Antibodies as Biosensors’ Key Components: State-of-the-Art in Russia 2020–2021
Abstract
:1. Introduction
2. Antibodies and Their Recombinant Analogs in Sensor Development
2.1. Monoclonal and Polyclonal Antibodies
2.2. Development of Recombinant Technology in Antibody Design
2.2.1. Recombinant Antibodies in Detection Systems
2.2.2. Phage–Antibody Conjugates in Detection Systems
2.2.3. Non-Immunoglobulin Alternative Protein Scaffolds in Detection Systems
3. Monoclonal Antibodies as Tools to Study Biological Molecules and Intermolecular Interactions
4. Production of Monoclonal Antibodies and Immunodiagnostic Kits in Russia
5. Immunodiagnostic Systems Based on Nanomaterials
5.1. Immunochromatographic Test Systems (LFIA)
5.2. Nucleic Acid Lateral Flow Immunoassay
5.3. Optical Methods of Immunodetection Based on Nanomaterials
6. Practical Topical Applications of Modern Immunosensor Systems
6.1. Screening of SARS-CoV-2 Antibodies
6.2. Immunochemical Detection Systems for Assaying Foodstuffs
6.3. Development and Application of Multiplex Test Systems Based on Analytical Microchips
6.4. Detection of Diagnostic Antibodies
7. The Use of Physico-Chemical Methods for the Development of Immunosensors
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Soleymani, L.; Li, F. Mechanistic challenges and advantages of biosensor miniaturization into the nanoscale. ACS Sens. 2017, 2, 458–467. [Google Scholar] [CrossRef]
- Vturina, I.Y.; Kuryatov, A.B.; Kiselev, A.V.; Khoroshilova, N.I.; Ovechkina, G.V.; Abdulaev, N.G.; Tsetlin, V.I.; Vasilov, R.G. Immunochemical study of bacteriorhodopsin using monoclonal antibodies. Biol. Membr. 1984, 1, 1161–1170. (In Russian) [Google Scholar]
- Ovchinnikov, Y.A.; Abdulaev, N.G.; Vasilov, R.G.; Vturina, J.Y.; Kuryatov, A.B.; Kiselev, A.V. The antigenic structure and topography of bacteriorhodopsin in purple membranes as determined by interaction with monoclonal antibodies. FEBS Lett. 1985, 179, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Tillib, S.V. Prospective applications of single-domain antibodies in biomedicine. Mol. Biol. 2020, 54, 317–326. [Google Scholar] [CrossRef]
- Razai, A.; Garcia-Rodriguez, C.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Robles, Y.; Tsai, R.; Smith, T.J.; Smith, L.A.; Siegel, R.W.; et al. Molecular evolution of antibody affinity for sensitive detection of botulinum neurotoxin type A. J. Mol. Biol. 2005, 351, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Varnum, S.M.; Warner, M.G.; Dockendorff, B.; Anheier, N.C., Jr.; Lou, J.; Marks, J.D.; Smith, L.A.; Feldhaus, M.J.; Grate, J.W.; Bruckner-Lea, C.J. Enzyme-amplified protein microarray and a fluidic renewable surface fluorescence immunoassay for botulinum neurotoxin detection using high-affinity recombinant antibodies. Anal. Chim. Acta 2006, 570, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Götzke, H.; Kilisch, M.; Martínez-Carranza, M.; Sograte-Idrissi, S.; Rajavel, A.; Schlichthaerle, T.; Engels, N.; Jungmann, R.; Stenmark, P.; Opazo, F.; et al. The ALFA-tag is a highly versatile tool for nanobody-based bioscience applications. Nat. Commun. 2019, 10, 4403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Shen, Z.; Mernaugh, R. Recombinant antibodies and their use in biosensors. Anal. Bioanal. Chem. 2012, 402, 3027–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokrushina, Y.A.; Golovin, A.V.; Smirnov, I.V.; Chatziefthimiou, S.D.; Stepanova, A.V.; Bobik, T.V.; Zalevsky, A.O.; Zlobin, A.S.; Konovalov, K.A.; Terekhov, S.S.; et al. Multiscale computation delivers organophosphorus reactivity and stereoselectivity to immunoglobulin scavengers. Proc. Natl. Acad. Sci. USA 2020, 117, 22841–22848. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, C.; Levy, R.; Arndt, J.W.; Forsyth, C.M.; Razai, A.; Lou, J.; Geren, I.; Stevens, R.C.; Marks, J.D. Molecular evolution of antibody cross-reactivity for two subtypes of type A botulinum neurotoxin. Nat. Biotechnol. 2007, 25, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Edfors, F.; Hober, A.; Linderbäck, K.; Maddalo, G.; Azimi, A.; Sivertsson, Å.; Tegel, H.; Hober, S.; Szigyarto, C.A.; Fagerberg, L.; et al. Enhanced validation of antibodies for research applications. Nat. Commun. 2018, 9, 4130. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The therapeutic potential of nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, D.; Mei, K.; Yan, T.; Wang, Y.; Wu, W.; Chen, Y.; Wang, J.; Zhang, Q.; Wu, S. Nanomechanical sensor for rapid and ultrasensitive detection of tumor markers in serum using nanobody. Nano Res. 2021. [Google Scholar] [CrossRef]
- Bastos-Soares, E.A.; Sousa, R.M.O.; Gómez, A.F.; Alfonso, J.; Kayano, A.M.; Zanchi, F.B.; Funes-Huacca, M.E.; Stábeli, R.G.; Soares, A.M.; Pereira, S.S.; et al. Single domain antibodies in the development of immunosensors for diagnostics. Int. J. Biol. Macromol. 2020, 165, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Applications of nanobodies. Annu. Rev. Anim. Biosci. 2021, 9, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Flicker, S.; Zettl, I.; Tillib, S.V. Nanobodies-useful tools for allergy treatment? Front. Immunol. 2020, 11, 576255. [Google Scholar] [CrossRef]
- Nosenko, M.A.; Atretkhany, K.N.; Mokhonov, V.V.; Chuvpilo, S.A.; Yanvarev, D.V.; Drutskaya, M.S.; Tillib, S.V.; Nedospasov, S.A. Generation and evaluation of bispecific anti-TNF antibodies based on single-chain VHH domains. Meth. Mol. Biol. 2021, 2248, 91–107. [Google Scholar] [CrossRef]
- Goryainova, O.S.; Ivanova, T.I.; Rutovskaya, M.V.; Tillib, S.V. A method for the parallel and sequential generation of single-domain antibodies for the proteomic analysis of human blood plasma. Mol. Biol. 2017, 51, 985–996. (In Russian) [Google Scholar] [CrossRef]
- Dias-Lopes, C.; Paiva, A.L.; Guerra-Duarte, C.; Molina, F.; Felicori, L. Venomous arachnid diagnostic assays, lessons from past attempts. Toxins 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Føns, S.; Ledsgaard, L.; Nikolaev, M.V.; Vassilevski, A.A.; Sørensen, C.V.; Chevalier, M.K.; Fiebig, M.; Laustsen, A.H. Discovery of a recombinant human monoclonal immunoglobulin G antibody against α-latrotoxin from the Mediterranean black widow spider (Latrodectus tredecimguttatus). Front. Immunol. 2020, 11, 587825. [Google Scholar] [CrossRef]
- Larionova, M.D.; Markova, S.V.; Tikunova, N.V.; Vysotski, E.S. The smallest isoform of Metridia longa luciferase as a fusion partner for hybrid proteins. Int. J. Mol. Sci. 2020, 21, 4971. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.; Viktorsson, K.; Paz Gomero, E.; Hååg, P.; Arapi, V.; Kaminskyy, V.O.; Kamali, C.; De Petris, L.; Ekman, S.; Lewensohn, R.; et al. Detection of tumor-associated membrane receptors on extracellular vesicles from non-small cell lung cancer patients via immuno-PCR. Cancers 2021, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Maerle, A.V.; Simonova, M.A.; Pivovarov, V.D.; Voronina, D.V.; Drobyazina, P.E.; Trofimov, D.Y.; Alekseev, L.P.; Zavriev, S.K.; Ryazantsev, D.Y. Development of the covalent antibody–DNA conjugates technology for detection of IgE and IgM antibodies by immuno-PCR. PLoS ONE 2019, 14, e0209860. [Google Scholar] [CrossRef]
- Kushnarova-Vakal, A.; Äärelä, A.; Huovinen, T.; Virta, P.; Lamminmäki, U. Site-specific linking of an oligonucleotide to mono- and bivalent recombinant antibodies with SpyCatcher-SpyTag system for immuno-PCR. ACS Omega 2020, 5, 24927–24934. [Google Scholar] [CrossRef] [PubMed]
- Artykov, A.A.; Fursova, K.K.; Ryazantsev, D.Y.; Shchannikova, M.P.; Loskutova, I.V.; Shepelyakovskaya, A.O.; Laman, A.G.; Zavriev, S.K.; Brovko, F.A. Detection of staphylococcal enterotoxin a by phage display mediated immuno-PCR method. Russ. J. Bioorg. Chem 2017, 43, 540–543. [Google Scholar] [CrossRef]
- Rezaei, Z.S.; Shahangian, S.S.; Hasannia, S.; Sajedi, R.H. Development of a phage display-mediated immunoassay for the detection of vascular endothelial growth factor. Anal. Bioanal. Chem. 2020, 412, 7639–7648. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Q.; Wu, W.; Yan, T.; Tang, X.; Zhang, W.; Yu, L.; Li, P. Anti-idiotypic nanobody-phage display-mediated real-time immuno-PCR for sensitive, simultaneous and quantitative detection of total aflatoxins and zearalenone in grains. Food Chem. 2019, 297, 124912. [Google Scholar] [CrossRef]
- Huang, W.; Tu, Z.; Ning, Z.; He, Q.; Li, Y. Development of real-time immuno-PCR based on phage displayed an anti-idiotypic nanobody for quantitative determination of citrinin in monascus. Toxins 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishina, I.A.; Filimonova, I.N.; Zakharova, M.Y.; Ovchinnikova, L.A.; Mamedov, A.E.; Lomakin, Y.A.; Belogurov, A.A., Jr. Exhaustive search of the receptor ligands by the CyCLOPS (Cytometry Cell-Labeling Operable Phage Screening) technique. Int. J. Mol. Sci. 2020, 21, 6258. [Google Scholar] [CrossRef]
- Stumpp, M.T.; Dawson, K.M.; Binz, H.K. Beyond antibodies: The DARPin® drug platform. BioDrugs 2020, 34, 423–433. [Google Scholar] [CrossRef]
- Gebauer, M.; Skerra, A. Engineered protein scaffolds as next-generation therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 391–415. [Google Scholar] [CrossRef]
- Frejd, F.Y.; Kim, K.-T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef] [Green Version]
- Becker, L.; Singh Badwal, J.; Brandl, F.; Verdurmen, W.P.R.; Plückthun, A. Thermodynamic stability is a strong predictor for the delivery of DARPins to the cytosol via anthrax toxin. Pharmaceutics 2021, 13, 1285. [Google Scholar] [CrossRef]
- Owens, B. Faster, deeper, smaller-The rise of antibody-like scaffolds. Nat. Biotechnol. 2017, 35, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Caputi, A.P.; Navarra, P. Beyond antibodies: Ankyrins and DARPins. From basic research to drug approval. Curr. Opin. Pharmacol. 2020, 51, 93–101. [Google Scholar] [CrossRef]
- Shilova, O.N.; Deyev, S.M. DARPins: Promising scaffolds for theranostics. Acta Natur. 2019, 11, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Bragina, O.; Chernov, V.; Schulga, A.; Konovalova, E.; Garbukov, E.; Vorobyeva, A.; Orlova, A.; Tashireva, L.; Sorensen, J.; Zelchan, R.; et al. Phase I trial of 99mTc-(HE)3-G3, a DARPin-based probe for imaging of HER2 expression in breast cancer. J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Garousi, J.; Rinne, S.S.; Schulga, A.; Deyev, S.; Vorobyeva, A. On the prevention of kidney uptake of radiolabeled DARPins. EJNMMI Res. 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guryev, E.L.; Smyshlyaeva, A.S.; Shilyagina, N.Y.; Sokolova, E.A.; Shanwar, S.; Kostyuk, A.B.; Lyubeshkin, A.V.; Schulga, A.A.; Konovalova, E.V.; Lin, Q.; et al. UCNP-Based photoluminescent nanomedicines for targeted imaging and theranostics of cancer. Molecules 2020, 25, 4302. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Kolesnikova, O.A.; Kotelnikova, P.A.; Soloviev, V.D.; Popov, A.A.; Proshkina, G.M.; Nikitin, M.P.; Deyev, S.M. Comparative evaluation of engineered polypeptide scaffolds in HER2-targeting magnetic nanocarrier delivery. ACS Omega 2021, 6, 16000–16008. [Google Scholar] [CrossRef] [PubMed]
- Deyev, S.M.; Xu, T.; Liu, Y.; Schulga, A.; Konovalova, E.; Garousi, J.; Rinne, S.S.; Larkina, M.; Ding, H.; Gräslund, T.; et al. Influence of the position and composition of radiometals and radioiodine labels on imaging of Epcam expression in prostate cancer model using the DARPin Ec1. Cancers 2021, 13, 3589. [Google Scholar] [CrossRef]
- Xu, T.; Vorobyeva, A.; Schulga, A.; Konovalova, E.; Vorontsova, O.; Ding, H.; Gräslund, T.; Tashireva, L.A.; Orlova, A.; Tolmachev, V.; et al. Imaging-guided therapy simultaneously targeting HER2 and EpCAM with trastuzumab and EpCAM-directed toxin provides additive effect in ovarian cancer model. Cancers 2021, 13, 3939. [Google Scholar] [CrossRef]
- Shramova, E.; Proshkina, G.; Shipunova, V.; Ryabova, A.; Kamyshinsky, R.; Konevega, A.; Schulga, A.; Konovalova, E.; Telegin, G.; Deyev, S. Dual targeting of cancer cells with DARPin-based toxins for overcoming tumor escape. Cancers 2020, 12, 3014. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles decorated with anti-HER2 affibody for targeted delivery and photoinduced cell death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef]
- Karyagina, T.S.; Ulasov, A.V.; Rosenkranz, A.A.; Slastnikova, T.A.; Khramtsov, Y.V.; Lupanova, T.N.; Georgiev, G.P.; Sobolev, A.S. New recombinant carriers binding specifically to the epidermal growth factor receptor. Dokl. Biochem. Biophys. 2020, 490, 22–24. [Google Scholar] [CrossRef]
- Bragina, O.; von Witting, E.; Garousi, J.; Zelchan, R.; Sandström, M.; Orlova, A.; Medvedeva, A.; Doroshenko, A.; Vorobyeva, A.; Lindbo, S.; et al. Phase I study of 99mTc-ADAPT6, a scaffold protein-based probe for visualization of HER2 expression in breast cancer. J. Nucl. Med. 2021, 62, 493–499. [Google Scholar] [CrossRef]
- Laman, A.G.; Shepelyakovskaya, A.O.; Brovko, F.A.; Sizova, S.V.; Artemyev, M.V.; Oleinikov, V.A. Application of monoclonal antibodies and phage display technology for YB-1 protein analysis. Russ. J. Bioorg. Chem. 2020, 46, 43–51. [Google Scholar] [CrossRef]
- Rudenko, N.V.; Karatovskaya, A.P.; Tsfasman, I.M.; Brovko, F.A.; Vasilyeva, N.V. Molecular forms of AlpA and AlpB lytic endopeptidases from Lysobacter sp. XL1: Immunochemical determination of their intra- and extracellular localization. Russ. J. Bioorg. Chem. 2017, 43, 526–530. [Google Scholar] [CrossRef]
- Demidova, N.A.; Klimova, R.R.; Kushch, A.A.; Lesnova, E.I.; Masalova, O.V.; Dorofeeva, A.D.; Nikonova, A.A.; Fedorova, N.E.; Zverev, V.V. Obtaining and characterization of the monoclonal antibodies against G-protein of the respiratory syncytial virus. J. Microbiol. Epidemiol. Immunobiol. 2020, 97, 7–14. [Google Scholar] [CrossRef]
- Rudenko, N.V.; Nagel, A.; Zamyatina, A.; Karatovskaya, A.; Salyamov, V.; Andreeva-Kovalevskaya, Z.; Siunov, A.; Kolesnikov, A.; Shepelyakovskaya, A.; Boziev, K.; et al. A monoclonal antibody against the C-terminal domain of Bacillus cereus hemolysin II inhibits HlyII cytolytic activity. Toxins 2020, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.V.; Karatovskaya, A.P.; Zamyatina, A.V.; Siunov, A.V.; Andreeva-Kovalevskaya, Z.I.; Nagel, A.S.; Brovko, F.A.; Solonin, A.S. C-Terminal domain of Bacillus cereus hemolysin II is able to interact with erythrocytes. Russ. J. Bioorg. Chem. 2020, 46, 321–326. [Google Scholar] [CrossRef]
- Zamyatina, A.V.; Rudenko, N.V.; Karatovskaya, A.P.; Shepelyakovskaya, A.O.; Siunov, A.V.; Andreeva-Kovalevskaya, J.I.; Nagel, A.S.; Salyamov, V.I.; Kolesnikov, A.S.; Brovko, F.A.; et al. HlyIIC-15 monoclonal antibody against the C-end domain of B. cereus HlyII interacts with the thrombin recognition site. Russ. J. Bioorg. Chem. 2020, 46, 1214–1220. [Google Scholar] [CrossRef]
- Antipova, N.V.; Larionova, T.D.; Siniavin, A.E.; Nikiforova, M.A.; Gushchin, V.A.; Babichenko, I.I.; Volkov, A.V.; Shakhparonov, M.I.; Pavlyukov, M.S. Establishment of murine hybridoma cells producing antibodies against spike protein of SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 9167. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.V.; Sokolov, A.V.; Rak, A.Y.; Ischenko, A.M.; Kudling, T.V.; Vakhrushev, A.V.; Gorbunov, A.A. Epitope specificity of two anti-morphine monoclonal antibodies: In vitro and in silico studies. J. Mol. Recognit. 2020, 33, e2846. [Google Scholar] [CrossRef] [PubMed]
- Mazina, M.Y.; Ziganshin, R.H.; Magnitov, M.D.; Golovnin, A.K.; Vorobyev, N.E. Proximity-dependent biotin labelling reveals CP190 as an EcR/Usp molecular partner. Sci. Rep. 2020, 10, 4793. [Google Scholar] [CrossRef]
- Konyshev, I.; Byvalov, A.; Ananchenko, B.; Fakhrullin, R.; Danilushkina, A.; Dudina, L. Force interactions between Yersiniae lipopolysaccharides and monoclonal antibodies: An optical tweezers study. J. Biomech. 2020, 99, 109504. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.L.; Krylov, V.B.; Khlusevich, Y.A.; Baykov, I.K.; Yashunsky, D.V.; Emelyanova, L.A.; Tsvetkov, Y.E.; Karelin, A.A.; Bardashova, A.V.; Wong, S.S.W.; et al. Novel mouse monoclonal antibodies specifically recognizing β-(1→3)-D-glucan antigen. PLoS ONE 2019, 14, e0215535. [Google Scholar] [CrossRef]
- Zurochka, A.; Dobrinina, M.; Zurochka, V.; Hu, D.; Solovyev, A.; Ryabova, L.; Kritsky, I.; Ibragimov, R.; Sarapultsev, A. Seroprevalence of SARS-CoV-2 antibodies in symptomatic individuals is higher than in persons who are at increased risk exposure: The results of the single-center, prospective, cross-sectional study. Vaccines 2021, 9, 627. [Google Scholar] [CrossRef]
- Kostinov, M.P.; Zhuravlev, P.I.; Gladkova, L.S.; Mashilov, K.V.; Polishchuk, V.B.; Shmitko, A.D.; Zorina, V.N.; Blagovidov, D.A.; Pahomov, D.V.; Vlasenko, A.E.; et al. Comparative analysis of the measles antibody levels in healthy medical personnel of maternity ward and women in labor. Front. Immunol. 2021, 12, 680506. [Google Scholar] [CrossRef] [PubMed]
- Spitsyn, A.N.; Utkin, D.V.; Kireev, M.N.; Ovchinnikova, M.V.; Kuznetsov, O.S.; Erokhin, P.S.; Kochubei, V.I. The spectrophotometric characteristic of immunoglobulin conjugates for diagnostics of causative agents of especially dangerous infections. Opt. Spectrosc. 2020, 128, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Alatortseva, G.I.; Dotsenko, V.V.; Nesterenko, L.N.; Luhverchik, L.N.; Kabargina, V.Y.; Amiantova, I.I.; Zhukina, M.V.; Zhavoronok, S.V.; Nurmatov, Z.S.; Nurmatov, A.Z.; et al. Line immunoassay for detection of IgG antibodies to hepatitis E virus (Hepeviridae, Orthohepevirus, Orthohepevirus A). Vopr. Virusol. 2020, 65, 132–142. (In Russian) [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Lateral flow serodiagnosis in the double-antigen sandwich format: Theoretical consideration and confirmation of advantages. Sensors 2020, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, A.; Koets, M. Simple and rapid bacterial protein and DNA diagnostic methods based on signal generation with colloidal carbon particles. In Rapid Methods for Biological and Chemical Contaminants in Food and Feed; Van Amerongen, A., Barug, D., Lauwaars, M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; pp. 105–126. [Google Scholar]
- Ivanov, A.V.; Shmyglya, I.V.; Zherdev, A.V.; Dzantiev, B.B.; Safenkova, I.V. The challenge for rapid detection of high-structured circular RNA: Assay of potato spindle tuber viroid based on recombinase polymerase amplification and lateral flow tests. Plants 2020, 9, 1369. [Google Scholar] [CrossRef]
- Zvereva, E.A.; Popravko, D.S.; Hendrickson, O.D.; Vostrikova, N.L.; Chernukha, I.M.; Dzantiev, B.B.; Zherdev, A.V. Lateral flow immunoassay to detect the addition of beef, pork, lamb, and horse muscles in raw meat mixtures and finished meat products. Foods 2020, 9, 1662. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Vostrikova, N.L.; Chernukha, I.M.; Dzantiev, B.B.; Zherdev, A.V. Lateral flow immunoassay for sensitive detection of undeclared chicken meat in meat products. Food Chem. 2021, 344, 128598. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Dzantiev, B.B.; Zherdev, A.V. Sensitive lateral flow immunoassay for the detection of pork additives in raw and cooked meat products. Food Chem. 2021, 359, 129927. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. J. Food Sci. Technol. 2021, 58, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Popravko, D.S.; Zherdev, A.V.; Xu, C.; Dzantiev, B.B. An immunochromatographic test system for the determination of lincomycin in foodstuffs of animal origin. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1141, 122014. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Zherdev, A.V.; Godjevargova, T.; Xu, C.; Dzantiev, B.B. Development of a double immunochromatographic test system for simultaneous determination of lincomycin and tylosin antibiotics in foodstuffs. Food Chem. 2020, 318, 126510. [Google Scholar] [CrossRef] [PubMed]
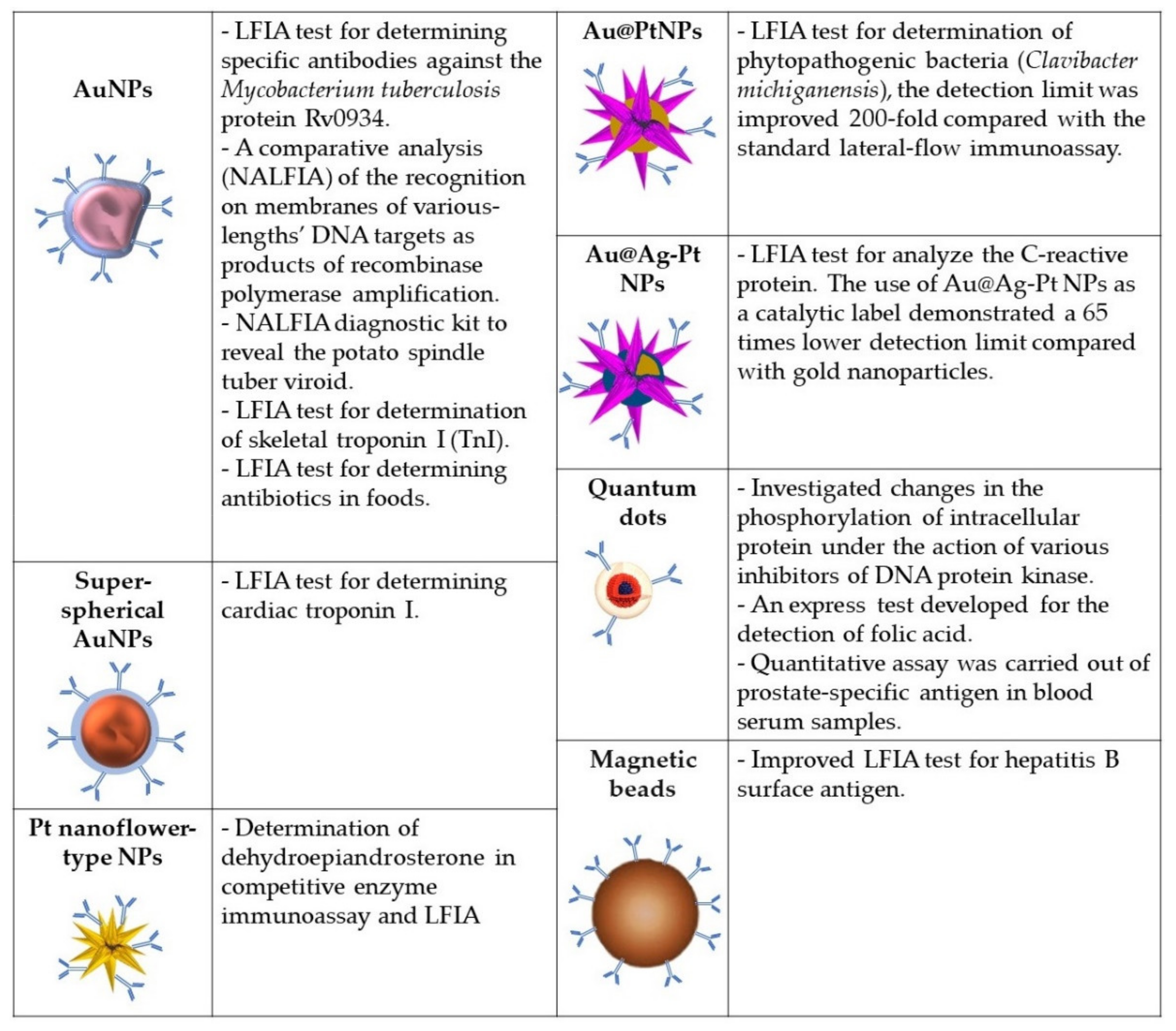
- Byzova, N.A.; Zherdev, A.V.; Khlebtsov, B.N.; Burov, A.M.; Khlebtsov, N.G.; Dzantiev, B.B. Advantages of highly spherical gold nanoparticles as labels for lateral flow immunoassay. Sensors 2020, 20, 3608. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, Q.; Chen, Y.; Shen, D.; Xiao, H.; Eremin, S.A.; Cui, X.; Zhao, S. Platinum nanoflowers with peroxidase-like property in a dual immunoassay for dehydroepiandrosterone. Microchim. Acta 2020, 187, 592. [Google Scholar] [CrossRef] [PubMed]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Urchin peroxidase-mimicking Au@Pt nanoparticles as a label in lateral flow immunoassay: Impact of nanoparticle composition on detection limit of Clavibacter michiganensis. Microchim. Acta 2020, 187, 268. [Google Scholar] [CrossRef] [PubMed]
- Panferov, V.G.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Peroxidase-mimicking nanozyme with surface-dispersed Pt atoms for the colorimetric lateral flow immunoassay of C-reactive protein. Microchim. Acta 2021, F8, 309. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, N.; Lafont, F.; Charlier, C.; Benhelli-Mokrani, H.; Sokolov, P.; Sukhanova, A.; Fleury, F.; Nabiev, I. Comparative advantages and limitations of quantum dots in protein array applications. Meth. Mol. Biol. 2020, 2135, 259–273. [Google Scholar] [CrossRef]
- Novikova, A.S.; Ponomaryova, T.S.; Goryacheva, I.Y. Fluorescent AgInS/ZnS quantum dots microplate and lateral flow immunoassays for folic acid determination in juice samples. Microchim. Acta 2020, 187, 427. [Google Scholar] [CrossRef]
- Tsoy, T.; Karaulov, A.; Nabiev, I.; Sukhanova, A. Multiplexed detection of cancer serum antigens with a quantum dot-based lab-on-bead system. In Quantum Dots: Applications in Biology, Methods in Molecular Biology, 3rd ed.; Fontes, A., Santos, B.S., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2020; Volume 2135, pp. 225–236. [Google Scholar] [CrossRef]
- Bragina, V.A.; Orlov, A.V.; Znoyko, S.L.; Pushkarev, A.V.; Novichikhin, D.O.; Guteneva, N.V.; Nikitin, M.P.; Gorshkov, B.G.; Nikitin, P.I. Nanobiosensing based on optically selected antibodies and superparamagnetic labels for rapid and highly sensitive quantification of polyvalent hepatitis B surface antigen. Anal. Methods 2021, 13, 2424–2433. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef] [PubMed]
- Calabria, D.; Calabretta, M.M.; Zangheri, M.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Michelini, E.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; et al. Recent Advancements in Enzyme-Based Lateral Flow Immunoassays. Sensors 2021, 21, 3358. [Google Scholar] [CrossRef]
- Safenkova, I.V.; Panferov, V.G.; Panferova, N.A.; Varitsev, Y.A.; Zherdev, A.V.; Dzantiev, B.B. Alarm lateral flow immunoassay for detection of the total infection caused by the five viruses. Talanta 2019, 195, 739–744. [Google Scholar] [CrossRef]
- Tang, R.H.; Liu, L.N.; Zhang, S.F.; He, X.C.; Li, X.J.; Xu, F.; Ni, Y.H.; Li, F. A review on advances in methods for modification of paper supports for use in point-of-care testing. Microchim. Acta 2019, 186, 521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ding, L.; Wu, Y.; Huang, X.; Lai, W.; Xiong, Y. Emerging strategies to develop sensitive AuNP-based ICTS nanosensors. TrAC Trend. Anal. Chem. 2019, 112, 147–160. [Google Scholar] [CrossRef]
- Safenkova, I.V.; Ivanov, A.V.; Slutskaya, E.S.; Samokhvalov, A.V.; Zherdev, A.V.; Dzantiev, B.B. Key significance of DNA-target size in lateral flow assay coupled with recombinase polymerase amplification. Anal. Chim. Acta 2020, 1102, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Shanwar, S.; Zvyagin, A.V.; Deyev, S.M.; Balalaeva, I.V. Photoluminescent nanomaterials for medical biotechnology. Acta Natur. 2021, 13, 16–31. [Google Scholar] [CrossRef]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional gold-based nanocomposites for theranostics. Biomaterials 2016, 108, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Smyshlyaeva, A.S.; Shilyagina, N.Y.; Shanwar, S.; Kostyuk, A.B.; Shulga, A.A.; Konovalova, E.V.; Zvyagin, A.V.; Deyev, S.M.; Petrov, R.V. Multifunctional Complexes Based on Photoluminescent Upconversion Nanoparticles for Theranostics of the HER2-Positive Tumors Dokl. Biochem. Biophys. 2020, 491, 73–76. [Google Scholar] [CrossRef]
- Shramova, E.I.; Kotlyar, A.B.; Lebedenko, E.N.; Deyev, S.M.; Proshkina, G.M. Near-Infrared Activated Cyanine Dyes As Agents for Photothermal Therapy and Diagnosis of Tumors. Acta Natur. 2020, 12, 102–113. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, I.M.; Tuzikov, A.B.; Nizovtsev, A.V.; Baidakova, L.K.; Galanina, O.E.; Shilova, N.V.; Ziganshina, M.M.; Dolgushina, N.V.; Bayramova, G.R.; Sukhikh, G.T.; et al. SARS-CoV-2 peptide bioconjugates designed for antibody diagnostics. Bioconjug. Chem. 2021, 32, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, R.; Flegel, W.A.; Srivastava, K.; Williams, E.C.; Ryzhov, I.; Tuzikov, A.; Galanina, O.; Shilova, N.; Sukhikh, G.; Perry, H.; et al. COVID-19 antibody screening with SARS-CoV-2 red cell kodecytes using routine serologic diagnostic platforms. Transfusion 2021, 61, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Bobik, T.V.; Kostin, N.N.; Skryabin, G.A.; Tsabai, P.N.; Simonova, M.A.; Knorre, V.D.; Stratienko, O.N.; Aleshenko, N.L.; Vorobiev, I.I.; Khurs, E.N.; et al. COVID-19 in Russia: Clinical and immunological features of the first-wave patients. Acta Natur. 2021, 13, 102–115. [Google Scholar] [CrossRef]
- Bobik, T.V.; Kostin, N.N.; Skryabin, G.A.; Tsabai, P.N.; Simonova, M.A.; Knorre, V.D.; Mokrushina, Y.A.; Smirnov, I.V.; Kosolapova, J.A.; Vtorushina, V.V.; et al. Epitope-specific response of human milk immunoglobulins in COVID-19 recovered women. Pathogens 2021, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, J.; Eremin, S.; Dias, A.C.P.; Zhang, X. Development of ELISA and chemiluminescence enzyme immunoassay for quantification of histamine in drug products and food samples. Anal. Bioanal. Chem. 2020, 412, 4739–4747. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.V.; Karatovskaya, A.P.; Noskov, A.N.; Shepelyakovskaya, A.O.; Shchannikova, M.P.; Loskutova, I.V.; Artyemieva, O.A.; Nikanova, D.A.; Gladyr, E.A.; Brovko, F.A. Immunochemical assay with monoclonal antibodies for detection of staphylococcal enterotoxin H. J. Food Drug Anal. 2018, 26, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Shepelyakovskaya, A.; Rudenko, N.; Karatovskaya, A.; Shchannikova, M.; Shulcheva, I.; Fursova, K.; Zamyatina, A.; Boziev, K.; Oleinikov, V.; Brovko, F. Development of a bead-based multiplex assay for the simultaneous quantification of three staphylococcal enterotoxins in food by flow cytometry. Food Anal. Meth. 2020, 13, 1202–1210. [Google Scholar] [CrossRef]
- Savvateeva, E.N.; Yukina, M.Y.; Nuralieva, N.F.; Filippova, M.A.; Gryadunov, D.A.; Troshina, E.A. Multiplex autoantibody detection in patients with autoimmune polyglandular syndromes. Int. J. Mol. Sci. 2021, 22, 5502. [Google Scholar] [CrossRef]
- Antipchik, M.; Polyakov, D.; Sinitsyna, E.; Dzhuzha, A.; Shavlovsky, M.; Korzhikova-Vlakh, E.; Tennikova, T. Towards the development of a 3-D biochip for the detection of hepatitis C virus. Sensors 2020, 20, 2719. [Google Scholar] [CrossRef] [PubMed]
- Runina, A.V.; Shpilevaya, M.V.; Katunin, G.L.; Kubanov, A.A. Differential serodiagnostics of latent stages of syphilis based on measuring IgG and IgM levels towards extended panel of recombinant antigens of T. pallidum. Bull. Exp. Biol. Med. 2020, 169, 470–473. [Google Scholar] [CrossRef]
- Shlyapnikov, Y.M.; Malakhova, E.A.; Shlyapnikova, E.A. Improving immunoassay performance with cleavable blocking of microarrays. Anal. Chem. 2021, 93, 1126–1134. [Google Scholar] [CrossRef]
- Shirokov, D.A.; Manuvera, V.A.; Miroshina, O.A.; Dubovoi, A.S.; Samuseva, G.N.; Dmitrieva, M.E.; Lazarev, V.N. Generation of recombinant VP3 protein of infectious bursal disease virus in three different expression systems, antigenic analysis of the obtained polypeptides and development of an ELISA test. Arch. Virol. 2020, 165, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Dobrochaeva, K.; Khasbiullina, N.; Shilova, N.; Antipova, N.; Obukhova, P.; Galanina, O.; Gorbach, M.; Popova, I.; Khaidukov, S.; Grishchenko, N.; et al. Human natural antibodies recognizing glycan Galβ1-3GlcNAc (LeC). Int. J. Mol. Sci. 2020, 21, 6511. [Google Scholar] [CrossRef]
- Orlov, A.V.; Pushkarev, A.V.; Znoyko, S.L.; Novichikhin, D.O.; Bragina, V.A.; Gorshkov, B.G.; Nikitin, P.I. Multiplex label-free biosensor for detection of autoantibodies in human serum: Tool for new kinetics-based diagnostics of autoimmune diseases. Biosens. Bioelectron. 2020, 159, 112187. [Google Scholar] [CrossRef] [PubMed]
- Pushkarev, A.V.; Orlov, A.V.; Znoyko, S.L.; Novichikhin, D.O.; Bragina, V.A.; Sizikov, A.A.; Alipour, E.; Ghourchian, H.; Nikitin, A.I.; Sorokin, G.M.; et al. Data on characterization of glass biochips and validation of the label-free biosensor for detection of autoantibodies in human serum. Data Brief 2020, 30, 105648. [Google Scholar] [CrossRef] [PubMed]
- Larina, E.N.; Karasev, V.S.; Shpilevaya, M.V.; Aliev, T.K.; Bochkova, O.P.; Karamova, A.E.; Balabashin, D.S.; Deryabin, D.G.; Bobik, T.V.; Smirnov, I.V.; et al. Recombinant fragment of the extracellular domain of human desmoglein 3 fused with the Fc-fragment of human IgG1 selectively adsorbs autoreactive antibodies from the sera of pemphigus patients. Dokl. Biochem. Biophys. 2021, 498, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Parshukova, D.A.; Smirnova, L.P.; Kornetova, E.G.; Semke, A.V.; Buneva, V.N.; Ivanova, S.A. IgG-Dependent hydrolysis of myelin basic protein of patients with different courses of schizophrenia. J. Immunol. Res. 2020, 2020, 8986521. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Kabirova, E.M.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the sera of patients with multiple sclerosis recognize and hydrolyze miRNAs. Int. J. Mol. Sci. 2021, 22, 2812. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Kabirova, E.M.; Sizikov, A.E.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the sera of patients with systemic lupus erythematosus hydrolyzed miRNAs. J. Inflamm. Res. 2020, 13, 681–699. [Google Scholar] [CrossRef]
- Kompaneets, I.Y.; Ermakov, E.A.; Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. Secretory immunoglobulin A from human milk hydrolyzes microRNA. J. Dairy Sci. 2020, 103, 6782–6797. [Google Scholar] [CrossRef]
- Svalova, T.S.; Medvedeva, M.V.; Saigushkina, A.A.; Kozitsin, I.V.; Malysheva, N.N.; Zhdanovskikh, V.O.; Okhokhonin, A.V.; Kozitsina, A.N. A label-free impedimetric immunosensor based on covalent immobilization of anti-E.Coli antibody via a copper-catalyzed azide-alkyne cycloaddition reaction. Anal. Bioanal. Chem. 2020, 412, 5077–5087. [Google Scholar] [CrossRef] [PubMed]
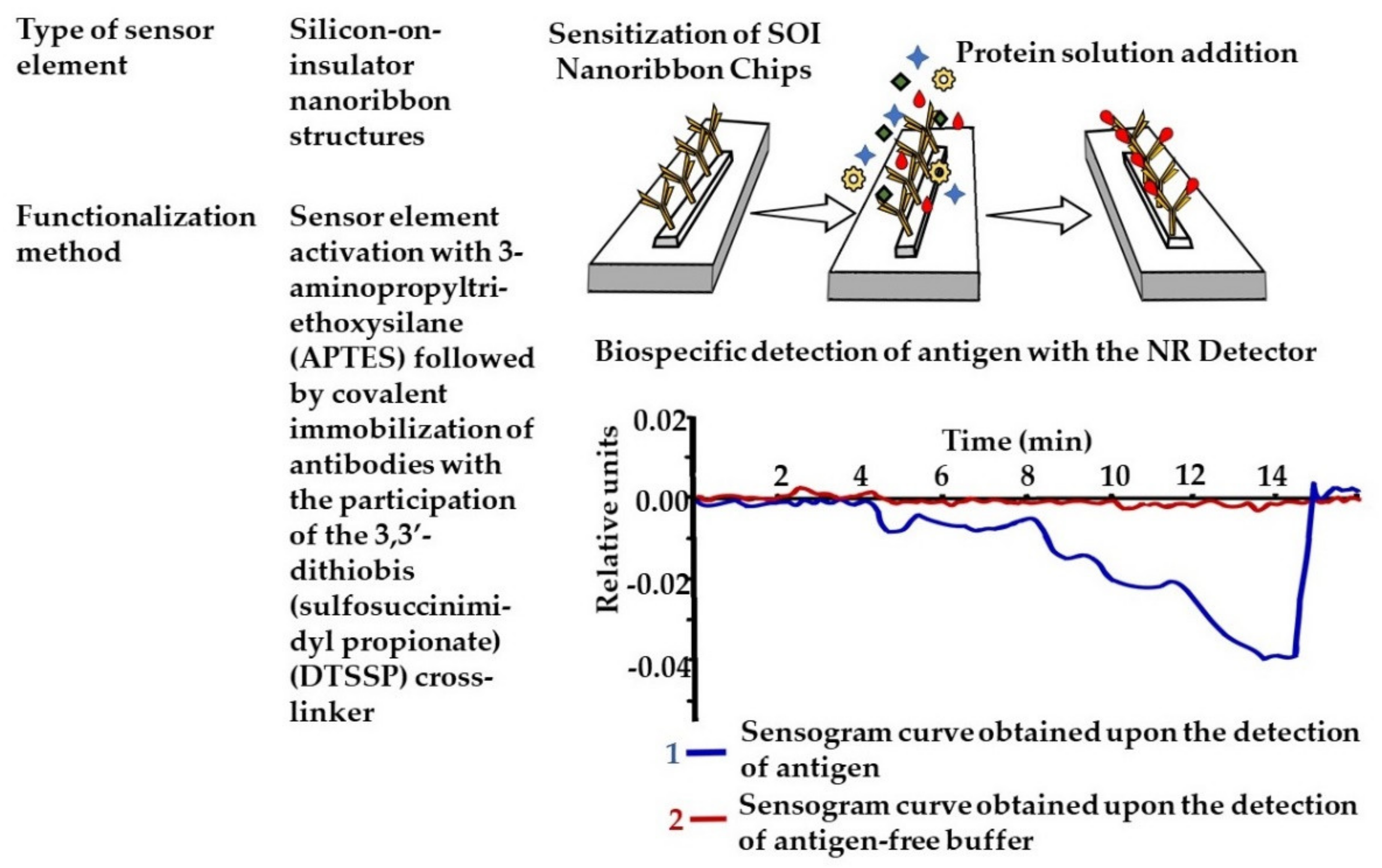
- Malsagova, K.A.; Pleshakova, T.O.; Kozlov, A.F.; Galiullin, R.A.; Popov, V.P.; Tikhonenko, F.V.; Glukhov, A.V.; Ziborov, V.S.; Shumov, I.D.; Petrov, O.F.; et al. Detection of influenza virus using a SOI-nanoribbon chip, based on an N-type field-effect transistor. Biosensors 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
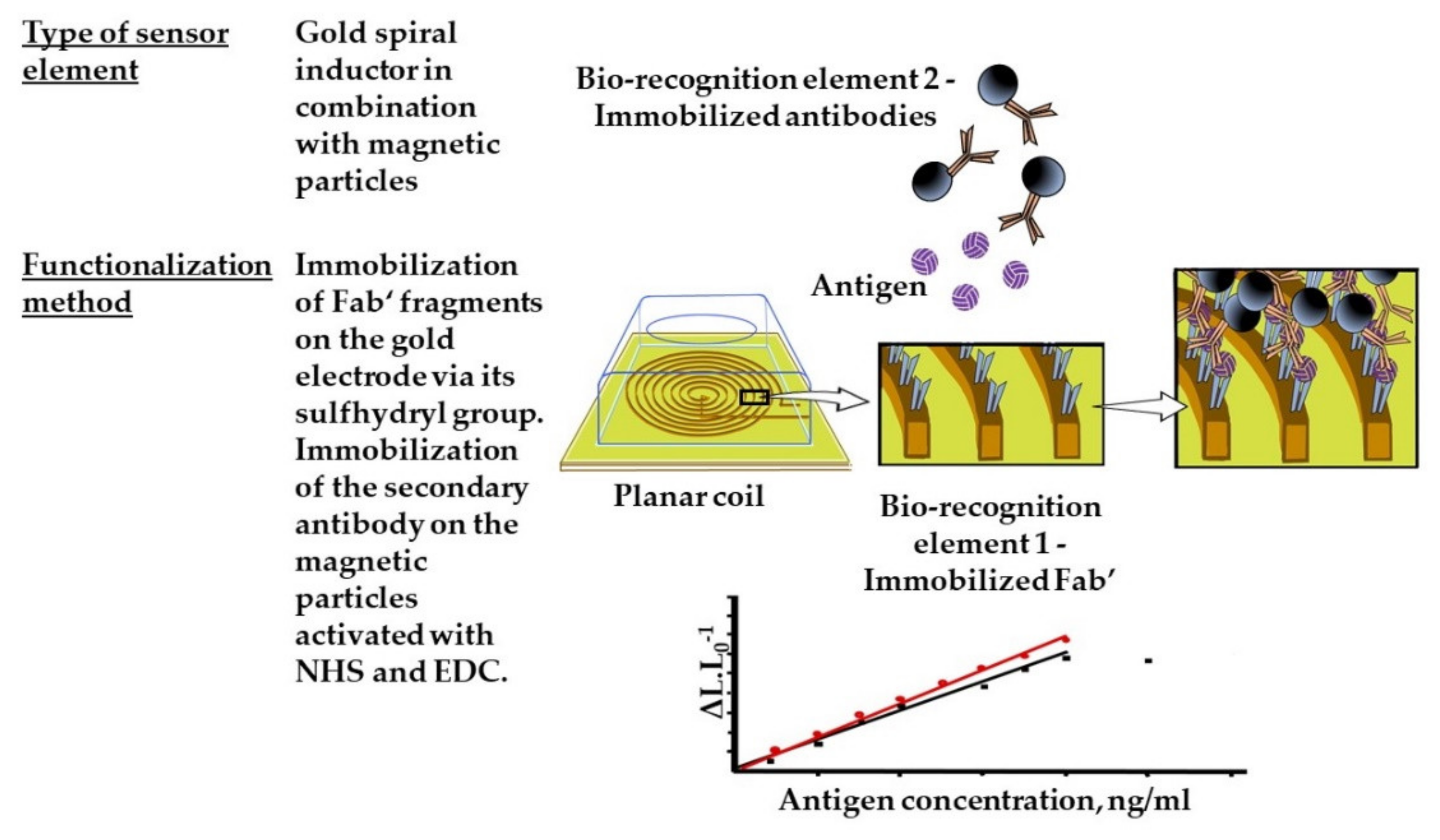
- Alipour, E.; Shariatpanahi, S.P.; Ghourchian, H.; Piro, B.; Fathipour, M.; Boutorabi, S.M.; Znoyko, S.L.; Nikitin, P.I. Designing a magnetic inductive micro-electrode for virus monitoring: Modelling and feasibility for hepatitis B virus. Microchim. Acta 2020, 187, 463. [Google Scholar] [CrossRef]
- Guliy, O.; Zaitsev, B.; Teplykh, A.; Balashov, S.; Fomin, A.; Staroverov, S.; Borodina, I. Acoustical slot mode sensor for the rapid coronaviruses detection. Sensors 2021, 21, 1822. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Dhiman, T.K.; Lakshmi, G.B.V.S.; Berlina, A.N.; Solanki, P.R. A highly sensitive label-free amperometric biosensor for norfloxacin detection based on chitosan-yttria nanocomposite. Int. J. Biol. Macromol. 2020, 151, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Shlyapnikov, Y.M.; Kanev, I.L.; Shlyapnikova, E.A. Rapid ultrasensitive gel-free immunoblotting with magnetic labels. Anal. Chem. 2020, 92, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Shlyapnikov, Y.M.; Malakhova, E.A.; Shlyapnikova, E.A. Rapid detection of femtogram amounts of protein by gel-free immunoblot. Bull. Exp. Biol. Med. 2020, 169, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Ringaci, A.; Alenichev, M.K.; Drozhzhennikova, E.B.; Shevchenko, K.G.; Cherkasov, V.R.; Nikitin, M.P.; Nikitin, P.I. Dynamic light scattering biosensing based on analyte-induced inhibition of nanoparticle aggregation. Anal. Bioanal. Chem. 2020, 412, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
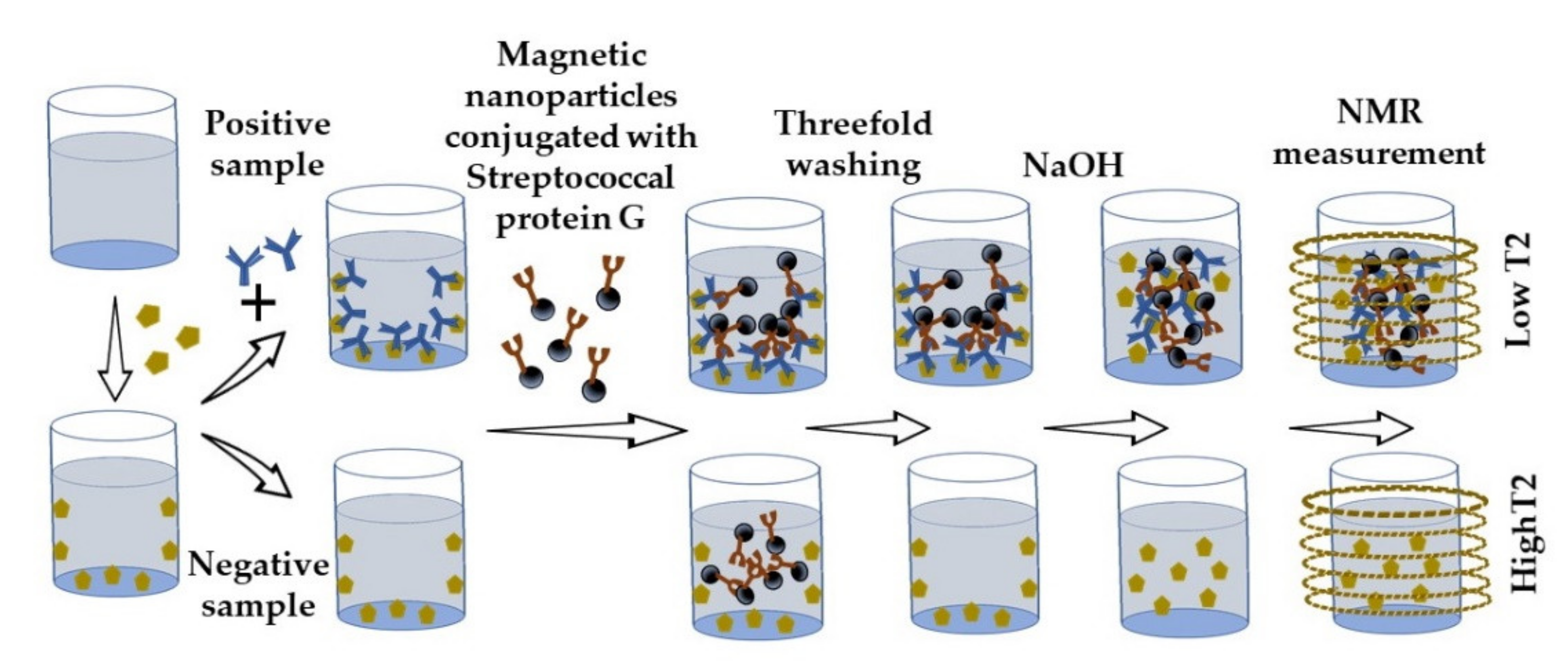
- Khramtsov, P.; Kropaneva, M.; Bochkova, M.; Kiselkov, D.; Timganova, V.; Zamorina, S.; Rayev, M. Nuclear magnetic resonance immunoassay of tetanus antibodies based on the displacement of magnetic nanoparticles. Anal. Bioanal. Chem. 2021, 413, 1461–1471. [Google Scholar] [CrossRef] [PubMed]


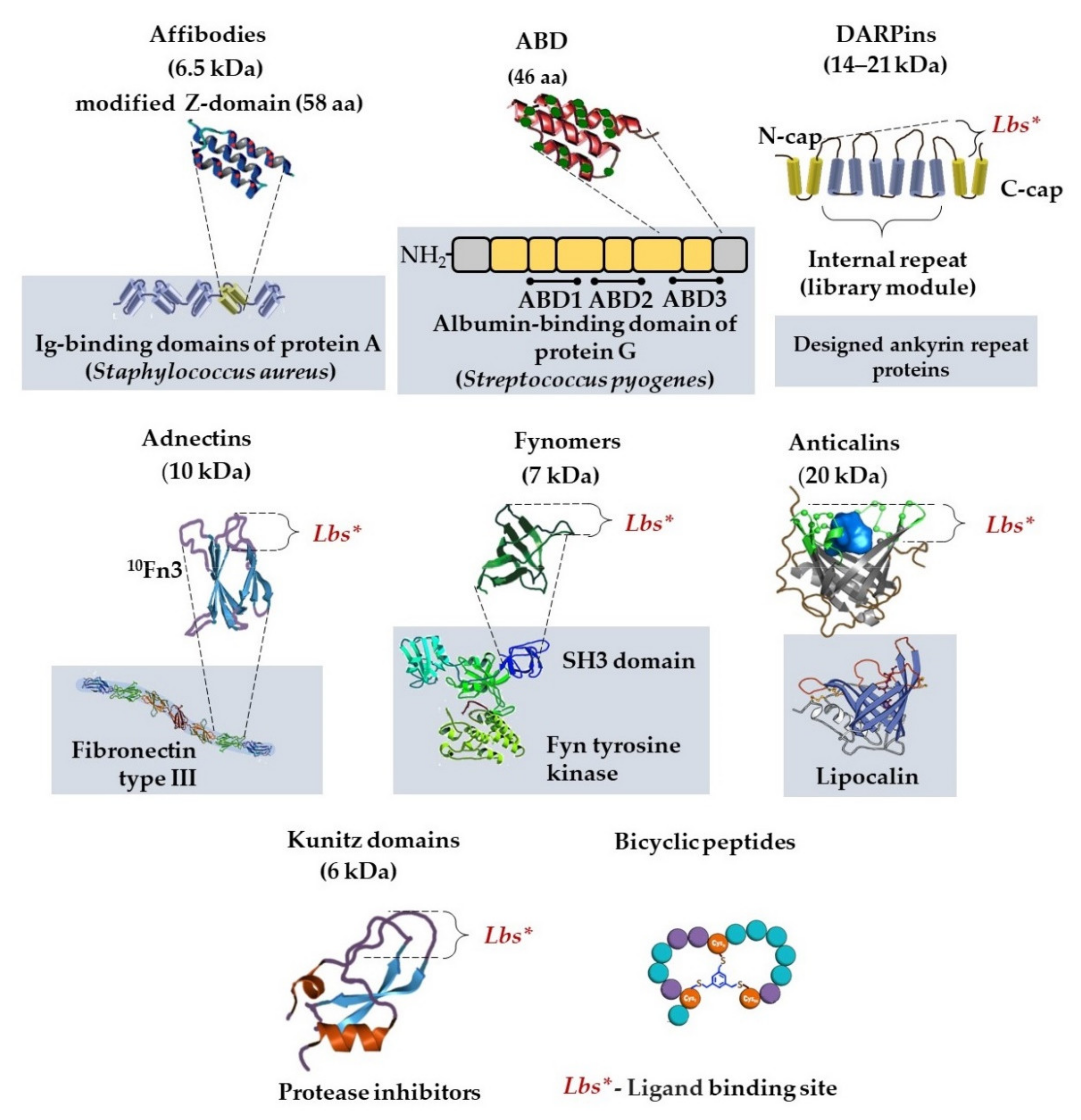

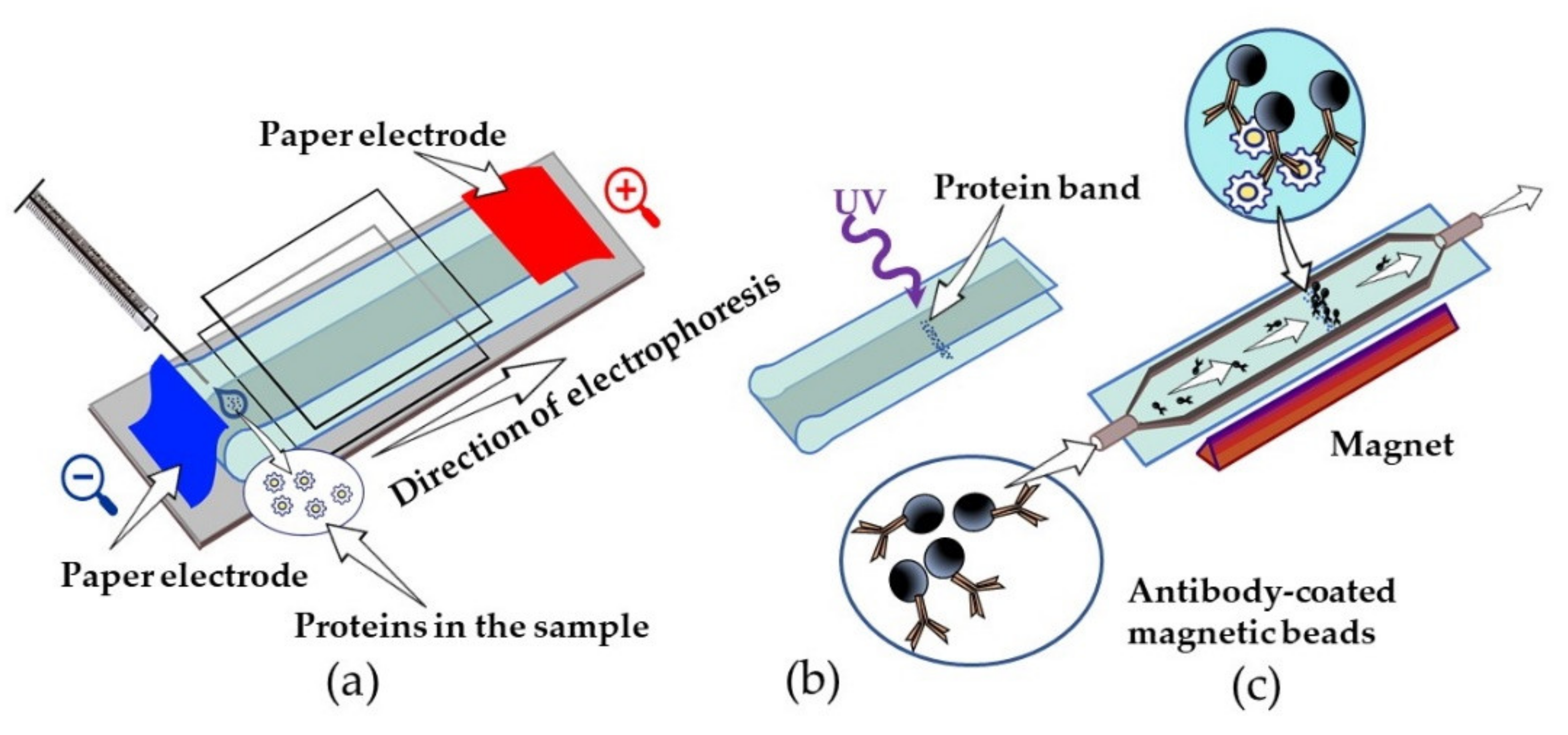
| Scaffold | Size, kDa | Original Protein | Structure | Source | Production System | Homogeneity | Affinity/Specificity |
|---|---|---|---|---|---|---|---|
| Polyclonal antibodies | 150, 900 | Full-size immunoglobulin | Globular proteins | Serum of immune animals | Animal | Heterogeneous with differing paratopes for an antigen | High affinity |
| Monoclonal antibodies | 150 | Full-size immunoglobulin | Globular proteins | Plasma cell of immune animals | Animal | Homogeneous with one paratope | High affinity for one epitope |
| scFv | 28 | Variable domain of IgG heavy and light chains | Predominant β-folding structure described for variable domain IgG | Combinatorial library-constructed-based B-cells | In both prokaryotic (mainly E. coli) and eukaryotic (yeast, insect cells, plant cells, and mammalian cells) systems | Homogeneous | Micromolar–nanomolar after first rounds of biopanning |
| VhH | 12–15 | Variable domain of heavy chain of camelid antibody | Folded β-sheet | Combinatorial library-based B-cells of Camelidae | In both eukaryotic and prokaryotic systems (E. coli, Saccharomyces cerevisiae) | Homogeneous | High target affinity and specificity |
| DARPins | 14–18 | Natural ankyrin repeats | α-helical + β-turn | Combinatorial library | E. coli (up to 200 mg/L) | Homogeneous | Nanomolar–picomolar affinity |
| Affibodies | 6 | Z domain of staphylococcal protein A | α-helical | Combinatorial library | Peptide synthesis and E. coli | Homogeneous | Nanomolar–picomolar affinity |
| ABD | 5 | Albumin-binding domain of streptococcal protein G | α-helical | Combinatorial library | Peptide synthesis and E. coli | Homogeneous | Nanomolar–picomolar affinity |
| Scaffold | Preferred Application | Advantages | Limitations |
|---|---|---|---|
| Polyclonal antibodies | For detecting proteins with low content; as secondary antibodies in immunoassays; as capture antibodies in sandwich EIA; detection in solutions with different pH and salt concentrations; immunoprecipitation | Cost-effective production compared to mAbs, about three months; easily labeled without loss of binding capacity; resistant to minor changes in antigens | High-probability immune cross-reactivity; contain non-target antibodies; background noise; variability of lots |
| Monoclonal antibodies | For quantitative analyses; for staining cells with less background | Stable preparations; production of highly concentrated quantities possible | Costly production, about 6 months; necessary gene engineering (humanization) to reduce immunogenicity |
| scFv | As a capture and/or detection agent in immunosensors and immunoassays: colorimetric (EIA); fluorescence (including FRET); chemiluminescense; luminescense; immuno-PCR; immunoelectron microscopy; electrochemical; quartz-crystal microbalance; SPR; piezoelectric microcantilever; simultaneous recognition of multiple epitopes; using bi- and tri-specific constructs; detection of toxins and venoms | Cost-effective production compared to mAbs; time-consuming procedure selection from combinatorial library (about 2 weeks for primary selection); fast tissue penetration; the possibility of genetically engineered labeling | Lack of effector functions; reduced thermal stability compared to mAbs |
| VhH | As a capture and/or detection agent in immunoassays: radiochemical; crystallography; ELISA; SPR; piezoelectric microcantilever; predominantly for cancer target recognition, bioimaging; drug delivery; detection and neutralization of toxins and venoms; definition of autoantigens | Cost-effective production compared to mAbs; time-consuming procedure selection from combinatorial library (about 1 month for primary selection); highly soluble; thermally and pH stable; fast tissue penetration; special conformational diversity; recognize epitopes, which are not immunogenic for conventional mAbs; low immunogenicity; the possibility of genetically engineered labeling | Lack of effector functions |
| DARPins | Predominantly for cancer target recognition, bioimaging; drug delivery | Cost-effective production compared to mAbs; time-consuming procedure selection from combinatorial library (about 1 month for primary selection); high thermal and pH stability; fast tissue penetration; the possibility of genetically engineered labeling | Lack of effector functions; required to increase the serum half-life by increasing their molecular size |
| Affibodies | Predominantly for cancer target recognition, particulary by electrochemical impedance spectroscopy; bioimaging; drug delivery; detection of toxins | Cost-effective production compared to mAbs; time-consuming procedure selection from combinatorial library (about 1 month for primary selection); high thermal and pH stability; fast tissue penetration; the possibility of genetically engineered labeling | Lack of effector functions; required to increase the serum half-life by increasing their molecular size; immunogenicity of protein A |
| ABD | Cancer target recognition, particulary by immuno-positron emission tomography; bioimaging; drug delivery | Cost-effective production compared to mAbs; time-consuming procedure selection from combinatorial library (about 1 month for primary selection); high thermal and pH stability; fast tissue penetration; the possibility of genetically engineered labeling | Lack of effector functions; immunogenicity of protein G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudenko, N.; Fursova, K.; Shepelyakovskaya, A.; Karatovskaya, A.; Brovko, F. Antibodies as Biosensors’ Key Components: State-of-the-Art in Russia 2020–2021. Sensors 2021, 21, 7614. https://doi.org/10.3390/s21227614
Rudenko N, Fursova K, Shepelyakovskaya A, Karatovskaya A, Brovko F. Antibodies as Biosensors’ Key Components: State-of-the-Art in Russia 2020–2021. Sensors. 2021; 21(22):7614. https://doi.org/10.3390/s21227614
Chicago/Turabian StyleRudenko, Natalia, Ksenia Fursova, Anna Shepelyakovskaya, Anna Karatovskaya, and Fedor Brovko. 2021. "Antibodies as Biosensors’ Key Components: State-of-the-Art in Russia 2020–2021" Sensors 21, no. 22: 7614. https://doi.org/10.3390/s21227614
APA StyleRudenko, N., Fursova, K., Shepelyakovskaya, A., Karatovskaya, A., & Brovko, F. (2021). Antibodies as Biosensors’ Key Components: State-of-the-Art in Russia 2020–2021. Sensors, 21(22), 7614. https://doi.org/10.3390/s21227614






