A Facile Fluorometric Assay of Orotate Phosphoribosyltransferase Activity Using a Selective Fluorogenic Reaction for Orotic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cell Culturing
2.3. Preparation of Cell Lysate
2.4. Condition of Enzyme Reaction for OPRT
2.5. FL Reaction and Measurement
3. Results and Discussion
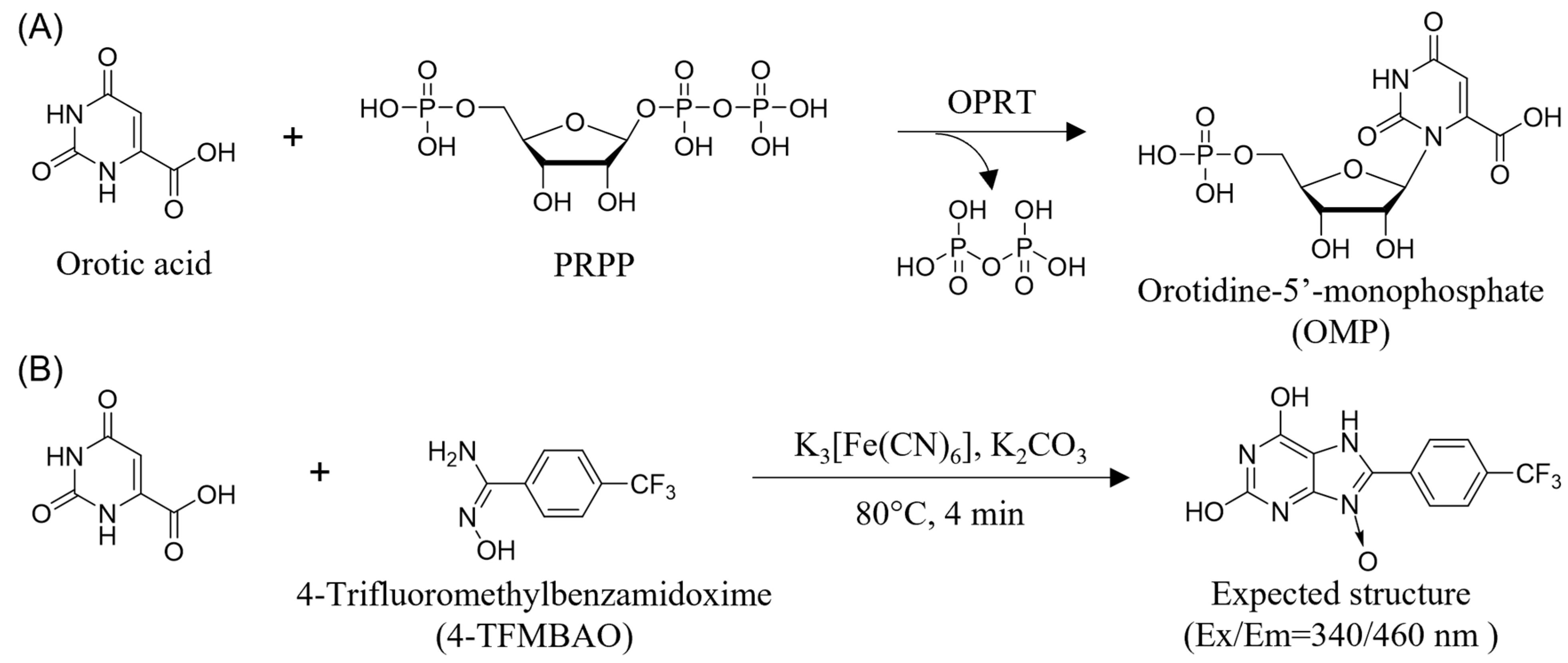
3.1. Principle of the Present Method
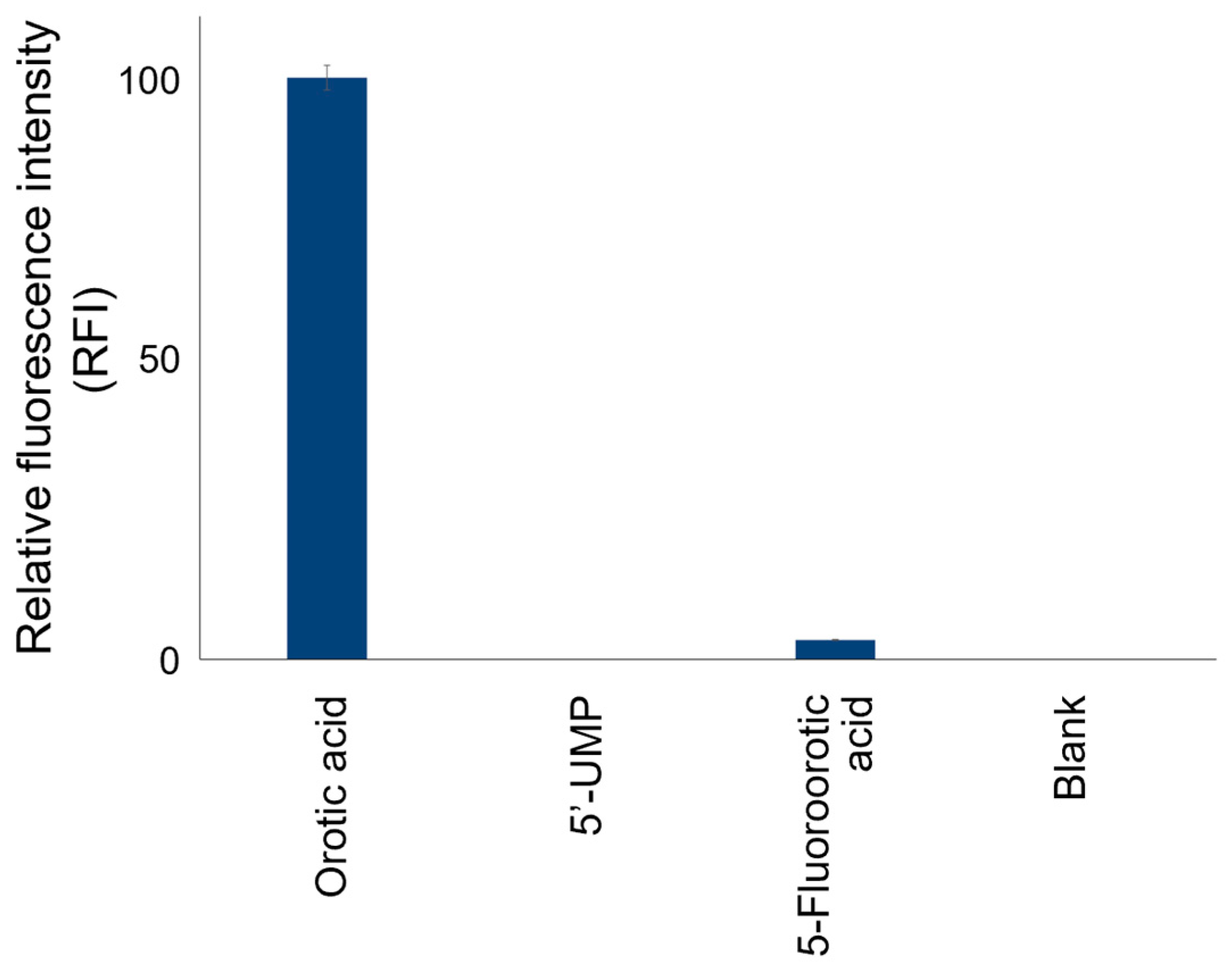
3.2. Detection of OPRT Activity by the Fluorogenic Reaction with 4-TFMBAO
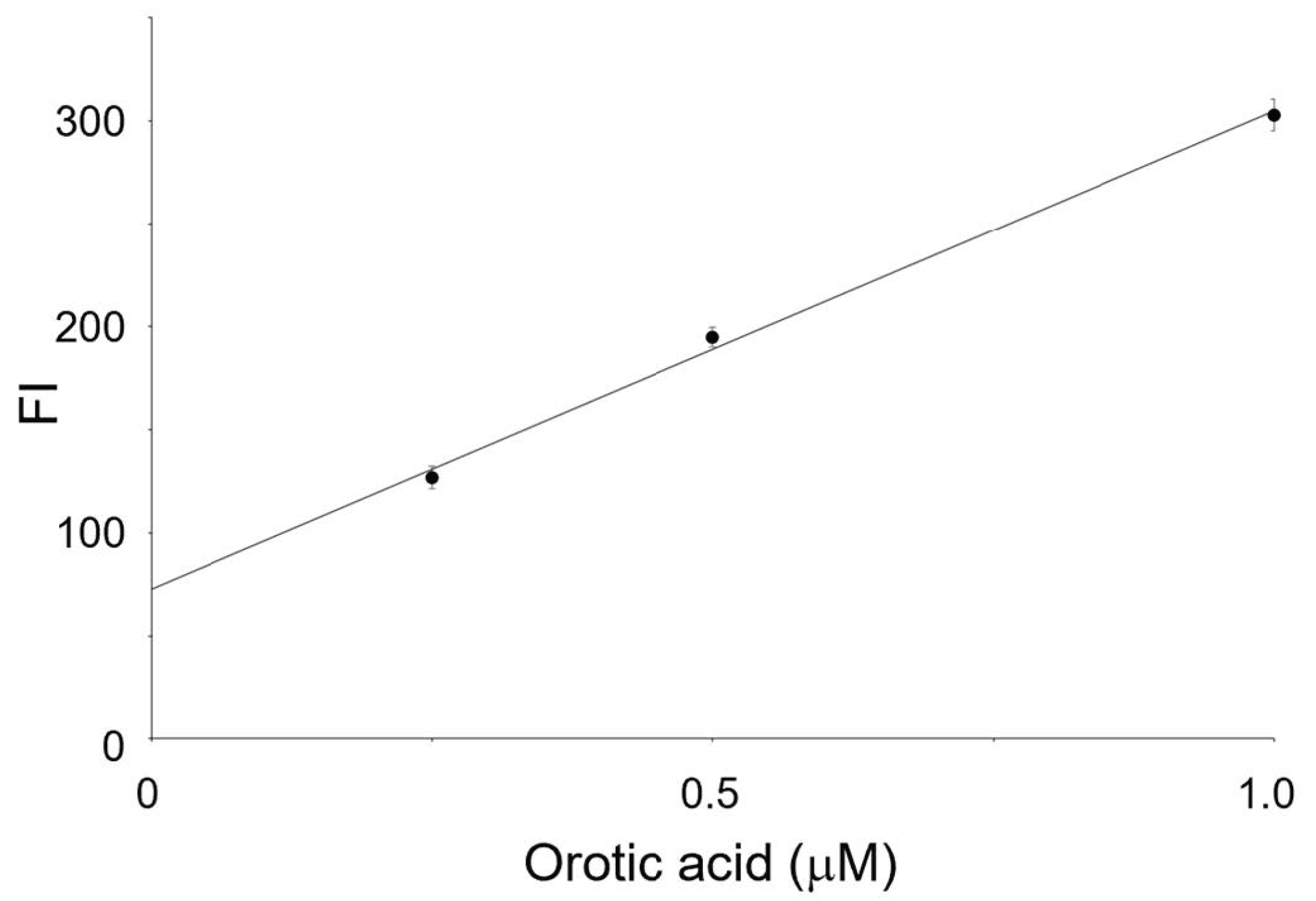
3.3. Optimization of the Substrate Concentration
3.4. Measurement of OPRT Activity in HeLa Cell
3.5. Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Donovan, G.A.; Neuhard, J.J. Pyrimidine metabolism in microorganisms. Bacteriol. Rev. 1970, 34, 278–343. [Google Scholar] [CrossRef] [PubMed]
- Gonza´lez-Segura, L.; Witte, J.F.; McClard, R.W.; Hurley, T.D. Ternary Complex Formation and Induced Asymmetry in Orotate Phosphoribosyltransferase. Biochem. 2007, 46, 14075–14086. [Google Scholar] [CrossRef] [PubMed]
- Musick, W.D.L.; Nyhan, W.L. Structural features of the phosphoribosyltransferases and their relationship to the human deficiency disorders of purine and pyrimidine metabolism. CRC Crit. Rev. Biochem. 1981, 11, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Lazcano, A.A.; Hassan, D.; Pourmadadi, M.; Shamsabadipour, A.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. 5-Fluorouracil nano-delivery systems as a cutting-edge for cancer therapy. Eur. J. Med. Chem. 2023, 246, 114995. [Google Scholar] [CrossRef]
- Abdullah, S.; El Hadad, S.; Aldahlawi, A. The development of a novel oral 5-Fluorouracil in-situ gelling nanosuspension to potentiate the anticancer activity against colorectal cancer cells. Int. J. Pharm. 2022, 613, 121406. [Google Scholar] [CrossRef]
- Ciaffaglione, V.; Modica, M.N.; Pittalà, V.; Romeo, G.; Salerno, L.; Intagliata, S. Mutual Prodrugs of 5-Fluorouracil: From a Classic Chemotherapeutic Agent to Novel Potential Anticancer Drugs. ChemMedChem. 2021, 16, 3496–3512. [Google Scholar] [CrossRef]
- Álvarez, P.; Marchal, J.A.; Boulaiz, H.; Carrillo, E.; Vélez, C.; Rodríguez-Serrano, F.; Melguizo, C.; Prados, J.; Madeddu, R.; Aranega, A. 5-Fluorouracil derivatives: A patent review. Expert Opin. Ther. Pat. 2012, 22, 107–123. [Google Scholar] [CrossRef]
- Hozumi, Y.; Tanaka, T.; Nakano, T.; Matsui, H.; Nasu, T.; Koike, S.; Kakehata, S.; Ito, T.; Goto, K. Orotate phosphoribosyltransferase localizes to the Golgi complex and its expression levels affect the sensitivity to anti-cancer drug 5-fluorouracil. Biomed. Res. 2015, 36, 403–409. [Google Scholar] [CrossRef]
- Matt, P.; van Zwieten-Boot, B.; Rojas, G.C.; Ter Hofstede, H.; Garcia-Carbonero, R.; Abadie, J.C.E.; Pignatti, F. The European Medicines Agency review of Tegafur/Gimeracil/Oteracil (Teysuno™) for the treatment of advanced gastric cancer when given in combination with cisplatin: Summary of the Scientific Assessment of the Committee for medicinal products for human use (CHMP). Oncologist 2011, 16, 1451–1457. [Google Scholar]
- Miyazaki, K.; Shibahara, T.; Sato, D.; Uchida, K.; Suzuki, H.; Matsui, H.; Yanaka, A.; Nakahara, A.; Matsuzaki, Y.; Miyazaki, K. Influence of chemotherapeutic agents and cytokines on the expression of 5-fluorouracil-associated enzymes in human colon cancer cell lines. J. Gastroenterol. 2006, 41, 140–150. [Google Scholar] [CrossRef]
- Bailey, C.J. Orotic aciduria and uridine monophosphate synthase: A reappraisal. J. Inherit. Metab. Dis. 2009, 32, S227–S233. [Google Scholar] [CrossRef] [PubMed]
- Suchi, M.; Mizuno, H.; Kawai, Y.; Tsuboi, T.; Sumi, S.; Okajima, K.; Hodgson, M.E.; Ogawa, H.; Wada, Y. Molecular cloning of the human UMP synthase gene and characterization of point mutations in two hereditary orotic aciduria families. Am. J. Hum. Genet. 1997, 60, 525–539. [Google Scholar] [PubMed]
- Leija, C.; Rijo-Ferreira, F.; Kinch, L.N.; Grishin, N.V.; Nischan, N.; Kohler, J.J.; Hu, Z.; Phillips, M.A. Pyrimidine Salvage Enzymes Are Essential for De Novo Biosynthesis of Deoxypyrimidine Nucleotides in Trypanosoma brucei. PLoS Pathog. 2016, 12, e1006010. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.B.; Sienkiewicz, N.; Wyllie, S.; Patterson, S.; Fairlamb, A.H. Trypanosoma brucei (UMP synthase null mutants) are avirulent in mice, but recover virulence upon prolonged culture in vitro while retaining pyrimidine auxotrophy. Mol. Microbiol. 2013, 90, 443–455. [Google Scholar] [CrossRef]
- Krungkrai, S.R.; Aoki, S.; Palacpac, N.M.Q.; Sato, D.; Mitamura, T.; Krungkrai, J.; Horii, T. Human malaria parasite orotate phosphoribosyltransferase: Functional expression, characterization of kinetic reaction mechanism and inhibition profile. Mol. Biochem. Parasitol. 2004, 134, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hupe, D.J.; Behrens, N.D. A method for assaying orotate phosphoribosyltransferase and measuring phosphoribosylpyrophosphate. Anal. Biochem. 1987, 161, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Umeki, M.; Miyake, H.; Iida, T.; Okumura, M.; Ohno, K.; Sakamoto, M.; Miyoshi, N.; Takahashi, M.; Tsumura, H.; et al. Impact of 5-fluorouracil metabolizing enzymes on chemotherapy in patients with resectable colorectal cancer. Oncol. Rep. 2014, 32, 887–892. [Google Scholar] [CrossRef]
- Krungkrai, J.J.; Wutipraditkul, N.; Prapunwattana, P.; Krungkrai, S.R.; Rochanakij, S. A nonradioactive high-performance liquid chromatographic microassay for uridine 5′-monophosphate synthase, orotate phosphoribosyltransferase, and orotidine 5′-monophosphate decarboxylase. Anal. Biochem. 2001, 299, 162–168. [Google Scholar] [CrossRef]
- Yin, S.; Dragusha, S.; Ejupi, V.; Shibata, T.; Kabashima, T.; Kai, M. Sensitive and Selective Determination of Orotic Acid in Biological Specimens Using a Novel Fluorogenic Reaction. J. Fluoresc. 2015, 25, 1005–1011. [Google Scholar] [CrossRef]
- Yin, S.; Kabashima, T.; Zhu, Q.; Shibata, T.; Kai, M. Fluorescence assay of dihydroorotate dehydrogenase that may become a cancer biomarker. Sci. Rep. 2017, 7, 40670. [Google Scholar] [CrossRef]
- Mizutani, Y.; Wada, H.; Fukushima, M.; Yoshida, O.; Nakanishi, H.; Li, Y.N.; Miki, T. Prognostic Significance of Orotate Phosphoribosyltransferase Activity in Bladder Carcinoma. Cancer 2004, 100, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W.; Jones, M.E. Inhibitors of orotate phosphoribosyl-transferase and orotidine-5′-phosphate decarboxylase from mouse Ehrlich ascites cells: A procedure for analyzing the inhibition of a multi-enzyme complex. Biochem. Pharmacol. 1977, 26, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kamiya, K.; Yoshimura, T.; Sasaki, K.; Tsutani, H.; Nakamura, T.; Ho, D.H. Activities of enzymes converting 5-fluorouracil to 5-fluorouridine-5′ monophosphate and 5-fluorodeoxyuridine-5′monophosphate in subcultured cell lines and solid tumor tissues. J. Jpm. Soc. Cancer Ther. 1990, 25, 990–996. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, T.; Narita, T.; Suto, Y.; Yasmin, H.; Kabashima, T. A Facile Fluorometric Assay of Orotate Phosphoribosyltransferase Activity Using a Selective Fluorogenic Reaction for Orotic Acid. Sensors 2023, 23, 2507. https://doi.org/10.3390/s23052507
Shibata T, Narita T, Suto Y, Yasmin H, Kabashima T. A Facile Fluorometric Assay of Orotate Phosphoribosyltransferase Activity Using a Selective Fluorogenic Reaction for Orotic Acid. Sensors. 2023; 23(5):2507. https://doi.org/10.3390/s23052507
Chicago/Turabian StyleShibata, Takayuki, Tomohiro Narita, Yutaka Suto, Hasina Yasmin, and Tsutomu Kabashima. 2023. "A Facile Fluorometric Assay of Orotate Phosphoribosyltransferase Activity Using a Selective Fluorogenic Reaction for Orotic Acid" Sensors 23, no. 5: 2507. https://doi.org/10.3390/s23052507
APA StyleShibata, T., Narita, T., Suto, Y., Yasmin, H., & Kabashima, T. (2023). A Facile Fluorometric Assay of Orotate Phosphoribosyltransferase Activity Using a Selective Fluorogenic Reaction for Orotic Acid. Sensors, 23(5), 2507. https://doi.org/10.3390/s23052507









