Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Characterization of Material Properties
2.4. Characterization of Sensing Performances
3. Results and Discussion
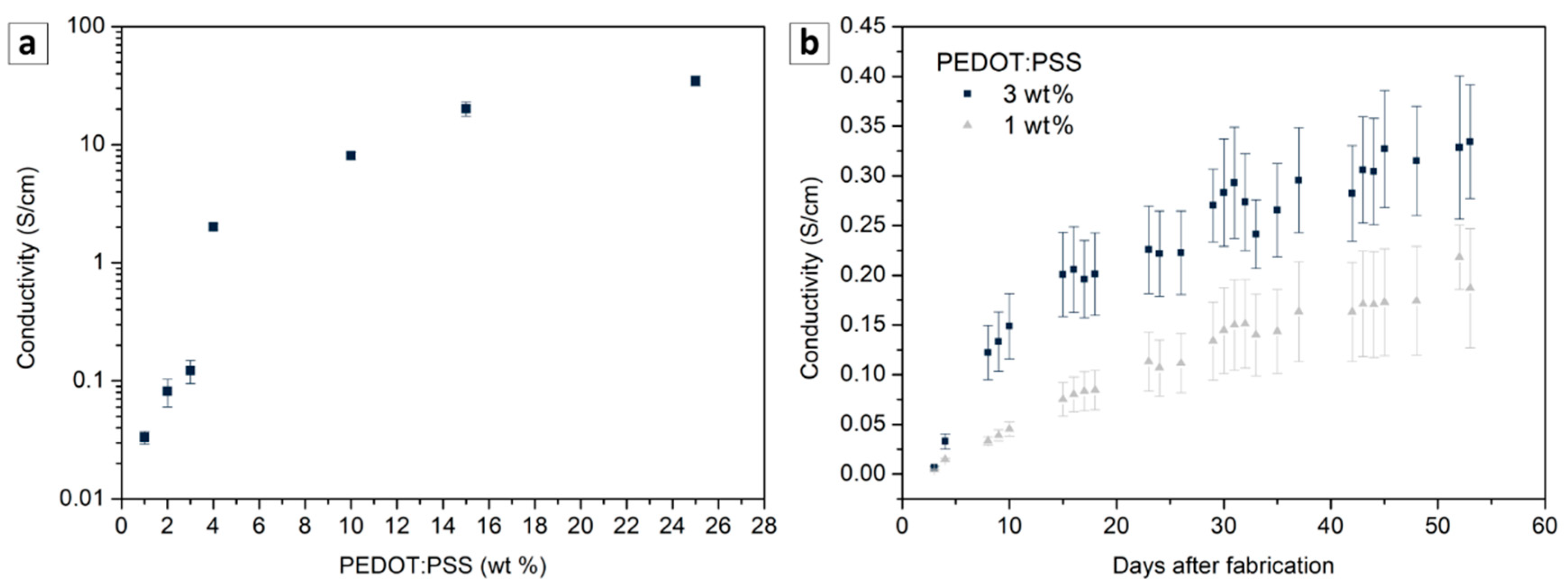
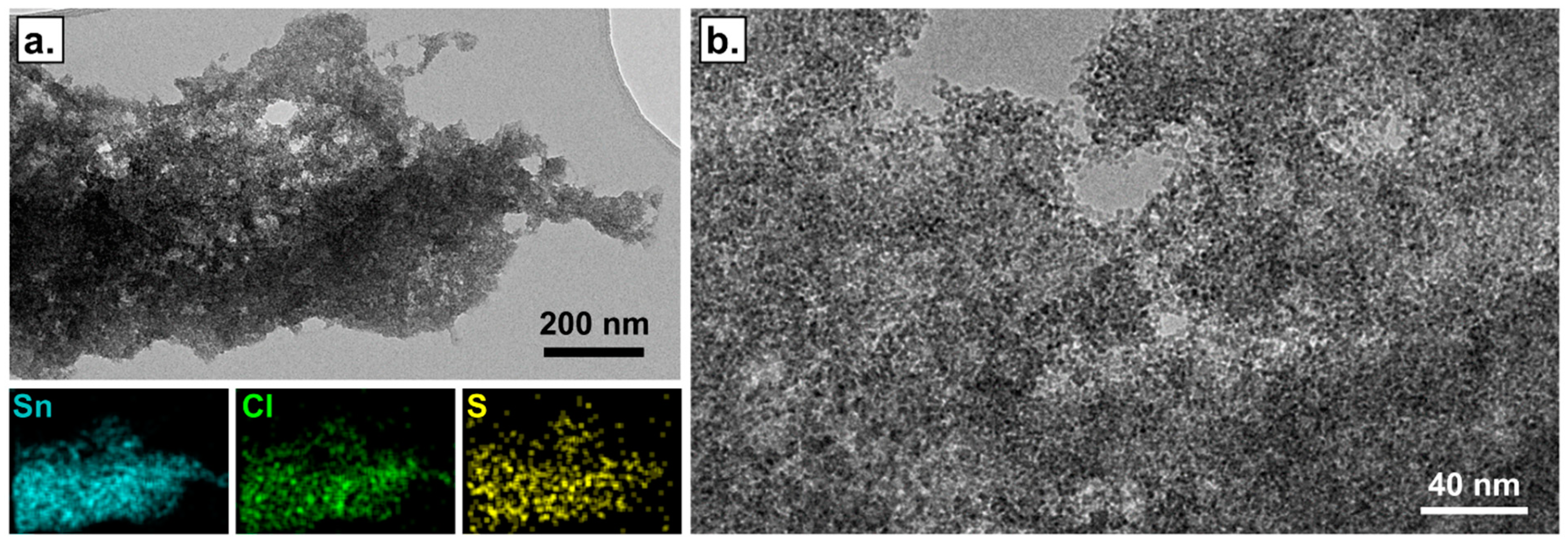
3.1. Material Characterization
3.2. Detetion Performance under 50% of Humidity
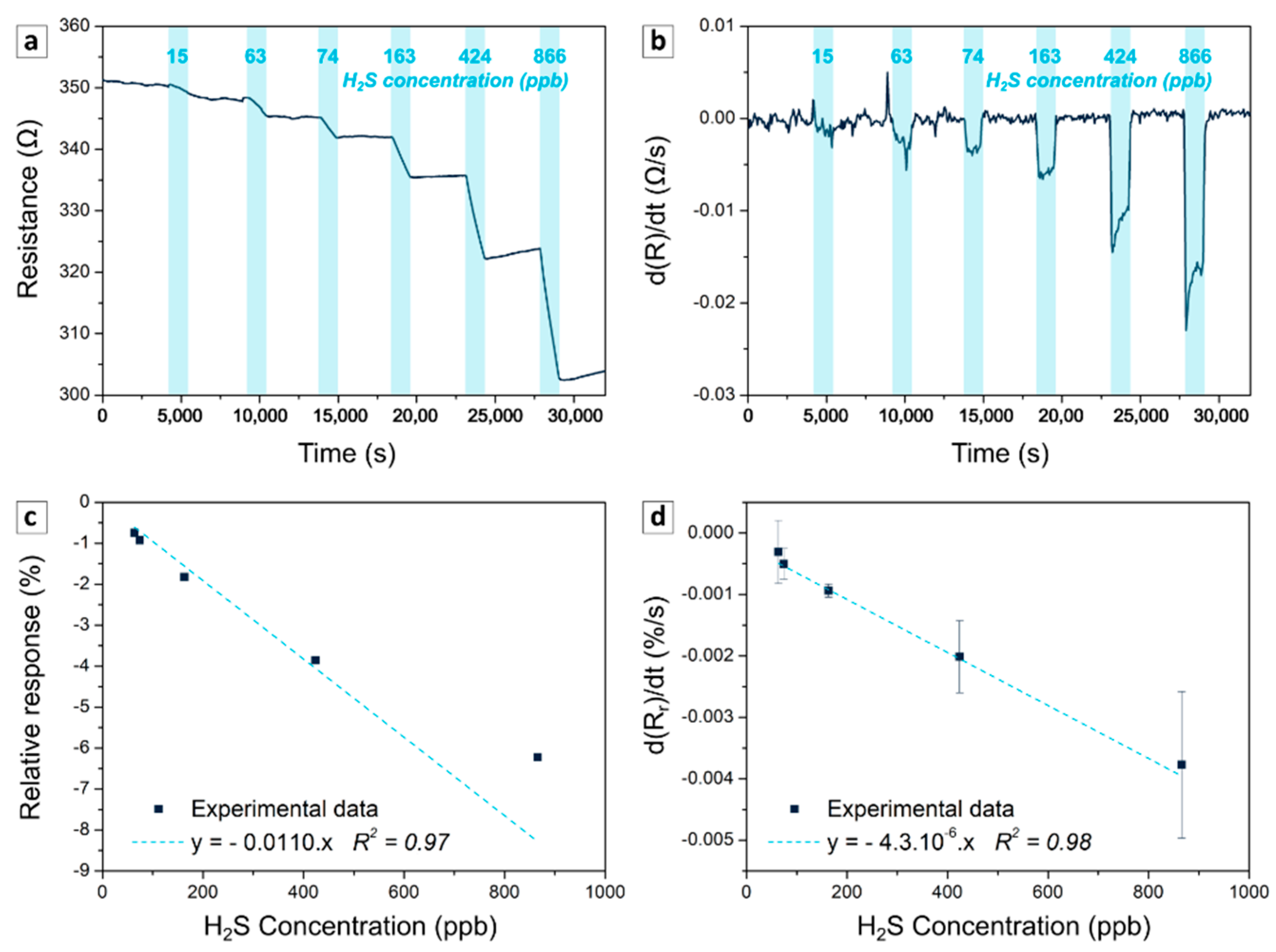
3.2.1. Calibration
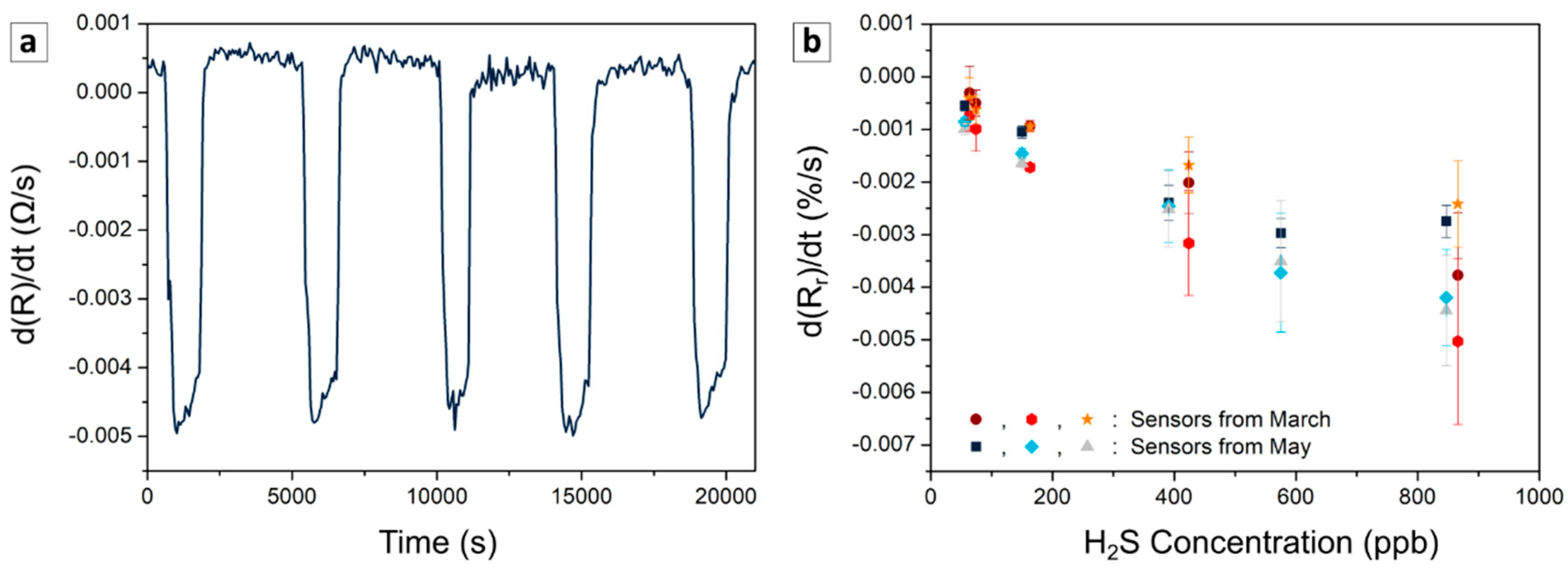
3.2.2. Repeatability, Reproducibility, Stability
3.2.3. Use Case: Detection under Continuous Variation of H2S Concentration
3.3. Impact of Interfering Species
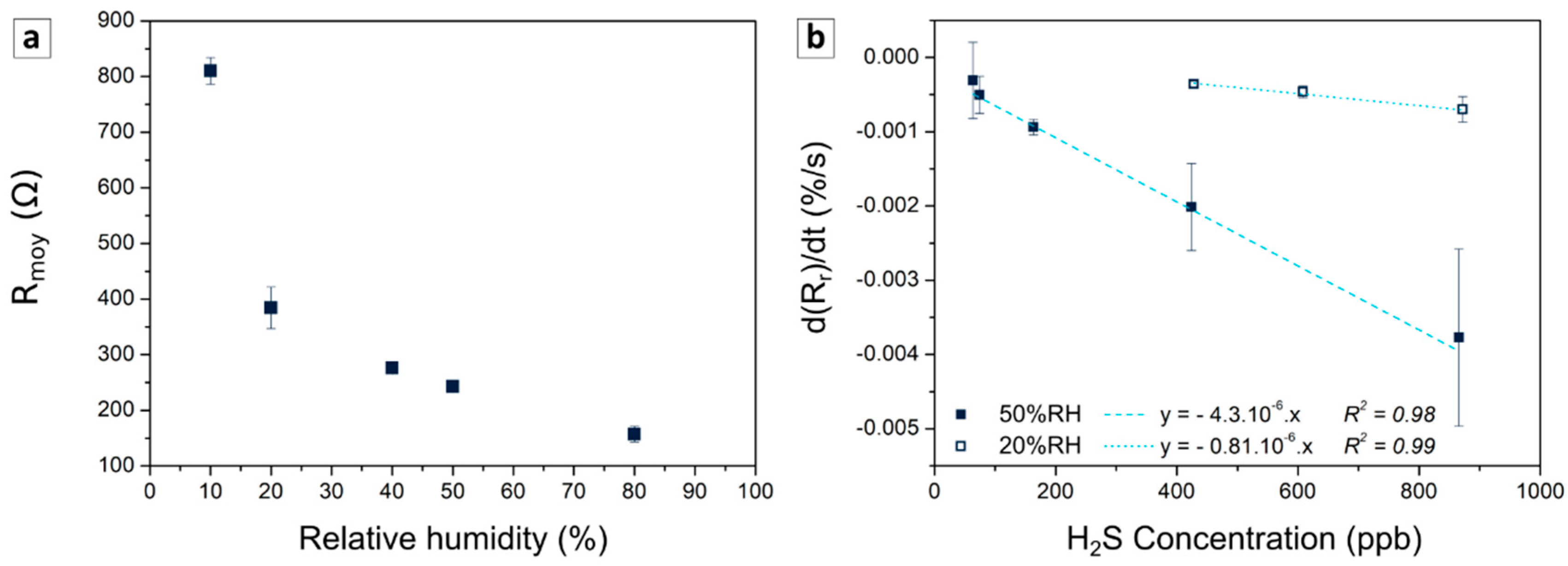
3.3.1. Humidity
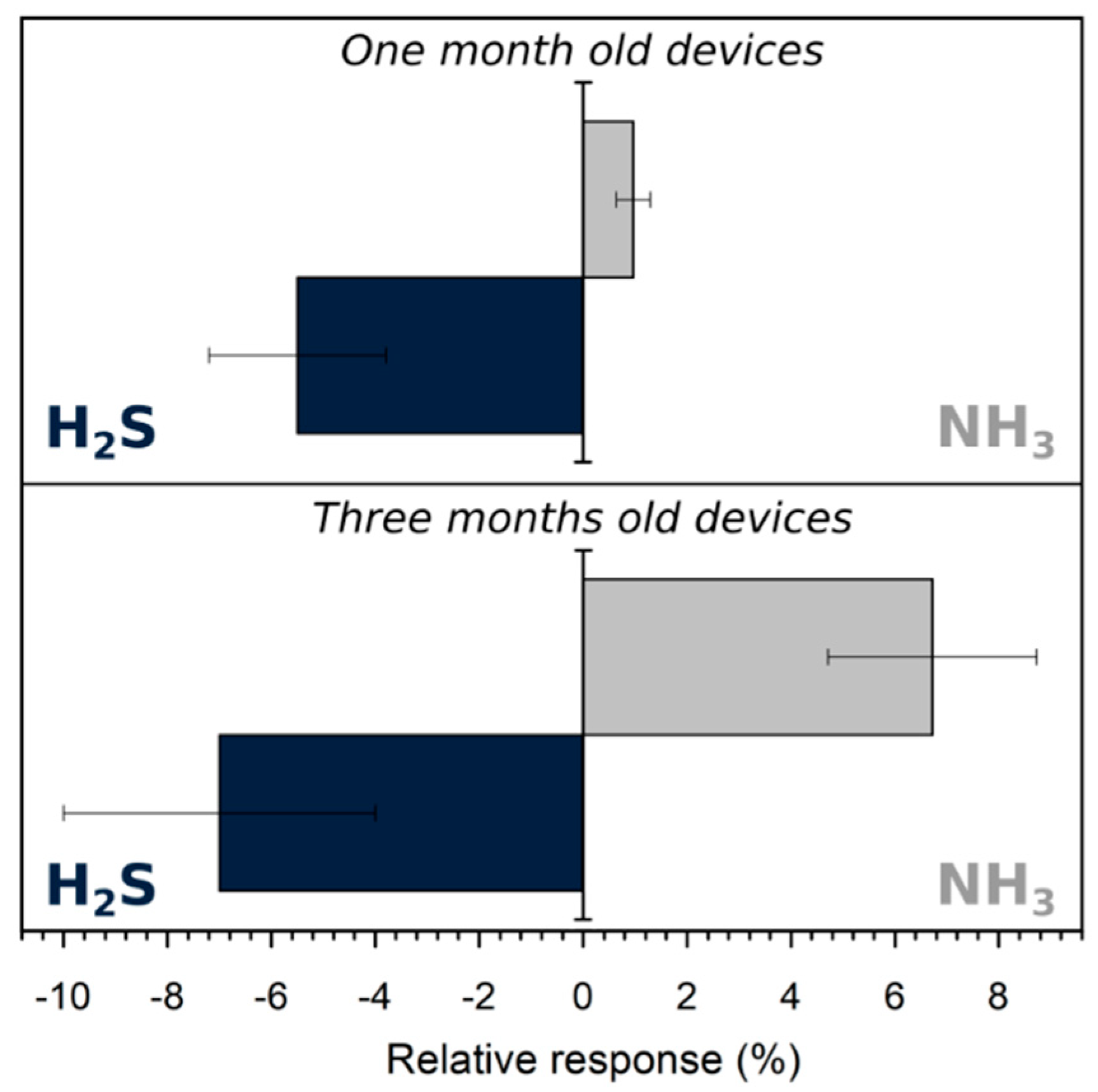
3.3.2. Oxidative Gas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rubright, S.L.M.; Pearce, L.L.; Peterson, J. Environmental toxicology of hydrogen sulfide. Nitric Oxide—Biol. Chem. 2017, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration, USA. OSHA Standards: Hydrogen Sulfide Exposure. Available online: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html (accessed on 29 September 2021).
- National Research Council (US) Committee on Acute Exposure Guideline Levels. 4 Hydrogen Sulfide Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2010; Volume 9, pp. 173–218. ISBN 9780309254816. [Google Scholar]
- Zoccali, M.; Tranchida, P.Q.; Mondello, L. Fast gas chromatography-mass spectrometry: A review of the last decade. TrAC—Trends Anal. Chem. 2019, 118, 444–452. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Franchina, F.A.; Dugo, P.; Mondello, L. Comprehensive two-dimensional gas chromatography- mass spectrometry: Recent evolution and current trends. Mass Spectrom. Rev. 2016, 35, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E.; Brunet, J.; Pauly, A.; Ndiaye, A.; Varenne, C. Nanomaterials for the selective detection of hydrogen sulfide in air. Sensors 2017, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Kim, K.H.; Tang, K.T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC—Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Serban, B.; Cobianu, C.; Brezeanu, M. Hydrogen sulphide sensing review. Ann. Acad. Rom. Sci. Ser. Sci. Technol. Inf. 2014, 7, 55–67. [Google Scholar]
- Wong, Y.C.Y.H.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.C.Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2020, 167, 37503. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, G.; Zeng, G.; Liu, L.; Zhang, C. Metal oxides and metal salt nanostructures for hydrogen sulfide sensing: Mechanism and sensing performance. RSC Adv. 2015, 5, 54793–54805. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Duc, C.; Boukhenane, M.-L.L.; Wojkiewicz, J.-L.L.; Redon, N. Hydrogen sulfide detection by sensors based on conductive polymers: A review. Front. Mater. 2020, 7, 215. [Google Scholar] [CrossRef]
- Virji, S.; Fowler, J.D.; Baker, C.O.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber composites with metal salts: Chemical sensors for hydrogen sulfide. Small 2005, 1, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.; Morrin, A.; Shepherd, R.L.; In Het Panhuis, M.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of polyaniline-based gas sensors using piezoelectric inkjet and screen printing for the detection of hydrogen sulfide. IEEE Sens. J. 2010, 10, 1419–1426. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Peltonen, J.; Lindén, M. Printed hydrogen sulfide gas sensor on paper substrate based on polyaniline composite. Thin Solid Film 2013, 534, 621–628. [Google Scholar] [CrossRef]
- Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Bollström, R.; Gulin-Sarfraz, T.; Peltonen, J.; Linden, M. Stable ink dispersions suitable for roll-to-roll printing with sensitivity towards hydrogen sulphide gas. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 401–407. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Rodner, M.; Eriksson, J. First-order time-derivative readout of epitaxial graphene-based gas sensors for fast analyte determination. Sens. Actuators Rep. 2020, 2, 100012. [Google Scholar] [CrossRef]
- Wu, C.H.; Jiang, G.J.; Chiu, C.C.; Chong, P.; Jeng, C.C.; Wu, R.J.; Chen, J.H. Fast gas concentration sensing by analyzing the rate of resistance change. Sens. Actuators B Chem. 2015, 209, 906–910. [Google Scholar] [CrossRef]
- Dimitriev, O.P. Doping of polyaniline by transition-metal salts. Macromolecules 2004, 37, 3388–3395. [Google Scholar] [CrossRef]
- Dimitriev, O.P. Doping of polyaniline by transition metal salts: Effect of metal cation on the film morphology. Synth. Met. 2004, 142, 299–303. [Google Scholar] [CrossRef]
- Pauw, L.J. van der A method of measuring specific resistivity and hall effect of disc of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Carquigny, S.; Redon, N.; Plaisance, H.; Reynaud, S. Development of a polyaniline/fluoral-P chemical sensor for gaseous formaldehyde detection. IEEE Sens. J. 2012, 12, 1300–1306. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.; Zhang, W.; Dai, Z.; Wei, W.; Luo, S.; Chen, X.; Chen, W.; Rao, F.; Wang, L.; et al. Conjugated System of PEDOT:PSS-Induced Self-Doped PANI for Flexible Zinc-Ion Batteries with Enhanced Capacity and Cyclability. ACS Appl. Mater. Interfaces 2019, 11, 30943–30952. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, S.; Chiolerio, A.; Porro, S.; Accardo, D.; Garino, N.; Bejtka, K.; Perrone, D.; Pirri, C.F. Synthesis of polyaniline-based inks, doping thereof and test device printing towards electronic applications. J. Mater. Chem. C 2013, 1, 5101. [Google Scholar] [CrossRef]
- Arenas, M.C.; Sánchez, G.; Nicho, M.E.; Elizalde-Torres, J.; Castaño, V.M. Engineered doped and codoped polyaniline gas sensors synthesized in N,N,dimethylformamide media. Appl. Phys. A Mater. Sci. Process. 2012, 106, 901–908. [Google Scholar] [CrossRef]
- Quillard, S.; Louarn, G.; Lefrant, S.; Macdiarmid, A.G. Vibrational analysis of polyaniline: A comparative study of leucoemeraldine, emeraldine, and pernigraniline bases. Phys. Rev. B 1994, 50, 12496–12508. [Google Scholar] [CrossRef] [PubMed]
- Trchová, M.; Šeděnková, I.; Konyushenko, E.N.; Stejskal, J.; Ćirić-Marjanović, G. Evolution of polyaniline nanotubes: The oxidation of aniline in water. J. Phys. Chem. B 2006, 110, 9461–9468. [Google Scholar] [CrossRef]
- Siddiqui, G.U.; Sajid, M.; Ali, J.; Kim, S.W.; Doh, Y.H.; Choi, K.H. Wide range highly sensitive relative humidity sensor based on series combination of MoS2 and PEDOT:PSS sensors array. Sens. Actuators B Chem. 2018, 266, 354–363. [Google Scholar] [CrossRef]
- Murugan, C.; Subramanian, E.; Padiyan, D.P. Enhanced sensor functionality of in situ synthesized polyaniline-SnO2 hybrids toward benzene and toluene vapors. Sens. Actuators B Chem. 2014, 205, 74–81. [Google Scholar] [CrossRef]
- Šeděnková, I.; Trchová, M.; Stejskal, J. Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water—FTIR and Raman spectroscopic studies. Polym. Degrad. Stab. 2008, 93, 2147–2157. [Google Scholar] [CrossRef]
- Kong, P.; Liu, P.; Ge, Z.; Tan, H.; Pei, L.; Wang, J.; Zhu, P.; Gu, X.; Zheng, Z.; Li, Z. Conjugated HCl-doped polyaniline for photocatalytic oxidative coupling of amines under visible light. Catal. Sci. Technol. 2019, 9, 753–761. [Google Scholar] [CrossRef]
- Ping, Z.; Nauer, G.E.; Neugebauer, H.; Theiner, J.; Neckel, A. Protonation and electrochemical redox doping processes of polyaniline in aqueous solutions: Investigations using in situ FTIR-ATR spectroscopy and a new doping system. J. Chem. Soc.—Faraday Trans. 1997, 93, 121–129. [Google Scholar] [CrossRef]
- Harada, I.; Furukawa, Y.; Ueda, F. Vibrational Spectra and Structure of Polyaniline and Related Compound. Synt. Met. 1989, 29, E303–E312. [Google Scholar] [CrossRef]
- Tao, S.; Hong, B.; Kerong, Z. An infrared and Raman spectroscopic study of polyanilines co-doped with metal ions and H+. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2007, 66, 1364–1368. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C.; Zeng, Y. Fourier transform infrared spectra of transition metal ion-containing polyanilines synthesized in different reaction conditions. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2007, 66, 37–41. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Bangar, M.A.; Deshusses, M.A.; Myung, N.V.; Mulchandani, A. Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor. Appl. Phys. Lett. 2009, 94, 2012–2015. [Google Scholar] [CrossRef] [Green Version]
- Su, P.G.; Peng, Y.T. Fabrication of a room-temperature H2S gas sensor based on PPy/WO3 nanocomposite films by in-situ photopolymerization. Sensors Actuators, B Chem. 2014, 193, 637–643. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.; Lv, S.; Zhang, K.; Tang, D. Cu2+-Doped SnO2 Nanograin/Polypyrrole Nanospheres with Synergic Enhanced Properties for Ultrasensitive Room-Temperature H2S Gas Sensing. Anal. Chem. 2017, 89, 11135–11142. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Zeng, F.W.; Liu, X.X.; Diamond, D.; Lau, K.T. Humidity sensors based on polyaniline nanofibres. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Cavallo, P.; Acevedo, D.F.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Barbero, C.A. Understanding the sensing mechanism of polyaniline resistive sensors. Effect of humidity on sensing of organic volatiles. Sens. Actuators B Chem. 2015, 210, 574–580. [Google Scholar] [CrossRef]
- Travers, J.P.; Nechtschein, M. Water effects in polyaniline: A new conduction process. Synth. Met. 1987, 21, 135–141. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonia detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]

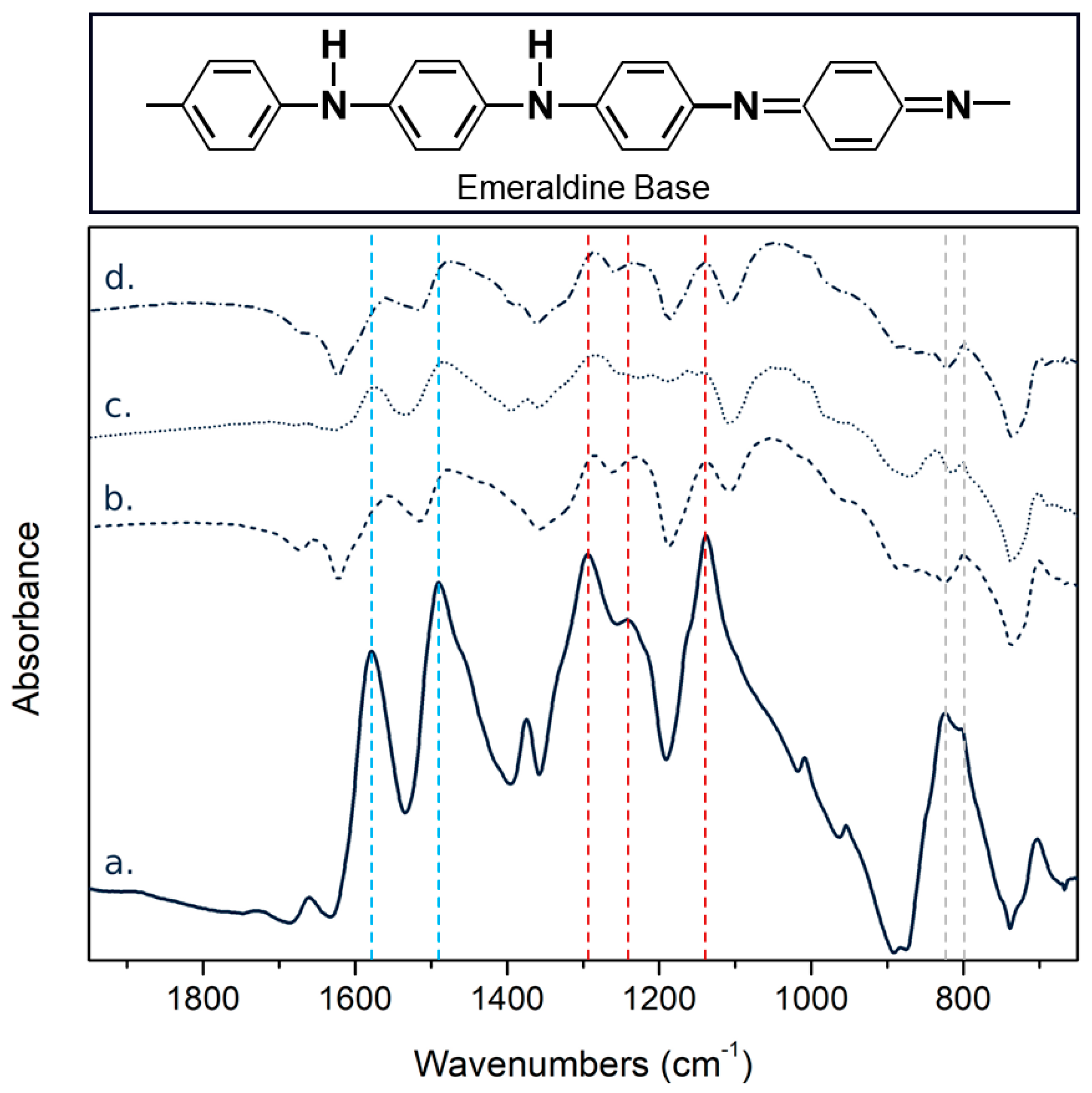

| Samples | PAni | PAni-SnCl2 | PAni-PEDOT:PSS | PAni-SnCl2-PEDOT:PSS |
|---|---|---|---|---|
| C=C (Quinioid, Q) | 1578 | 1556 | 1575 | 1559 |
| C=C (Benzenoid, B) | 1490 | 1478 | 1484 | 1476 |
| C-N or –N= | 1293 | 1284 | 1284 | 1287 |
| C-N+ or C=N+ | 1241 | 1230 | - | 1236 |
| -NH+= | 1138 | 1138 | 1141 | 1141 |
| N=Q=N, SO3− | - | 1055 | 1052 | 1053 |
| C-H out-of-plane for different mode of aromatic rings | 823 | 800 | 835/801 | 800 |
| Ratio IQ/IB | 0.87 | 0.92 | 0.93 | 0.95 |
| Ratio IN+/IN | 0.90 | 1.0 | - | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duc, C.; Boukhenane, M.L.; Fagniez, T.; Khouchaf, L.; Redon, N.; Wojkiewicz, J.-L. Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature. Sensors 2021, 21, 6529. https://doi.org/10.3390/s21196529
Duc C, Boukhenane ML, Fagniez T, Khouchaf L, Redon N, Wojkiewicz J-L. Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature. Sensors. 2021; 21(19):6529. https://doi.org/10.3390/s21196529
Chicago/Turabian StyleDuc, Caroline, Mohamed Lamine Boukhenane, Thomas Fagniez, Lahcen Khouchaf, Nathalie Redon, and Jean-Luc Wojkiewicz. 2021. "Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature" Sensors 21, no. 19: 6529. https://doi.org/10.3390/s21196529
APA StyleDuc, C., Boukhenane, M. L., Fagniez, T., Khouchaf, L., Redon, N., & Wojkiewicz, J.-L. (2021). Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature. Sensors, 21(19), 6529. https://doi.org/10.3390/s21196529







