Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intraoperative HS Image Acquisition System
2.2. In Vivo Human-Brain HS Dataset
2.3. HS Data Preprocessing and Band Selection
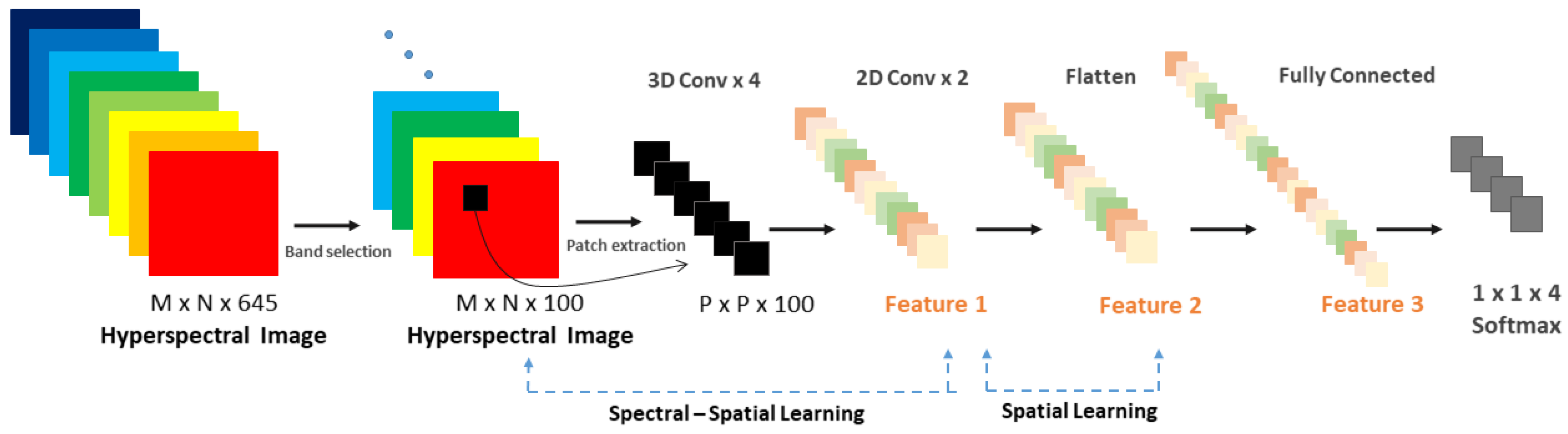
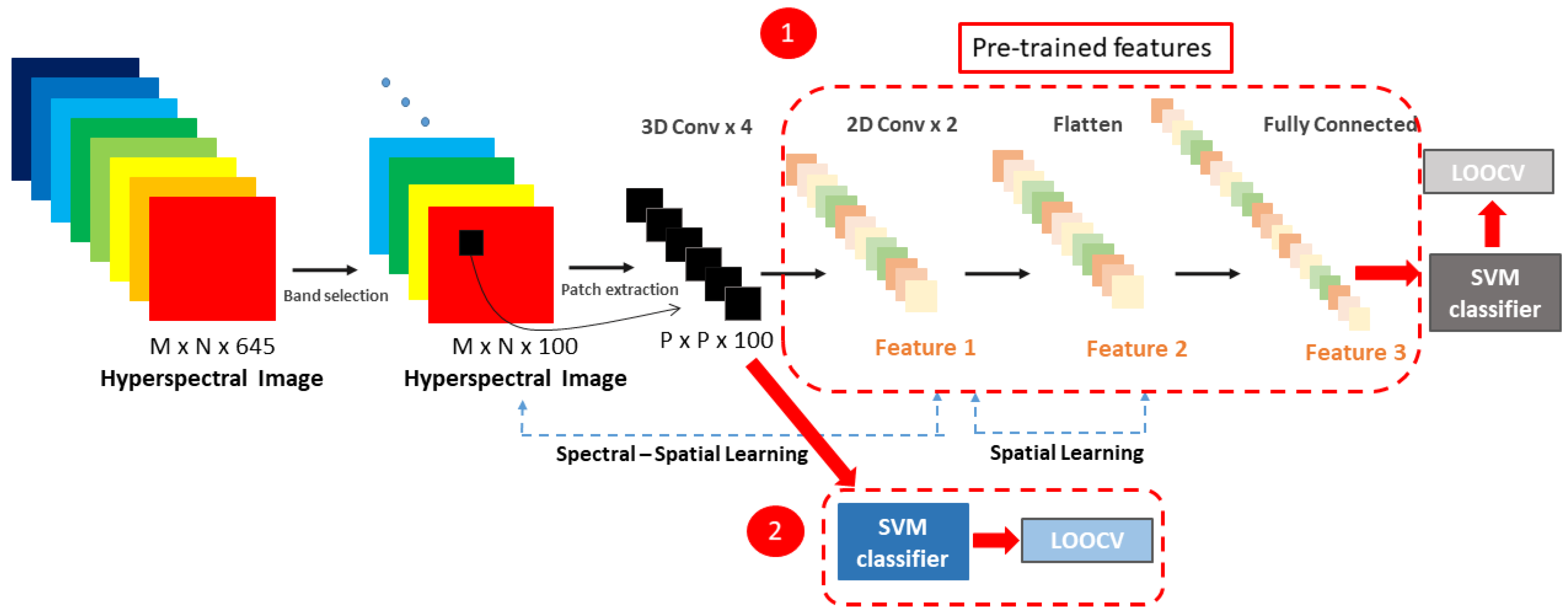
2.4. HS Image Classification: A Deep Spectral-Spatial Approach
2.5. Traditional Supervised Classification Methods
3. Experiments
3.1. Evaluation Protocols
3.2. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| 3D–2D Hybrid CNN | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image ID | Accuracy | Sensitivity | Specificity | AUC | |||||||||
| NT | TT | BV | BG | NT | TT | BV | BG | NT | TT | BV | BG | ||
| 008-01 | 0.79 | 0.87 | 1.00 | 0.35 | 0.98 | 0.99 | 0.81 | 1.00 | 0.90 | 0.72 | 0.48 | 0.78 | 0.92 |
| 008-02 | 0.97 | 0.89 | 1.00 | 0.94 | 0.99 | 1.00 | 0.99 | 0.98 | 0.99 | 0.84 | 0.84 | 0.89 | 0.93 |
| 010-03 | 0.91 | 0.97 | - | 0.74 | 0.88 | 0.84 | - | 0.98 | 0.99 | 0.82 | - | 0.87 | 0.97 |
| 012-01 | 0.78 | 0.93 | 0.92 | 0.66 | 0.95 | 0.74 | 0.98 | 1.00 | 0.99 | 0.86 | 0.93 | 0.86 | 0.93 |
| 012-02 | 0.79 | 0.82 | 0.84 | 0.74 | 0.95 | 0.80 | 1.00 | 0.97 | 0.95 | 0.87 | 0.73 | 0.86 | 0.81 |
| 014-01 | 0.97 | - | - | 1.00 | 0.97 | 0.97 | - | 1.00 | 1.00 | - | - | 0.95 | 0.99 |
| 015-01 | 0.87 | 0.82 | 0.98 | 0.78 | 0.98 | 0.91 | 0.99 | 0.97 | 0.95 | 0.90 | 0.95 | 0.89 | 0.97 |
| 016-04 | 0.91 | 0.89 | - | 0.90 | 0.95 | 0.97 | - | 0.91 | 0.99 | 0.80 | - | 0.77 | 0.94 |
| 016-05 | 0.82 | 0.73 | - | 0.90 | 0.94 | 0.97 | - | 0.91 | 0.85 | 0.80 | - | 0.90 | 0.97 |
| 017-01 | 0.83 | 0.48 | - | 1.00 | 1.00 | 1.00 | - | 0.84 | 0.93 | 0.69 | - | 0.90 | 0.99 |
| 020-01 | 0.56 | 0.94 | - | 0.69 | 0.87 | 0.51 | 0.99 | 0.99 | 0.95 | 0.76 | 0.50 | 0.87 | 0.93 |
| 025-02 | 0.35 | 0.01 | - | 0.20 | 1.00 | 0.96 | 1.00 | 1.00 | 0.08 | 0.50 | 0.49 | 0.57 | 0.54 |
| Mean | 0.80 | 0.76 | 0.68 | 0.74 | 0.96 | 0.87 | 0.98 | 0.92 | 0.87 | 0.78 | 0.70 | 0.84 | 0.91 |
| Std.D. | 0.18 | 0.28 | 0.47 | 0.25 | 0.04 | 0.15 | 0.02 | 0.08 | 0.26 | 0.11 | 0.21 | 0.10 | 0.13 |
| 3D–2D Hybrid CNN + SVM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image ID | Accuracy | Sensitivity | Specificity | AUC | |||||||||
| NT | TT | BV | BG | NT | TT | BV | BG | NT | TT | BV | BG | ||
| 008-01 | 0.60 | 0.72 | 0.07 | 0.57 | 0.96 | 0.68 | 0.99 | 0.83 | 0.81 | 0.98 | 0.89 | 0.67 | 0.99 |
| 008-02 | 0.88 | 0.72 | 0.79 | 0.92 | 0.93 | 0.97 | 0.96 | 0.94 | 0.96 | 0.84 | 0.88 | 0.93 | 0.95 |
| 010-03 | 0.90 | 0.95 | - | 0.69 | 0.91 | 0.84 | - | 0.97 | 0.99 | 0.90 | - | 0.83 | 0.95 |
| 012-01 | 0.82 | 0.93 | 0.84 | 0.76 | 0.86 | 0.80 | 0.99 | 0.96 | 0.99 | 0.87 | 0.92 | 0.86 | 0.93 |
| 012-02 | 0.74 | 0.93 | 0.36 | 0.79 | 0.69 | 0.77 | 0.98 | 0.86 | 0.97 | 0.86 | 0.67 | 0.84 | 0.84 |
| 014-01 | 0.96 | - | - | 0.91 | 0.93 | 0.97 | - | 0.96 | 1.00 | - | - | 0.95 | 0.98 |
| 015-01 | 0.87 | 0.91 | 0.88 | 0.84 | 0.94 | 0.89 | 1.00 | 0.94 | 0.99 | 0.91 | 0.94 | 0.89 | 0.96 |
| 016-04 | 0.69 | 0.63 | - | 0.67 | 0.83 | 0.98 | - | 0.76 | 0.88 | 0.81 | - | 0.73 | 0.87 |
| 016-05 | 0.83 | 0.67 | - | 0.87 | 0.97 | 0.97 | - | 0.89 | 0.90 | 0.82 | - | 0.88 | 0.95 |
| 017-01 | 0.78 | 0.38 | - | 0.93 | 0.99 | 1.00 | - | 0.78 | 0.99 | 0.69 | - | 0.86 | 0.99 |
| 020-01 | 0.57 | 0.90 | - | 0.73 | 0.89 | 0.53 | 1.00 | 0.98 | 0.91 | 0.72 | 0.50 | 0.86 | 0.93 |
| 025-02 | 0.32 | - | - | 0.09 | 1.00 | 0.96 | 0.93 | 1.00 | 0.04 | 0.49 | 0.49 | 0.54 | 0.54 |
| Mean | 0.75 | 0.68 | 0.42 | 0.73 | 0.91 | 0.86 | 0.98 | 0.91 | 0.87 | 0.81 | 0.76 | 0.82 | 0.91 |
| Std.D. | 0.18 | 0.30 | 0.41 | 0.23 | 0.09 | 0.15 | 0.03 | 0.08 | 0.27 | 0.13 | 0.20 | 0.12 | 0.12 |
| SVM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image ID | Accuracy | Sensitivity | Specificity | AUC | |||||||||
| NT | TT | BV | BG | NT | TT | BV | BG | NT | TT | BV | BG | ||
| 008-01 | 0.61 | 0.73 | - | 0.66 | 0.96 | 0.64 | 0.96 | 0.86 | 0.81 | 0.72 | 0.48 | 0.78 | 0.92 |
| 008-02 | 0.87 | 0.70 | 0.72 | 0.85 | 0.93 | 0.99 | 0.95 | 0.93 | 0.91 | 0.84 | 0.84 | 0.89 | 0.93 |
| 010-03 | 0.92 | 0.96 | - | 0.76 | 0.88 | - | 0.98 | 1.00 | 0.82 | - | 0.87 | 0.97 | |
| 012-01 | 0.81 | 0.93 | 0.87 | 0.74 | 0.91 | 0.79 | 1.00 | 0.97 | 0.98 | 0.86 | 0.93 | 0.86 | 0.93 |
| 012-02 | 0.74 | 0.94 | 0.49 | 0.81 | 0.65 | 0.76 | 0.97 | 0.89 | 0.97 | 0.87 | 0.73 | 0.86 | 0.81 |
| 014-01 | 0.98 | - | - | 0.91 | 0.98 | 0.98 | - | 1.00 | 1.00 | - | - | 0.95 | 0.99 |
| 015-01 | 0.87 | 0.89 | 0.90 | 0.82 | 0.97 | 0.89 | 1.00 | 0.96 | 0.98 | 0.90 | 0.95 | 0.89 | 0.97 |
| 016-04 | 0.73 | 0.61 | - | 0.53 | 0.94 | 0.98 | - | 0.78 | 0.91 | 0.80 | - | 0.77 | 0.94 |
| 016-05 | 0.82 | 0.61 | - | 0.95 | 0.99 | 0.98 | - | 0.84 | 0.93 | 0.80 | - | 0.90 | 0.97 |
| 017-01 | 0.80 | 0.39 | - | 1.00 | 1.00 | 1.00 | - | 0.79 | 0.96 | 0.69 | - | 0.90 | 0.99 |
| 020-01 | 0.59 | 0.95 | - | 0.75 | 0.91 | 0.55 | 1.00 | 0.99 | 0.89 | 0.76 | 0.50 | 0.87 | 0.93 |
| 025-02 | 0.33 | - | - | 0.14 | 1.00 | 0.99 | 0.96 | 1.00 | 0.06 | 0.50 | 0.49 | 0.57 | 0.54 |
| Mean | 0.76 | 0.70 | 0.43 | 0.74 | 0.93 | 0.87 | 0.98 | 0.92 | 0.87 | 0.78 | 0.70 | 0.84 | 0.91 |
| Std | 0.18 | 0.30 | 0.42 | 0.23 | 0.09 | 0.15 | 0.02 | 0.08 | 0.26 | 0.11 | 0.21 | 0.10 | 0.13 |
| 2D CNN | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image ID | Accuracy | Sensitivity | Specificity | AUC | |||||||||
| NT | TT | BV | BG | NT | TT | BV | BG | NT | TT | BV | BG | ||
| 008-01 | 0.66 | 0.89 | - | 0.63 | 1.00 | 1.00 | 0.88 | 0.71 | 0.93 | 0.99 | 0.50 | 0.81 | 1.00 |
| 008-02 | 0.83 | 0.68 | 0.19 | 1.00 | 0.90 | 0.93 | 0.99 | 0.93 | 0.82 | 0.92 | 0.87 | 1.00 | 0.94 |
| 010-03 | 0.83 | 0.82 | - | 0.84 | 0.84 | 0.82 | - | 0.91 | 1.00 | 0.92 | - | 0.93 | 0.98 |
| 012-01 | 0.66 | 0.92 | 0.38 | 0.56 | 0.96 | 0.64 | 0.93 | 1.00 | 0.91 | 0.82 | 0.87 | 0.97 | 0.99 |
| 012-02 | 0.74 | 0.96 | 0.15 | 0.81 | 0.75 | 0.77 | 0.97 | 0.87 | 0.98 | 0.97 | 0.91 | 0.95 | 0.97 |
| 014-01 | 0.99 | - | - | 0.91 | 0.91 | 1.00 | - | 1.00 | 1.00 | - | - | 1.00 | 1.00 |
| 015-01 | 0.64 | 0.77 | 0.23 | 0.79 | 0.98 | 0.67 | 1.00 | 0.88 | 0.91 | 0.83 | 0.90 | 0.93 | 0.99 |
| 016-04 | 0.76 | 0.65 | - | 0.90 | 0.86 | 0.95 | - | 0.74 | 0.99 | 0.94 | - | 0.89 | 1.00 |
| 016-05 | 0.71 | 0.33 | - | 1.00 | 1.00 | 1.00 | - | 0.68 | 1.00 | 0.93 | - | 0.93 | 1.00 |
| 017-01 | 0.88 | 0.64 | - | 1.00 | 1.00 | 1.00 | - | 0.91 | 0.88 | 0.99 | - | 0.99 | 1.00 |
| 020-01 | 0.59 | 0.88 | - | 0.78 | 0.93 | 0.79 | 1.00 | 1.00 | 0.51 | 0.93 | 0.35 | 0.99 | 0.90 |
| 025-02 | 0.30 | - | - | 0.04 | 1.00 | 1.00 | 1.00 | 1.00 | 0.02 | 0.40 | 0.58 | 0.81 | 0.44 |
| Mean | 0.72 | 0.69 | 0.14 | 0.77 | 0.93 | 0.88 | 0.97 | 0.89 | 0.83 | 0.88 | 0.71 | 0.93 | 0.93 |
| Std.D. | 0.17 | 0.29 | 0.15 | 0.27 | 0.08 | 0.14 | 0.05 | 0.12 | 0.29 | 0.17 | 0.23 | 0.07 | 0.16 |
| 1D DNN | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image ID | Accuracy | Sensitivity | Specificity | AUC | |||||||||
| NT | TT | BV | BG | NT | TT | BV | BG | NT | TT | BV | BG | ||
| 008-01 | 0.67 | 0.99 | 0.02 | 0.57 | 1.00 | 0.99 | 0.87 | 0.71 | 0.99 | 1.00 | 0.82 | 0.82 | 1.00 |
| 008-02 | 0.89 | 0.84 | 0.32 | 0.89 | 0.92 | 0.96 | 0.98 | 0.97 | 0.87 | 0.97 | 0.96 | 0.99 | 0.96 |
| 010-03 | 0.90 | 0.86 | - | 1.00 | 0.96 | 0.98 | - | 0.90 | 1.00 | 0.98 | - | 1.00 | 1.00 |
| 012-01 | 0.90 | 0.85 | 0.65 | 0.93 | 1.00 | 0.92 | 0.96 | 1.00 | 0.99 | 0.95 | 0.96 | 1.00 | 1.00 |
| 012-02 | 0.70 | 1.00 | 0.03 | 0.95 | 0.54 | 0.73 | 0.97 | 0.79 | 1.00 | 0.91 | 0.85 | 0.97 | 0.90 |
| 014-01 | 1.00 | - | - | 1.00 | 1.00 | 1.00 | - | 1.00 | 1.00 | - | - | 0.24 | - |
| 015-01 | 0.78 | 0.99 | 0.29 | 0.93 | 1.00 | 0.80 | 1.00 | 1.00 | 0.92 | 0.97 | 0.83 | 1.00 | 0.98 |
| 016-04 | 0.72 | 0.92 | - | 0.93 | 0.23 | 1.00 | - | 0.61 | 1.00 | 1.00 | - | 0.82 | 1.00 |
| 016-05 | 0.96 | 0.88 | - | 1.00 | 1.00 | 1.00 | - | 0.96 | 1.00 | 0.99 | - | 1.00 | 1.00 |
| 017-01 | 0.77 | 0.41 | - | 1.00 | 0.92 | 1.00 | - | 0.89 | 0.64 | 0.99 | - | 0.99 | 0.96 |
| 020-01 | 0.59 | 0.94 | - | 0.86 | 1.00 | 0.90 | 1.00 | 1.00 | 0.46 | 0.98 | 0.82 | 1.00 | 1.00 |
| 025-02 | 0.45 | - | 0.02 | - | 1.00 | 1.00 | 1.00 | 1.00 | 0.01 | 0.21 | 0.99 | 0.88 | 0.70 |
| Mean | 0.78 | 0.79 | 0.19 | 0.84 | 0.88 | 0.94 | 0.97 | 0.90 | 0.82 | 0.91 | 0.89 | 0.89 | 0.87 |
| Std.D. | 0.16 | 0.31 | 0.25 | 0.29 | 0.24 | 0.09 | 0.05 | 0.13 | 0.31 | 0.23 | 0.08 | 0.22 | 0.29 |
References
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar] [PubMed]
- Stummer, W.; Tonn, J.C.; Mehdorn, H.M.; Nestler, U.; Franz, K.; Goetz, C.; Bink, A.; Pichlmeier, U.; ALA-Glioma Study Group. Counterbalancing risks and gains from extended resections in malignant glioma surgery: A supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J. Neurosurg. 2011, 114, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Ganser, K.A.; Dickhaus, H.; Staubert, A.; Bonsanto, M.M.; Wirtz, C.R.; Tronnier, V.M.; Kunze, S. Quantification of brain shift effects in MRI images. Biomed. Technik. Biomed. Eng. 1997, 42, 247–248. [Google Scholar] [CrossRef]
- Nimsky, C.; Ganslandt, O.; Cerny, S.; Hastreiter, P.; Greiner, G.; Fahlbusch, R. Quantification of, Visualization of, and Compensation for Brain Shift Using Intraoperative Magnetic Resonance Imaging. Neurosurgery 2000, 47, 1070–1080. [Google Scholar] [CrossRef]
- Floeth, F.W.; Sabel, M.; Ewelt, C.; Stummer, W.; Felsberg, J.; Reifenberger, G.; Steiger, H.J.; Stoffels, G.; Coenen, H.H.; Langen, K.J. Comparison of (18)F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 731–741. [Google Scholar] [CrossRef]
- Li, Q.; He, X.; Wang, Y.; Liu, H.; Xu, D.; Guo, F. Review of spectral imaging technology in biomedical engineering: Achievements and challenges. J. Biomed. Opt. 2013, 18, 100901. [Google Scholar] [CrossRef] [Green Version]
- Kamruzzaman, M.; Sun, D.-W. Introduction to hyperspectral imaging technology. In Computer Vision Technology for Food Quality; Academic Press: Cambridge, MA, USA, 2016; pp. 111–139. [Google Scholar]
- Guolan, L.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar]
- Halicek, M.; Fabelo, H.; Ortega, S.; Callico, G.M.; Fei, B. In-Vivo and Ex-Vivo Tissue Analysis through Hyperspectral Imaging Techniques: Revealing the Invisible Features of Cancer. Cancers 2019, 11, 756. [Google Scholar] [CrossRef] [Green Version]
- Ortega, S.; Fabelo, H.; Iakovidis, D.K.; Koulaouzidis, A.; Callico, G.M. Use of Hyperspectral/Multispectral Imaging in Gastroenterology. Shedding Some–Different–Light into the Dark. J. Clin. Med. 2019, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Akbari, H.; Halig, L.V.; Schuster, D.M.; Osunkoya, A.; Master, V.; Nieh, P.T.; Chen, G.Z.; Fei, B. Hyperspectral imaging and quantitative analysis for prostate cancer detection. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Aboughaleb, I.H.; Aref, M.H.; El-Sharkawy, Y.H. Hyperspectral imaging for diagnosis and detection of ex-vivo breast cancer. Photodiagnosis Photodyn Ther. 2020, 101922. [Google Scholar] [CrossRef] [PubMed]
- Pourreza-Shahri, R.; Saki, F.; Kehtarnavaz, N.; Leboulluec, P.; Liu, H. Classification of ex-vivo breast cancer positive margins measured by hyperspectral imaging. In Proceedings of the 2013 IEEE International Conference on Image Processing, Melbourne, VIC, Australia, 15–18 September 2013; pp. 1408–1412. [Google Scholar]
- Manni, F.; Fonollà, R.; Sommen, F.; Zinger, S.; Shan, C.; Kho, E.; de Koning, S.B.; Ruers, T.; de With, P.H.N. Hyperspectral imaging for colon cancer classification in surgical specimens: Towards optical biopsy during image-guided surgery. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Liu, Z.; Wang, H.; Li, Q. Tongue Tumor Detection in Medical Hyperspectral Images. Sensors 2012, 12, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Little, J.V.; Wang, X.; Zhang, H.; Patel, M.R.; Griffith, C.C.; El-Deiry, M.W.; Chen, A.Y.; Fei, B. Detection of Head and Neck Cancer in Surgical Specimens Using Quantitative Hyperspectral Imaging. Clin. Cancer Res. 2017, 23, 5426–5436. [Google Scholar] [CrossRef] [Green Version]
- Manni, F.; Sommen, F.; Zinger, S.; Kho, E.; de Koning, S.B.; Ruers, T.; Shan, C.; Schleipen, J.; de With, H.N.P. Automated tumor assessment of squamous cell carcinoma on tongue cancer patients with hyperspectral imaging. In Medical Imaging 2019: Image-Guided Procedures, Robotic Interventions, and Modeling; International Society for Optics and Photonics: San Diego, CA, USA, 2019; Volume 10951. [Google Scholar]
- Trajanovski, S.; Shan, C.; Weijtmans, P.J.C.; Brouwer de Koning, S.G.; Ruers, T.J.M. Tongue tumor detection in hyperspectral images using deep learning semantic segmentation. IEEE Trans. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Brouwer de Koning, S.G.; Weijtmans, P.J.C.; Karakullukcu, B.; Shan, C.; Baltussen, E.J.M.; Smit, L.A.; van Veen, R.L.P.; Hendriks, B.H.W.; Sterenborg, H.; Ruers, T.J.M. Toward assessment of resection margins using hyperspectral diffuse reflection imaging (400–1.700 nm) during tongue cancer surgery. Lasers Surg. Med. 2020, 56, 496–502. [Google Scholar] [CrossRef]
- Weijtmans, P.J.C.; Shan, C.; Tan, T.; Brouwer de Koning, S.G.; Ruers, T.J.M. A Dual Stream network for tumor detection in Hyperspectral images. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging, Venice, Italy, 8–11 April 2019; pp. 1256–1259. [Google Scholar]
- Parker, M.F.; Mooradian, G.C.; Karins, J.P.; O’Connor, D.M.; Speer, B.A.; Owensby, P.D.; Velasco, A. Hyperspectral diagnostic imaging of the cervix: Report on a new investigational device. J. Low Genit. Tract. Dis. 2000, 119. [Google Scholar] [CrossRef]
- Johansen, T.H.; Møllersen, K.; Ortega, S.; Fabelo, H.; Callicò, G.M.; Godtliebsen, F.A. Recent advances in hyperspectral imaging for melanoma detection. Wiley Interdiscip. Rev. Comput. Stat. 2020, 12, e1465. [Google Scholar] [CrossRef]
- Leon, R.; Martinez-Vega, B.; Fabelo, H.; Ortega, S.; Melian, V.; Castaño, I.; Carretero, G.; Almeida, P.; Garcia, A.; Quevedo, E.; et al. Non-Invasive Skin Cancer Diagnosis Using Hyperspectral Imaging for In-Situ Clinical Support. J. Clin. Med. 2020, 9, 1662. [Google Scholar] [CrossRef]
- Fabelo, H.; Ortega, S.; Szolna, A.; Bulters, D.; Piñeiro, J.F.; Kabwama, S.; O’Shanahan, A.J.; Bulstrode, H.; Bisshopp, S.; Ravi Kiran, B.; et al. In-vivo hyperspectral human brain image database for brain cancer detection. IEEE Access 2019, 7, 39098–39116. [Google Scholar] [CrossRef]
- Nader, S.; Berger, S.M. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar]
- Fabelo, H.; Ortega, S.; Ravi, D.; Kiran, B.R.; Sosa, C.; Bulters, D.; Callicó, G.M.; Bulstrode, H.; Szolna, A.; Piñeiro, J.F.; et al. Spatio-spectral classification of hyperspectral images for brain cancer detection during surgical operations. PLoS ONE 2018, 13, e0193721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabelo, H.; Halicek, M.; Ortega, S.; Shahedi, M.; Szolna, A.; Piñeiro, J.F.; Sosa, C.; O’Shanahan, A.J.; Bisshopp, S.; Espino, C.; et al. Deep Learning-Based Framework for In Vivo Identification of Glioblastoma Tumor using Hyperspectral Images of Human Brain. Sensors 2019, 19, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, B.; Leon, R.; Fabelo, H.; Ortega, S.; Piñeiro, J.F.; Szolna, A.; Hernandez, M.; Espino, C.; O’Shanahan, A.J.; Carrera., D.; et al. Most Relevant Spectral Bands Identification for Brain Cancer Detection Using Hyperspectral Imaging. Sensors 2019, 19, 5481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.K.; Krishna, G.; Dubey, S.R.; Chaudhuri, B.B. HybridSN: Exploring 3-D–2-D CNN Feature Hierarchy for Hyperspectral Image Classification. IEEE Geosci. Remote Sens. Lett. 2020, 17. [Google Scholar] [CrossRef] [Green Version]
- Fabelo, H.; Ortega, S.; Lazcano, R.; Madroñal, D.; Callicó, G.M.; Juárez, E.; Salvador, R.; Bulters, D.; Bulstrode, H.; Szolna, A.; et al. An Intraoperative Visualization System Using Hyperspectral Imaging to Aid in Brain Tumor Delineation. Sensors 2018, 18, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasubramanian, K.; Jones, S.; Sarkar, S.; Singh, A.K.; Singh, A.; Ganapathysubramanian, B. Hyperspectral band selection using genetic algorithm and support vector machines for early identification of charcoal rot disease in soybean stems. Plant Methods 2018, 14, 86. [Google Scholar] [CrossRef]
- Gao, J.; Du, Q.; Gao, L.; Sun, X.; Wu, Y.; Zhang, B. Ant colony optimization for supervised and unsupervised hyperspectral band selection. In Proceedings of the 5th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Gainesville, FL, USA, 26–28 June 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Xie, F.; Li, F.; Lei, C.; Ke, L. Representative band selection for hyperspectral image classification. ISPRS Int. J. Geo-Inf. 2018, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Dorigo, M.; Birattari, M.; Stutzle, T. Ant colony optimization. IEEE Comput. Intell. Mag. 2006, 1, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, M.E.; Haut, J.M.; Fernandez-Beltran, R.; Plaza, J.; Plaza, A.; Li, J.; Pla, F. Capsule networks for hyperspectral image classification. IEEE Trans. Geosci. Remote Sens. 2019, 57, 2145–2160. [Google Scholar] [CrossRef]
- Fang, L.; Liu, Z.; Song, W. Deep hashing neural networks for hyperspectral image feature extraction. IEEE Geosci. Remote. Sens. Lett. 2019, 16, 1412–1416. [Google Scholar] [CrossRef]
- Luo, Y.; Zou, J.; Yao, C.; Zhao, X.; Li, T.; Bai, G. HSI-CNN: A Novel Convolution Neural Network for Hyperspectral Image. In Proceedings of the International Conference on Audio, Language and Image Processing (ICALIP), Shanghai, China, 16–17 July 2018; pp. 464–469. [Google Scholar] [CrossRef] [Green Version]
- Manni, F.; van der Sommen, F.; Zinger, S.; Shan, C.; Holthuizen, R.; Lai, M.; Buström, G.; Hoveling, R.J.M.; Edström, E.; Elmi-Terander, A.; et al. Hyperspectral Imaging for Skin Feature Detection: Advances in Markerless Tracking for Spine Surgery. Appl. Sci. 2020, 10, 4078. [Google Scholar] [CrossRef]
- Paoletti, M.E.; Haut, J.M.; Plaza, J.; Plaza, A. Deep learning classifiers for hyperspectral imaging: A review. ISPRS J. Photogramm. Remote Sens. 2019, 158, 279–317. [Google Scholar] [CrossRef]
- Lai, M.; Skyrman, S.; Shan, C.; Paulussen, E.; Manni, F.; Swamy, A.; Babic, D.; Edstrom, E.; Persson, O.; Burstrom, G.; et al. Automated classification of brain tissue: Comparison between hyperspectral imaging and diffuse reflectance spectroscopy. In Medical Imaging 2020: Image-Guided Procedures, Robotic Interventions, and Modeling, 113151X; International Society for Optics and Photonics: Houston, TX, USA, 2019; Volume 11315. [Google Scholar] [CrossRef]





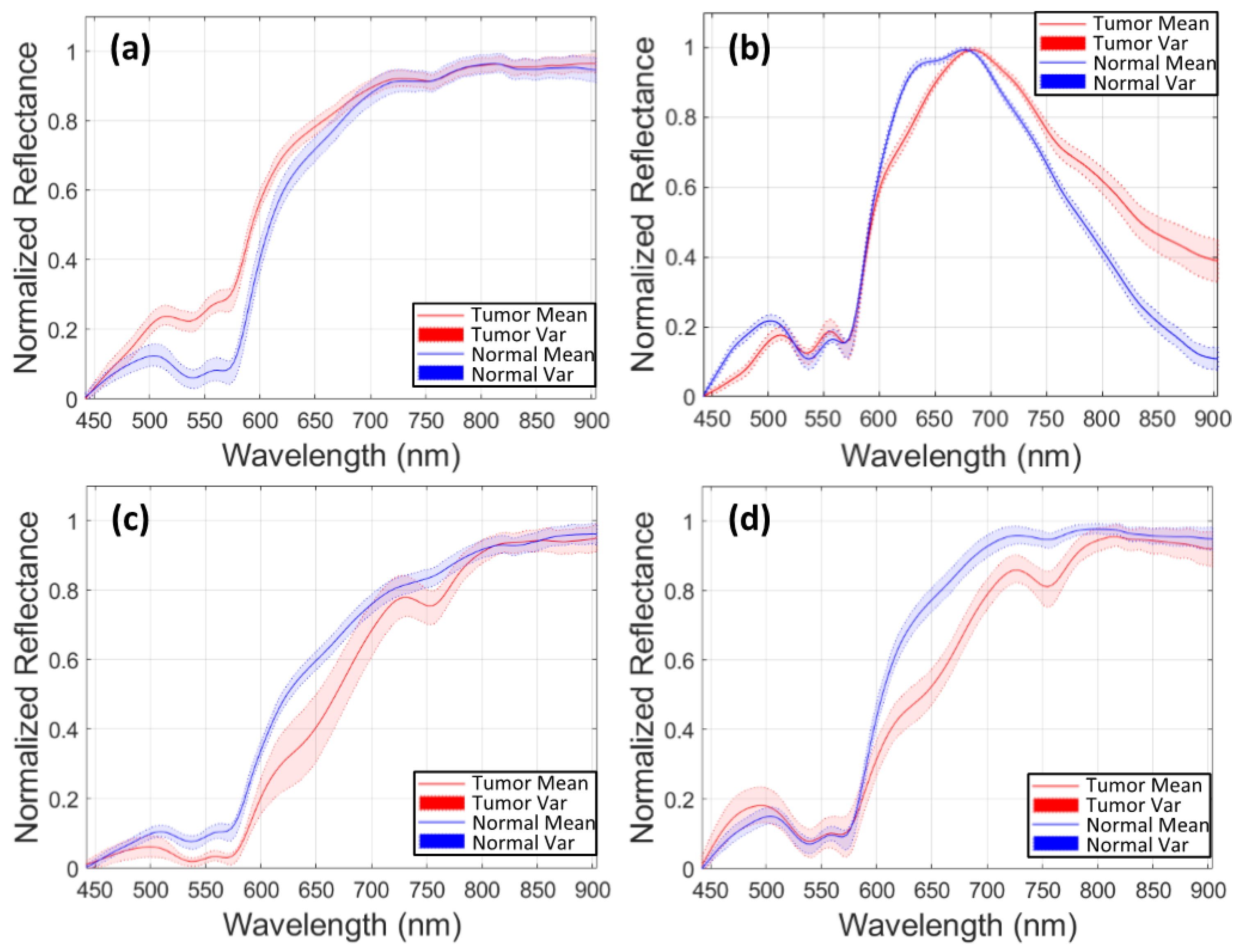

| Patient ID-Image ID | Number of Pixels | |||
|---|---|---|---|---|
| NT | TT | BV | BG | |
| 1 = 008-01 | 2295 | 1221 | 1331 | 630 |
| 2 = 008-02 | 2187 | 138 | 1000 | 7444 |
| 3 = 010-03 | 10,626 | 0 | 2332 | 3972 |
| 4 = 012-01 | 4516 | 855 | 8697 | 1685 |
| 5 = 012-02 | 6553 | 3139 | 6041 | 8731 |
| 6 = 014-01 | 0 | 30 | 64 | 1866 |
| 7 = 015-01 | 1251 | 2046 | 4089 | 696 |
| 8 = 016-04 | 1178 | 0 | 1064 | 956 |
| 9 = 016-05 | 2643 | 0 | 452 | 5125 |
| 10 = 017-01 | 1328 | 0 | 68 | 3069 |
| 11 = 020-01 | 1842 | 3655 | 1513 | 2625 |
| 12 = 025-02 | 977 | 1282 | 907 | 3687 |
| Total | 35,396 | 12,366 | 27,558 | 40,486 |
| Approach | Accuracy | Sensitivity | Specificity | AUC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT | TT | BV | BG | NT | TT | BV | BG | NT | TT | BV | BG | |||
| Proposed 3D–2D CNN | Mean | 0.80 | 0.76 | 0.68 | 0.74 | 0.96 | 0.87 | 0.98 | 0.92 | 0.87 | 0.78 | 0.70 | 0.84 | 0.91 |
| Std.D. | 0.18 | 0.28 | 0.47 | 0.25 | 0.04 | 0.15 | 0.02 | 0.08 | 0.26 | 0.11 | 0.21 | 0.10 | 0.13 | |
| 3D–2D-CNN + SVM | Mean | 0.75 | 0.68 | 0.42 | 0.73 | 0.91 | 0.86 | 0.98 | 0.91 | 0.87 | 0.81 | 0.76 | 0.82 | 0.91 |
| Std.D. | 0.18 | 0.30 | 0.41 | 0.23 | 0.09 | 0.15 | 0.03 | 0.08 | 0.27 | 0.13 | 0.20 | 0.12 | 0.12 | |
| SVM | Mean | 0.76 | 0.70 | 0.43 | 0.74 | 0.93 | 0.87 | 0.98 | 0.92 | 0.87 | 0.78 | 0.70 | 0.84 | 0.91 |
| Std.D. | 0.18 | 0.30 | 0.42 | 0.23 | 0.09 | 0.15 | 0.02 | 0.08 | 0.26 | 0.11 | 0.21 | 0.10 | 0.13 | |
| 2D CNN | Mean | 0.72 | 0.69 | 0.14 | 0.77 | 0.93 | 0.88 | 0.97 | 0.89 | 0.83 | 0.88 | 0.71 | 0.93 | 0.93 |
| Std.D. | 0.17 | 0.29 | 0.15 | 0.27 | 0.08 | 0.14 | 0.05 | 0.12 | 0.29 | 0.17 | 0.23 | 0.07 | 0.16 | |
| 1D DNN | Mean | 0.78 | 0.79 | 0.19 | 0.84 | 0.88 | 0.94 | 0.97 | 0.90 | 0.82 | 0.91 | 0.89 | 0.89 | 0.87 |
| Std.D. | 0.16 | 0.31 | 0.25 | 0.29 | 0.24 | 0.09 | 0.05 | 0.13 | 0.31 | 0.23 | 0.08 | 0.22 | 0.29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manni, F.; van der Sommen, F.; Fabelo, H.; Zinger, S.; Shan, C.; Edström, E.; Elmi-Terander, A.; Ortega, S.; Marrero Callicó, G.; de With, P.H.N. Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach. Sensors 2020, 20, 6955. https://doi.org/10.3390/s20236955
Manni F, van der Sommen F, Fabelo H, Zinger S, Shan C, Edström E, Elmi-Terander A, Ortega S, Marrero Callicó G, de With PHN. Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach. Sensors. 2020; 20(23):6955. https://doi.org/10.3390/s20236955
Chicago/Turabian StyleManni, Francesca, Fons van der Sommen, Himar Fabelo, Svitlana Zinger, Caifeng Shan, Erik Edström, Adrian Elmi-Terander, Samuel Ortega, Gustavo Marrero Callicó, and Peter H. N. de With. 2020. "Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach" Sensors 20, no. 23: 6955. https://doi.org/10.3390/s20236955
APA StyleManni, F., van der Sommen, F., Fabelo, H., Zinger, S., Shan, C., Edström, E., Elmi-Terander, A., Ortega, S., Marrero Callicó, G., & de With, P. H. N. (2020). Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach. Sensors, 20(23), 6955. https://doi.org/10.3390/s20236955









