MSE-VGG: A Novel Deep Learning Approach Based on EEG for Rapid Ischemic Stroke Detection
Abstract
1. Introduction
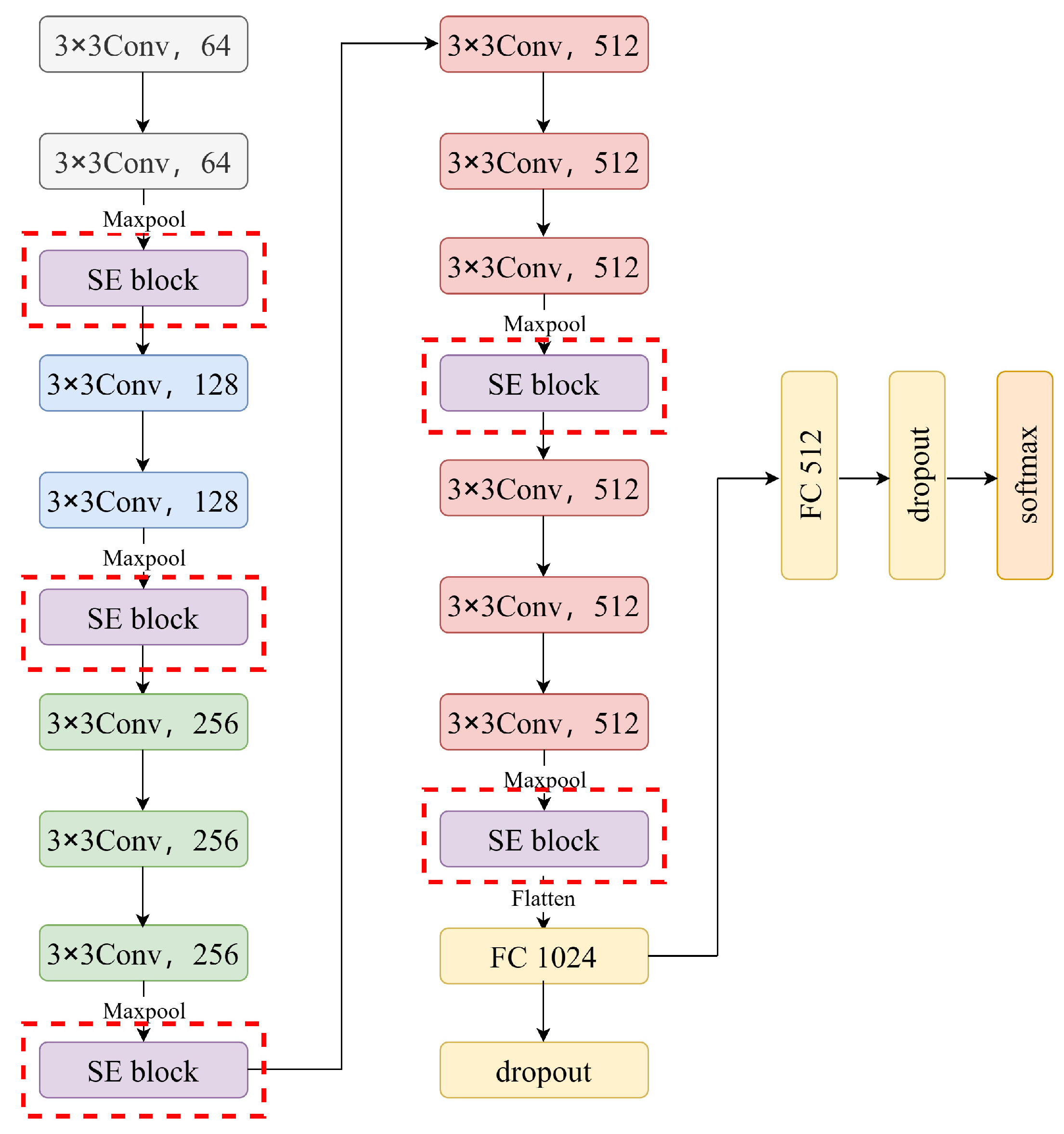
- A new deep learning model, MSE-VGG, was developed to perform the ischemic stroke detection task based on EEG signals. We constructed the model by introducing multiple squeeze-and-excitation (SE) modules into the traditional VGG model, which enhanced its ability to capture ischemic stroke features from EEG signals, allowing it to effectively distinguish between patients with ischemic stroke and non-stroke individuals.
- The functional connectivity information implied in EEG channels and complexity information are extracted by using the correlation-weighted Phase Lag Index (cwPLI) and Sample Entropy (SaEn), respectively. A fusion feature is proposed by combining these two types of information, which can not only capture the spatio-temporal relationships among multi-channel EEG electrodes but also learn the nonlinear dynamic changes in EEG signals of ischemic stroke patients.
- Extensive experiments were conducted on the private ZJU4H dataset to validate our model. The experimental results prove the superior performance of our method in the rapid identification of ischemic stroke based on EEG data, indicating that it is suitable for potential real-time application.
2. Materials and Methods
2.1. Data Acquisition
2.2. Methods
2.2.1. Preprocessing
2.2.2. Feature Extraction
| Algorithm 1 Algorithm for feature extraction |
|
2.2.3. The MSE-VGG Model
3. Experiments and Results
3.1. Experimental Requirements and Metrics
- True positive (TP): The model correctly predicts a positive case, i.e., the prediction matches the actual positive conditions.
- False negative (FN): The model incorrectly predicts a negative case, i.e., the prediction does not match the actual positive conditions.
- False positive (FP): The model erroneously predicts a positive case, i.e., the prediction does not match the actual negative conditions.
- True negative (TN): The model correctly predicts a negative case, i.e., the prediction matches the actual negative conditions.
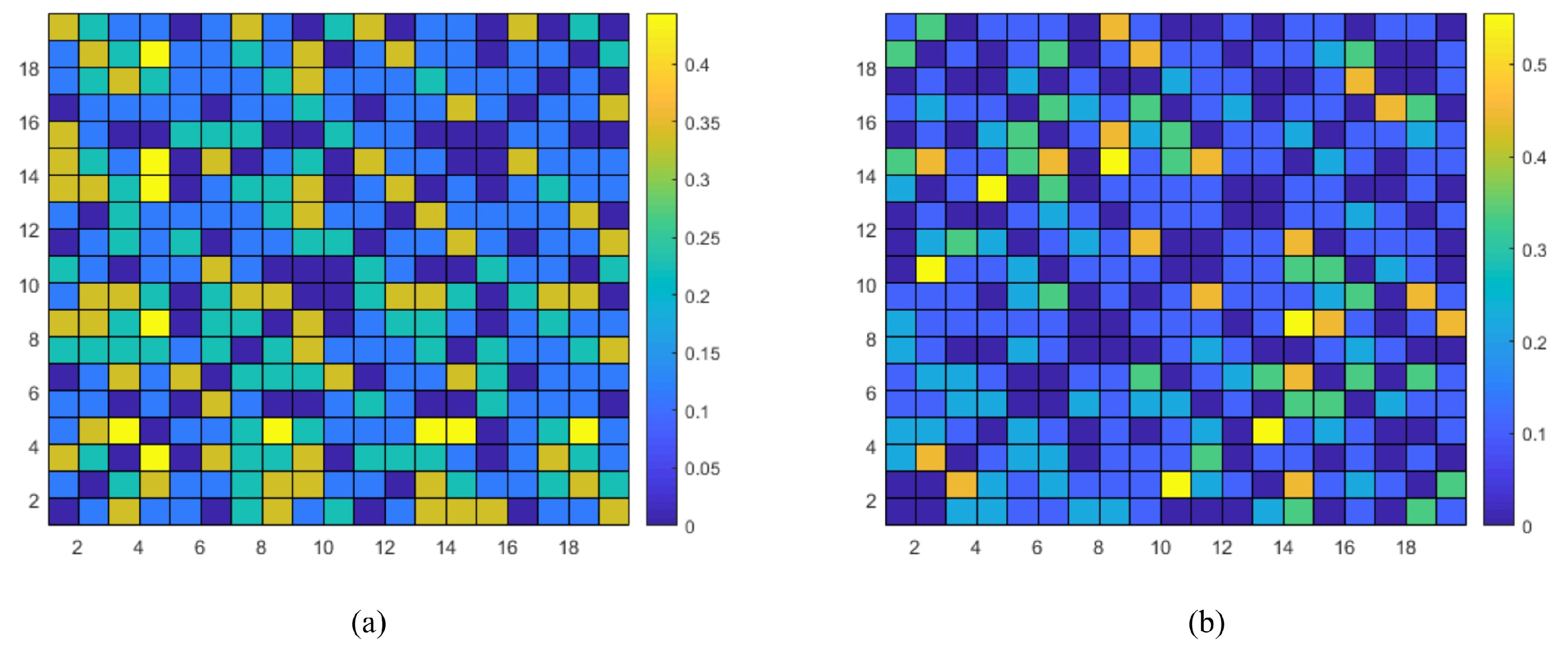
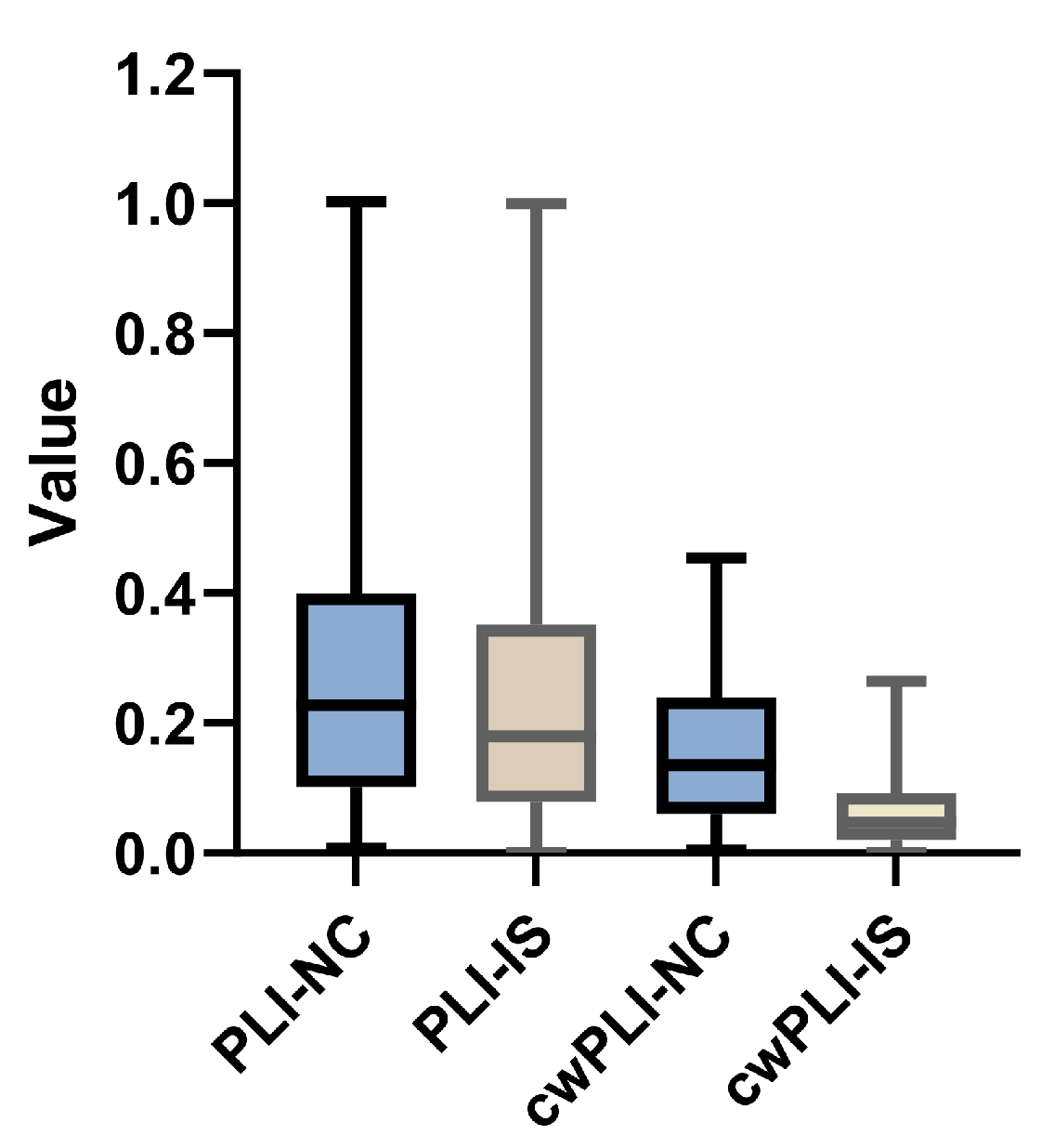
3.2. Verification of Proposed cwPLI Feature
3.3. Results
3.3.1. Comparative Analysis of Different Features
- PCC [23] determines whether there is a positive or negative correlation between two signals and the strength of that correlation.
- MI [24] is used to measure the amount of information about one signal contained in another and is capable of detecting both linear and nonlinear correlations between two signals.
- PLV [25] is a phase-based functional connectivity method used to measure the degree of phase synchronization between two channel signals, with higher values indicating stronger synchronization.
- PLI [26] measures the phase synchronization between two channel signals and is not sensitive to volume conduction effects but may be sensitive to noise.
3.3.2. Comparative Analysis of Different Classifiers
- Logistic Regression (LR) [48]: A classical linear discrimination model widely used for various classification tasks.
- AlexNet [49]: An iconic deep convolutional neural network known for its groundbreaking results in the 2012 ImageNet competition, marking a significant milestone in the field of deep learning for image recognition.
- RCF (Richer Convolutional Features) [50]: An improved method based on the VGG16 framework, aiming to enhance edge detection accuracy by capturing multi-scale and multi-level information from images.
- LSTM (Long Short-Term Memory) [51]: A special type of recurrent neural network structure that excels at handling and predicting long-term dependencies in time-series data.
- VGG [46]: A deep convolutional network that improves image recognition accuracy by using multiple small convolutional kernels () and increasing the number of network layers, widely applied in the field of computer vision.
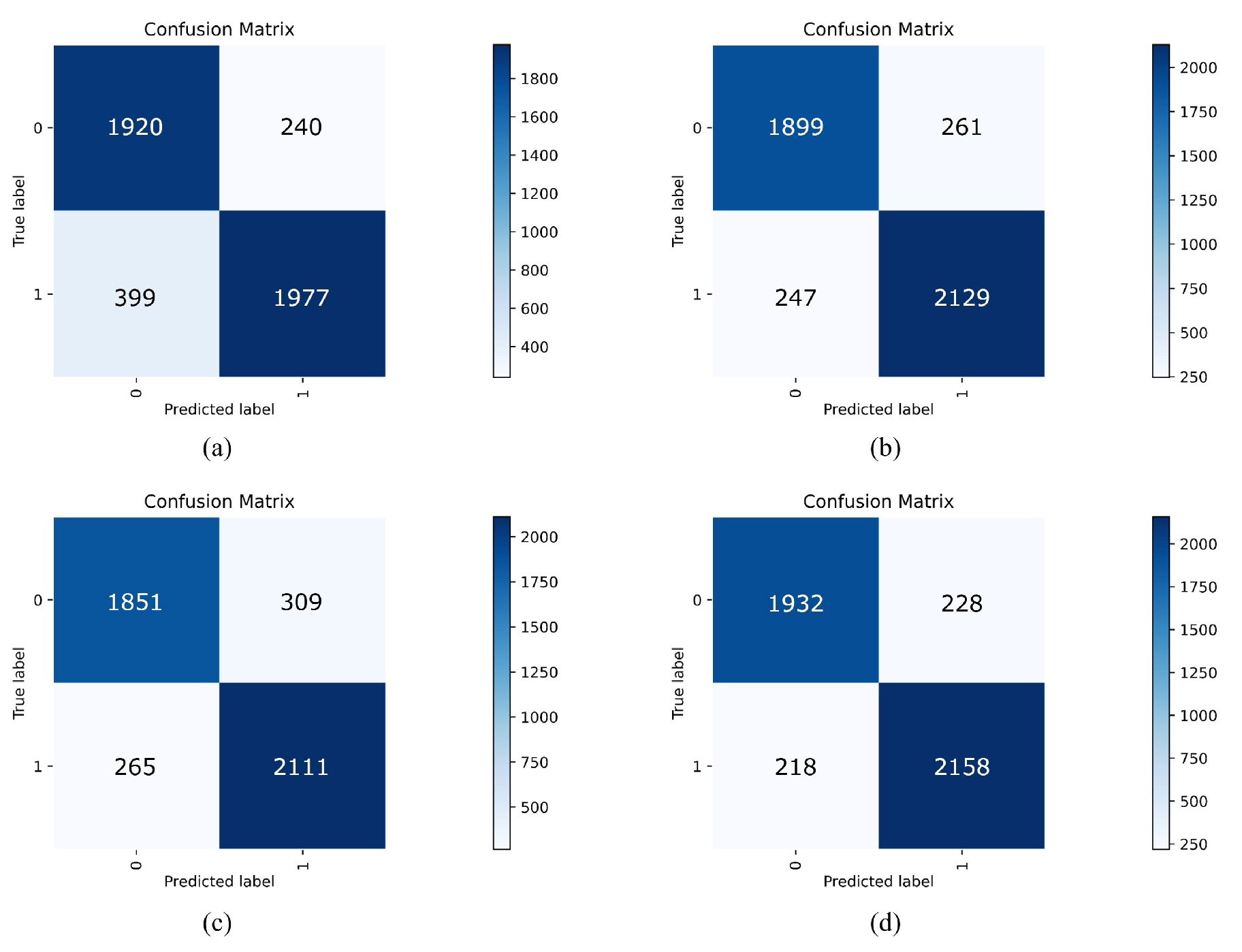
3.3.3. Ablation Experiments
3.3.4. Feasibility Analysis
- CT (computed tomography): A technology that uses X-rays to penetrate the human body and form images by receiving signals from detectors, providing detailed cross-sectional images to help doctors observe internal structures and abnormalities.
- MRI (magnetic resonance imaging): A technique that uses a strong magnetic field and radio waves to obtain detailed images of the internal body, suitable for imaging soft tissues, such as brain tissue.
- fMRI (functional magnetic resonance imaging): A neuroimaging technique that infers brain activity by measuring changes in blood flow during brain function, focusing on brain functional activity rather than anatomical structure.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Søyland, M.H.; Tveiten, A.; Eltoft, A.; Øygarden, H.; Varmdal, T.; Indredavik, B.; Mathiesen, E.B. Wake-up stroke and unknown-onset stroke; occurrence and characteristics from the nationwide Norwegian Stroke Register. Eur. Stroke J. 2022, 7, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Ebaid, D.; Kramer, S.; Werden, E.; Baxter, H.; Campbell, B.C.; Brodtmann, A. Pre-stroke physical activity and admission stroke severity: A systematic review. Int. J. Stroke 2021, 16, 1009–1018. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G. Embolic stroke of undetermined source: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Bersano, A.; Kraemer, M.; Burlina, A.; Mancuso, M.; Finsterer, J.; Sacco, S.; Salvarani, C.; Caputi, L.; Chabriat, H.; Oberstein, S.L.; et al. Heritable and non-heritable uncommon causes of stroke. J. Neurol. 2021, 268, 2780–2807. [Google Scholar] [CrossRef] [PubMed]
- Flach, C.; Muruet, W.; Wolfe, C.D.; Bhalla, A.; Douiri, A. Risk and secondary prevention of stroke recurrence: A population-base cohort study. Stroke 2020, 51, 2435–2444. [Google Scholar] [CrossRef]
- Rücker, V.; Heuschmann, P.U.; O’Flaherty, M.; Weingärtner, M.; Hess, M.; Sedlak, C.; Schwab, S.; Kolominsky-Rabas, P.L. Twenty-year time trends in long-term case-fatality and recurrence rates after ischemic stroke stratified by etiology. Stroke 2020, 51, 2778–2785. [Google Scholar] [CrossRef]
- Singletary, E.M.; Zideman, D.A.; Bendall, J.C.; Berry, D.C.; Borra, V.; Carlson, J.N.; Cassan, P.; Chang, W.T.; Charlton, N.P.; Djärv, T.; et al. 2020 international consensus on first aid science with treatment recommendations. Circulation 2020, 142, S284–S334. [Google Scholar] [CrossRef]
- Bentes, C.; Peralta, A.R.; Viana, P.; Martins, H.; Morgado, C.; Casimiro, C.; Franco, A.C.; Fonseca, A.C.; Geraldes, R.; Canhão, P.; et al. Quantitative EEG and functional outcome following acute ischemic stroke. Clin. Neurophysiol. 2018, 129, 1680–1687. [Google Scholar] [CrossRef]
- van Meenen, L.C.; van Stigt, M.N.; Siegers, A.; Smeekes, M.D.; van Grondelle, J.A.; Geuzebroek, G.; Marquering, H.A.; Majoie, C.B.; Roos, Y.B.; Koelman, J.H.; et al. Detection of large vessel occlusion stroke in the prehospital setting: Electroencephalography as a potential triage instrument. Stroke 2021, 52, e347–e355. [Google Scholar] [CrossRef]
- Vilou, I.; Varka, A.; Parisis, D.; Afrantou, T.; Ioannidis, P. EEG-neurofeedback as a potential therapeutic approach for cognitive deficits in patients with dementia, multiple sclerosis, stroke and traumatic brain injury. Life 2023, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Shreve, L.; Kaur, A.; Vo, C.; Wu, J.; Cassidy, J.M.; Nguyen, A.; Zhou, R.J.; Tran, T.B.; Yang, D.Z.; Medizade, A.I.; et al. Electroencephalography measures are useful for identifying large acute ischemic stroke in the emergency department. J. Stroke Cerebrovasc. Dis. 2019, 28, 2280–2286. [Google Scholar] [CrossRef]
- Burghaus, L.; Hilker, R.; Dohmen, C.; Bosche, B.; Winhuisen, L.; Galldiks, N.; Szelies, B.; Heiss, W.D. Early electroencephalography in acute ischemic stroke: Prediction of a malignant course? Clin. Neurol. Neurosurg. 2007, 109, 45–49. [Google Scholar] [CrossRef]
- Assenza, G.; Zappasodi, F.; Squitti, R.; Altamura, C.; Ventriglia, M.; Ercolani, M.; Quattrocchi, C.C.; Lupoi, D.; Passarelli, F.; Vernieri, F.; et al. Neuronal functionality assessed by magnetoencephalography is related to oxidative stress system in acute ischemic stroke. Neuroimage 2009, 44, 1267–1273. [Google Scholar] [CrossRef]
- van Meenen, L.C.; van Stigt, M.N.; Marquering, H.A.; Majoie, C.B.; Roos, Y.B.; Koelman, J.H.; Potters, W.V.; Coutinho, J.M. Detection of large vessel occlusion stroke with electroencephalography in the emergency room: First results of the ELECTRA-STROKE study. J. Neurol. 2022, 269, 2030–2038. [Google Scholar] [CrossRef]
- Aminov, A.; Rogers, J.M.; Johnstone, S.J.; Middleton, S.; Wilson, P.H. Acute single channel EEG predictors of cognitive function after stroke. PLoS ONE 2017, 12, e0185841. [Google Scholar] [CrossRef]
- Finnigan, S.P.; Rose, S.E.; Walsh, M.; Griffin, M.; Janke, A.L.; McMahon, K.L.; Gillies, R.; Strudwick, M.W.; Pettigrew, C.M.; Semple, J.; et al. Correlation of quantitative EEG in acute ischemic stroke with 30-day NIHSS score: Comparison with diffusion and perfusion MRI. Stroke 2004, 35, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, S.P.; Walsh, M.; Rose, S.E.; Chalk, J.B. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin. Neurophysiol. 2007, 118, 2525–2532. [Google Scholar] [CrossRef]
- Tasci, I.; Tasci, B.; Barua, P.D.; Dogan, S.; Tuncer, T.; Palmer, E.E.; Fujita, H.; Acharya, U.R. Epilepsy detection in 121 patient populations using hypercube pattern from EEG signals. Inf. Fusion 2023, 96, 252–268. [Google Scholar] [CrossRef]
- Modir, A.; Shamekhi, S.; Ghaderyan, P. A systematic review and methodological analysis of EEG-based biomarkers of Alzheimer’s disease. Measurement 2023, 220, 113274. [Google Scholar] [CrossRef]
- Maura, R.M.; Rueda Parra, S.; Stevens, R.E.; Weeks, D.L.; Wolbrecht, E.T.; Perry, J.C. Literature review of stroke assessment for upper-extremity physical function via EEG, EMG, kinematic, and kinetic measurements and their reliability. J. Neuroeng. Rehabil. 2023, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Huang, Y.; Chen, J.; Benesty, J.; Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer Science & Business Media: Berlin, Germany, 2009; pp. 1–4. [Google Scholar]
- Kraskov, A.; Stögbauer, H.; Grassberger, P. Estimating mutual information. Phys. Rev. E 2004, 69, 066138. [Google Scholar] [CrossRef] [PubMed]
- Aydore, S.; Pantazis, D.; Leahy, R.M. A note on the phase locking value and its properties. Neuroimage 2013, 74, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xia, C.; Wang, Z.; Zhu, J.; Zhang, X.; Sun, S.; Li, J.; Huo, X.; Li, X. Multivariate pattern analysis of EEG-based functional connectivity: A study on the identification of depression. IEEE Access 2019, 7, 92630–92641. [Google Scholar] [CrossRef]
- Wang, B.; Han, X.; Yang, S.; Zhao, P.; Li, M.; Zhao, Z.; Wang, N.; Ma, H.; Zhang, Y.; Zhao, T.; et al. An integrative prediction algorithm of drug-refractory epilepsy based on combined clinical-EEG functional connectivity features. J. Neurol. 2022, 269, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Catherine Joy, R.; Thomas George, S.; Albert Rajan, A.; Subathra, M. Detection of ADHD from EEG signals using different entropy measures and ANN. Clin. EEG Neurosci. 2022, 53, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Crowcroft, J.; Zhang, J. Automatic epileptic seizure detection in EEGs based on optimized sample entropy and extreme learning machine. J. Neurosci. Methods 2012, 210, 132–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, J.; Kong, W.; Liu, Y.; Wang, Q.; Jiang, T. Epilepsy prediction through optimized multidimensional sample entropy and Bi-LSTM. Biomed. Signal Process. Control 2021, 64, 102293. [Google Scholar] [CrossRef]
- Altaheri, H.; Muhammad, G.; Alsulaiman, M.; Amin, S.U.; Altuwaijri, G.A.; Abdul, W.; Bencherif, M.A.; Faisal, M. Deep learning techniques for classification of electroencephalogram (EEG) motor imagery (MI) signals: A review. Neural Comput. Appl. 2023, 35, 14681–14722. [Google Scholar] [CrossRef]
- Supriya, S.; Siuly, S.; Wang, H.; Zhang, Y. Epilepsy detection from EEG using complex network techniques: A review. IEEE Rev. Biomed. Eng. 2021, 16, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z. A review of artificial intelligence for EEG-based brain- computer interfaces and applications. Brain Sci. Adv. 2020, 6, 162–170. [Google Scholar] [CrossRef]
- Morabito, F.C.; Ieracitano, C.; Mammone, N. An explainable Artificial Intelligence approach to study MCI to AD conversion via HD-EEG processing. Clin. EEG Neurosci. 2023, 54, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Parashar, A.; Joshi, A.M. DepHNN: A novel hybrid neural network for electroencephalogram (EEG)-based screening of depression. Biomed. Signal Process. Control 2021, 66, 102393. [Google Scholar] [CrossRef]
- Wang, F.; Wu, S.; Zhang, W.; Xu, Z.; Zhang, Y.; Wu, C.; Coleman, S. Emotion recognition with convolutional neural network and EEG-based EFDMs. Neuropsychologia 2020, 146, 107506. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.U.; Alsulaiman, M.; Muhammad, G.; Mekhtiche, M.A.; Hossain, M.S. Deep Learning for EEG motor imagery classification based on multi-layer CNNs feature fusion. Future Gener. Comput. Syst. 2019, 101, 542–554. [Google Scholar] [CrossRef]
- Li, H.; Ding, M.; Zhang, R.; Xiu, C. Motor imagery EEG classification algorithm based on CNN-LSTM feature fusion network. Biomed. Signal Process. Control 2022, 72, 103342. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, Y.K.; Eskandarian, A. EEG-inception: An accurate and robust end-to-end neural network for EEG-based motor imagery classification. J. Neural Eng. 2021, 18, 046014. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, W.; Zhang, S.; Wang, P. Mild cognitive impairment classification convolutional neural network with attention mechanism. In Proceedings of the 2020 IEEE 16th International Conference on Control & Automation (ICCA), Singapore, 9–11 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1074–1078. [Google Scholar]
- Ding, X.; Nie, W.; Liu, X.; Wang, X.; Yuan, Q. Compact convolutional neural network with multi-headed attention mechanism for seizure prediction. Int. J. Neural Syst. 2023, 33, 2350014. [Google Scholar] [CrossRef]
- Erani, F.; Zolotova, N.; Vanderschelden, B.; Khoshab, N.; Sarian, H.; Nazarzai, L.; Wu, J.; Chakravarthy, B.; Hoonpongsimanont, W.; Yu, W.; et al. Electroencephalography might improve diagnosis of acute stroke and large vessel occlusion. Stroke 2020, 51, 3361–3365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, L.; Fan, S.Z.; Abbod, M.F.; Shieh, J.S. Sample entropy analysis for the estimating depth of anaesthesia through human EEG signal at different levels of unconsciousness during surgeries. PeerJ 2018, 6, e4817. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Lake, D.E.; Moorman, J.R. Sample entropy. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 384, pp. 172–184. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 7132–7141. [Google Scholar]
- Menard, S. Applied Logistic Regression Analysis; Number 106; Sage: Newcastle upon Tyne, UK, 2002. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. In Proceedings of the 36th Annual Conference on Neural Information Processing Systems (NeurIPS 2022), New Orleans, LA, USA, 28 Novemver–9 December 2022. [Google Scholar]
- Liu, Y.; Cheng, M.M.; Hu, X.; Wang, K.; Bai, X. Richer convolutional features for edge detection. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 3000–3009. [Google Scholar]
- Graves, A.; Graves, A. Long short-term memory. In Supervised Sequence Labelling with Recurrent Neural Networks; Technical University of Munich: Munich, Germany, 2012; pp. 37–45. [Google Scholar]





| Subjects | IS | NC | |
|---|---|---|---|
| MRI diagnosis | 39 | 20 | 19 |
| Age—mean ± SD | 63 ± 13 | 65 ± 13 | 60 ± 10 |
| Sex—No. of males/total | 23/39 | 11/20 | 12/19 |
| Duration of EEG (min) | 252.6 | 132.5 | 120.1 |
| Feature | Accuracy | Sensitivity | Specificity |
|---|---|---|---|
| PCC [23] | 81.17% | 76.02% | 87.56% |
| MI [24] | 81.17% | 78.82% | 83.52% |
| PLV [25] | 83.82% | 79.83% | 88.26% |
| PLI [26] | 84.10% | 80.02% | 88.61% |
| cwPLI | 88.80% | 88.49% | 89.08% |
| cwPLI+SaEn | 90.17% | 89.86% | 90.41% |
| Classifier | Accuracy | Sensitivity | Specificity |
|---|---|---|---|
| LR [48] | 78.31% | 74.67% | 82.34% |
| AlexNet [49] | 85.64% | 85.41% | 85.86% |
| RCF [50] | 87.57% | 84.58% | 90.34% |
| LSTM [51] | 85.98% | 84.45% | 87.43% |
| VGG [46] | 87.35% | 87.48% | 87.23% |
| MSE-VGG | 90.17% | 89.86% | 90.41% |
| Feature Extraction | Classification | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| PLI | MSE-VGG | 84.10% | 80.02% | 88.61% |
| cwPLI | VGG | 85.91% | 82.79% | 89.17% |
| cwPLI | MSE-VGG | 88.80% | 88.49% | 89.08% |
| cwPLI+SaEn | VGG | 87.35% | 87.48% | 87.23% |
| cwPLI+SaEn | MSE-VGG | 90.17% | 89.86% | 90.41% |
| Method | Portability | Low Cost | Simple Operation | Time (avg) |
|---|---|---|---|---|
| CT | × | × | × | Within 30 min |
| MRI | × | × | × | More than 20 min |
| fMRI | × | × | × | Within 20 min |
| Proposed | √ | √ | √ | Within 10 min + 4.62 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, W.; Yue, W.; Chen, F.; Shi, W.; Zhang, L.; Wan, J. MSE-VGG: A Novel Deep Learning Approach Based on EEG for Rapid Ischemic Stroke Detection. Sensors 2024, 24, 4234. https://doi.org/10.3390/s24134234
Tong W, Yue W, Chen F, Shi W, Zhang L, Wan J. MSE-VGG: A Novel Deep Learning Approach Based on EEG for Rapid Ischemic Stroke Detection. Sensors. 2024; 24(13):4234. https://doi.org/10.3390/s24134234
Chicago/Turabian StyleTong, Wei, Weiqi Yue, Fangni Chen, Wei Shi, Lei Zhang, and Jian Wan. 2024. "MSE-VGG: A Novel Deep Learning Approach Based on EEG for Rapid Ischemic Stroke Detection" Sensors 24, no. 13: 4234. https://doi.org/10.3390/s24134234
APA StyleTong, W., Yue, W., Chen, F., Shi, W., Zhang, L., & Wan, J. (2024). MSE-VGG: A Novel Deep Learning Approach Based on EEG for Rapid Ischemic Stroke Detection. Sensors, 24(13), 4234. https://doi.org/10.3390/s24134234







