Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Characteristics
2.2. Brief Description of the FMSF Technique and the Measurement Protocol
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Therianou, A.; Vasiadi, M.; Delivanis, D.A.; Petrakopoulou, T.; Katsarou-Katsari, A.; Antoniou, C.; Stratigos, A.; Tsilioni, I.; Katsambas, A.; Rigopoulos, D.; et al. Mitochondrial dysfunction in affected skin and increased mitochondrial DNA in serum from patients with psoriasis. Exp. Dermatol. 2019, 28, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Alwehaidah, M.S.; AlFadhli, S.; Al-Kafaji, G. Leukocyte mitochondrial DNA copy number is a potential non-invasive biomarker for psoriasis. PLoS ONE 2022, 17, e0270714. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, P.M.; Kalinina, S.; Rueck, A.; von Arnim, C.A.; von Einem, B. NADH autofluorescence—A marker on its way to boost bioenergetic research. Cytom. Part A J. Int. Soc. Anal. Cytol. 2019, 95, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Schmidt, W.-D.; Koch, A.; Scheibe, A.; Erfurth, F.; Fassler, D. Fluorescence remission spectroscopy of psoriatic lesions and the effect of topical anthralin therapy. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Dremin, V.V.; Dunaev, A.V. How the melanin concentration in the skin affects the fluorescence-spectroscopy signal formation. J. Opt. Technol. 2016, 83, 43. [Google Scholar] [CrossRef]
- Heikal, A.A.; Johnston, D.S.; Su, A.Y.; Alesci, S.; Galasko, D.; Montine, T.J.; Su, Y.-C.; Qi, X.; Liddell, J.R.; Properzi, F.; et al. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med. 2010, 4, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Katarzynska, J.; Lipinski, Z.; Cholewinski, T.; Piotrowski, L.; Dworzynski, W.; Urbaniak, M.; Borkowska, A.; Cypryk, K.; Purgal, R.; Marcinek, A.; et al. Non-invasive evaluation of microcirculation and metabolic regulation using flow mediated skin fluorescence (FMSF): Technical aspects and methodology. Rev. Sci. Instrum. 2019, 90, 104104. [Google Scholar] [CrossRef]
- Katarzynska, J.; Zielinski, J.; Marcinek, A.; Gebicki, J. New approach to non-invasive assessment of vascular circulation based on the response to transient ischemia. Vasc. Health Risk Manag. 2022, 18, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-invasive assessment of vascular circulation based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Chomicka, R.; Chomicki, W.; Krauze, T.; Uruski, P.; Guzik, M.; Piskorski, J.; Tykarski, A.; Guzik, P. Investigating the ischaemic PHASE of skin NADH fluorescence dynamics in recently diagnosed primary hypertension: A time series analysis. J. Clin. Med. 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Li, X.; Zhou, Q.; Quan, C.; Xue, F.; Zheng, J.; Yu, Y. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br. J. Dermatol. 2017, 176, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Yan, J. Identifying biomarkers in human psoriasis: Revealed by a systems metabolomics approach. Br. J. Dermatol. 2017, 176, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Wozniacka, A.; Szajerski, P.; Adamus, J.; Gebicki, J.; Sysa-Jedrzejowska, A. In search for new antipsoriatic agents: NAD+ topical composition. Ski. Pharmacol. Physiol. 2007, 20, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.-J.; Oh, G.-S.; Lee, S.-B.; Khadka, D.; Cao, W.; Choe, S.-K.; Shim, H.; Kim, C.-D.; Kwak, T.H.; et al. Augmentation of NAD+ by dunnione ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J. Inflamm. Res. 2022, 15, 4623–4636. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Michaels, J.J.C.; Al-Waili, N.; Finkelstein, M.; Allen, M.; Petrillo, R.; Carrey, Z.; Kolanuvada, B.; Lee, B.Y.; Riera, A.G.; et al. Therapeutic effect of hyperbaric oxygen in psoriasis vulgaris: Two case reports and a review of the literature. J. Med. Case Rep. 2009, 3, 7023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by Increased levels of Reactive Oxygen Species (ROS). Dose-Response 2014, 12, 288–341. [Google Scholar] [CrossRef] [PubMed]
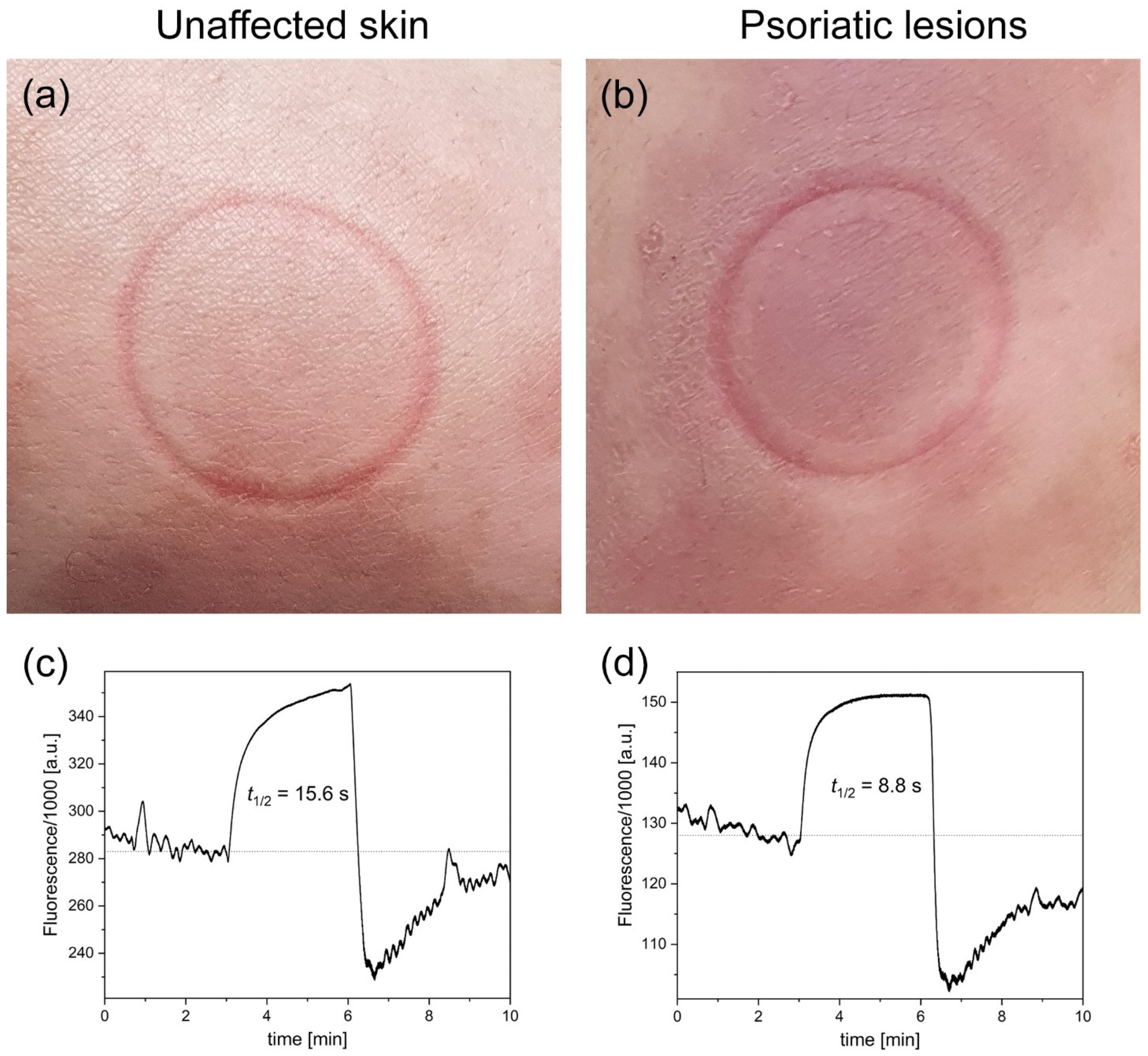
| Patient’s Code | Fluorescence Parameters | FMSF Parameters | ||
|---|---|---|---|---|
| Fluorescence at Baseline (a.u.) | t1/2 (s) | RHR (%) | log (HS) | |
| 004L unaffected skin | 284,000 | 15.6 | 43.9 | 1.59 |
| 004L psoriatic lesions | 128,000 | 8.8 | 38.5 | 1.08 |
| 005L unaffected skin | 412,000 | 12.9 | 37.5 | 2.08 |
| 005L psoriatic lesions | 261,000 | 8.4 | 36.4 | 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebicki, J.; Filipiak, T.; Marcinek, A.; Wozniacka, A. Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects. Sensors 2023, 23, 8718. https://doi.org/10.3390/s23218718
Gebicki J, Filipiak T, Marcinek A, Wozniacka A. Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects. Sensors. 2023; 23(21):8718. https://doi.org/10.3390/s23218718
Chicago/Turabian StyleGebicki, Jerzy, Tomasz Filipiak, Andrzej Marcinek, and Anna Wozniacka. 2023. "Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects" Sensors 23, no. 21: 8718. https://doi.org/10.3390/s23218718
APA StyleGebicki, J., Filipiak, T., Marcinek, A., & Wozniacka, A. (2023). Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects. Sensors, 23(21), 8718. https://doi.org/10.3390/s23218718










