Sonification for Personalised Gait Intervention
Abstract
1. Introduction
2. Assessing Gait
3. Personalising Interventions
3.1. Biofeedback
3.2. Personalised Cueing
4. Sound and Sonification Theory
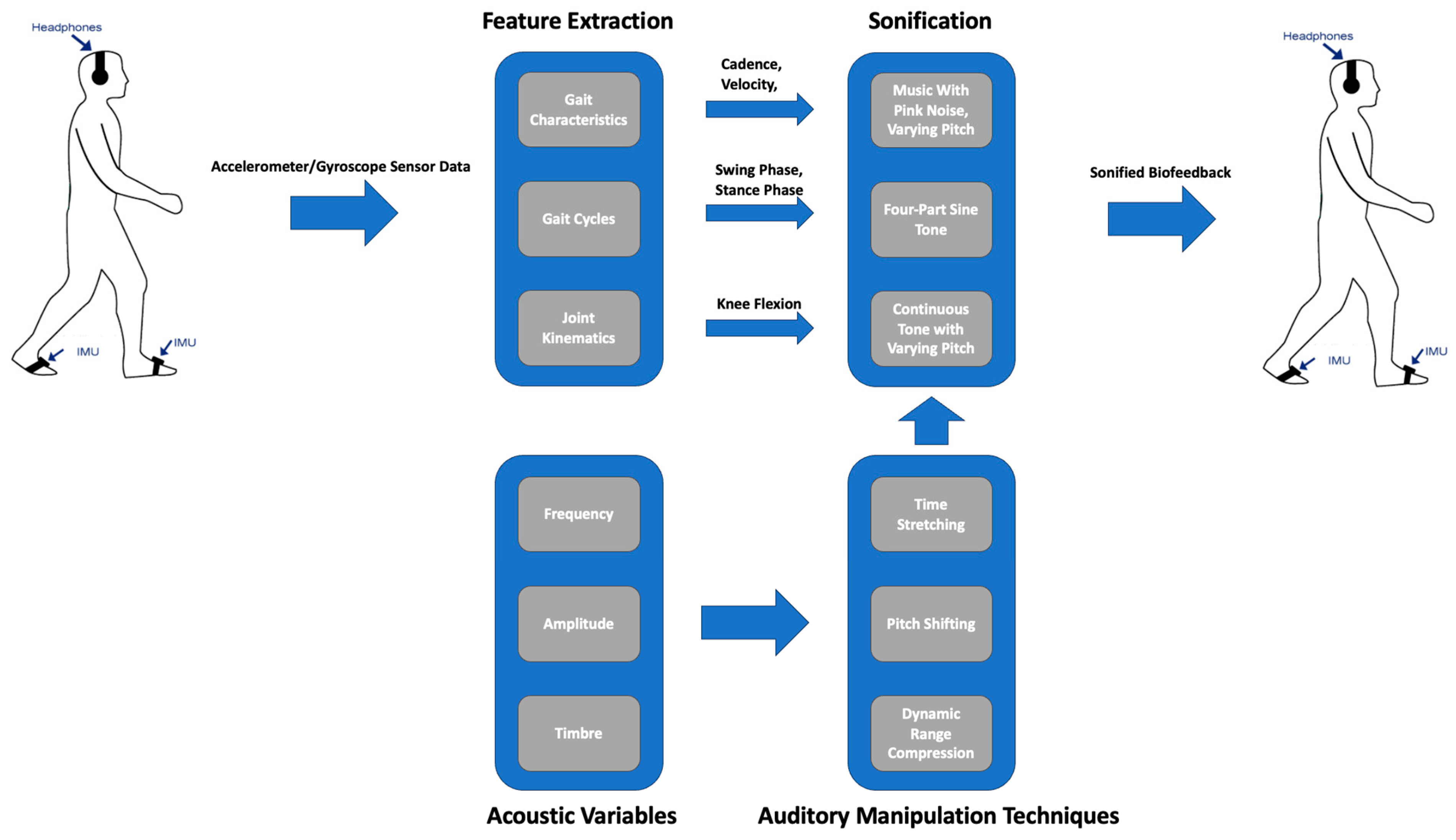
4.1. Acoustic Variables
4.1.1. Frequency
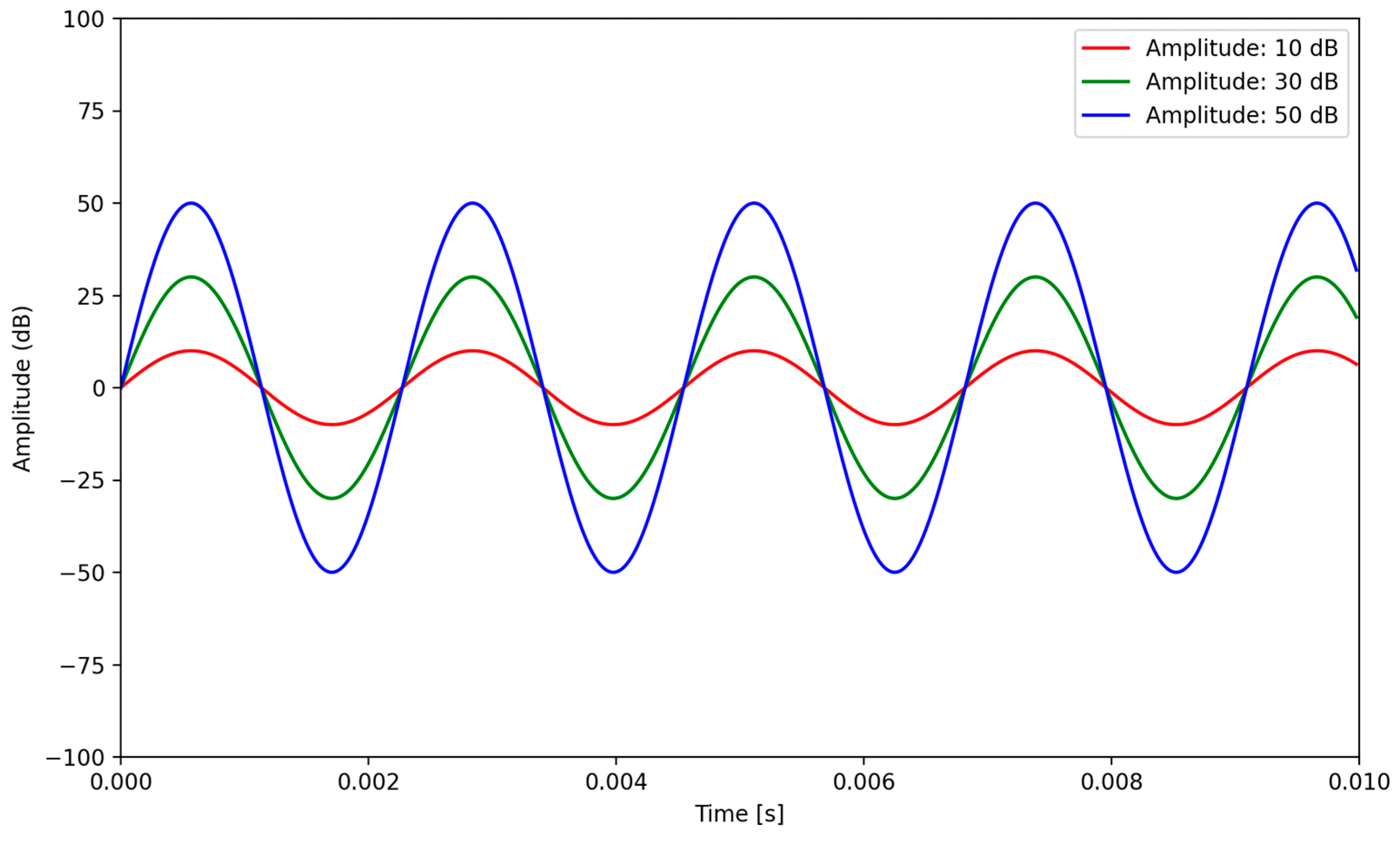
4.1.2. Amplitude
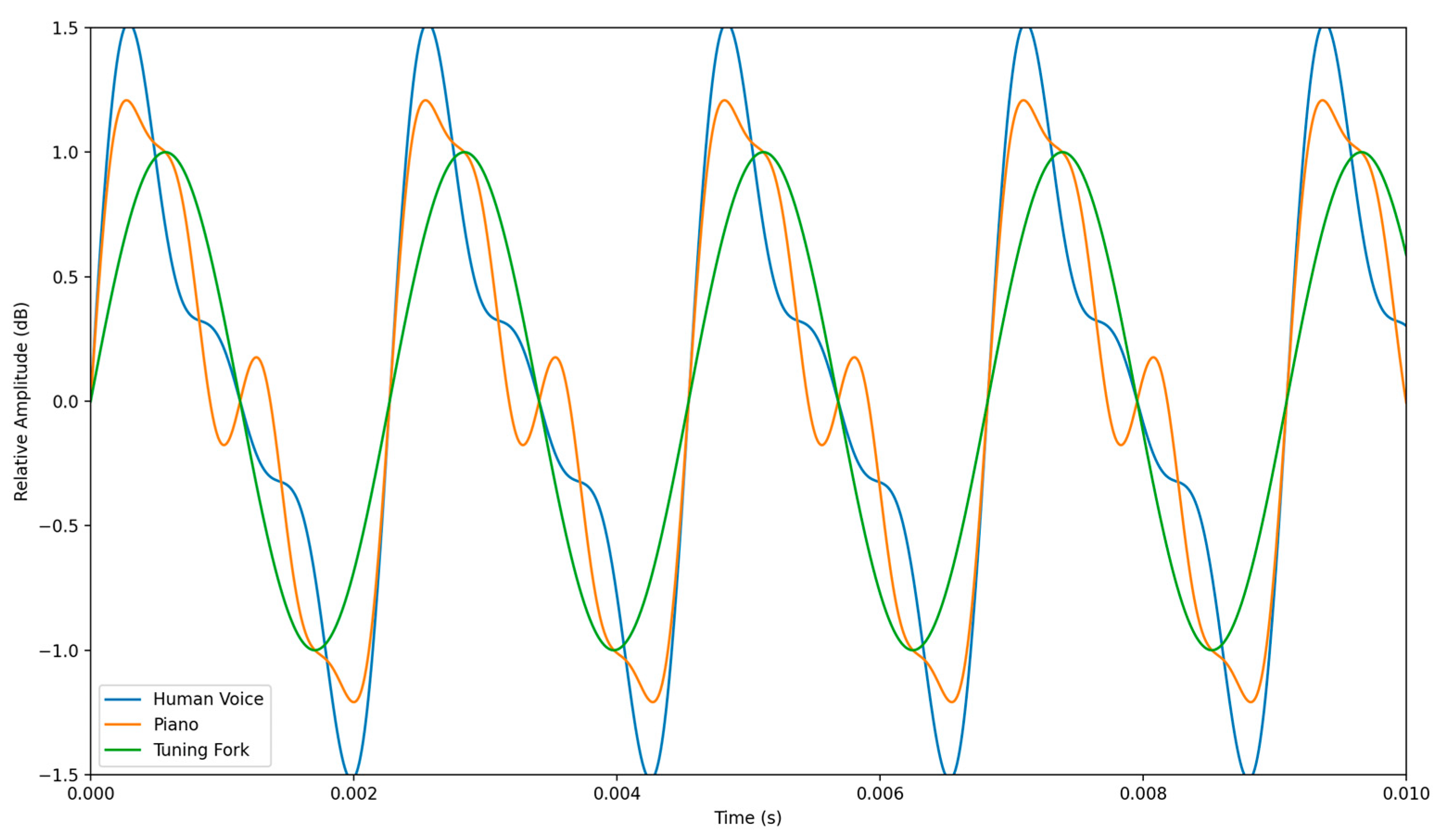
4.1.3. Timbre
4.2. Manipulating Acoustic Variables
4.2.1. Time Stretching
4.2.2. Pitch Shifting
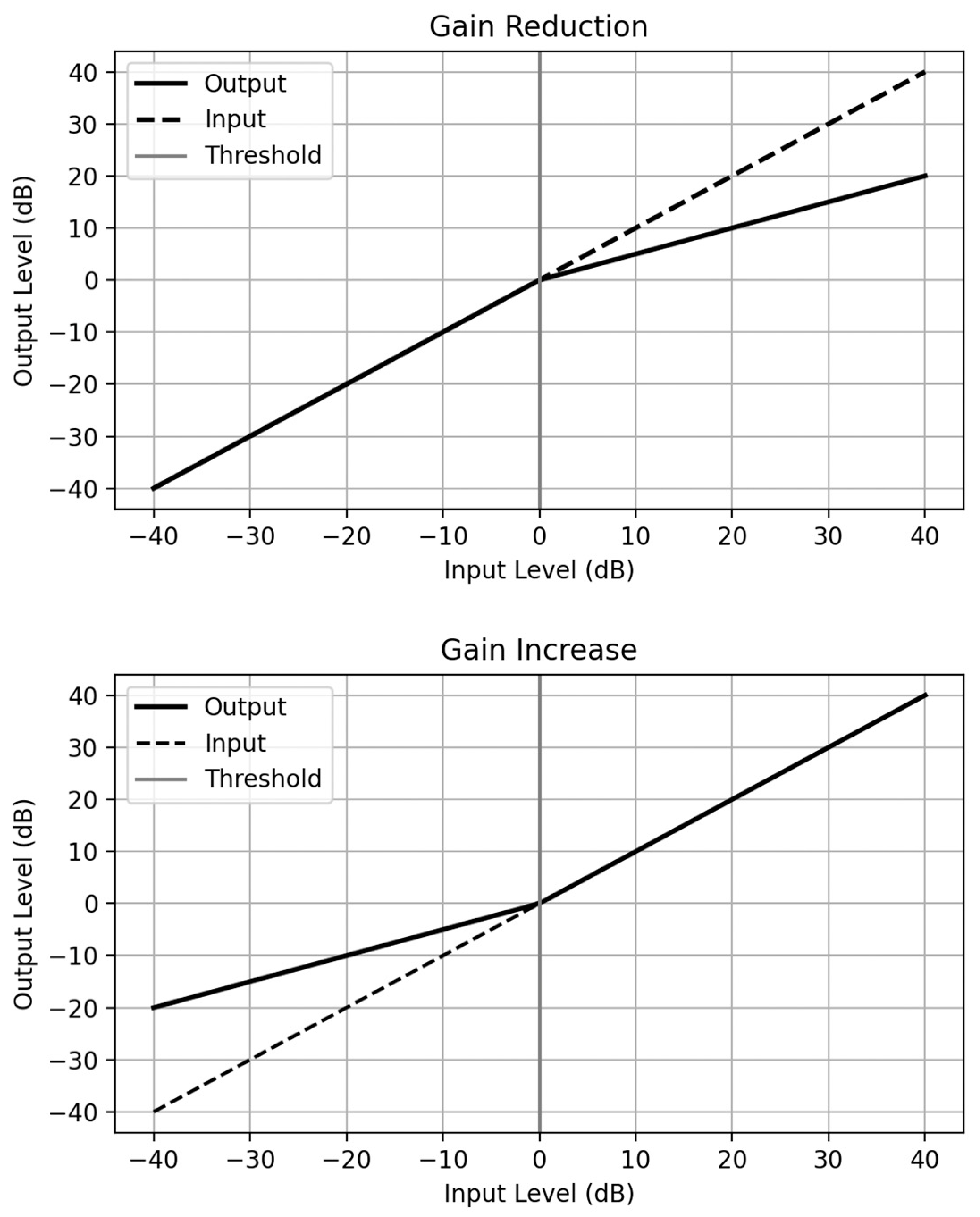
4.2.3. Dynamic Range Compression (DRC)
4.3. Influence of Acoustic Variables on Auditory Rehabilitation
4.3.1. Pitch
4.3.2. Amplitude
4.3.3. Timbre
5. Sonification as a Gait Rehabilitation Tool
5.1. Sonification of Gait Characteristics
5.1.1. Cadence Sonification
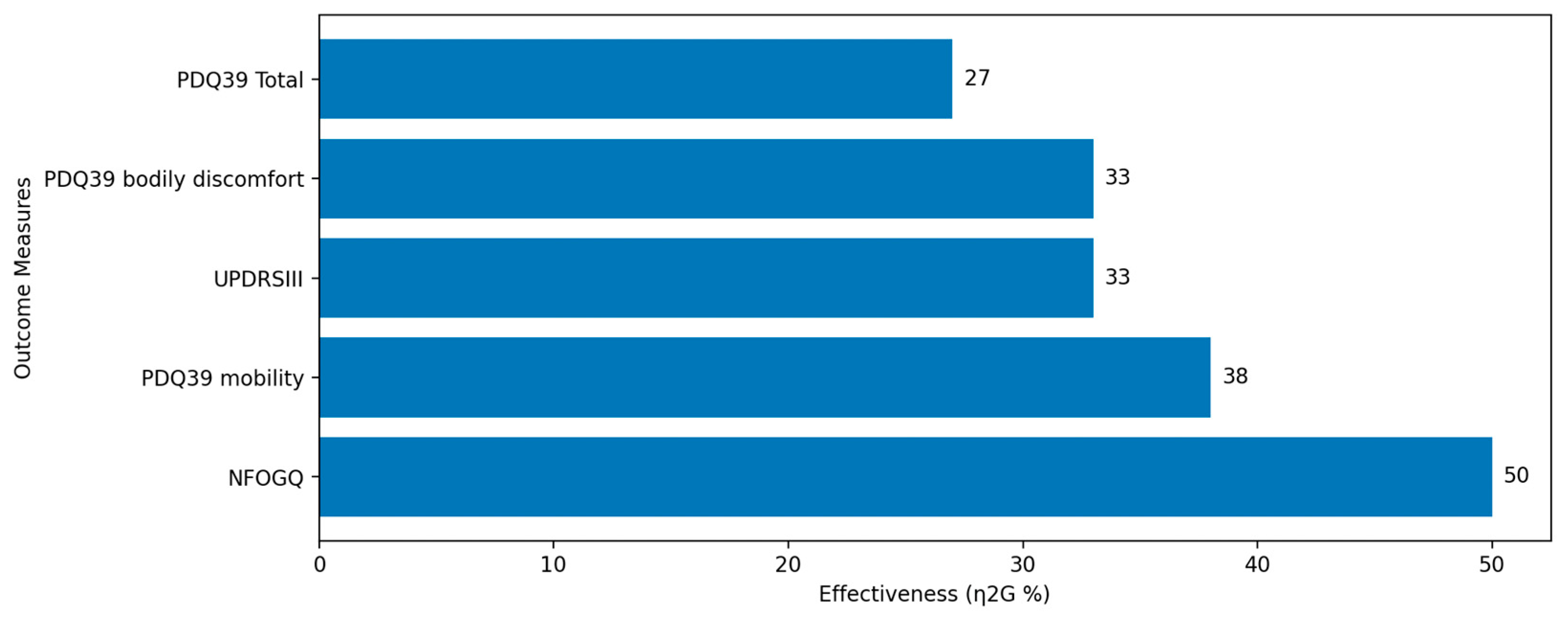
5.1.2. Velocity Sonification
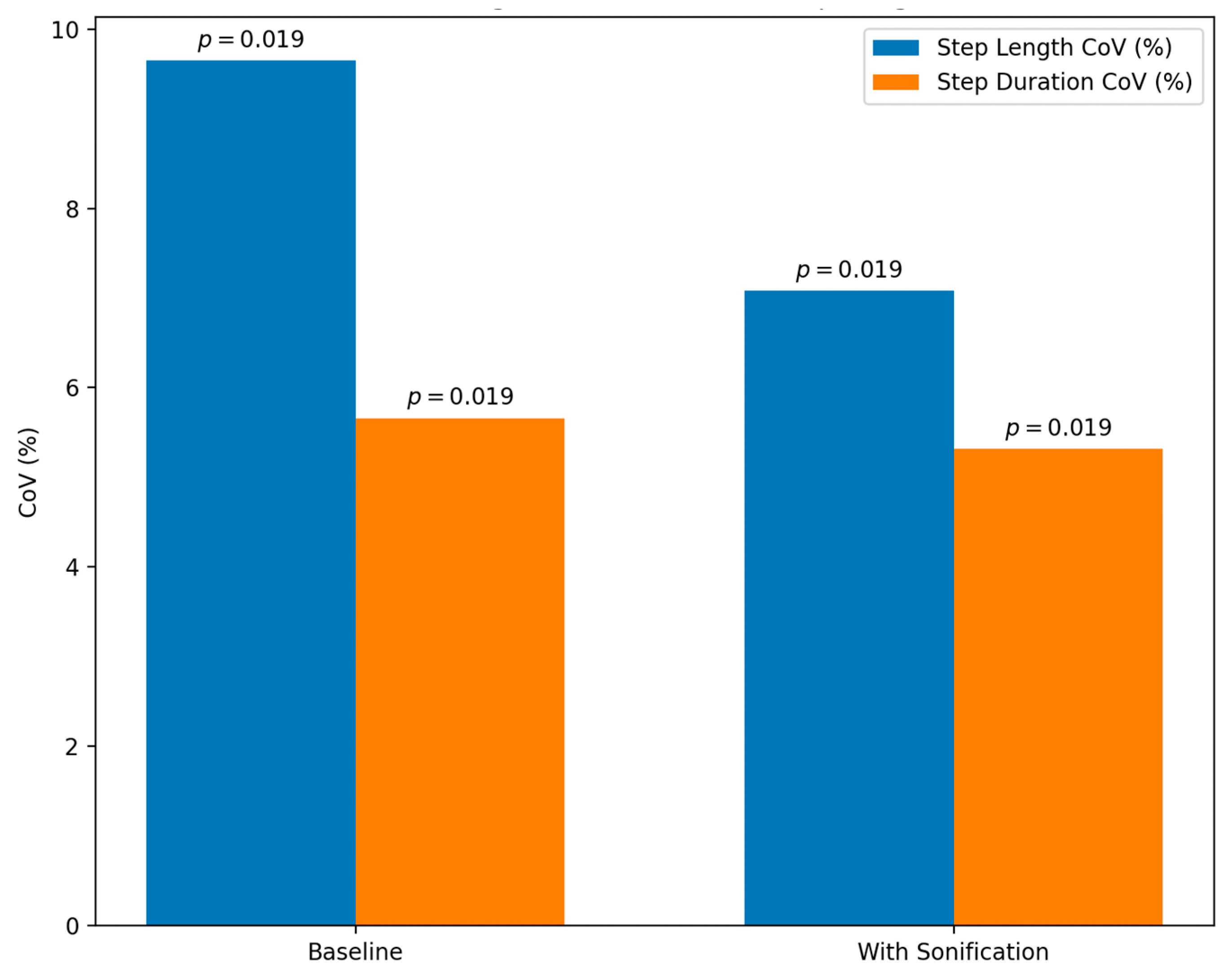
5.2. Sonification of Gait Cycles
5.3. Sonification of Joint Kinematics
5.4. The Unmet Clinical Need
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, A.; Budd, L.; Ison, S.; Timmis, A. Airports and Ageing Passengers: A Study of the UK. Res. Transp. Bus. Manag. 2019, 30, 100380. [Google Scholar] [CrossRef]
- Frank, J. Balance and Mobility Challenges in Older adultsImplications for Preserving Community Mobility. Am. J. Prev. Med. 2003, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Mancini, M. Objective Biomarkers of Balance and Gait for Parkinson’s Disease Using Body-Worn Sensors: Balance and Gait Biomarkers. Mov. Disord. 2013, 28, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.P.; Harvey, A.H. Fall Injuries in the Elderly. Clin. Geriatr. Med. 1985, 1, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Trombini-Souza, F.; Nogueira, R.T.d.S.A.; Serafim, A.C.B.; Lima, T.M.M.d.; Xavier, M.K.A.; Perracini, M.R.; de Araújo, R.C.; Sacco, I.C.; Nascimento, M.d.M. Concern About Falling, Confidence in Balance, Quality of Life, and Depression Symptoms in Community-Dwelling Older Adults After a 24-Week Dual-Task Training With Variable and Fixed Priority: A Randomized Controlled Trial. Res. Aging 2022, 44, 658–668. [Google Scholar] [CrossRef]
- Rantanen, T. Promoting Mobility in Older People. J. Prev. Med. Public Health 2013, 46, S50–S54. [Google Scholar] [CrossRef]
- Palacios-Navarro, G.; Albiol-Pérez, S.; García-Magariño García, I. Effects of Sensory Cueing in Virtual Motor Rehabilitation. A Review. J. Biomed. Inform. 2016, 60, 49–57. [Google Scholar] [CrossRef]
- Spaulding, S.J.; Barber, B.; Colby, M.; Cormack, B.; Mick, T.; Jenkins, M.E. Cueing and Gait Improvement Among People With Parkinson’s Disease: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2013, 94, 562–570. [Google Scholar] [CrossRef]
- Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. [Google Scholar] [CrossRef]
- Fiorente, N.; Calabrò, R.S. Music in Parkinson’s Disease Rehabilitation: Are We Heading in the Right Direction? Innov. Clin. Neurosci. 2023, 20, 11–13. [Google Scholar]
- Hermann, T.; Hunt, A.; Neuhoff, J.G. (Eds.) The Sonification Handbook; Logos Verlag: Berlin, Germany, 2011; ISBN 978-3-8325-2819-5. [Google Scholar]
- Ballora, M.; Pennycook, B.; Ivanov, P.C.; Glass, L.; Goldberger, A.L. Heart Rate Sonification: A New Approach to Medical Diagnosis. Leonardo 2004, 37, 41–46. [Google Scholar] [CrossRef]
- Blanco, A.L.A.; Grautoff, S.; Hermann, T. CardioSounds: A Portable System to Sonify ECG Rhythm Disturbances in Real-Time. In Proceedings of the 24th International Conference on Auditory Display—ICAD 2018, Houghton, MI, USA, 10–15 June 2018; The International Community for Auditory Display: Houghton, MI, USA, 2018; pp. 20–27. [Google Scholar]
- Sanderson, P.; Shek, V.; Watson, M. The Effect of Music on Monitoring a Simulated Anaesthetised Patient with Sonfication. In Proceedings of the 2004 Annual Conference of the Computer-Human Interaction Special Interest Group of the Human Factors and Ergonomics Society of Australia (OzCHI 2004), Wollongong, Australia, 22–24 November 2004. [Google Scholar]
- Özcan, E.; Chou, C.; Bogers, K.; van der Helm, A. Caretunes: 18th Sound and Music Computing Conference, SMC 2021. In Proceedings of the SMC 2021—18th Sound and Music Computing Conference, Virtual, 29 June–1 July 2021; pp. 276–283. [Google Scholar] [CrossRef]
- Särkämö, T.; Altenmüller, E.; Rodríguez-Fornells, A.; Peretz, I. Editorial: Music, Brain, and Rehabilitation: Emerging Therapeutic Applications and Potential Neural Mechanisms. Front. Hum. Neurosci. 2016, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, V.; Maes, P.-J.; Van Den Berghe, P.; De Clercq, D.; De Bie, T.; Leman, M. A Biofeedback Music-Sonification System for Gait Retraining. In Proceedings of the 5th International Conference on Movement and Computing, Genoa, Italy, 28–30 June 2018; pp. 1–5. [Google Scholar]
- Rodger, M.W.M.; Young, W.R.; Craig, C.M. Synthesis of Walking Sounds for Alleviating Gait Disturbances in Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Nown, T.H.; Upadhyay, P.; Kerr, A.; Andonovic, I.; Tachtatzis, C.; Grealy, M.A. A Mapping Review of Real-Time Movement Sonification Systems for Movement Rehabilitation. IEEE Rev. Biomed. Eng. 2023, 16, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Van der Marck, M.A.; Klok, M.P.C.; Okun, M.S.; Giladi, N.; Munneke, M.; Bloem, B.R. Consensus-Based Clinical Practice Recommendations for the Examination and Management of Falls in Patients with Parkinson’s Disease. Park. Relat. Disord. 2014, 20, 360–369. [Google Scholar] [CrossRef]
- Van Uden, C.J.; Besser, M.P. Test-Retest Reliability of Temporal and Spatial Gait Characteristics Measured with an Instrumented Walkway System (GAITRite®). BMC Musculoskelet. Disord. 2004, 5, 13. [Google Scholar] [CrossRef]
- Celik, Y.; Stuart, S.; Woo, W.L.; Godfrey, A. Gait Analysis in Neurological Populations: Progression in the Use of Wearables. Med. Eng. Phys. 2021, 87, 9–29. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM&r 2018, 10, S220–S232. [Google Scholar] [CrossRef]
- Young, F.; Mason, R.; Morris, R.E.; Stuart, S.; Godfrey, A. IoT-Enabled Gait Assessment: The Next Step for Habitual Monitoring. Sensors 2023, 23, 4100. [Google Scholar] [CrossRef]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Ballester, B.R.; Verschure, P.F.M.J. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front. Syst. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Belkacem, A.N.; Jamil, N.; Palmer, J.A.; Ouhbi, S.; Chen, C. Brain Computer Interfaces for Improving the Quality of Life of Older Adults and Elderly Patients. Front. Neurosci. 2020, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Persson, U.; Caulfield, B. Biofeedback in Rehabilitation. J. NeuroEng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef]
- Lim, I.; van Wegen, E.; de Goede, C.; Deutekom, M.; Nieuwboer, A.; Willems, A.; Jones, D.; Rochester, L.; Kwakkel, G. Effects of External Rhythmical Cueing on Gait in Patients with Parkinson’s Disease: A Systematic Review. Clin. Rehabil. 2005, 19, 695–713. [Google Scholar] [CrossRef]
- Bella, S.D.; Dotov, D.; Bardy, B.; de Cock, V.C. Individualization of Music-Based Rhythmic Auditory Cueing in Parkinson’s Disease: Rhythmic Auditory Cueing in Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2018, 1423, 308–317. [Google Scholar] [CrossRef]
- Ivkovic, V.; Fisher, S.; Paloski, W.H. Smartphone-Based Tactile Cueing Improves Motor Performance in Parkinson’s Disease. Park. Relat. Disord. 2016, 22, 42–47. [Google Scholar] [CrossRef]
- Barrios, J.A.; Crossley, K.M.; Davis, I.S. Gait Retraining to Reduce the Knee Adduction Moment through Real-Time Visual Feedback of Dynamic Knee Alignment. J. Biomech. 2010, 43, 2208–2213. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Lowenthal, J.; Herman, T.; Gruendlinger, L.; Peretz, C.; Giladi, N. Rhythmic Auditory Stimulation Modulates Gait Variability in Parkinson’s Disease: Effects of RAS on Gait Variability in PD. Eur. J. Neurosci. 2007, 26, 2369–2375. [Google Scholar] [CrossRef]
- Nees, M.; Walker, B. Theory of Sonification. In The Sonification Handbook; Logos Publishing House: Berlin, Germany, 2012; pp. 9–39. ISBN 978-3-8325-2819-5. [Google Scholar]
- Sawe, N.; Chafe, C.; Treviño, J. Using Data Sonification to Overcome Science Literacy, Numeracy, and Visualization Barriers in Science Communication. Front. Commun. 2020, 5, 46. [Google Scholar] [CrossRef]
- Vickers, P.; Alty, J.L. Using Music to Communicate Computing Information. Interact. Comput. 2002, 14, 435–456. [Google Scholar] [CrossRef]
- Franklin, K.M.; Roberts, J.C. Pie Chart Sonification. In Proceedings of the Seventh International Conference on Information Visualization, London, UK, 18 July 2003; pp. 4–9. [Google Scholar]
- Vickers, P. Sonification for Process Monitoring. In The Sonification Handbook; Logos Verlag: Berlin, Germany, 2011. [Google Scholar]
- Torres, A.V.; Kluckner, V.; Franinovic, K. Development of a Sonification Method to Enhance Gait Rehabilitation. In Proceedings of the ISon 2013, Erlangen, Germany, 10 December 2013; pp. 37–43. [Google Scholar]
- Gray PhD, L. Properties of Sound. J. Perinatol. 2000, 20, S6–S11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vickers, P. Sonification and Music, Music and Sonification. In The Routledge Companion to Sounding Art; Routledge: London, UK, 2016. [Google Scholar]
- Dubus, G.; Bresin, R. A Systematic Review of Mapping Strategies for the Sonification of Physical Quantities. PLoS ONE 2013, 8, e82491. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, S.A.; Podos, J.; Nemeth, E.; Goller, F.; Brumm, H. On the Relationship between, and Measurement of, Amplitude and Frequency in Birdsong. Anim. Behav. 2012, 84, e1–e9. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Holt, G. Sonication Amplitude and Processing Time Influence the Cellulose Nanocrystals Morphology and Dispersion. Nanocomposites 2020, 6, 41–46. [Google Scholar] [CrossRef]
- Siedenburg, K.; Saitis, C.; McAdams, S. The Present, Past, and Future of Timbre Research. In Timbre: Acoustics, Perception, and Cognition; Siedenburg, K., Saitis, C., McAdams, S., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research; Springer International Publishing: Cham, Switzerland, 2019; Volume 69, pp. 1–19. ISBN 978-3-030-14831-7. [Google Scholar]
- Vickers, P.; Hogg, B. Sonification Abstraite/Sonification Concr\`ete: An “Aesthetic Perspective Space” for Classifying Auditory Displays in the Ars Musica Domain. arXiv 2013, arXiv:1311.5426. [Google Scholar]
- Wessel, D. Timbre and the Perceptual Organization of Musical Patterns. J. Acoust. Soc. Am. 1989, 86, S58–S59. [Google Scholar] [CrossRef]
- Cochen De Cock, V.; Dotov, D.; Damm, L.; Lacombe, S.; Ihalainen, P.; Picot, M.C.; Galtier, F.; Lebrun, C.; Giordano, A.; Driss, V.; et al. BeatWalk: Personalized Music-Based Gait Rehabilitation in Parkinson’s Disease. Front. Psychol. 2021, 12, 655121. [Google Scholar] [CrossRef]
- Laroche, J.; Dolson, M. Improved Phase Vocoder Time-Scale Modification of Audio. IEEE Trans. Speech Audio Process. 1999, 7, 323–332. [Google Scholar] [CrossRef]
- Sklar, A.G. A Wavelet-Based Pitch-Shifting Method. Unpublished. 2006. Available online: https://www.semanticscholar.org (accessed on 1 June 2023).
- Hjortkjær, J.; Walther-Hansen, M. Perceptual Effects of Dynamic Range Compression in Popular Music Recordings. J. Audio Eng. Soc. 2014, 62, 37–41. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Rutledge, J.C. Multichannel Dynamic Range Compression for Music Signals. In Proceedings of the 1996 IEEE International Conference on Acoustics, Speech, and Signal Processing Conference Proceedings, Atlanta, GA, USA, 9 May 1996; Volume 2, pp. 1013–1016. [Google Scholar]
- Ley-Flores, J.; Alshami, E.; Singh, A.; Bevilacqua, F.; Bianchi-Berthouze, N.; Deroy, O.; Tajadura-Jiménez, A. Effects of Pitch and Musical Sounds on Body-Representations When Moving with Sound. Sci. Rep. 2022, 12, 2676. [Google Scholar] [CrossRef] [PubMed]
- Avanzino, L.; Lagravinese, G.; Abbruzzese, G.; Pelosin, E. Relationships between Gait and Emotion in Parkinson’s Disease: A Narrative Review. Gait Posture 2018, 65, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gómez-González, J.; Martín-Casas, P.; Cano-de-la-Cuerda, R. Effects of Auditory Cues on Gait Initiation and Turning in Patients with Parkinson’s Disease. Neurología 2019, 34, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Del-Olmo, M.; Bello, O.; Lopez-Alonso, V.; Andrés Sanchez, J.; Santos-García, D.; Valls-Solé, J. The Effects of Auditory Startle and Nonstartle Stimuli on Step Initiation in Parkinson’s Disease. Mov. Disord. 2012, 27, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yoo, G.E.; Shin, Y.; Cho, S. Gait Training for Adults with Cerebral Palsy Following Harmonic Modification in Rhythmic Auditory Stimulation. Ann. N. Y. Acad. Sci. 2020, 1473, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mezzarobba, S.; Grassi, M.; Pellegrini, L.; Catalan, M.; Kruger, B.; Furlanis, G.; Manganotti, P.; Bernardis, P. Action Observation Plus Sonification. A Novel Therapeutic Protocol for Parkinson’s Patient with Freezing of Gait. Front. Neurol. 2018, 8, 723. [Google Scholar] [CrossRef]
- Motion Capture—Mocap. Available online: https://www.qualisys.com/ (accessed on 2 June 2023).
- Kharb, A.; Saini, V.; Jain, Y.K.; Dhiman, S. A Review of Gait Cycle and Its Parameters. IJCEM Int. J. Comput. Eng. Manag. 2011, 13, 78–83. [Google Scholar]
- Linnhoff, D.; Ploigt, R.; Mattes, K. Sofigait—A Wireless Inertial Sensor-Based Gait Sonification System. Sensors 2022, 22, 8782. [Google Scholar] [CrossRef]
- Morris, R.; Hickey, A.; Del Din, S.; Godfrey, A.; Lord, S.; Rochester, L. A Model of Free-Living Gait: A Factor Analysis in Parkinson’s Disease. Gait Posture 2017, 52, 68–71. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Coleman, S.; Yarnall, A.; Burn, D.; Rochester, L. Cognition and Gait Show a Selective Pattern of Association Dominated by Phenotype in Incident Parkinson’s Disease. Front. Aging Neurosci. 2014, 6, 249. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J. Gerontol. Ser. A 2013, 68, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R.; Xue, X. Quantitative Gait Dysfunction and Risk of Cognitive Decline and Dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Lord, S.; Lawson, R.A.; Coleman, S.; Galna, B.; Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; Burn, D.J.; Rochester, L. Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease. J. Gerontol. Ser. A 2017, 72, 1656–1662. [Google Scholar] [CrossRef] [PubMed]



| Attribute | Soni 1.1 | Soni 1.2 | Soni 2.1 | Soni 2.2 |
|---|---|---|---|---|
| Phase of Action | Swing Phase | Swing Phase | Stance Phase | Stance Phase |
| Tone Type | Continuous Sine | Continuous Sine | Continuous Sine | Continuous Sine |
| Angle Range | 35° to −45° to +90° | 35° to −45° to +90° | Up to 35° | Up to 35° |
| Frequency Range | 220 Hz to 1760 Hz | Lower by half an octave (165 Hz to 1320 Hz) | 220 Hz to 1760 Hz | Lower by half an octave (165 Hz to 1320 Hz) |
| Approach | Method | Population | Main Outcomes |
|---|---|---|---|
| Cadence Sonification [17]. | A music-sonification system with pink noise, runs under various conditions. Real-time adjustment based on the runner’s cadence. | 10 healthy participants | Pink noise feedback significantly better than verbal instruction for altering cadence. Effective for both increasing and decreasing cadence. |
| Velocity Sonification [59]. | Action Observation plus Sonification (AOS) using visual and enhanced auditory cues. | 37 PD patients | Reduced severity and duration of FoG. Improved motor function and daily activity comfort. Lasting benefits observed. |
| Sonification of Swing Phase [18]. | Real-time sonification of the swing phase of the gait cycle with specific pitch tones. | 9 PD patients | Statistically significant reduction in the CoV for step length. Improved gait consistency. |
| Sonification of Knee Flexion [62]. | Prototype system Sofigait for sonification during walking. Different versions of sonification techniques. | 24 healthy individuals | Lower pitch (Soni 1.2) was found to be more pleasant. Limited data on the effectiveness in reducing gait asymmetry. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wall, C.; McMeekin, P.; Walker, R.; Hetherington, V.; Graham, L.; Godfrey, A. Sonification for Personalised Gait Intervention. Sensors 2024, 24, 65. https://doi.org/10.3390/s24010065
Wall C, McMeekin P, Walker R, Hetherington V, Graham L, Godfrey A. Sonification for Personalised Gait Intervention. Sensors. 2024; 24(1):65. https://doi.org/10.3390/s24010065
Chicago/Turabian StyleWall, Conor, Peter McMeekin, Richard Walker, Victoria Hetherington, Lisa Graham, and Alan Godfrey. 2024. "Sonification for Personalised Gait Intervention" Sensors 24, no. 1: 65. https://doi.org/10.3390/s24010065
APA StyleWall, C., McMeekin, P., Walker, R., Hetherington, V., Graham, L., & Godfrey, A. (2024). Sonification for Personalised Gait Intervention. Sensors, 24(1), 65. https://doi.org/10.3390/s24010065








