Halochromic Polystyrene Nanofibers Obtained by Solution Blow Spinning for Wine pH Sensing
Abstract
1. Introduction
2. Experimental
2.1. Materials
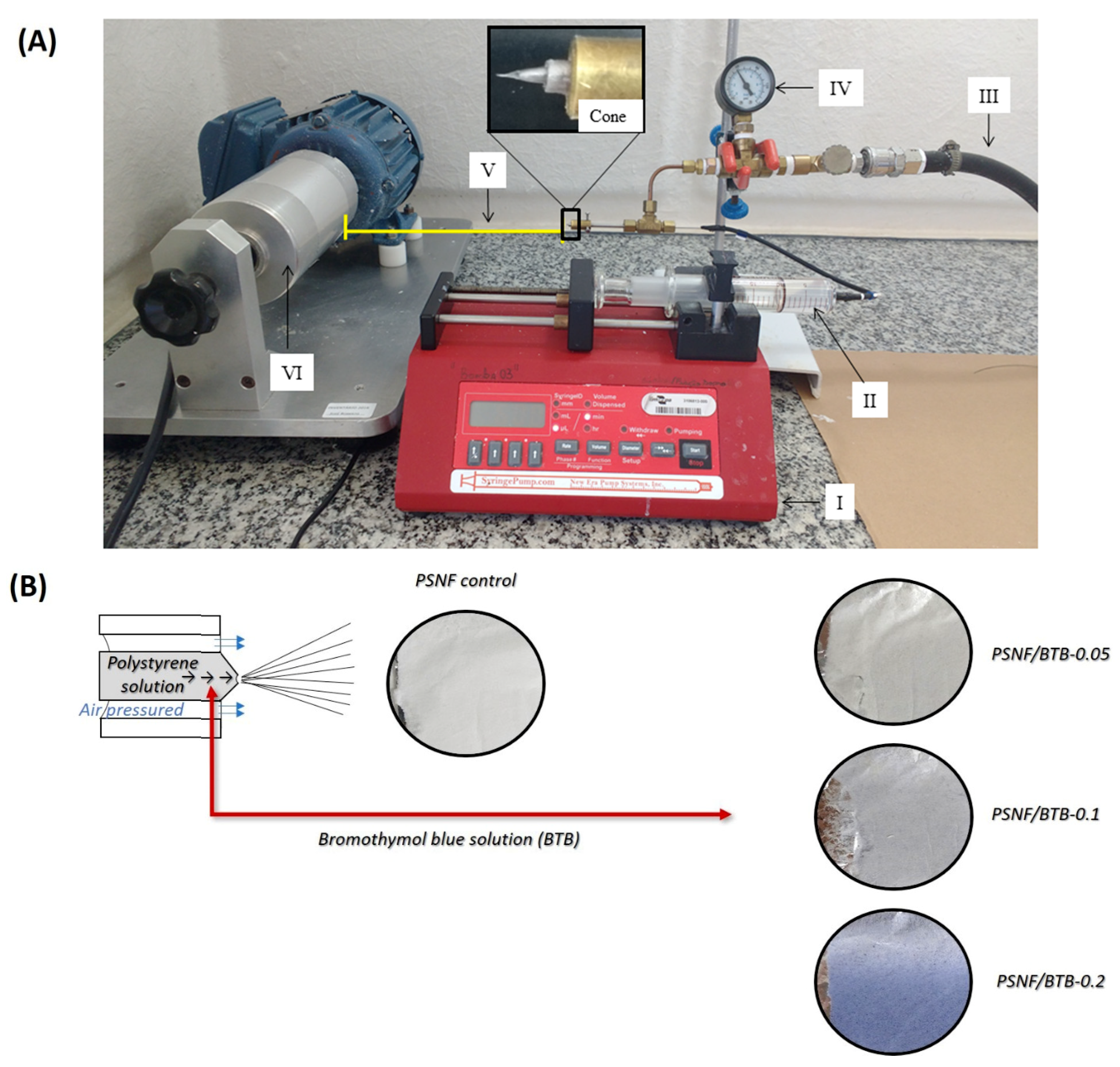
2.2. Solution Blow Spinning (SBS)
2.3. Characterization of the Nanofibers
2.4. Study of BTB Migration and Colorimetric Analysis
2.5. Halochromic Evaluation of PSNF/BTB-0.2 Mats in Wine Samples
2.6. Sensitivity and Limit of Detection (LOD)
3. Results and Discussion
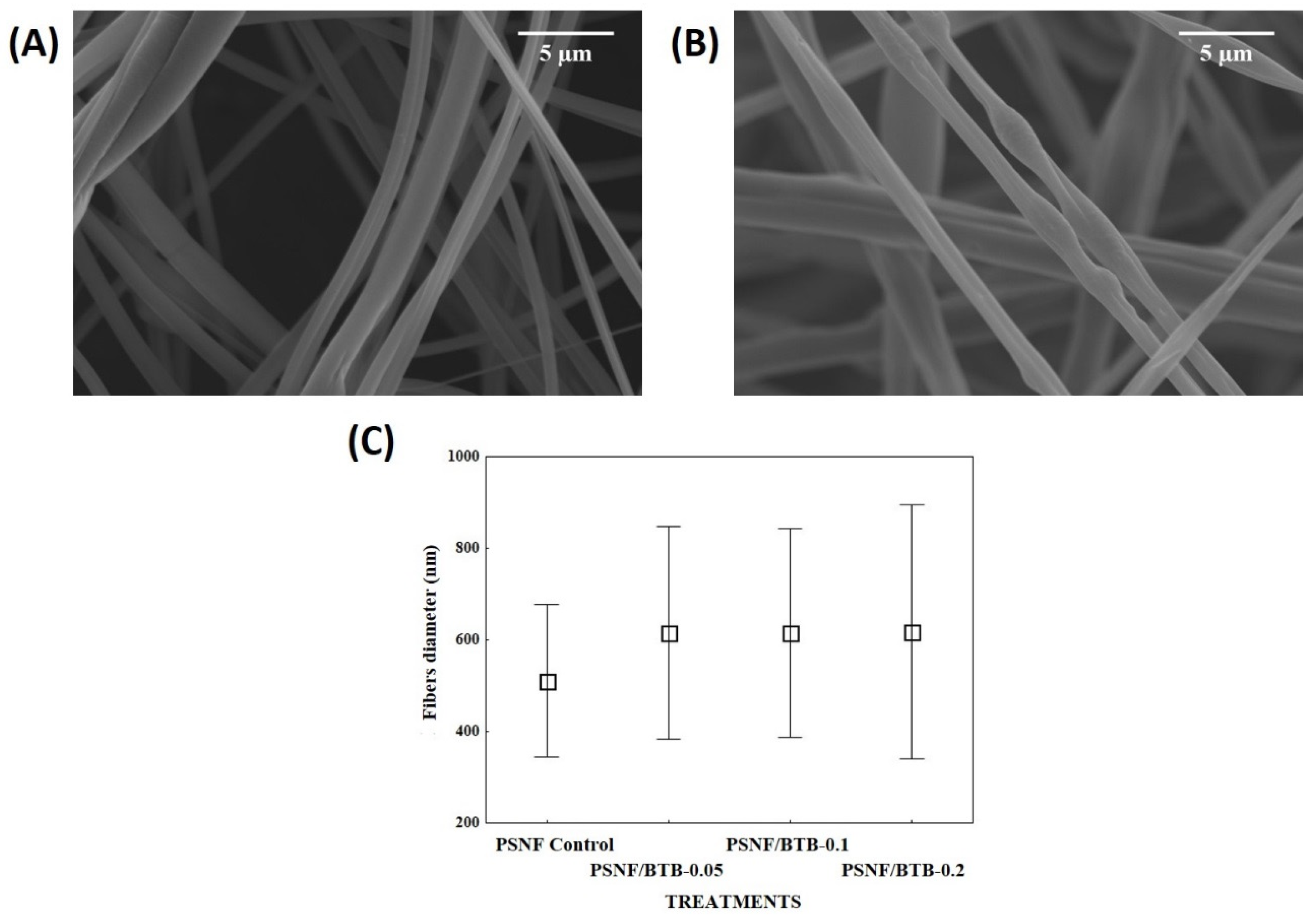
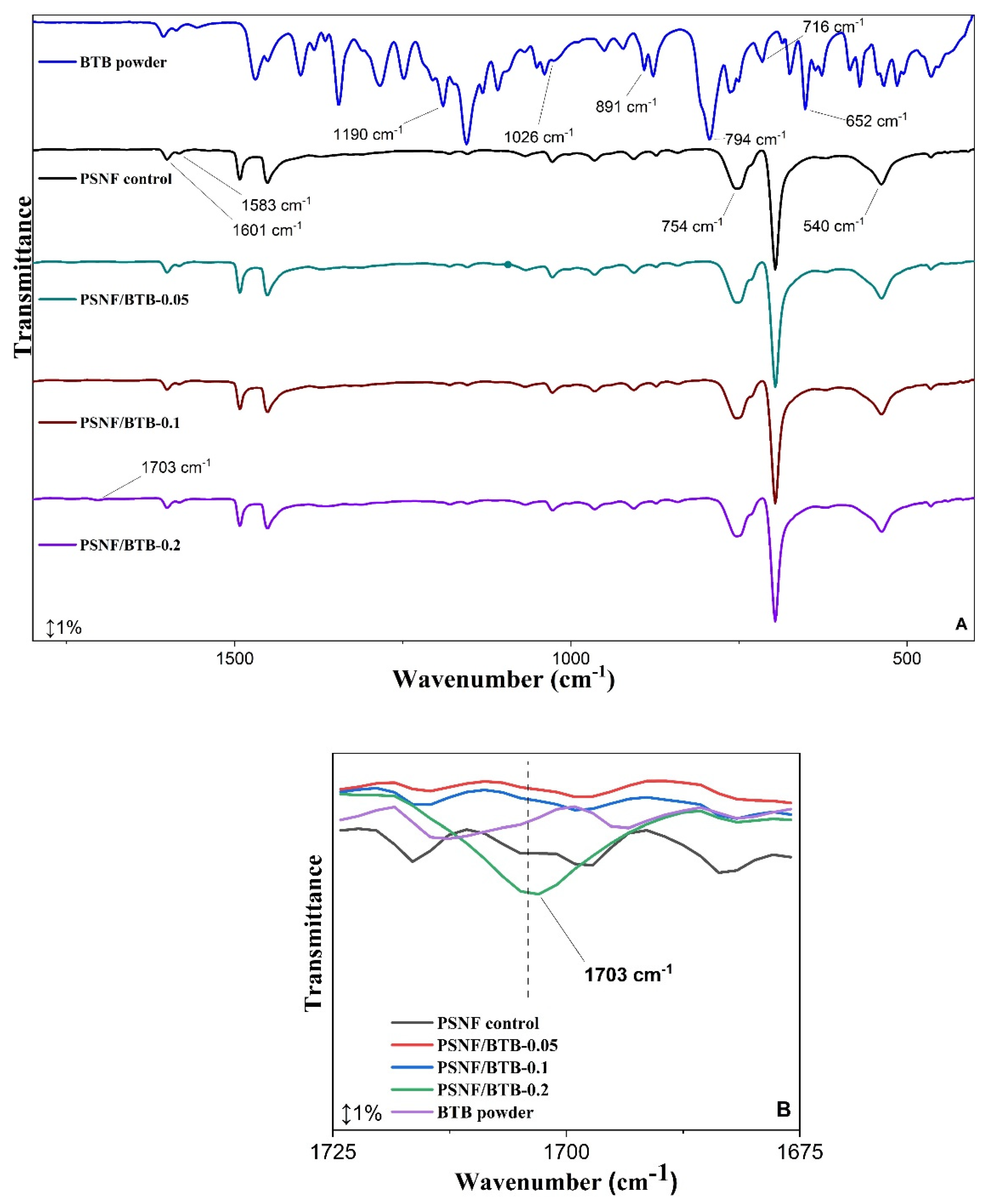
3.1. Morphological and Structural Characterization of Nanofibrous Mats
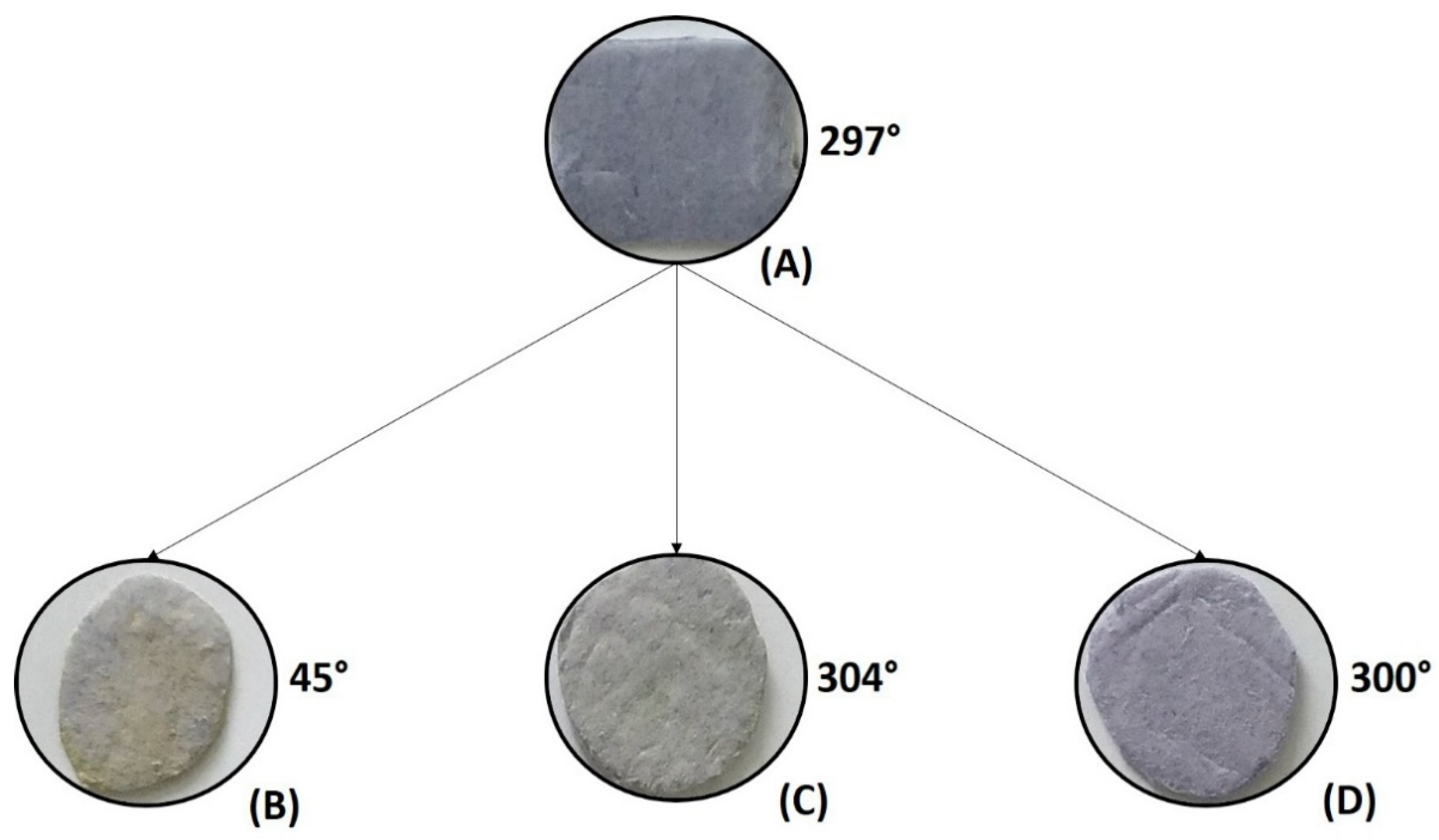
3.2. Analysis of the PSNF/BTB-0.2 pH Sensing Capacity by Immersion
3.3. Application of Halochromic PSNF/BTB-0.2 to Wine (Volatile Acidity)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haque, F.; Pi, F.; Zhao, Z.; Gu, S.; Hu, H.; Yu, H.; Guo, P. RNA versatility, flexibility, and thermostability for practice in RNA nanotechnology and biomedical applications. Wiley Interdiscip. Rev. RNA 2017, 9, e1452. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, N.H.; Gleize, P.J.P. Effect of silicon carbide nanowhiskers on hydration and mechanical properties of a Portland cement paste. Constr. Build. Mater. 2018, 169, 388–395. [Google Scholar] [CrossRef]
- Barbieux, D.; Padula, A.D. Paths and Challenges of New Technologies: The Case of Nanotechnology-Based Cosmetics Development in Brazil. Adm. Sci. 2018, 8, 16. [Google Scholar] [CrossRef]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology Applications and Implications of Agrochemicals toward Sustainable Agriculture and Food Systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An Overview of Nanotechnology in Food Science: Preparative Methods, Practical Applications, and Safety. J. Chem. 2018, 2018, 5427978. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Han, S.G.; Chin, K.B. Nanotechnology in Meat Processing and Packaging: Potential Applications—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M. Nanotechnology in Food Packaging: Opportunities and Challenges. In Nanomaterials for Food Packaging; Cerqueira, M.Â.P.R., Lagaron, J.M., Pastrana Castro, L.M., de Oliveira Soares Vicente, A.A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–11. ISBN 978-0-323-51271-8. [Google Scholar]
- Ai, K.; Liu, Y.; Lu, L. Hydrogen-bonding recognition-induced color change of gold nanoparticles for visual detection of melamine in raw milk and infant formula. J. Am. Chem. Soc. 2009, 131, 9496–9497. [Google Scholar] [CrossRef]
- Cheng, G.; Fa, J.-Q.; Xi, Z.-M.; Zhang, Z.-W.; Cheng, G.; Fa, J.-Q.; Xi, Z.-M.; Zhang, Z.-W. Research on the quality of the wine grapes in corridor area of China. Food Sci. Technol. 2015, 35, 38–44. [Google Scholar] [CrossRef]
- Khattab, T.A.; Rehan, M.; Aly, S.A.; Hamouda, T.; Haggag, K.M.; Klapötke, T.M. Fabrication of PAN-TCF-hydrazone nanofibers by solution blowing spinning technique: Naked-eye colorimetric sensor. J. Environ. Chem. Eng. 2017, 5, 2515–2523. [Google Scholar] [CrossRef]
- Golmohammadi Rostami, S.; Sorayani Bafqi, M.S.; Bagherzadeh, R.; Latifi, M.; Gorji, M. Multi-layer electrospun nanofiber mats with chemical agent sensor function. J. Ind. Text. 2015, 45, 467–480. [Google Scholar] [CrossRef]
- Shivakumar, N.; Madhusudan, P.; Kiruba Daniel, S.C.G. Nanomaterials for smart food packaging. In Nanomaterials for Industrial Applications; Micro & Nano Technologies Series; Elsevier: Madrid, Spain, 2018; ISBN 978-0-12-813352-1. [Google Scholar]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent Packaging: Concepts and Applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications—Review. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Mao, J.; Lu, Y.; Chang, N.; Yang, J.; Zhang, S.; Liu, Y. Multidimensional colorimetric sensor array for discrimination of proteins. Biosens. Bioelectron. 2016, 86, 56–61. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Smart microfibrillated cellulose as swab sponge-like aerogel for real-time colorimetric naked-eye sweat monitoring. Talanta 2019, 205, 120166. [Google Scholar] [CrossRef]
- Skim, J.R.; Suslick, K.S. Hand-Held Reader for Colorimetric Sensor Arrays. Anal. Chem. 2015, 87, 7810–7816. [Google Scholar]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Development of microporous cellulose-based smart xerogel reversible sensor via freeze drying for naked-eye detection of ammonia gas. Carbohydr. Polym. 2019, 210, 196–203. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Al Fassam, H.; Allam, A.A. Co-encapsulation of enzyme and tricyanofuran hydrazone into alginate microcapsules incorporated onto cotton fabric as a biosensor for colorimetric recognition of urea. React. Funct. Polym. 2019, 142, 199–206. [Google Scholar] [CrossRef]
- Diaz, Y.J.; Page, Z.A.; Knight, A.S.; Treat, N.J.; Hemmer, J.R.; Hawker, C.J.; Read de Alaniz, J. A Versatile and Highly Selective Colorimetric Sensor for the Detection of Amines. Chemistry A Eur. J. 2017, 23, 3562–3566. [Google Scholar] [CrossRef] [PubMed]
- Khattab, T.A.; Fouda, M.M.G.; Allam, A.A.; Othman, S.I.; Bin-Jumah, M.; Al-Harbi, H.M.; Rehan, M. Selective Colorimetric Detection of Fe (III) Using Metallochromic Tannin-Impregnated Silica Strips. ChemistrySelect 2018, 3, 12065–12071. [Google Scholar] [CrossRef]
- Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. Gold-Nanoparticle-Based Colorimetric Sensor Array for Discrimination of Organophosphate Pesticides. Anal. Chem. 2016, 88, 8099–8106. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.G.; Teno, J.; Torres, D.; Díaz, M. Solution Blow Spinning and Obtaining Submicrometric Fibers of Different Polymers. Int. J. Nanopart. Nanotechnol. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Mollet, T.; Ceylan, Ö.; De Clerck, K. The development of polyamide 6.6 nanofibres with a pH-sensitive function by electrospinning. Eur. Polym. J. 2010, 46, 2229–2239. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution blow spinning: A new method to produce micro- and nanofibers from polymer solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Mattoso, L.H.C.; Medeiros, E.S.; Zucolotto, V. Poly(lactic acid)/Carbon Nanotube Fibers as Novel Platforms for Glucose Biosensors. Biosensors 2012, 2, 70–82. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef]
- da S. Parize, D.D.; Foschini, M.M.; de Oliveira, J.E.; Klamczynski, A.P.; Glenn, G.M.; Marconcini, J.M.; Mattoso, L.H.C. Solution blow spinning: Parameters optimization and effects on the properties of nanofibers from poly(lactic acid)/dimethyl carbonate solutions. J. Mater. Sci. 2016, 51, 4627–4638. [Google Scholar]
- Li, Y.; Jiao, M.; Zhao, H.; Yang, M. Humidity sensing properties of the composite of electrospun crosslinked polyelectrolyte nanofibers decorated with Ag nanoparticles. Sens. Actuators B Chem. 2018, 273, 133–142. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Formo, E.; Lee, E.; Campbell, D.; Xia, Y. Functionalization of Electrospun TiO2 Nanofibers with Pt Nanoparticles and Nanowires for Catalytic Applications. Nano Lett. 2008, 8, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Schoolaert, E.; Steyaert, I.; Vancoillie, G.; Geltmeyer, J.; Lava, K.; Hoogenboom, R.; Clerck, K.D. Blend electrospinning of dye-functionalized chitosan and poly(ε-caprolactone): towards biocompatible pH-sensors. J. Mater. Chem. B 2016, 4, 4507–4516. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- European Comission. Commission Regulation (EU) NO 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, L12, 1–89. [Google Scholar]
- Lozano, J.; Álvarez, F.; Santos, J.P.; Horrillo, C. Detection of Acetic Acid in wine by means of an electronic nose. AIP Conf. Proc. 2011, 1362, 176–177. [Google Scholar]
- Hopfer, H.; Nelson, J.; Ebeler, S.E.; Heymann, H. Correlating wine quality indicators to chemical and sensory measurements. Molecules 2015, 20, 8453–8483. [Google Scholar] [CrossRef]
- Loock, H.-P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Gimenes, D.T.; dos Santos, W.T.P.; Tormin, T.F.; Munoz, R.A.A.; Richter, E.M. Flow-Injection Amperometric Method for Indirect Determination of Dopamine in the Presence of a Large Excess of Ascorbic Acid. Electroanalysis 2010, 22, 74–78. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Huan, S.; Liu, G.; Han, G.; Cheng, W.; Fu, Z.; Wu, Q.; Wang, Q. Effect of Experimental Parameters on Morphological, Mechanical and Hydrophobic Properties of Electrospun Polystyrene Fibers. Materials 2015, 8, 2718–2734. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Vainilovitch, I.S.; Magonov, S.N. FTIR spectroscopy of polymer films under uniaxial stretching. Polym. Bull. 1991, 25, 499–506. [Google Scholar] [CrossRef]
- Rodbari, R.J.; Wendelbo, R.; Jamshidi, L.C.L.A.; Hernández, E.P.; Nascimento, L. Study of physical and chemical characterization of nanocomposite polystyrene/graphene oxide high acidity can be applied in thin films. J. Chil. Chem. Soc. 2016, 61, 3120–3124. [Google Scholar] [CrossRef][Green Version]
- Nyquist, R.A.; Putzig, C.L.; Leugers, M.A.; McLachlan, R.D.; Thill, B. Comparison of the Vibrational Spectra and Assignments for α-Syndiotactic, β-Syndiotactic, Isotactic, and Atactic Polystyrene and Toluene, Comparison of the Vibrational Spectra and Assignments for α-Syndiotactic, β-Syndiotactic, Isotactic, and Atactic Polystyrene and Toluene. Appl. Spectrosc. 1992, 46, 981–987. [Google Scholar]
- Bhutto, A.A.; Vesely, D.; Gabrys, B.J. Miscibility and interactions in polystyrene and sodium sulfonated polystyrene with poly(vinyl methyl ether) PVME blends. Part II. FTIR. Polymer 2003, 44, 6627–6631. [Google Scholar] [CrossRef]
- Krasovskii, A.N.; Novikov, D.V.; Osmolovskaya, N.A.; Borisova, S.V. ATR IR spectra and structure of boundary layers of atactic polystyrene. Polym. Sci. Ser. A 2012, 54, 451–458. [Google Scholar] [CrossRef]
- Rahman, N.; Manirul Haque, S.; Azmi, S.N.H.; Rahman, H. Optimized and validated spectrophotometric methods for the determination of amiodarone hydrochloride in commercial dosage forms using N-bromosuccinimide and bromothymol blue. J. Saudi Chem. Soc. 2017, 21, 25–34. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al-Ghafri, L.T.; Al-Ghafri, S.S.; Al-Haribi, M.M. Determination of Buspirone HCl in Commercial Dosage Forms by Extractive Spectrophotometric Method and Comparison by HPLC Method. Sci. J. Anal. Chem. 2015, 3, 91. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 978-0-470-02731-8. [Google Scholar]
- Qi, Z.; Matsuda, N.; Santos, J.; Itoh, K.; Takatsu, A.; Kato, K. A Study of Molecular Adsorption of Bromothymol Blue by Optical Waveguide Spectroscopy. Langmuir 2003, 19, 214–217. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Crowe, J.H. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 1989, 28, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Dowding, C.E.; Borda, M.J.; Fey, M.V.; Sparks, D.L. A new method for gaining insight into the chemistry of drying mineral surfaces using ATR-FTIR. J. Colloid Interface Sci. 2005, 292, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Hernández, P.; Ramírez, M.T.; Rojas-Hernández, A.; Gutiérrez, A. Determination of pK(a)’s for thymol blue in aqueous medium: Evidence of dimer formation. Talanta 1998, 46, 1439–1452. [Google Scholar] [CrossRef]
- Tassanawat, S.; Phandee, A.; Magaraphan, R.; Nithitanakul, M.; Manuspiya, H. pH-Sensitive PP/Clay Nanocomposites for Beverage Smart Packaging. In Proceedings of the 2007 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007; pp. 478–482. [Google Scholar]
- Lakshmi, D.; Rajendran, S.; Sathiyabama, J.; Rathis, R.J.; Prabha, S.S. Application of infra-red spectroscopy in corrosion inhibition studies. Int. J. Nano Corros. Sci. Eng. 2016, 3, 181–203. [Google Scholar]
- Sahil, K.; Prashant, B.; Akanksha, M.; Premjeet, S.; Devashish, R. Interpretation of infrared spectra. Int. J. Pharm. Chem. Sci. 2012, 1, 174–200. [Google Scholar]
- Wang, S.; Copeland, L. Effect of acid hydrolysis on starch structure and functionality: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Lazzaro, F.; Borzì, M.A.; Sabatino, L.; Traulo, P.; Gagliano, G. Dehydroacetic acid in cheese and cheese coating, results of official control in Italy. Food Addit. Contam. Part B 2018, 11, 75–81. [Google Scholar] [CrossRef]
- Costa, M.A.S.; Cerri, B.C.; Ceccato-Antonini, S.R. Ethanol addition enhances acid treatment to eliminate Lactobacillus fermentum from the fermentation process for fuel ethanol production. Lett. Appl. Microbiol. 2018, 66, 77–85. [Google Scholar] [CrossRef]
- Lourenço, A.S.; Nascimento, R.F.; Silva, A.C.; Ribeiro, W.F.; Araujo, M.C.U.; Oliveira, S.C.B.; Nascimento, V.B. Voltammetric determination of tartaric acid in wines by electrocatalytic oxidation on a cobalt(II)-phthalocyanine-modified electrode associated with multiway calibration. Anal. Chim. Acta 2018, 1008, 29–37. [Google Scholar] [CrossRef]
- FAO. Agribusiness Handbook—Grapes Wines; Agribusiness Manuals; FAO Investiment Center Division: Rome, Italy, 2009. [Google Scholar]
- MAPA. Instrução Normativa NO 14, de 08 de fevereiro de 2018, Complementação dos Padrões de Identidade e Qualidade do Vinho e Derivados da Uva e do Vinho; MAPA: Brasília, Brazil, 2018; pp. 1–27.
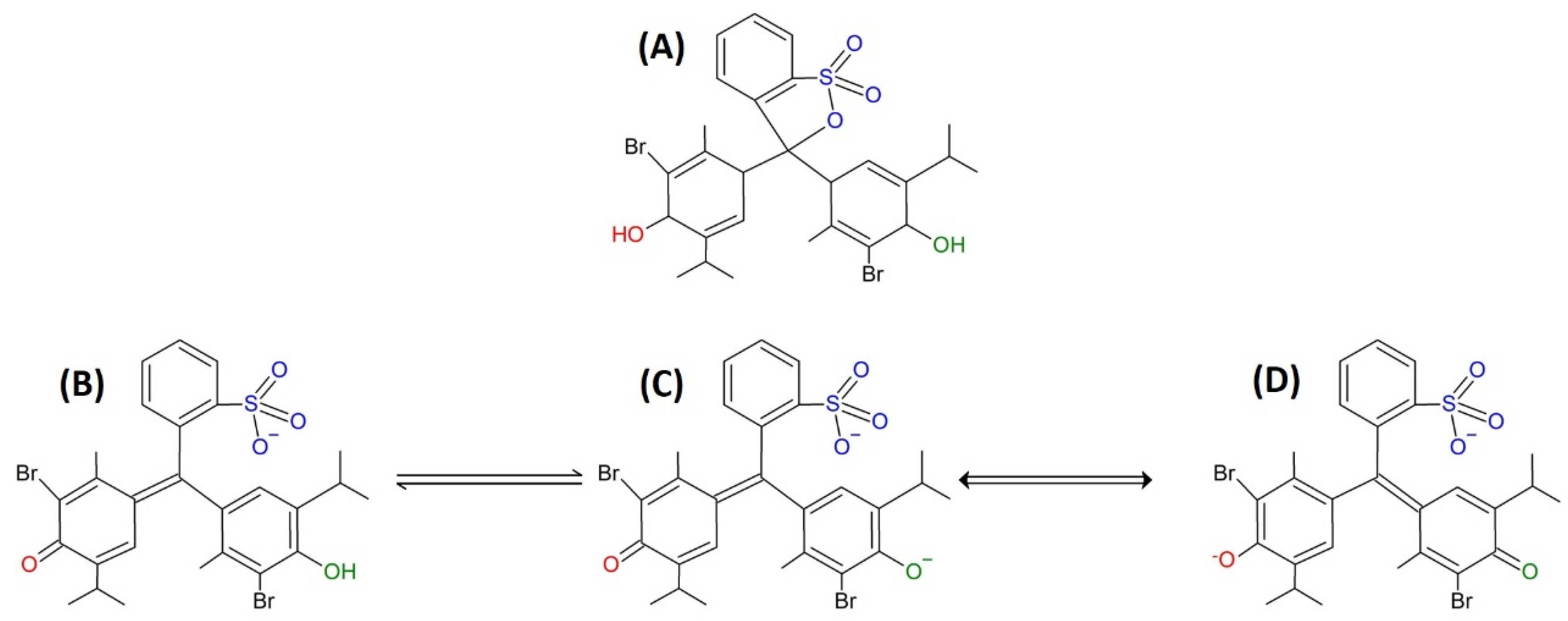
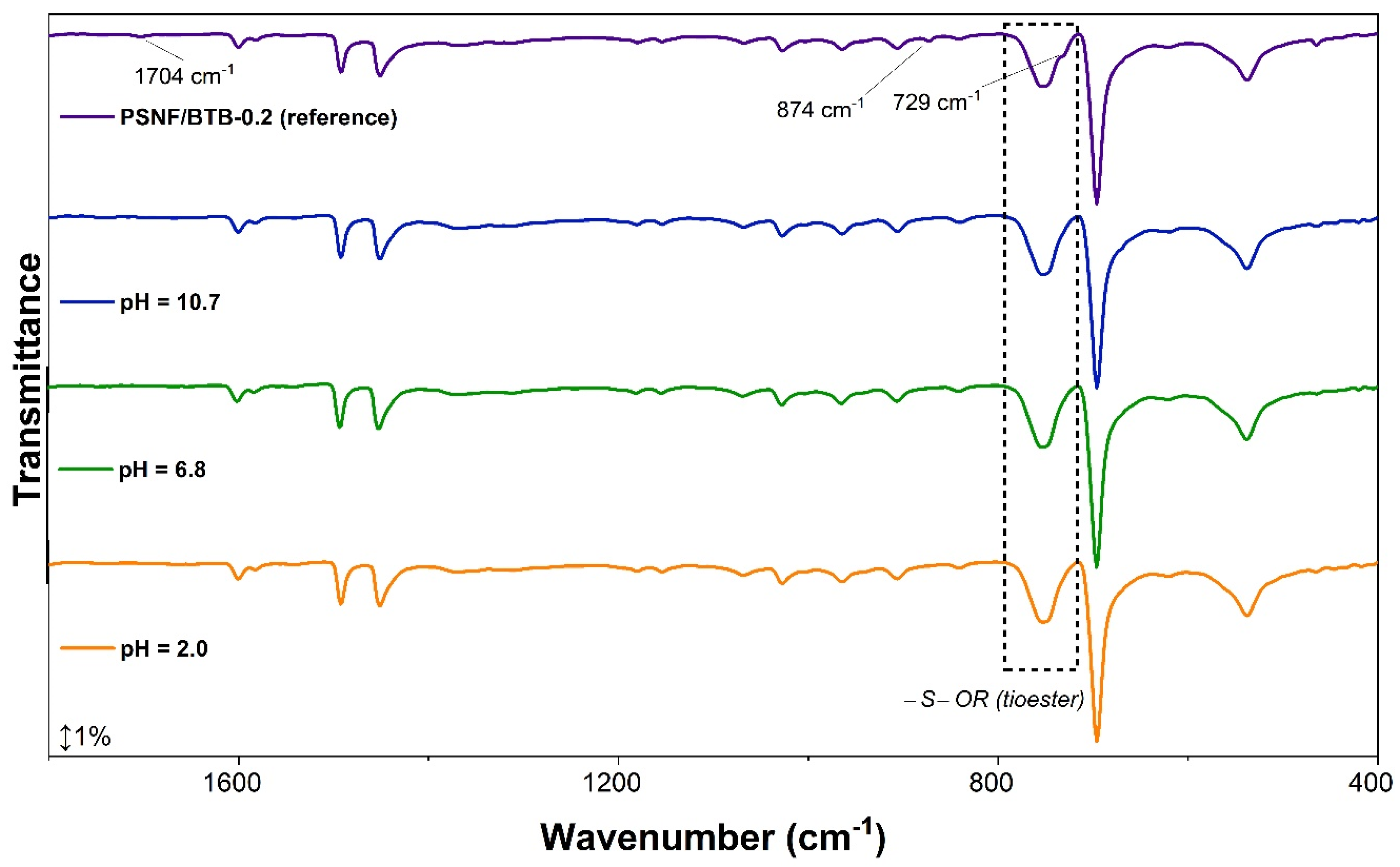
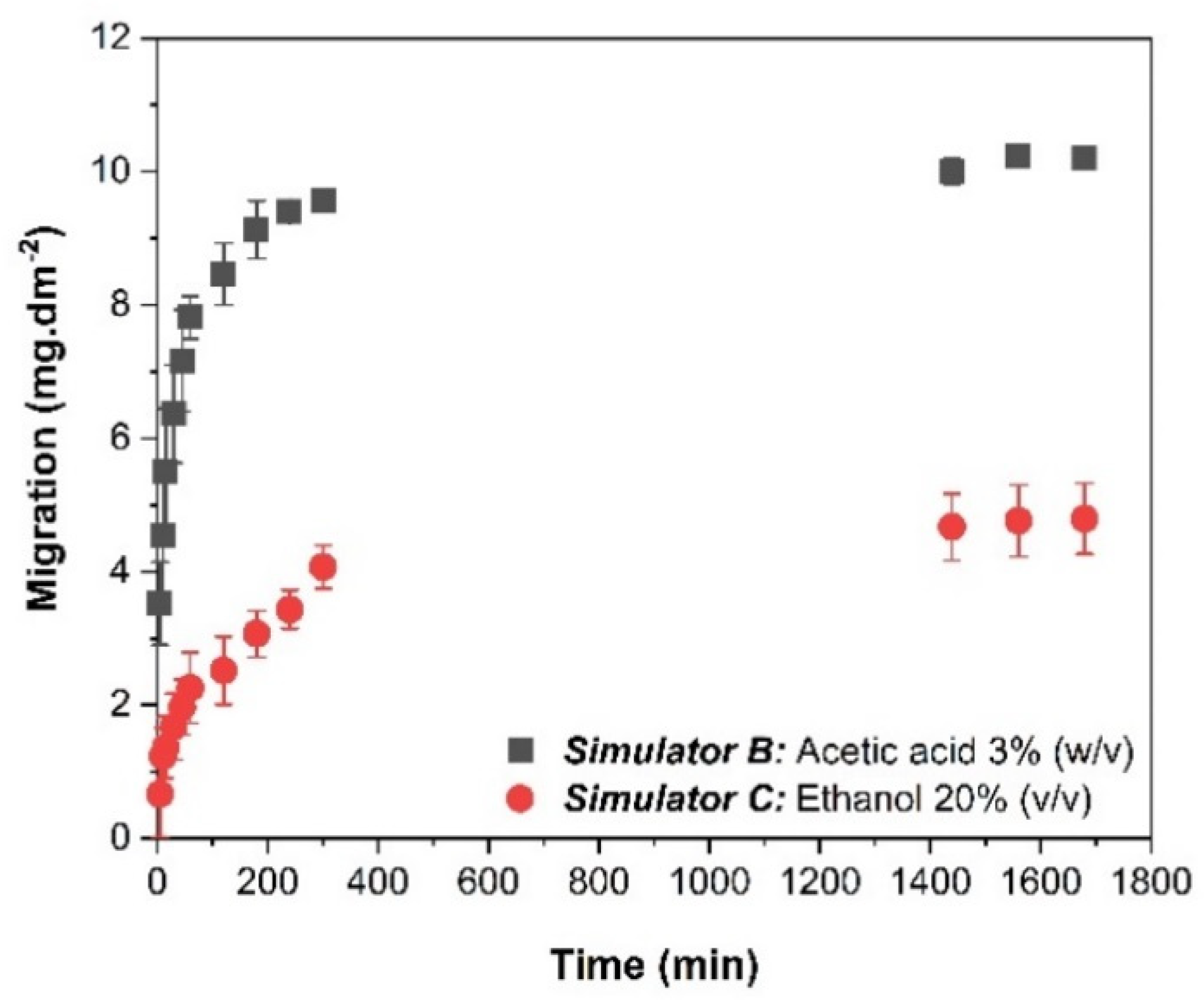
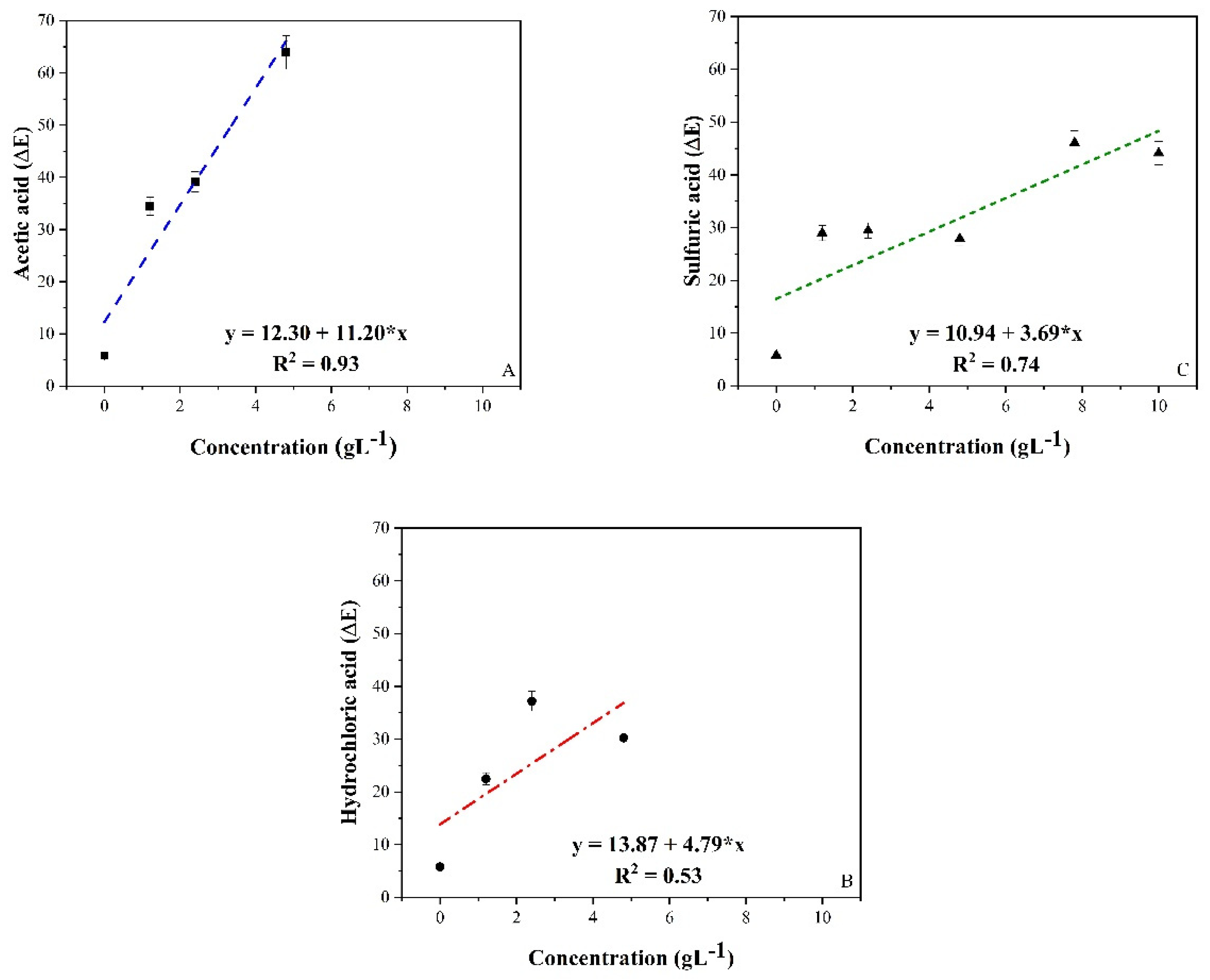
| Mediums Compared | ΔE | |
|---|---|---|
| Nanofibers | Films | |
| Acid—Basic | 14.66 | 1.87 |
| Acid—Neutral | 8.26 | 5.30 |
| Basic—Neutral | 7.45 | 6.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, K.W.E.; Natarelli, C.V.L.; Thomazi, A.C.; Ferreira, G.M.D.; Frota, M.M.; Bastos, M.d.S.R.; Mattoso, L.H.C.; Oliveira, J.E. Halochromic Polystyrene Nanofibers Obtained by Solution Blow Spinning for Wine pH Sensing. Sensors 2020, 20, 417. https://doi.org/10.3390/s20020417
Miranda KWE, Natarelli CVL, Thomazi AC, Ferreira GMD, Frota MM, Bastos MdSR, Mattoso LHC, Oliveira JE. Halochromic Polystyrene Nanofibers Obtained by Solution Blow Spinning for Wine pH Sensing. Sensors. 2020; 20(2):417. https://doi.org/10.3390/s20020417
Chicago/Turabian StyleMiranda, Kelvi W.E., Caio V. L. Natarelli, Adriana C. Thomazi, Guilherme M. D. Ferreira, Maryana M. Frota, Maria do Socorro R. Bastos, Luiz H. C. Mattoso, and Juliano E. Oliveira. 2020. "Halochromic Polystyrene Nanofibers Obtained by Solution Blow Spinning for Wine pH Sensing" Sensors 20, no. 2: 417. https://doi.org/10.3390/s20020417
APA StyleMiranda, K. W. E., Natarelli, C. V. L., Thomazi, A. C., Ferreira, G. M. D., Frota, M. M., Bastos, M. d. S. R., Mattoso, L. H. C., & Oliveira, J. E. (2020). Halochromic Polystyrene Nanofibers Obtained by Solution Blow Spinning for Wine pH Sensing. Sensors, 20(2), 417. https://doi.org/10.3390/s20020417







