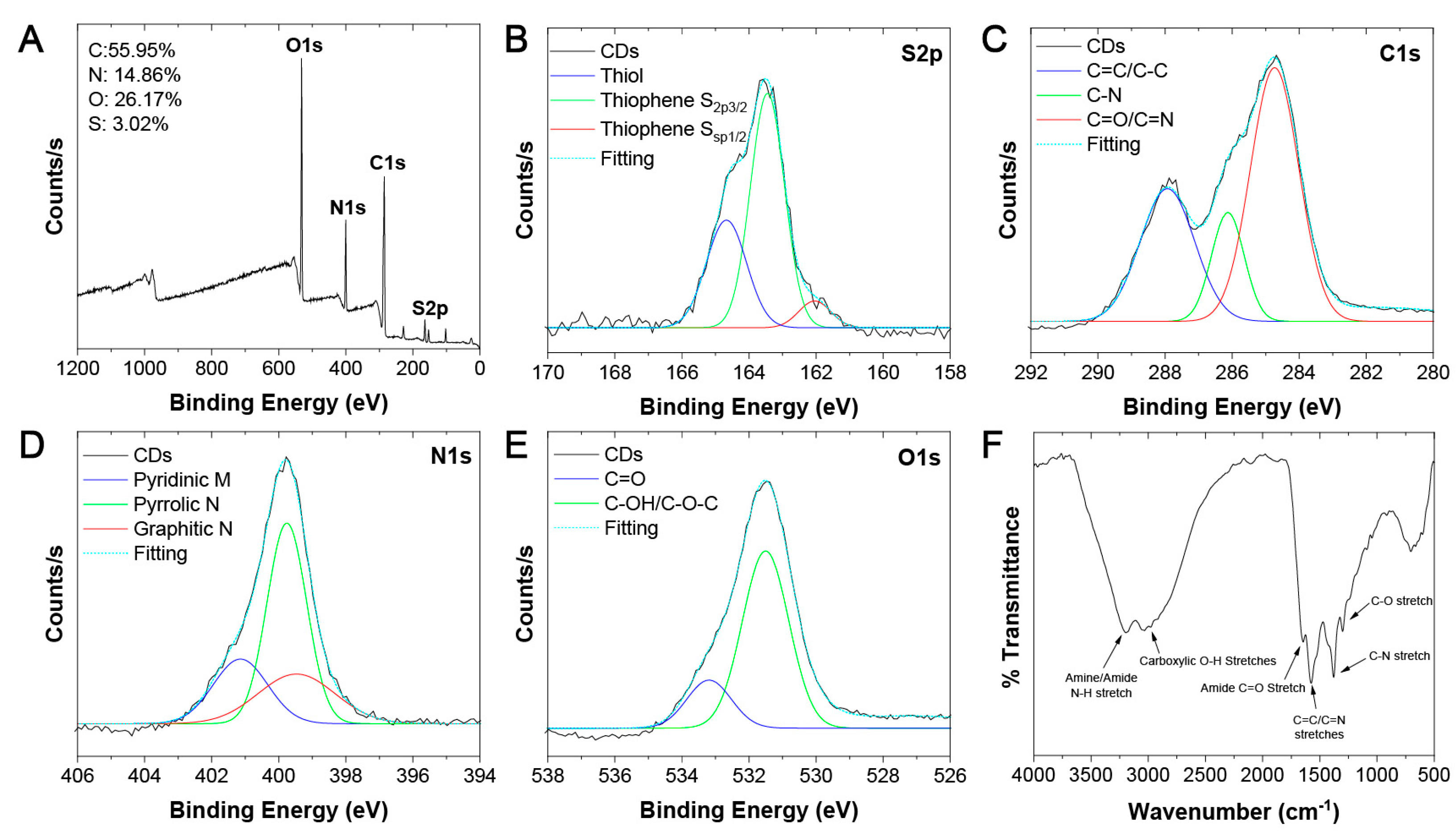
3.1. Physico-Chemical and Optical Characterization
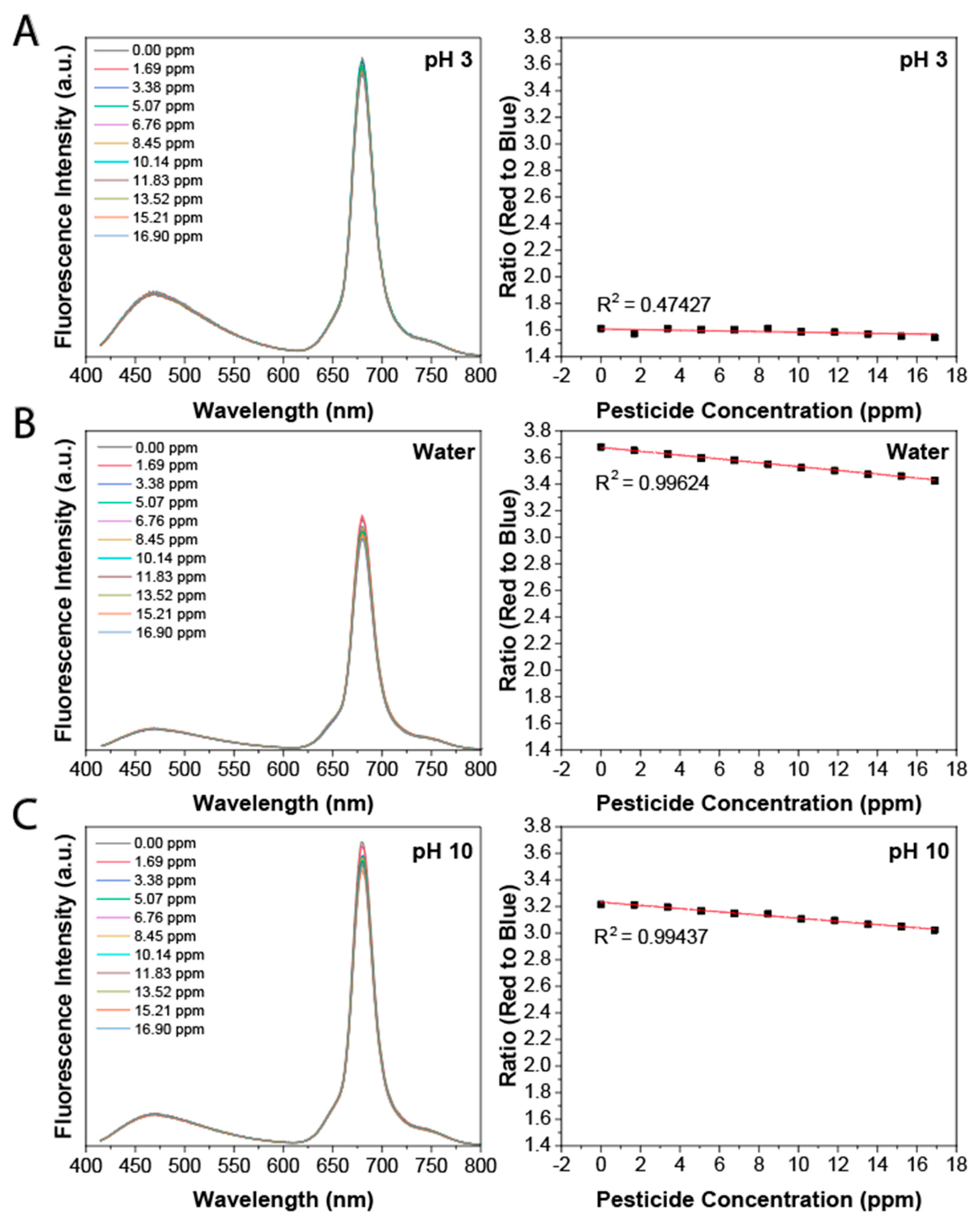
3.2. Detection of Glyphosate Using a Ratiometric Approach
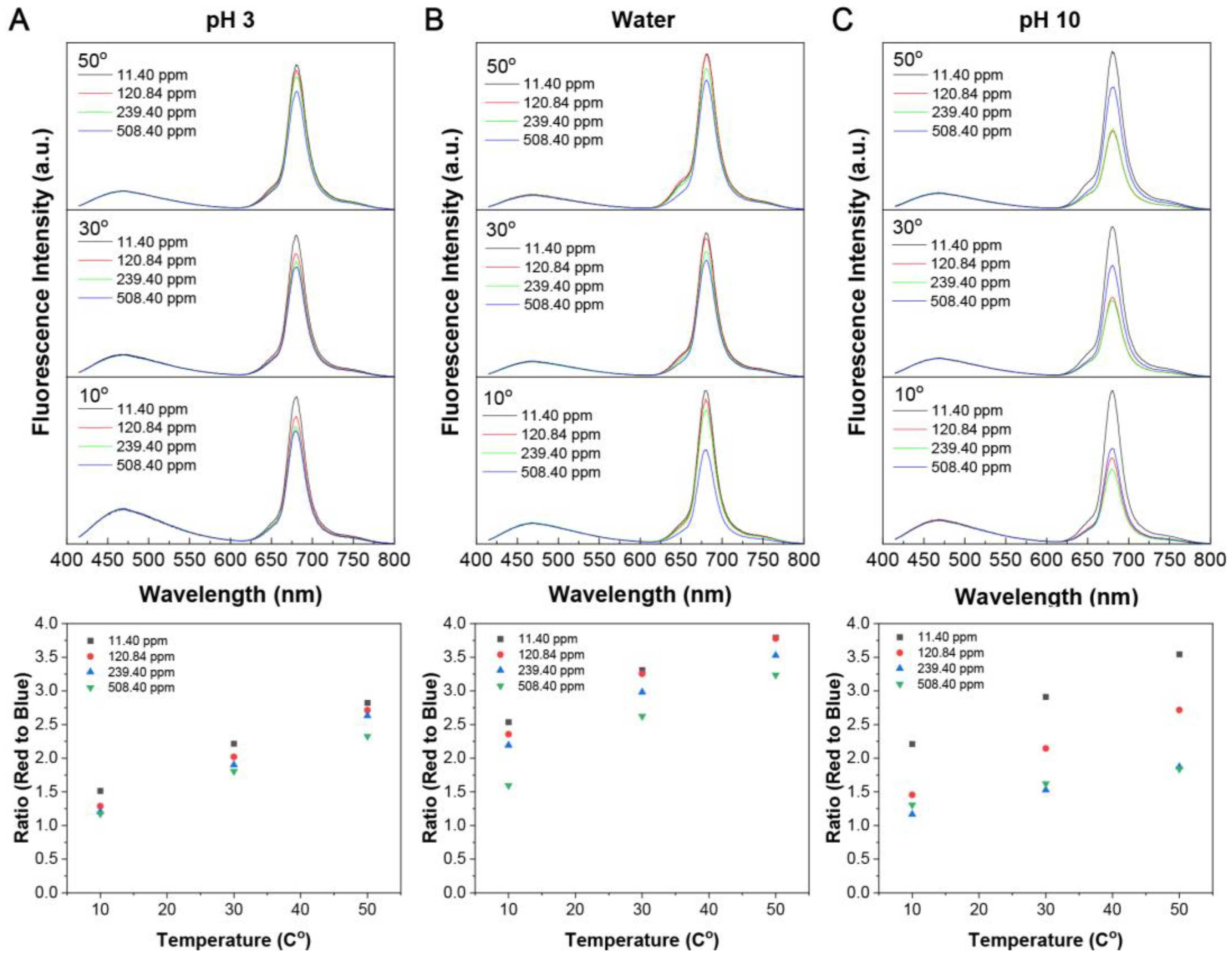
3.3. Dynamic Quenching of the Red Fluorescence
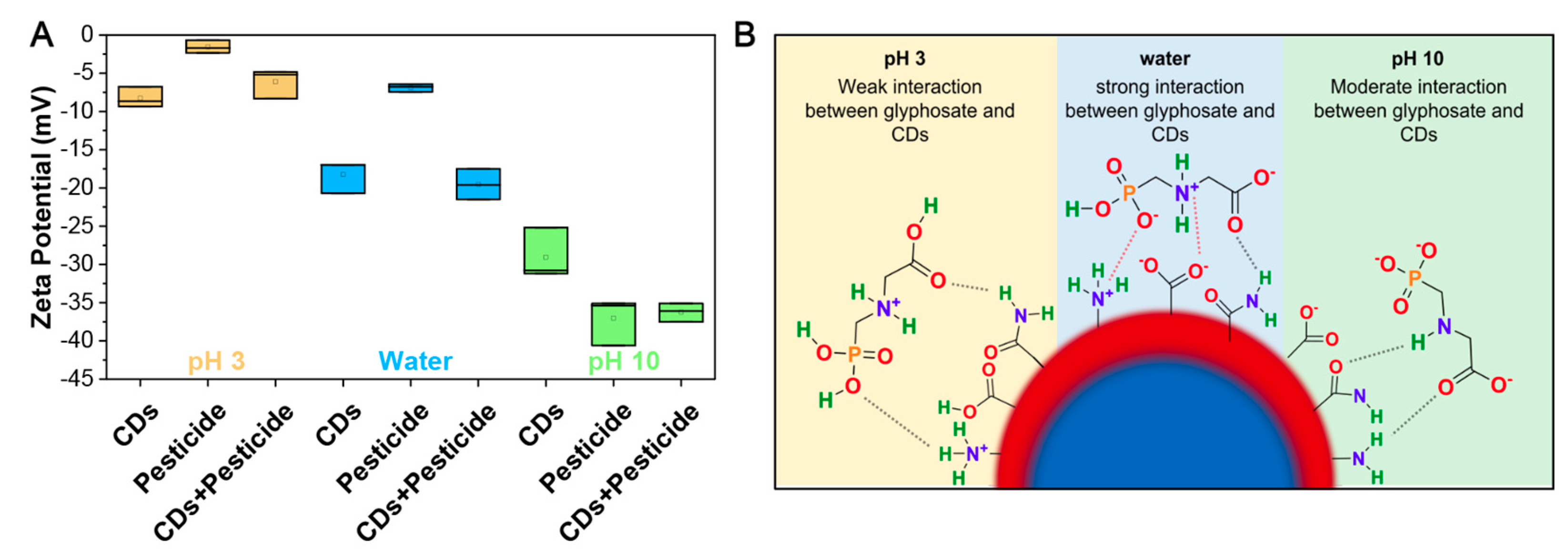
3.4. Ionic Interactions of CDs with Glyphosate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Seneff, S.; Samsel, A. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg. Neurol. Int. 2015, 6, 45. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef]
- Tzanetou, E.; Karasali, H. Glyphosate Residues in Soil and Air: An Integrated Review. Available online: www.intechopen.com (accessed on 5 October 2022).
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lyu, Z.; Li, S.; Ruan, X.; Fei, M.; Zhou, Y.; Niu, X.; Zhu, W.; Du, D.; Lin, Y. Molecularly imprinted polypyrrole nanotubes based electrochemical sensor for glyphosate detection. Biosens. Bioelectron. 2021, 191, 113434. [Google Scholar] [CrossRef]
- Goodwin, L.; Startin, J.R.; Keely, B.J.; Goodall, D.M. A nalysis of glyphosate and glufosinate by capillary electrophoresis–mass spectrometry utilising a sheathless microelectrospray interface. J. Chromatogr. A 2003, 1004, 107–119. [Google Scholar] [CrossRef]
- Royer, A.; Beguin, S.; Tabet, J.C.; Hulot, S.; Reding, M.A.; Communal, P.Y. Determination of Glyphosate and Aminomethylphosphonic Acid Residues in Water by Gas Chromatography with Tandem Mass Spectrometry after Exchange Ion Resin Purification and Derivatization. Appl. Veg. Matrixes 2000, 72, 3826–3832. [Google Scholar] [CrossRef]
- Bernal, J.; Martin, M.; Nozal, M.; Anadón, A.; Martínez-Larrañaga, M.; Martínez, M. Development and validation of a liquid chromatography—Fluorescence—Mass spectrometry method to measure glyphosate and aminomethylphosphonic acid in rat plasma. J. Chromatogr. B 2010, 878, 3290–3296. [Google Scholar] [CrossRef]
- Chen, Z.; He, W.; Beer, M.; Megharaj, M.; Naidu, R. Speciation of glyphosate, phosphate and aminomethylphosphonic acid in soil extracts by ion chromatography with inductively coupled plasma mass spectrometry with an octopole reaction system. Talanta 2009, 78, 852–856. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Lan, S.; Zhang, C.; Hou, C.; He, Q.; Huo, D. A turn-on fluorescent sensor based on carbon dots from: Sophora japonica leaves for the detection of glyphosate. Anal Methods 2020, 12, 4130–4138. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Fu, Z.; Gao, W.; Zhang, C.; Zhao, Q.; Deng, F.; Lu, X. Review on carbon dots in food safety applications. Talanta 2019, 194, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-dependent fluorescence in carbon dots. J. Phys. Chem. C. 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Zor, E.; Morales-Narváez, E.; Zamora-Gálvez, A.; Bingol, H.; Ersoz, M.; Merkoçi, A. Graphene quantum dots-based photoluminescent sensor: A multifunctional composite for pesticide detection. ACS Appl. Mater. Interfaces 2015, 7, 20272–20279. [Google Scholar] [CrossRef] [PubMed]
- Desmond, L.J.; Phan, A.N.; Gentile, P. Critical overview on the green synthesis of carbon quantum dots and their application for cancer therapy. Environ. Sci. Nano 2021, 8, 848–862. [Google Scholar] [CrossRef]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Frontiers in carbon dots: Design, properties and applications. Mater. Chem. Front. 2019, 3, 2571–2601. [Google Scholar] [CrossRef]
- Anwar, S.; Ding, H.; Xu, M.; Hu, X.; Li, Z.; Wang, J.; Liu, L.; Jiang, L.; Wang, D.; Dong, C.; et al. Recent Advances in Synthesis, Optical Properties, and Biomedical Applications of Carbon Dots. ACS Appl. Bio. Mater. 2019, 2, 2317–2338. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Rubio, F.; Veldhuis, L.J.; Clegg, B.S.; Fleeker, J.R.; Hall, J.C. Comparison of a direct ELISA and an HPLC method for glyphosate determinations in water. J. Agric. Food Chem. 2003, 51, 691–696. [Google Scholar] [CrossRef]
- Lundgren, L.N. A New Method for the Determination of Glyphosate and (Aminomethyl)phosphonic Acid Residues in Soils. J. Agric. Food Chem. 1986, 34, 535–538. [Google Scholar] [CrossRef]
- Corbera, M.; Hidalgo, M.; Salvadó, V.; Wieczorek, P. Determination of glyphosate and aminomethylphosphonic acid in natural water using the capillary electrophoresis combined with enrichment step. Anal. Chim. Acta. 2005, 540, 3–7. [Google Scholar] [CrossRef]
- Guo, Z.-X.; Cai, Q.; Yang, Z. Determination of glyphosate and phosphate in water by ion chromatography—Inductively coupled plasma mass spectrometry detection. J. Chromatogr. A 2005, 1100, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sens. Actuators B Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.; Hou, J.; Li, H.; Xu, Y.; Wang, B.; Ding, H.; Ding, L. Facile, green and clean one-step synthesis of carbon dots from wool: Application as a sensor for glyphosate detection based on the inner filter effect. Talanta 2016, 160, 268–275. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Llorent-Martínez, E.; Ortega-Barrales, P.; Ruiz-Medina, A. Graphene quantum dots-silver nanoparticles as a novel sensitive and selective luminescence probe for the detection of glyphosate in food samples. Talanta 2020, 207, 120344. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Zhang, Z.; Zhang, J. “Off-on” fluorescence probe based on green emissive carbon dots for the determination of Cu2+ ions and glyphosate and development of a smart sensing film for vegetable packaging. Microchim. Acta 2022, 189, 1–9. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, J.; Liu, S.; Yang, J.; Zhang, H.; Yan, J.; Hu, X. Fluorescent carbon dots for glyphosate determination based on fluorescence resonance energy transfer and logic gate operation. Sens. Actuators B Chem. 2017, 242, 545–553. [Google Scholar] [CrossRef]
- Yarur, F.; Macairan, J.R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. Nano 2019, 6, 1121–1130. [Google Scholar] [CrossRef]
- Macairan, J.R.; Jaunky, D.B.; Piekny, A.; Naccache, R. Intracellular ratiometric temperature sensing using fluorescent carbon dots. Nanoscale Adv. 2019, 1, 105–113. [Google Scholar] [CrossRef]
- Macairan, J.R.; Zhang, I.; Clermont-Paquette, A.; Naccache, R.; Maysinger, D. Ratiometric pH Sensing in Living Cells Using Carbon Dots. Part. Part. Syst. Charact. 2020, 37, 1900430. [Google Scholar] [CrossRef]
- An, J.; Chen, M.; Hu, N.; Hu, Y.; Chen, R.; Lyu, Y.; Guo, W.; Li, L.; Liu, Y. Carbon dots-based dual-emission ratiometric fluorescence sensor for dopamine detection. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 243, 118804. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, G.; Li, Y.; Guo, J.; Chen, X.; Tan, Y.; Tang, W.; Li, Z. Dual-modes of ratiometric fluorescent and smartphone-integrated colorimetric detection of glyphosate by carbon dots encapsulated porphyrin metal–organic frameworks. Appl. Surf. Sci. 2022, 602, 154368. [Google Scholar] [CrossRef]
- Li, H.; Su, D.; Gao, H.; Yan, X.; Kong, D.; Jin, R.; Liu, X.; Wang, C.; Lu, G. Design of Red Emissive Carbon Dots: Robust Performance for Analytical Applications in Pesticide Monitoring. Anal. Chem. 2020, 92, 3198–3205. [Google Scholar] [CrossRef]
- Tang, J.; Ma, X.; Yang, J.; Feng, D.-D.; Wang, X.-Q. Recent advances in metal-organic frameworks for pesticide detection and adsorption. Dalton Trans. 2020, 49, 14361–14372. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, G.; Luo, X.; Lin, W.; Han, Y.; Huang, J.; Li, Z. Carbon dots@Cu metal–organic frameworks hybrids for ratiometric fluorescent determination of pesticide thiophanate-methyl. Microchim. Acta 2022, 189, 325. [Google Scholar] [CrossRef]
- Macairan, J.-R.; de Medeiros, T.V.; Gazzetto, M.; Villanueva, F.Y.; Cannizzo, A.; Naccache, R. Elucidating the mechanism of dual-fluorescence in carbon dots. J. Colloid. Interface Sci. 2022, 606, 67–76. [Google Scholar] [CrossRef]
- Kremen, A. Glyphosate Residue in Canada. 2014. Available online: http://www.canada-organic.ca/sites/default/files/glyphosate_report-_august_2014.pdf (accessed on 13 February 2022).
- Acuña, A.U.; Adam, W.; Amat, F.; Armesto, D.; Atvars, T.D.Z.; Bard, A.; Bill, E.; Björn, L.O.; Bohne, C.; Bolton, J.; et al. Glossary of Terms used in Photochemistry. J. Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar]
- Lakowicz, J.R. (Ed.) Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. [Google Scholar]
- William, R.; Laws, P.B.C. Fluorescence quenching studies: Analysis of nonlinear Stern-Volmer data. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1992; Volume 210, pp. 448–463. [Google Scholar] [CrossRef]
| Pesticide | Analytical Methods | Detection Limit | References |
|---|---|---|---|
| Glyphosate | High-performance chromatography (HPLC) | 50 ng/mL (0.05 ppm) | [21] |
| Gas chromatography–mass spectrometry (GC-MS) | 0.1 ug/mL (0.0001 ppm) | [22] | |
| Capillary electrophoresis (CE) | 85 ng/mL (0.085 ppm) | [23] | |
| Inductively coupled plasma mass spectrometry (ICP-MS) | 0.7 ug/mL (0.7 ppm) | [24] | |
| Carbon dots | 0.4 ng/mL (0.0004 ppm) 0.8 ng/mL (0.0008 ppm) 9 ng/mL (0.009 ppm) 12 ng/mL (0.012 ppm) 0.6 umol/L (0.10 ppm) | [25] [26] [27] [28] [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clermont-Paquette, A.; Mendoza, D.-A.; Sadeghi, A.; Piekny, A.; Naccache, R. Ratiometric Sensing of Glyphosate in Water Using Dual Fluorescent Carbon Dots. Sensors 2023, 23, 5200. https://doi.org/10.3390/s23115200
Clermont-Paquette A, Mendoza D-A, Sadeghi A, Piekny A, Naccache R. Ratiometric Sensing of Glyphosate in Water Using Dual Fluorescent Carbon Dots. Sensors. 2023; 23(11):5200. https://doi.org/10.3390/s23115200
Chicago/Turabian StyleClermont-Paquette, Adryanne, Diego-Andrés Mendoza, Amir Sadeghi, Alisa Piekny, and Rafik Naccache. 2023. "Ratiometric Sensing of Glyphosate in Water Using Dual Fluorescent Carbon Dots" Sensors 23, no. 11: 5200. https://doi.org/10.3390/s23115200
APA StyleClermont-Paquette, A., Mendoza, D.-A., Sadeghi, A., Piekny, A., & Naccache, R. (2023). Ratiometric Sensing of Glyphosate in Water Using Dual Fluorescent Carbon Dots. Sensors, 23(11), 5200. https://doi.org/10.3390/s23115200







