A New Boron–Rhodamine-Containing Carboxylic Acid as a Sugar Chemosensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Synthesis of BRhoC
2.4. Spectral Measurement of the pH and Sugar Response of BRhoC
3. Results and Discussion
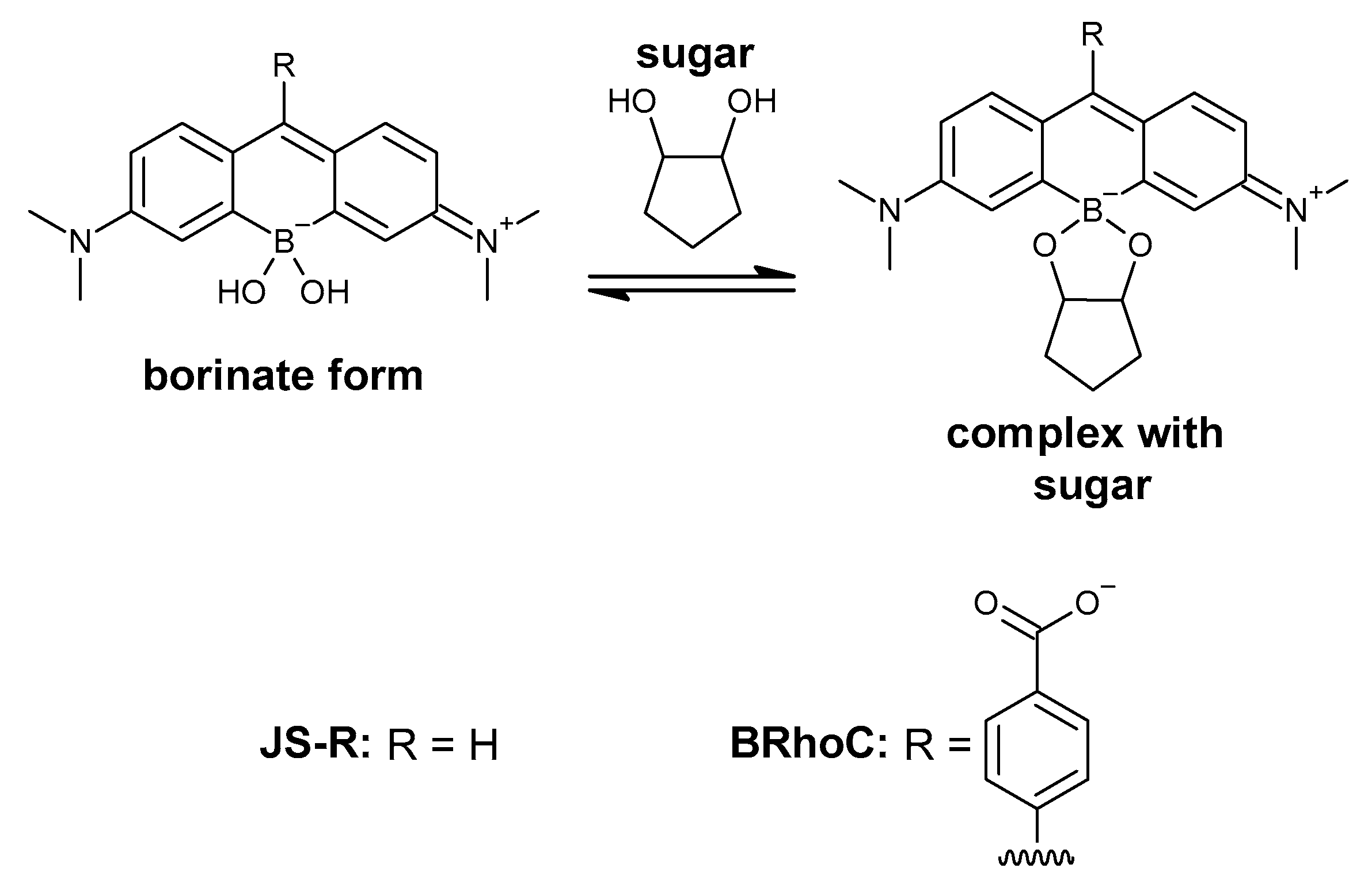
3.1. Synthesis of BRhoC
3.2. Optical Properties of BRhoC
3.3. pH Response of BRhoC
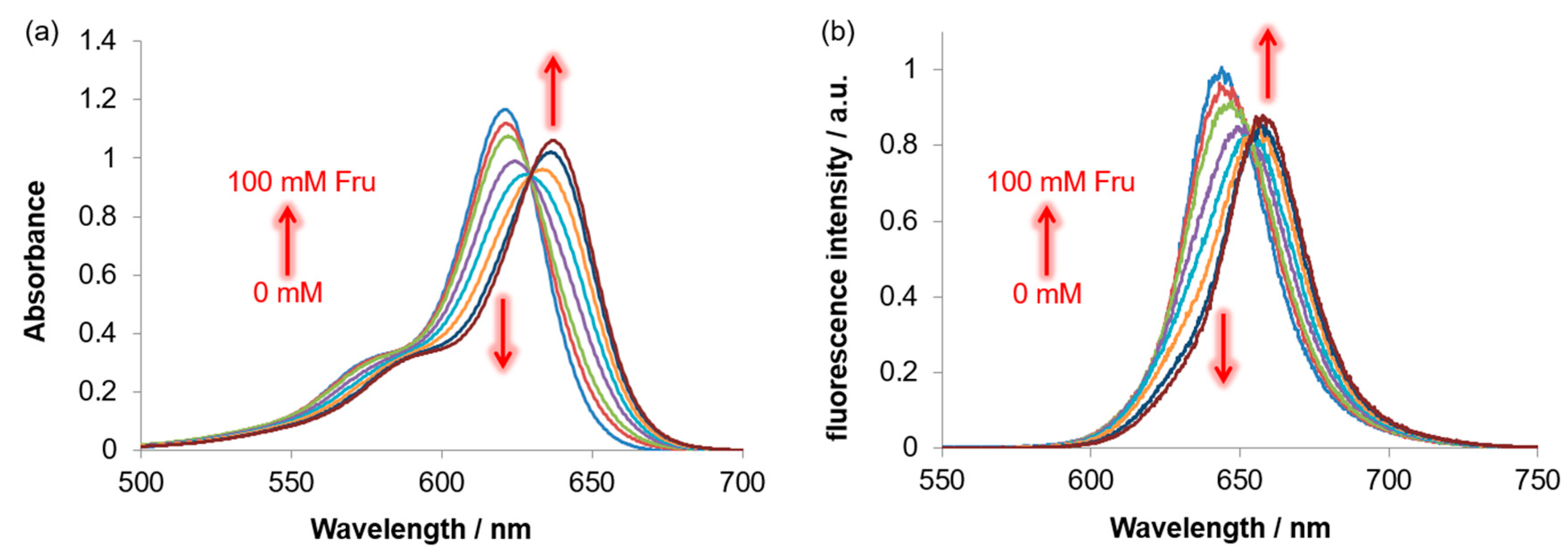
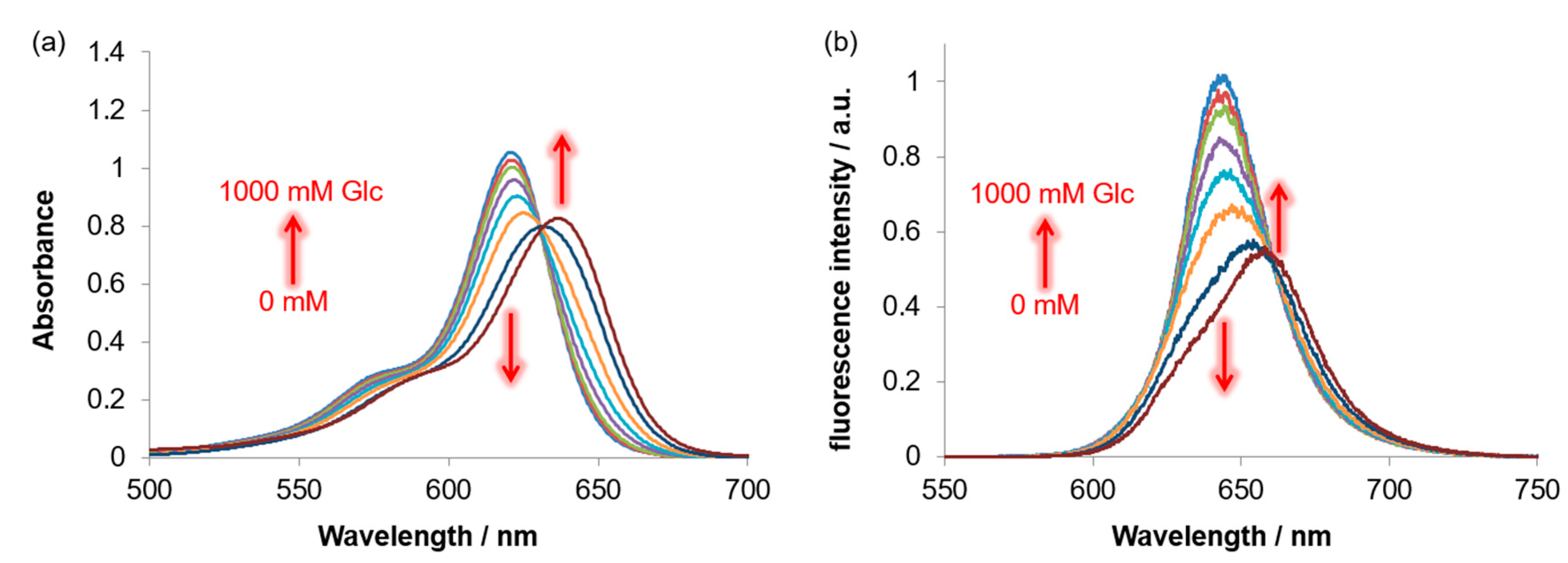
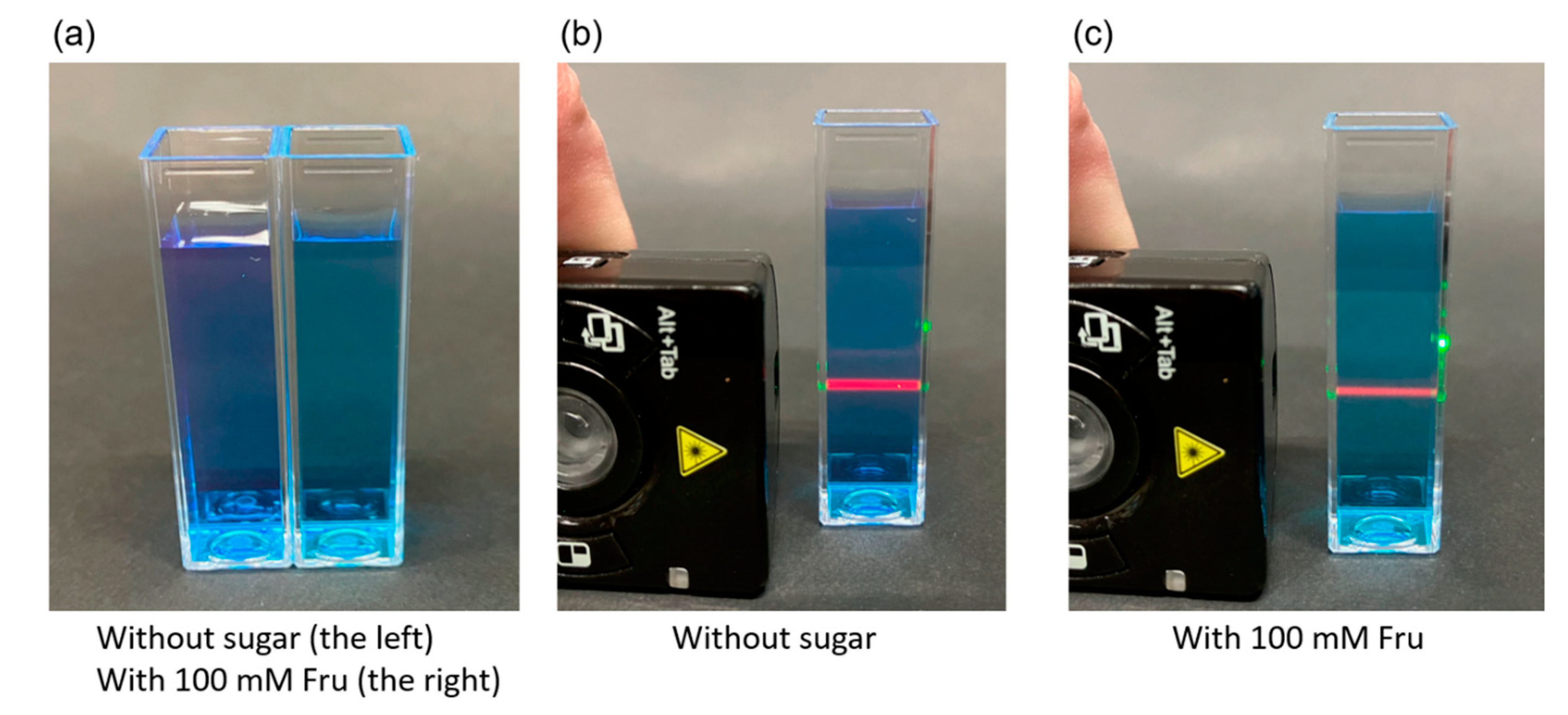
3.4. Response of BRhoC to Sugar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Zhai, W.; Fossey, J.S.; James, T.D. Boronic acids for fluorescence imaging of carbohydrates. Chem. Commun. 2016, 52, 3456–3469. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chapin, B.M.; Metola, P.; Collins, B.; Wang, B.; James, T.D.; Anslyn, E.V. The mechanisms of boronate ester formation and fluorescent turn-on in ortho-aminomethylphenylboronic acids. Nat. Chem. 2019, 11, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.T.; Kedge, J.L.; Fossey, J.S. Molecular boronic acid-based saccharide sensors. ACS Sens. 2021, 6, 1508–1528. [Google Scholar] [CrossRef] [PubMed]
- Tsuchido, Y.; Fujiwara, S.; Hashimoto, T.; Hayashita, T. Development of supramolecular saccharide sensors based on cyclodextrin complexes and self-assembling systems. Chem. Pharm. Bull. 2017, 65, 318–325. [Google Scholar] [CrossRef]
- Bian, Z.; Liu, A.; Li, Y.; Fang, G.; Yao, Q.; Zhang, G.; Wu, Z. Boronic acid sensors with double recognition sites: A review. Analyst 2020, 145, 719–744. [Google Scholar] [CrossRef]
- Mortellaro, M.; DeHennis, A. Performance characterization of an abiotic and fluorescent-based continuous glucose monitoring system in patients with type 1 diabetes. Biosens. Bioelectron. 2014, 61, 227–231. [Google Scholar] [CrossRef]
- Crane, B.C.; Barwell, N.P.; Gopal, P.; Gopichand, M.; Higgs, T.; James, T.D.; Jones, C.M.; Mackenzie, A.; Mulavisala, K.P.; Paterson, W. The development of a continuous intravascular glucose monitoring sensor. J. Diabetes Sci. Technol. 2015, 9, 751–761. [Google Scholar] [CrossRef]
- Davis, A.P.; Wareham, R.S. A tricyclic polyamide receptor for carbohydrates in organic media. Angew. Chem.-Int. Ed. 1998, 37, 2270–2273. [Google Scholar] [CrossRef]
- Davis, A.P.; Wareham, R.S. Carbohydrate recognition through noncovalent interactions: A challenge for biomimetic and supramolecular chemistry. Angew. Chem.-Int. Ed. 1999, 38, 2978–2996. [Google Scholar] [CrossRef]
- Sun, X.; James, T.D. Glucose sensing in supramolecular chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar] [CrossRef]
- Egawa, Y.; Miki, R.; Seki, T. Colorimetric sugar sensing using boronic acid-substituted azobenzenes. Materials (Basel) 2014, 7, 1201–1220. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, N.; Egawa, Y.; Miki, R.; Fujihara, T.; Ishimaru, Y.; Seki, T. A red fluorophore comprising a borinate-containing xanthene analogue as a polyol sensor. Org. Biomol. Chem. 2016, 14, 10031–10036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lesiak, L.; Lai, R.; Beck, J.R.; Zhao, J.; Elowsky, C.G.; Li, H.; Stains, C.I. Chemoselective alteration of fluorophore scaffolds as a strategy for the development of ratiometric chemodosimeters. Angew. Chem.-Int. Ed. 2017, 56, 4197–4200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, T.; Lin, X.; Fan, M.; Zho, Y.; Li, N.; Cui, X. Boron-substituted rhodamine for ratiometric monitoring dynamic of H2O2 and HOCl in vivo. Sens. Actuators B Chem. 2021, 331, 129411. [Google Scholar] [CrossRef]
- Mottram, L.F.; Forbes, S.; Ackley, B.D.; Peterson, B.R. Hydrophobic analogues of rhodamine B and rhodamine 101: Potent fluorescent probes of mitochondria in living C. elegans. Beilstein J. Org. Chem. 2012, 8, 2156–2165. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, F.; Huang, Y.; Kong, D.; Ye, Q.; Xu, J.; Chen, L. Rhodamine-based ratiometric fluorescent probes based on excitation energy transfer mechanisms: Construction and applications in ratiometric sensing. RSC Adv. 2016, 6, 50732–50760. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.Y.; Liu, J.; Dong, D. Recent developments in rhodamine salicylidene hydrazone chemosensors. Anal. Methods 2016, 8, 2863–2871. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Ma, W.; Lu, R.; Zhou, W.; Gao, H. Recent developments in rhodamine-based chemosensors: A review of the years 2018–2022. Chemosensors 2022, 10, 399. [Google Scholar] [CrossRef]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer. ACS Chem. Biol. 2011, 6, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, T.; Nagano, T.; Hanaoka, K. Silicon-substituted xanthene dyes and their unique photophysical properties for fluorescent probes. Chem. Asian J. 2017, 12, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Xu, Z. Heteroatom-substituted rhodamine dyes: Structure and spectroscopic properties. Chin. Chem. Lett. 2018, 30, 1667–1681. [Google Scholar] [CrossRef]
- Brøndsted, F.; Stains, C.I. Heteroatom-substituted xanthene fluorophores enter the shortwave-infrared region. Photochem. Photobiol. 2022, 98, 400–403. [Google Scholar] [CrossRef]
- Khan, Z.; Sekar, N. Far-red to NIR emitting xanthene-based fluorophores. Dye. Pigment. 2022, 208, 110735. [Google Scholar] [CrossRef]
- Fu, M.; Xiao, Y.; Qian, X.; Zhao, D.; Xu, Y. A design concept of long-wavelength fluorescent analogs of rhodamine dyes: Replacement of oxygen with silicon atom. Chem. Commun. 2008, 15, 1780. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Q.J.; Hu, H.G.; Yu, S.C.; Liu, X.; Liu, L.; Wu, Q.Y. Spirolactonized Si-rhodamine: A novel NIR fluorophore utilized as a platform to construct Si-rhodamine-based probes. Chem. Commun. 2012, 48, 8781–8783. [Google Scholar] [CrossRef]
- Kim, E.; Yang, K.S.; Giedt, R.J.; Weissleder, R. Red Si–rhodamine drug conjugates enable imaging in GFP cells. Chem. Commun. 2014, 50, 4504. [Google Scholar] [CrossRef]
- Kushida, Y.; Nagano, T.; Hanaoka, K. Silicon-substituted xanthene dyes and their applications in bioimaging. Analyst 2015, 140, 685–695. [Google Scholar] [CrossRef]
- Nie, H.; Qiao, L.; Yang, W.; Guo, B.; Xin, F.; Jing, J.; Zhang, X. UV-assisted synthesis of long-wavelength Si-pyronine fluorescent dyes for real-time and dynamic imaging of glutathione fluctuation in living cells. J. Mater. Chem. B 2016, 4, 4826–4831. [Google Scholar] [CrossRef]
- Grimm, J.B.; Brown, T.A.; Tkachuk, A.N.; Lavis, L.D. General synthetic method for Si-fluoresceins and Si-rhodamines. ACS Cent. Sci. 2017, 3, 975–985. [Google Scholar] [CrossRef]
- Zhou, X.; Lai, R.; Beck, J.R.; Li, H.; Stains, C.I. Nebraska Red: A phosphinate-based near-infrared fluorophore scaffold for chemical biology applications. Chem. Commun. 2016, 52, 12290–12293. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Usuba, J.; Adler, R.A.; Yamaguchi, S. Synthesis of seminaphtho-phospha-fluorescein dyes based on the consecutive arylation of aryldichlorophosphines. Chem. Commun. 2017, 53, 8565–8568. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, M.; Taki, M.; Senda, K.; Sato, Y.; Ariyoshi, T.; Okada, Y.; Kawakami, R.; Imamura, T.; Yamaguchi, S. A highly photostable near-infrared labeling agent based on a phospha-rhodamine for long-term and deep imaging. Angew. Chem.-Int. Ed. 2018, 57, 10137–10141. [Google Scholar] [CrossRef]
- Lin, X.; Fan, M.; Li, N.; Yang, J.; Zhu, H.; Chen, B.; Zhu, J.; Zhang, D.; Wang, T.; Cui, X. Phosphorus-substituted rhodamines for bioimaging of the lysosomal peroxynitrite in vivo. Dye. Pigment. 2022, 201, 110201. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.Q.; Zhang, H.; Shi, H.; Shi, Y.; Guo, W. Sulfone-rhodamines: A new class of near-infrared fluorescent dyes for bioimaging. ACS Appl. Mater. Interfaces 2016, 8, 22953–22962. [Google Scholar] [CrossRef]
- Raliski, B.K.; Mun, D.M.; Miller, E.W. Sulfone rhodamines for voltage imaging. Chem.-Asian J. 2022, 17, e2022009. [Google Scholar] [CrossRef] [PubMed]
- “Seitai-kinou Kanren Kagaku Jikken-hou”; Division of Biofunctional Chemistry, The Chemical Society of Japan, Editor-in-chief: K. Kano, Kagakudojin: Tokyo, Japan, 2003; ISBN 9784759809251.
- Ward, C.J.; Patel, P.; James, T.D. Boronic acid appended azo dyes–colour sensors for saccharides. J. Chem. Soc. Perkin Trans. 1 2002, 2, 462–470. [Google Scholar] [CrossRef]
- Isak, S.J.; Eyring, E.M. Fluorescence quantum yield of cresyl violet in methanol and water as a function of concentration. J. Phys. Chem. 1992, 96, 1738–1742. [Google Scholar] [CrossRef]
- Nowicka-Jankowska, T. Some properties of isosbestic points. J. Inorg. Nucl. Chem. 1971, 33, 2043–2050. [Google Scholar] [CrossRef]
- Lotfy, H.M.; Saleh, S.S.; Hassan, N.Y.; Salem, H. A comparative study of novel spectrophotometric methods based on isosbestic points; Application on a pharmaceutical ternary mixture. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 126, 112–121. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komori, Y.; Sugimoto, S.; Sato, T.; Okawara, H.; Watanabe, R.; Takano, Y.; Kitaoka, S.; Egawa, Y. A New Boron–Rhodamine-Containing Carboxylic Acid as a Sugar Chemosensor. Sensors 2023, 23, 1528. https://doi.org/10.3390/s23031528
Komori Y, Sugimoto S, Sato T, Okawara H, Watanabe R, Takano Y, Kitaoka S, Egawa Y. A New Boron–Rhodamine-Containing Carboxylic Acid as a Sugar Chemosensor. Sensors. 2023; 23(3):1528. https://doi.org/10.3390/s23031528
Chicago/Turabian StyleKomori, Yuta, Shun Sugimoto, Toranosuke Sato, Honoka Okawara, Ryo Watanabe, Yuki Takano, Satoshi Kitaoka, and Yuya Egawa. 2023. "A New Boron–Rhodamine-Containing Carboxylic Acid as a Sugar Chemosensor" Sensors 23, no. 3: 1528. https://doi.org/10.3390/s23031528
APA StyleKomori, Y., Sugimoto, S., Sato, T., Okawara, H., Watanabe, R., Takano, Y., Kitaoka, S., & Egawa, Y. (2023). A New Boron–Rhodamine-Containing Carboxylic Acid as a Sugar Chemosensor. Sensors, 23(3), 1528. https://doi.org/10.3390/s23031528






