Abstract
Many (if not most) biosensors rely on functional silane coatings as a first step toward covalent immobilization of specific capture molecules. While methods for silanization of silica (SiO2) surfaces are very well developed, less has been done to develop and characterize silanization methods for alternative substrates, such as alumina (Al2O3). In particular, the behavior of Al2O3 coatings grown on aluminum under ambient conditions has not been studied. To address this issue, we have tested solution-phase deposition of two silanes on Al2O3 (3-aminopropyl triethoxysilane and 3-triethoxysilyl)propylsuccinic anhydride) and their applicability to analyte-specific biosensing. Contact angle measurements and imaging via Scanning Electron Microsopy (SEM) were employed to characterize surfaces. We find that 3-aminopropyl triethoxysilane produces well-behaved films and demonstrate that this surface can undergo further reaction with glutaraldehyde followed by an anti-Bacillus subtilis antibody to yield functionalized Al2O3 surfaces capable of specific capture of B. subtilis spores (a model of B. anthracis, the causative organism of Anthrax). In contrast, 3-triethoxysilyl)propylsuccinic anhydride did not behave well with Al/Al2O3 under the reaction conditions tested. In addition to providing specific protocols for Al/Al2O3 functionalization, this work highlights the importance of surface chemistry assessment in the development of new sensors.
1. Introduction
There is a continuing need for new methods for the detection of pathogenic organisms [1,2,3,4]. While extensive research effort on pathogen detection has been expended in the sensing field, optical and electrical sensing methods able to provide increased speed, sensitivity, and selectivity at low cost are desirable [5,6,7]. In the context of inexpensive electrical or optical sensors, aluminum is particularly attractive as a base material for new device development [8,9]. Aluminum is an abundant material, and its extensive commercial use has led to the development of manufacturing-scale methods for its deposition in a broad range of formats. However, for aluminum to be useful as the starting point for the production of sensors, methods must be available for reliable, activity-preserving covalent attachment of biomolecules used for specific analyte capture to its surface.
On exposure to oxygen, including in ambient atmosphere, Al metal forms a thin (1–1.5 nm) surface layer of aluminum oxide (Al2O3, or alumina) [10,11,12]. Porous anodic alumina has been studied extensively as a substrate for sensor production, and requisite methods for immobilization of capture molecules on porous alumina are well developed [13]. Likewise, silanization of Al2O3 nanostructures (a typical precursor to biomolecule immobilization) has been studied [14,15]. In contrast, as far as we are aware, there are no studies available regarding the immobilization of capture biomolecules on aluminum metal substrates with ambient Al2O3 as a precursor to their use as biosensors. We sought to address this gap, and in particular to assess (1) whether the thin layer of ambient Al2O3 would be sufficiently robust to allow for functionalization, and (2) whether antibodies covalently attached to such substrates via methods we have previously developed for Si/SiO2 retained their analyte binding activity and specificity. Here, we describe successful attachment of antibodies specific for Bacillus subtilis (a commonly used, safe to handle surrogate for Bacillus anthracis, the causative agent of Anthrax) [16,17] spores to Al/Al2O3 substrates and specific detection of spores as visualized via scanning electron microscopy (SEM). B. anthracis remains a pathogen of particular concern, primarily because of its potential as a biowarfare agent [18]. Thus, sensors and diagnostics able to detect B. anthracis spores are the subject of considerable continuing research [19,20], leading us to choose this pathogen as a model system.
2. Methods
2.1. Materials
15 × 15 mm substrates consisting of an aluminum layer deposited on glass (SiO2) were obtained from BiSenTech, Inc. (Rochester, NY, USA). The aluminum layer is covered with approximately 1.0 to 1.5 nm of aluminum oxide (Al2O3). Toluene was purchased from Fisher chemical (Hampton, NH, USA) (Cat. T324-4). 3-Aminopropyl triethoxysilane and 3-triethoxysilyl)propylsuccinic anhydride were purchased from Gelest, Inc. (Morrisville, PA, USA). Bacillus subtilis spores were obtained from Sigma Aldrich (Saint Louis, MO, USA) (Cat. 110649). Anti-Bacillus subtilis was obtained from MyBioSource (San Diego, CA, USA) (Cat. #MBS612878); anti-human interleukin-6 (IL-6) was obtained from Biolegend, (San Diego, CA. USA) (Cat. 501110).
2.2. Substrate Functionalization
The thin layer of Al2O3 produced under ambient conditions on aluminum is too fragile to permit cleaning with caustic solutions such as piranha (H2SO4/H2O2). Instead, prior to silanization, substrates were washed with 100% ethanol, followed by 100% isopropanol, followed by distilled, deionized water (ddH2O). This wash procedure was repeated 3 times sequentially. The substrates were then dried with a stream of nitrogen, and UV-Ozone treated for 30 min (UV-Ozone cleaner: Procleaner™, Bioforce nanoscience, Virginia Beach, VA, USA). This step was taken because Sun et al. have shown that exposure of Al2O3 to UV-Ozone cleaners increases the ratio of Al-OH, required for reaction with silanes, within the oxide and decreases the ratio of Al-O-Al groups [21].
2.3. Silanization
After UV/Ozone treatment, substrates were placed in a 1% solution of either 3-aminopropyltriethoxysilane (APTES) or (3-triethoxysilyl)propylsuccinic anhydride (TESP-SA) in dry toluene (v/v; toluene dried via distillation over Na metal under N2) for 30 min at ambient temperature (21–22 °C) with shaking on a rotating platform. The substrates were then rinsed in dry toluene for 5 min, dried with a stream of nitrogen and then finally cured in an oven at 110 °C for 1 h.
2.4. Glutaraldehyde and Antibody Functionalization
APTES-functionalized Al/Al2O3 substrates intended for antibody deposition were placed in an 8% (w/w) glutaraldehyde solution in water for 30 min. The chips were then washed three times in 1× PBS, pH 7.5, for 5 min in each solution. The substrates were then transferred to a solution of 100 µg/mL anti-Bacillus subtilis antibody in 1× PBS, pH 7.5, or 100 μg/mL of anti-IL-6 in PBS solution pH 7.5, to be used as a negative control. Substrates were allowed to react with the respective antibody solutions for 30 min and were then washed 3 times in washing buffer consisting of 150 mM NaCl, 10 Na2HPO4, 10 NaH2PO4-2H2O, 3 mM EDTA, and 0.005% TWEEN-20.
2.5. Target Incubation
The substrates were transferred to a solution of Bacillus subtilis spores (Sigma Aldrich, 110649) at a concentration of 107 CFU/mL for 2 h. In order to test the “worst case scenario” for discriminating specific, antibody-mediated spore capture vs. nonspecific adhesion of spores, substrates were not blocked prior to their incubation with the spore solution. Following spore exposure, substrates were then washed in washing buffer, followed by water, and finally dried with nitrogen. These substrates served as experimental substrates to assess the specific binding of antibodies to Bacillus subtilis spores in solution. Unfunctionalized substrates as well as substrates functionalized with APTES alone or APTES followed by glutaraldehyde served as a control for non-specific spore binding to a chemically functionalized surface.
2.6. Contact Angle Measurements
Contact angles were measured using a Ramé-hart Model 100-00-115, NRL C.A Goniometer (Mountain Lake, NJ, USA). Bare Si/SiO2 and Al/Al2O3 substrates were treated with UV/ozone plasma prior to evaluation; functionalized substrates were tested immediately after silane deposition. For each measurement, the horizontal line (stationary arm) was aligned parallel to the surface of the substrate. A single water droplet was dispensed on the substrate surface. The height of the stage was adjusted such that the horizontal marker line in the goniometer window aligned to the droplet−surface interface. The stage was then moved so that the fulcrum (intersection) of the stationary and moving arm was at the outermost (right side) point of contact between the droplet and the surface. The moving arm was then adjusted to measure the contact angle. The stage was moved to the left side of the droplet and the measurement was repeated. These steps were repeated for three separate droplets, the results of which were averaged and the standard deviations calculated.
2.7. SEM Analysis
Scanning electron microscopy (SEM) was conducted using a Zeiss Auriga Scanning Electron Microscope (Oberkochen, Germany). Images were obtained using the secondary electron detector, and at 20 kV acceleration voltage, using the standard 30 µm aperture. To initiate analysis, the edge of the chip was first located. The image was then focused, and stigmation and aperture alignment were optimized for the best image quality. The scan speed was increased until it matched the electron beam scan raster speed. This allowed for easier and faster scanning. Next, the magnification was adjusted to around 12k. Several fields of view were obtained to evaluate silanization quality. For assessment of spore capture, the sample was moved to scan for spores from the top edge to the bottom edge of the sample. A total of ~54 mm2 were scanned for each chip. Any spores observed were imaged at higher magnification.
3. Results
Covalent attachment of antibodies requires that the substrate carry a functional group able to react irreversibly with surface functionality on the antibody. Most commonly, this means providing an electrophile on the substrate, since nucleophilic amines as represented by lysine side chains are typically present on the antibody surface. Here, we tested two strategies: a one-step process in which deposition of TESP-SA would yield surface-bound anyhydrides, and the other a two-step process requiring silanization with APTES followed by reaction with glutaraldehyde (Figure 1). We and others have successfully used both silanes in the production of active sensors [22,23]. However, in addition to providing an operationally simpler approach, use of TESP-SA was expected to provide a more uniform reactive layer, given our past experience with these two silanes on Si/SiO2, and the known propensity of surface-bound glutaraldehyde to polymerize, forming a non-uniform, irregular surface [24].
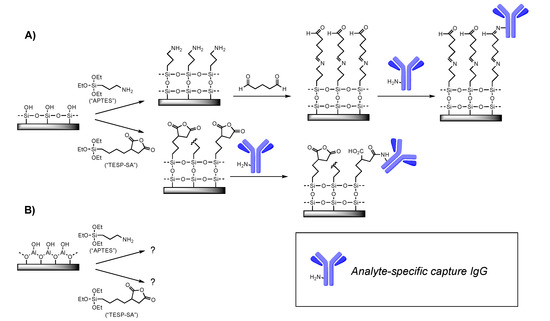
Figure 1.
Surface chemistry tested. (A) Previous work from our lab and others has demonstrated the utility of APTES followed by glutaraldehyde, or TESP-SA, as reagents for depositing amine-reactive (electrophilic) functional groups on Si/SiO2 surfaces. (B) This work. For clarity, only one representative surface amine is shown on the IgG cartoon.
Spectroscopic ellipsometry is one of the most common methods for examining thin film formation on substrates [25] and is a technique we have used extensively in the optimization of surface chemistry for Si/SiO2. However, spectroscopic ellipsometry relies on the availability of a well-validated model for fitting observed data. Lacking such a model for assessing silanized Al/Al2O3 substrates, we turned to SEM, as that would allow us to both observe silane-dependent changes in chip surface directly and also visualize individual B. subtilis spores during that phase of experiments. We immediately observed that TESP-SA behaved poorly on this surface, not producing a uniform film but rather yielding islands of deposited silane (Figure 2B). In contrast, APTES-functionalized chips had a much more uniform appearance, indicating either a complete lack of reaction, or formation of a contiguous film under the deposition conditions used (Figure 2C). As may be seen in Figure 2, APTES-functionalized surfaces were not pristine, however, with silane forming “folds” or aggregates in places. A close-up of this phenomenon is shown in Figure 2D. Since these structures were absent in SEM images of Al/Al2O3 substrates obtained from the manufacturer, we interpreted this as evidence of surface reaction. Given the known propensity of APTES to polymerize [19], it is likely that they are the result of localized APTES self-reaction. Reaction of Al/Al2O3 substrates with higher concentrations of APTES (5% or 10%) under analogous conditions produced greater amounts of surface aggregates (Supplementary Figure S1), leading us to use the 1% silane treatment for remaining studies. ATR-IR measurements of unfunctionalized and APTES-functionalized Al/Al2O3 substrates confirmed the presence of an -NH2 stretch in the APTES-functionalized material (Supplementary Figure S2). Finally, SEM images also confirmed that Si/SiO2 substrates were highly uniform in appearance following silanization with TESP-SA (Figure 2E) and APTES (Figure 2F), consistent with our previous work.
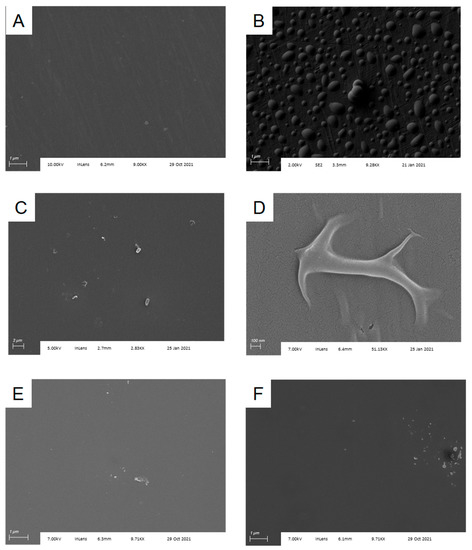
Figure 2.
Silanization of Al/Al2O3 substrates. (A) Cleaned substrate as obtained from the supplier; (B) substrate following silanization with TESP-SA; (C) substrate following silanization with APTES; (D) higher magnification image of silanization “fold” from (C); (E) Si/SiO2 substrate following silanization with TESP-SA; (F) Si/SiO2 substrate following silanization with APTES.
Surface hydrophilicity or hydrophobicity is an important component of successful sensor functionalization. To examine differences between Al/Al2O3 and Si/SiO2 before and after silanization, we measured water contact angles for each surface (Table 1). Both surfaces were sufficiently hydrophilic immediately after UV-ozone treatment that a contact angle could not be measured and remained hydrophilic (contact angle < 90°) throughout the process of silanization (for either APTES or TESP-SA) and glutaraldehyde treatment (after APTES). However, the Al2O3 surfaces were found to have contact angles that were between 12.7° and 22.4° higher (more hydrophobic) than the equivalent SiO2 surfaces.

Table 1.
Contact angles measured for Si/SiO2 and Al/Al2O3 surfaces before and after reaction with TESP-SA silane or APTES silane followed by glutaraldehyde. All values are in degrees. “NM” = not measurable.
Having chosen APTES-silanized Al/Al2O3 substrates for further experiments, we then treated silanized substrates with aqueous glutaraldehyde followed by an anti-B. subtilis antibody to facilitate specific capture of B. subtilis spores. After a 2 h incubation with spore solution followed by water rinse, chips with a covalently attached anti-B. subtilis spore antibody captured approximately 64,000 spores per chip, based on observed densities of 3 spores per 3500 μm2 in multiple SEM images. Representative chip images are shown in Figure 3. Spores are readily distinguishable from surface debris (Figure S2) and have sizes and morphologies consistent with literature SEM data [26].
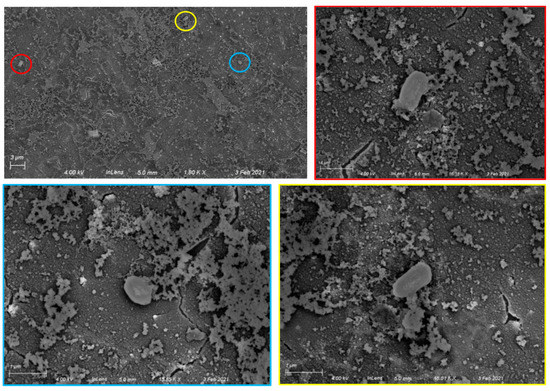
Figure 3.
Specific capture of Bacillus subtilis spores on Al/Al2O3 surfaces with covalently attached anti-B. subtilis antibodies. Top left: 1800× image showing three spores (circled). Top right and bottom: higher magnification images (15,850×–16,180×) showing individual spores. The color framing each high-magnification image corresponds to the circled area in the lower-magnification image.
To control for nonspecific binding of spores to the chip surface, we also examined Al/Al2O3 chips functionalized only with APTES, APTES plus glutaraldehyde, or APTES plus glutaraldehyde chips onto which an anti-IL-6 antibody had been attached, after exposure to B. subtilis spores under identical conditions. SEM imaging of these three substrates (Figure 4) showed capture of various forms of dust and debris (likely part of the spore preparation), but no spores. Finally, Bacillus atrophaeus spores were not captured by anti-B. subtilis functionalized substrates (Figure S4). These results confirm specific spore capture by the anti-B. subtilis functionalized surfaces and indicate that the antibody retains both its activity and selectivity when immobilized.
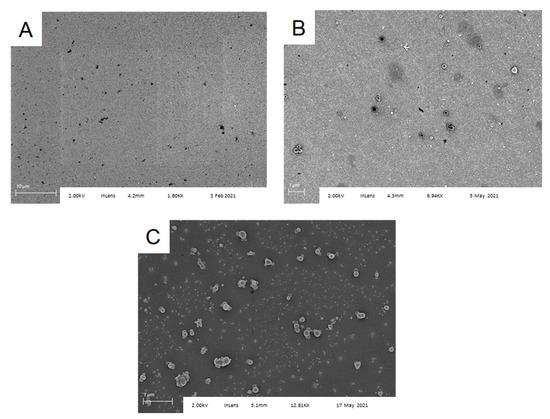
Figure 4.
Bacillus subtilis spores are not captured by control surfaces functionalized with (A) APTES; (B) APTES followed by glutaraldehyde; and (C) APTES followed by glutaraldehyde, then anti-IL-6. Ambient dust or solution particulates coat the surface, but no spores are observed.
4. Conclusions
We have successfully demonstrated that antibodies may be covalently attached to silanized, glutaraldehyde-treated ambient Al2O3 grown on an aluminum surface, and that these antibodies retain their ability to specifically bind a target of interest (here, B. subtilis spores). This result confirms that these substrates may be used effectively in the production of new biosensors. Silanization with APTES proceeded analogously to APTES silanization of Si/SiO2; however, reaction with TEPSA produced a highly nonuniform surface. This observation, coupled with the observation that functionalized Al/Al2O3 surfaces were consistently more hydrophobic than equivalent Si/SiO2 surfaces, despite both materials having high hydrophilicity prior to silanization, highlights the importance of carefully characterizing each step in the fabrication of sensors using new materials. While APTES deposited efficiently on the Al/Al2O3 surface, as noted above, it was not pristine. Vapor-phase silanization [11] would potentially produce a higher-quality silane film. We are currently investigating this possibility.
We were gratified to observe that Al/Al2O3 surfaces to which anti-B. subtilis antibodies were attached following silanization and glutaraldehyde treatment were capable of specific capture of B. anthracis spores. In contrast, substrates carrying anti-IL-6 antibodies, as well as substrates at each earlier step of the functionalization process (bare Al2O3, APTES, APTES + glutaraldehyde) did not capture spores. The lack of nonspecific capture on unfunctionalized, APTES, and APTES + glutaraldehyde substrates is likely assisted by the structure of the B. subtilis endospore [27]: the outer crust is heavily glycosylated [23] and therefore less susceptible to ionic interactions with the APTES amine group or covalent reaction with glutaraldehyde than a simple protein would be. In sum, these studies provide a starting point for future development of Al substrates carrying an ambient layer of Al2O3 as optical and electrical biosensors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s22093441/s1, Figure S1: Comparison of silanization with 1%, 5%, and 10% solutions of APTES in dry toluene. Significantly greater numbers of aggregates are observed in substrates treated with the 5% or 10% solutions. Arrows highlight representative aggregates, with their measured size; Figure S2: ATR-IR spectrum of APTES-functionalized Al/Al2O3 substrate. A band for the APTES-NH2 group is readily visible at 3240 cm−1; this band is not visible in an unfunctionalized Al/Al2O3 substrate; Figure S3: Spores have a distinct size and shape, and are readily distinguished from debris on the surface of the chip; Figure S4: Al/Al2O3 substrate functionalized with an anti-Bacillus subtilis antibody does not capture Bacillus atrophaeus spores, confirming specificity.
Author Contributions
Conceptualization, E.P.L. and B.L.M.; methodology, E.P.L. and B.L.M.; validation, E.P.L. and B.L.M.; formal analysis, E.P.L. and B.L.M.; investigation, E.P.L. and B.L.M.; resources, B.L.M.; writing—original draft preparation, E.P.L. and B.L.M.; writing—review and editing, E.P.L. and B.L.M.; visualization, E.P.L. and B.L.M.; supervision, B.L.M.; project administration, B.L.M.; funding acquisition, B.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by BiSenTech, Inc., Rochester, NY, USA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is available from the corresponding author on request.
Acknowledgments
We thank Jalil Shojaie for obtaining ATR-IR for this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, A.; Mishra, R.K.; Goud, K.Y.; Mohamed, M.A.; Kummari, S.; Tiwari, S.; Li, Z.; Narayan, R.; Stanciu, L.A.; Marty, J.L. Optical biosensors for diagnostics of infectious viral disease: A recent update. Diagnostics 2021, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Monteil, S.; Casson, A.J.; Jones, S.T. Electronic and electrochemical viral detection for the detection of infectious diseases. Talanta 2021, 230, 122026. [Google Scholar]
- Khan, S.; Burciu, B.; Filipe, C.D.M.; Li, Y.; Dellinger, K.; Didar, T. DNAzyme-based biosensors: Immobilization strategies, applications, and future prospective. ACS Nano 2021, 15, 13943–13969. [Google Scholar] [CrossRef] [PubMed]
- Monteil, S.; Casson, A.J.; Jones, S.T. Electronic and electrochemical viral detection for point-of-care use: A systematic review. PLoS ONE 2021, 16, e0258002. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Rho, D.; Breaux, C.; Kim, S. Label-free optical resonator-based biosensors. Sensors 2020, 20, 5901. [Google Scholar] [CrossRef] [PubMed]
- Ligler, F.S.; Gooding, J.J. Lighting up biosensors: Now and the decade to come. Anal. Chem. 2019, 91, 8732–8738. [Google Scholar] [CrossRef]
- Natasha, N.Z.; Rajapaksha, R.D.A.A.; Uda, M.N.A.; Hashim, U. Electrical DNA biosensor using aluminium interdigitated electrode for E. coli O157:H7 detection. AIP Conf. Proc. 2017, 1885, 020235. [Google Scholar]
- Lambert, A.S.; Valiulis, S.N.; Malinick, A.S.; Tanabe, I.; Cheng, Q. Plasmonic biosensing with aluminum thin films under the Kretschmann configuration. Anal. Chem. 2020, 92, 8654–8659. [Google Scholar] [CrossRef]
- Nguyen, L.; Hashimoto, T.; Zakharov, D.N.; Stach, E.A.; Rooney, A.P.; Berkels, B.; Thompson, G.E.; Haigh, S.J.; Burnett, T.L. Atomic-scale insights into the oxidation of aluminum. ACS Appl. Mater. Interfaces 2018, 10, 2230–2235. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Growth kinetics and mechanisms of aluminum-oxide films formed by thermal oxidation of aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]
- Evertsson, J.; Bertram, F.; Zhang, F.; Rullik, L.; Merte, L.R.; Shipilin, M.; Soldemo, M.; Ahmadi, S.; Vinogradov, N.; Carla, F.; et al. The thickness of native oxides on aluminum alloys and single crystals. Appl. Surf. Sci. 2015, 349, 826–832. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferre-Borrull, J.; Marsal, L.F. Advances in optical biosensors and sensors using nanoporous anodic alumina. Sensors 2020, 20, 5068. [Google Scholar] [CrossRef]
- Nayak, N.; Huertas, R.; Crespo, J.G.; Portugal, C.A.M. Surface modification of alumina monolithic columns with 3-aminopropyltetraethoxysilane (APTES) for protein attachment. Sep. Purif. Technol. 2019, 229, 115674. [Google Scholar] [CrossRef]
- Simunin, M.M.; Voronin, A.S.; Fadeev, Y.V.; Mikhlin, Y.L.; Lizunov, D.A.; Samoilo, A.S.; Chirkov, D.Y.; Voronina, S.Y.; Khartov, S.V. Features of functionalization of the surface of alumina nanofibers by hydrolysis of organosilanes on surface hydroxyl groups. Polymers 2021, 13, 4374. [Google Scholar] [CrossRef]
- Chan, S.; Horner, S.R.; Miller, B.L.; Fauchet, P.M. Identification of gram negative bacteria using nanoscale silicon microcavities. J. Am. Chem. Soc. 2001, 123, 11797–11798. [Google Scholar] [CrossRef]
- Greenberg, D.L.; Busch, J.D.; Keim, P.; Wagner, D.M. Identifying experimental surrogates for Bacillus anthracis spores: A review. Investig. Genet. 2010, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Spencer, R.C. Bacillus anthracis. J. Clin. Pathol. 2003, 56, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Pohanka, M. Current trends in the biosensors for biological warfare agents assay. Materials 2019, 12, 2303. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-B.; Cui, M.-M.; Li, M.; Zhang, X.-E. Biosensors for the detection of Bacillus anthracis. Acc. Chem. Res. 2021, 54, 4451–4461. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, R.; Zhang, J.; Qiu, Z.-J.; Wu, D. Effects of UV-oxone treatment on sensing behaviours of EGFETs with Al2O3 sensing film. Materials 2017, 10, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, A.R.; Sriram, R.; Carter, J.A.; Miller, B.L. Comparative study of solution phase and vapor phase deposition of aminosilanes on silicon dioxide surfaces. Mater. Sci. Eng. C 2014, 35, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cognetti, J.S.; Steiner, D.J.; Abedin, M.; Bryan, M.R.; Shanahan, C.; Tokranova, N.; Young, E.; Klose, A.M.; Zavriyev, A.; Judy, N.; et al. Disposable photonics for cost-effective clinical bioassays: Application to COVID-19 antibody testing. Lab Chip 2021, 21, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Flounders, A.W.; Volponi, J.V.; Ashley, C.S.; Wally, K.; Schoeniger, J.S. Development of sensors for direct detection of organophosphates. Part I: Immobilization, characterization and stabilization of acetylcholinesterase and organophosphate hydrolase on silica supports. Biosens. Bioelectron. 1999, 8–9, 703–713. [Google Scholar] [CrossRef]
- Aspnes, D.E. Spectroscopic ellipsometry–past, present, and future. Thin Solid Film. 2014, 571, 334–344. [Google Scholar] [CrossRef]
- Bartels, J.; Blüher, A.; Castellanos, S.L.; Richter, M.; Günther, M.; Mascher, T. The Bacillus subtilis endospore crust: Protein interaction network, architecture and glycosylation state of a potential glycoprotein layer. Mol. Microbiol. 2019, 112, 1576–1592. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).