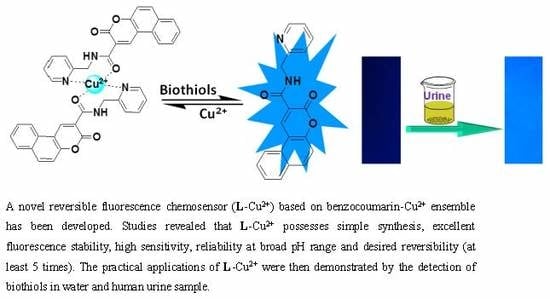
A Copper (II) Ensemble-Based Fluorescence Chemosensor and Its Application in the ‘Naked–Eye’ Detection of Biothiols in Human Urine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Fluorescent Ligand L
2.3. Preparation of the Stock Solutions of L
2.4. Quantum Yield Measurement
2.5. The Study of Reversibility of L-Cu2+
2.6. Visualization of Biothiols in Human Urine
3. Results
3.1. Design, Synthesis of Fluorescent Ligand (L)
3.2. Spectroscopic Properties of L-Cu2+ Ensemble
3.3. Spectroscopic Responses of L-Cu2+ towards Biothiols
3.4. ‘Naked-Eye’ Detection of Biothiols in Human Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Wang, J.; Liu, J.; Ning, L.; Zhu, X.; Yu, B.; Liu, X.; Yao, X.; Zhang, H. Near-infrared and naked-eye fluorescence probe for direct and highly selective detection of cysteine and its application in living cells. Anal. Chem. 2015, 87, 4856–4863. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoon, J. Recent progress in fluorescent and colorimetric chemosensors for detection ofamino acids. Chem. Soc. Rev. 2012, 41, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, L.; Chen, W.; Huang, J.; Huang, C.; Sheng, J.; Song, X. A lysosome-targetable fluorescent probe for simultaneously sensing Cys/Hcy, GSH, and H2S from different signal patterns. ACS Sens. 2018, 3, 2513–2517. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Tsay, O.G.; Lee, K.M.; Churchill, D.G. Selective and competitive cysteine chemosensing: Resettable fluorescent “turn on” aqueous detection via Cu2+ displacement and salicylaldimine hydrolysis. New J. Chem. 2012, 36, 1949–1952. [Google Scholar] [CrossRef]
- Yin, C.; Guo, F.; Zhang, J.; Martinez-Manez, R.; Yang, Y.; Lv, H.; Li, S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Zhang, Y.; Zhang, C.; Jin, J.; Li, H.; Yao, S. A novel colorimetric/fluorescence dual-channel sensor based on NBD for the rapid and highly sensitive detection of cysteine and homocysteine in living cells. Anal. Methods 2016, 8, 2420–2426. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Zhao, C.; Wang, R.; Fei, Q.; Luo, S.; Guo, Z.; Tian, H.; Zhu, W. A dual-response BODIPY-based fluorescent probe for the discrimination of glutathione from cystein and homocystein. Chem. Sci. 2015, 6, 2584–2589. [Google Scholar] [CrossRef]
- He, L.; Xu, Q.; Liu, Y.; Wei, H.; Tang, Y.; Lin, W. Coumarin-Based Turn-On Fluorescence Probe for Specific Detection of Glutathione over Cysteine and Homocysteine. ACS Appl. Mater. Interfaces 2015, 7, 12809–12813. [Google Scholar] [CrossRef]
- Lee, D.; Jeong, K.; Luo, X.; Kim, G.; Yang, Y.; Chen, X.; Kim, S.; Yoon, J. Near-infrared fluorescent probes for the detection of glutathione and their application in the fluorescence imaging of living cells and tumor-bearing mice. J. Mater. Chem. B 2018, 6, 2541–2546. [Google Scholar] [CrossRef]
- Liu, K.; Shang, H.; Kong, X.; Lin, W. A novel near-infrared fluorescent probe with a large Stokes shift for biothiol detection and application in In Vitro and In Vivo fluorescence imaging. J. Mater. Chem. B 2017, 5, 3836–3841. [Google Scholar] [CrossRef]
- Singh, G.; Bains, D.; Singh, H.; Kaur, N.; Singh, N. Polydentate aromatic nanoparticles complexed with Cu2+ for the detection of cysteamine using a smartphone as a portable diagnostic tool. ACS Appl. Nano Mater. 2019, 2, 5841–5849. [Google Scholar] [CrossRef]
- Arabali, V.; Karimi-Maleh, H. Electrochemical determination of cysteamine in the presence of guanine and adenine using a carbon paste electrode modified with N-(4-hydroxyphenyl)-3,5-dinitrobenzamide and magnesium oxide nanoparticles. Anal. Method 2016, 8, 5604–5610. [Google Scholar] [CrossRef]
- Soriano, B.D.; Tam, L.T.; Lu, H.S.; Valladares, V.G. A fluorescent-based HPLC assay for quantification of cysteine and cysteamine adducts in Escherichia coli-derived proteins. J. Chromatogr. B 2012, 880, 27–33. [Google Scholar] [CrossRef]
- Vacek, J.; Klejdus, B.; Petrlova, J.; Lojkova, L.; Kuban, V. A hydrophilic interaction chromatography coupled to a mass spectrometry for the determination of glutathione in plant somatic embryos. Analyst 2006, 131, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Imamura, Y.; Tanaka, H.; Makita, M. Determination of cysteamine and cystamine by gas chromatography with flame photometric detection. J. Pharm. Biomed. Anal. 1993, 11, 963–969. [Google Scholar] [CrossRef]
- Kataoka, H.; Tanaka, H.; Makita, M. Determination pf total cysteamine in urine and plasma samples by gas chromatography with flame photometric detection. J. Chromatogr. B Biomed. Sci. Appl. 1994, 657, 9–13. [Google Scholar] [CrossRef]
- Burford, N.; Eelman, M.D.; Mahony, D.E.; Morash, M. Definitive identification of cysteine and glutathione complexes of bismuth by mass spectrometry: Assessing the biochemical fate of bismuth pharmaceutical agents. Chem. Commun. 2003, 146–147. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Hou, M.; Shi, Y.; Guo, W. Selective fluorescence detection of cysteine over homocysteine and glutathione based on a cysteine-triggered dual michael addition/retro-aza-aldol cascade reaction. Anal. Chem. 2015, 87, 11475–11483. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Gardiner, J.E.; Kim, G.; Yevglevskis, M.; Lloyd, M.D.; Jenkins, A.T.A.; Bull, S.D.; Yoon, J.; James, T.D. Long-wavelength TCF-based fluorescence probes for the detection and intracellular imaging of biological thiols. Chem. Commun. 2018, 54, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Qiao, L.; Yang, W.; Guo, B.; Xin, F.; Jing, J.; Zhang, X. UV-assisted synthesis of long-wavelength Si-pyronine fluorescent dyes for real-time and dynamic imaging of glutathione fluctuation in living cells. J. Mater. Chem. B 2016, 4, 4826–4831. [Google Scholar] [CrossRef]
- Cao, M.; Chen, H.; Chen, D.; Xu, Z.; Liu, S.H.; Chen, X.; Yin, J. Naphthalimide-based fluorescent probe for selectively and specifically detecting glutathione in the lysosomes of living cells. Chem. Commun. 2016, 52, 721–724. [Google Scholar] [CrossRef]
- Guo, F.; Tian, M.; Miao, F.; Zhang, W.; Song, G.; Liu, Y.; Yu, X.; Sun, J.Z.; Wong, W.Y. Lighting up cysteine and homocysteine in sequence based on the kinetic difference of the cyclization/addition reaction. Org. Biomol. Chem. 2013, 11, 7721–7728. [Google Scholar] [CrossRef]
- Liu, T.; Lin, J.; Li, Z.; Lin, L.; Shen, Y.; Zhu, H.; Qian, Y. Imaging of living cells and zebrafish In Vivo using a ratiometric fluorescent probe for hydrogen sulfide. Analyst 2015, 140, 7165–7169. [Google Scholar] [CrossRef]
- Manna, S.; Karmakar, S.; Ali, S.S.; Guria, U.N.; Sarkar, U.N.; Datta, P.; Mandalc, D.; Mahapatra, D. A Michael addition-cyclization-based switch-on fluorescent chemodosimeter for cysteine and its application in live cell imaging. New J. Chem. 2018, 42, 4951–4958. [Google Scholar] [CrossRef]
- Tong, L.; Qian, Y. A NIR rhodamine fluorescent chemodosimeter specific for glutathione: Knoevenagel condensation, detection of intracellular glutathione and living cell imaging. J. Mater. Chem. B 2018, 6, 1791–1798. [Google Scholar] [CrossRef]
- Xu, G.; Tang, Y.; Lin, W. A multi-signal fluorescent probe for the discrimination of cysteine/homocysteine and glutathione and application in living cells and zebrafish. New J. Chem. 2018, 42, 12615–12620. [Google Scholar] [CrossRef]
- Malwal, S.R.; Labade, A.; Andhalkar, A.S.; Sengupta, K.; Chakrapani, H. A highly selective sulfinate ester probe for thiol bioimaging. Chem. Commun. 2014, 50, 11533–11535. [Google Scholar] [CrossRef]
- Ge, C.; Wang, H.; Zhang, B.; Yao, J.; Li, X.; Feng, W.; Zhou, W.; Wang, Y.; Fang, J. A thiol–thiosulfonate reaction providing a novel strategy for turn-on thiol sensing. Chem. Commun. 2015, 51, 14913–14916. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, W.; Wang, W.; Dong, W.; Tong, Y.; Dong, C.; Shuang, S. A two-photon ratiometric fluorescent probe for highly selective sensing of mitochondrial cysteine in live cells. Analyst 2019, 144, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, C.; Li, Y.; Guo, J.; Xiao, J.; Qing, Z.; Li, J.; Yang, R. A Ratiometric two-photon fluorescent cysteine probe with well-resolved dual emissions based on intramolecular charge transfer-mediated two-photon-fret integration mechanism. ACS Sens. 2018, 3, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhou, Y.; Qu, Y.; Xu, Y.; Wang, X.; Liu, X.; Zhang, Q.; Peng, X. A novel AIE plus ESIPT fluorescent probe with a large stokes shift for cysteine and homocysteine: Application in cell imaging and portable kit. Ind. Eng. Chem. Res. 2018, 57, 15216–15223. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, C.S.; Tian, Y.S.; Han, J.H.; Cho, B.R. A two-photon fluorescent probe for thiols in live cells and tissues. J. Am. Chem. Soc. 2010, 132, 1216–1217. [Google Scholar] [CrossRef]
- Han, X.; Song, X.; Yu, F.; Chen, L. A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress. Chem. Sci. 2017, 8, 6991–7002. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, F.; Lin, W. Single fluorescent probe for reversibly detecting copper ions and cysteine in a pure water system. RSC Adv. 2016, 6, 30951–30955. [Google Scholar] [CrossRef]
- Gao, B.; Cui, L.; Pan, Y.; Zhang, G.; Zhou, Y.; Zhang, C.; Shuang, S.; Dong, C. A highly selective ratiometric fluorescent probe for biothiol and imaging in live cells. RSC Adv. 2016, 6, 43028–43033. [Google Scholar] [CrossRef]
- Zhang, M.; Han, H.; Zhang, H.; Wang, C.; Lu, Y.; Zhu, W. A new colorimetric and fluorescent probe with a large stokes shift for rapid and specific detection of biothiols and its application in living cells. J. Mater. Chem. B 2017, 5, 8780–8785. [Google Scholar] [CrossRef]
- Guo, Z.; Nam, S.; Park, S.; Yoon, J. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging. Chem. Sci. 2012, 3, 2760–2765. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Hou, K.; Wu, M.; Huang, Z.; Yu, X. Binol-based fluorescent sensor for recognition of Cu (II) and sulfite anion in water. J. Org. Chem. 2012, 77, 8350–8354. [Google Scholar] [CrossRef]
- Das, P.; Mandal, A.K.; Reddy, U.; Baidya, M.; Ghosh, S.K.; Das, A. Designing a thiol specific fluorescent probe for possible use as a reagent for intracellular detection and estimation in blood serum: Kinetic analysis to probe the role of intramolecular hydrogen bonding. Org. Biomol. Chem. 2013, 11, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Song, H.; Lv, H. Turn-on persistent luminescence probe based on graphitic carbon nitride for imaging detection of biothiols in biological fluids. Anal. Chem. 2013, 85, 11876–11884. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Ou, D.; Li, Q.; Li, Z. An indirect approach for anion detection: The displacement strategy and its application. Chem. Commun. 2012, 48, 8462–8477. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Jung, S.H.; Lee, J.-H.; Lee, S.S.; Jung, J.H. Imidazole-appended p-phenylene-Cu(II) ensemble as a chemoprobe for histidine in biological samples. Chem. Commun. 2014, 50, 15243–15246. [Google Scholar] [CrossRef] [PubMed]
- You, Q.H.; Lee, A.W.M.; Chan, W.H.; Zhua, X.M.; Leung, K.C.F. A coumarin-based fluorescent probe for recognition of Cu2+ and fast detection of histidine in hard-to-transfect cells by a sensing ensemble approach. Chem. Commun. 2014, 50, 6207–6210. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Jia, H.; Succar, P.; Zhao, L.; Zhang, R.; Duan, C.; Zhang, Z. A highly selective and sensitive ON-OFF-ON fluorescence chemosensor for cysteine detection in endoplasmic reticulum. Biosens. Bioelectron. 2015, 74, 461–468. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Q.; Han, Q.; He, G.; Hu, Y.; Feng, H.; Jia, H.; Zhang, R.; Zhang, Z. Selective and sensitive detection of cysteine in water and live cells using a coumarin—Cu2+ fluorescent ensemble. New J. Chem. 2018, 42, 15839–15846. [Google Scholar] [CrossRef]
- Jia, H.; Yang, M.; Meng, Q.; He, G.; Wang, Y.; Hu, Y.; Zhang, R.; Zhang, Z. Synthesis and application of an aldazine-based fluorescence chemosensor for the sequential detection of Cu2+ and biological thiols in aqueous solution and living cells. Sensors 2016, 16, 79. [Google Scholar] [CrossRef]
- Zhang, F.; Liang, X.; Zhang, W.; Wang, Y.; Wang, H.; Mohammed, H.Y.; Song, B.; Zhang, R.; Yuan, J. A unique iridium (III) complex-based chemosensor for multi-signal detection and multi-channel imaging of hypochlorous acid in liver injury. Biosens. Bioelectron. 2017, 87, 1005–1011. [Google Scholar] [CrossRef]
- Xie, X.; Fan, J.; Liang, M.; Li, Y.; Jiao, X.; Wang, X.; Tang, B. A two-photon excitable and ratiometric fluorogenic nitric oxide photoreleaser and its biological applications. Chem. Commun. 2017, 53, 11941–11944. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Tian, M.; Kong, X.; Song, W.; Lu, Y.; Lin, W. Forster resonance energy transfer-based fluorescent probe for the selective imaging of hydroxylamine in living cells. Anal. Chem. 2019, 91, 11397–11402. [Google Scholar] [CrossRef] [PubMed]
- Wi, Y.; Le, H.T.; Verwilst, P.; Sunwoo, K.; Kim, S.J.; Song, J.E.; Yoon, H.Y.; Han, G.; Kim, J.S.; Kang, C.; et al. Modulating the GSH/Trx selectivity of a fluorogenic disulfide-based thiol sensor to reveal diminished GSH levels under ER stress. Chem. Commun. 2018, 54, 8897–8900. [Google Scholar]
- Zhang, H.; Chen, J.; Xiong, H.; Zhang, Y.; Chen, W.; Sheng, J.; Song, X. An endoplasmic reticulum-targetable fluorescent probe for highly selective detection of hydrogen sulfide. Org. Biomol. Chem. 2019, 17, 1436–1441. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; Zhao, Y.; Fan, D.; Qian, L.; Yang, C.; Zhang, K. Synthesis, characterization and fluorescent properties of two triethylene-glycol dicoumarin-3-carboxylates. Spectrochim. Acta A 2007, 68, 725–727. [Google Scholar] [CrossRef]
- Xie, F.; Tan, H.; Li, Z.; Yang, H. A europium-based fluorescence probe for detection of thiols in urine. Anal. Methods 2014, 6, 6990–6996. [Google Scholar] [CrossRef]
- Sun, S.; Tu, k.; Yan, X. An indicator-displacement assay for naked-eye detection and quantification of histidine in human urine. Analyst 2012, 137, 2124–2128. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; CD-ROM; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Yang, L.; Wang, J.; Yang, L.; Zhang, C.; Zhang, R.; Zhang, Z.; Liu, B.; Jiang, C. Fluorescent paper sensor fabricated by carbazolebasedbprobes for dual visual detection of Cu2+ and gaseous H2S. RSC Adv. 2016, 6, 56384–56391. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Meng, Q.; Jia, H.; Wang, Y.; Kong, X.; Duan, C.; Zhang, Z. A Coumarin-based colorimetric and fluorescent chemosensor for the “Naked-eye” detection of fluoride ion in 100% natural water medium using coated chromatography plates. ChemistrySelect 2016, 1, 4397–4402. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, W.; Liu, S.; Wu, S. A coumarin-based sensitive and selective fluorescent sensor for copper (ii) ions. New J. Chem. 2014, 38, 4434–4439. [Google Scholar] [CrossRef]
- Abel, A.S.; Averin, A.D.; Cheprakov, A.V.; Roznyatovsky, V.A.; Denat, F.; Bessmertnykh-Lemeune, A.; Beletskaya, I.P. 6-Polyamino-substituted quinolines: Synthesis and multiple metal (CuII, HgII and ZnII) monitoring in aqueous media. Org. Biomol. Chem. 2019, 17, 4243–4260. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Frémond, L.; Espinosa, E.; Guilard, R.; Ou, Z.; Kadish, K.M. Synthesis, characterization, and x-ray crystal structures of cyclam derivatives. 5. copper (II) binding studies of a pyridine-strapped 5, 12-dioxocyclam-based macrobicycle. Inorg. Chem. 2004, 43, 5572–5587. [Google Scholar] [CrossRef] [PubMed]
- DujolsFrancis, V.; Czarnik, F.W. A Long-wavelength fluorescent chemodosimeter selective for Cu (II) ion in water. J. Am. Chem. Soc. 1997, 119, 7386–7387. [Google Scholar]
- Yang, Z.; She, M.; Zhang, J.; Chen, X.; Huang, Y.; Zhu, H.; Liu, P.; Li, J.; Shi, Z. Highly sensitive and selective rhodamine Schiff base “off-on” chemosensors for Cu2+ imaging in living cells. Sens. Actuators B 2013, 176, 482–487. [Google Scholar] [CrossRef]
- Cao, X.; Lin, W.; He, L. A Near-infrared fluorescence turn-on sensor for sulfide anions. Org. Lett. 2011, 13, 4716–4719. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Singh, A.; Gupta, P.; Mittal, A.; Jose, D.A. Selective detection of cyanide in water and biological samples by an off-the-shelf compound. ACS Sens. 2016, 1, 1265–1271. [Google Scholar] [CrossRef]
- Feng, H.; Wang, Y.; Liu, J.; Zhang, Z.Q.; Yang, X.Y.; Chen, R.; Meng, Q.T.; Zhang, R. A highly specific fluorescent probe for rapid detection of hypochlorous acid In Vivo and in water samples. J. Mater. Chem. B 2019, 7, 3909–3916. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Z.Q.; Meng, Q.T.; Jia, H.M.; Wang, Y.; Zhang, R. Rapid response fluorescence probe enabled In Vivo diagnosis and assessing treatment response of hypochlorous acid-mediated rheumatoid arthritis. Adv. Sci. 2018, 5, 1800397. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, H.; Li, H.; Yang, X.; Jia, H.; Kang, W.; Meng, Q.; Zhang, Z.; Zhang, R. A Copper (II) Ensemble-Based Fluorescence Chemosensor and Its Application in the ‘Naked–Eye’ Detection of Biothiols in Human Urine. Sensors 2020, 20, 1331. https://doi.org/10.3390/s20051331
Wang Y, Feng H, Li H, Yang X, Jia H, Kang W, Meng Q, Zhang Z, Zhang R. A Copper (II) Ensemble-Based Fluorescence Chemosensor and Its Application in the ‘Naked–Eye’ Detection of Biothiols in Human Urine. Sensors. 2020; 20(5):1331. https://doi.org/10.3390/s20051331
Chicago/Turabian StyleWang, Yue, Huan Feng, Haibo Li, Xinyi Yang, Hongmin Jia, Wenjun Kang, Qingtao Meng, Zhiqiang Zhang, and Run Zhang. 2020. "A Copper (II) Ensemble-Based Fluorescence Chemosensor and Its Application in the ‘Naked–Eye’ Detection of Biothiols in Human Urine" Sensors 20, no. 5: 1331. https://doi.org/10.3390/s20051331
APA StyleWang, Y., Feng, H., Li, H., Yang, X., Jia, H., Kang, W., Meng, Q., Zhang, Z., & Zhang, R. (2020). A Copper (II) Ensemble-Based Fluorescence Chemosensor and Its Application in the ‘Naked–Eye’ Detection of Biothiols in Human Urine. Sensors, 20(5), 1331. https://doi.org/10.3390/s20051331