One-Step Assembly of Fluorescence-Based Cyanide Sensors from Inexpensive, Off-The-Shelf Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Thin Layer Chromatography
2.3. Attenuated Total Reflectance-Fourier Transform Infrared Studies
3. Results and Discussion
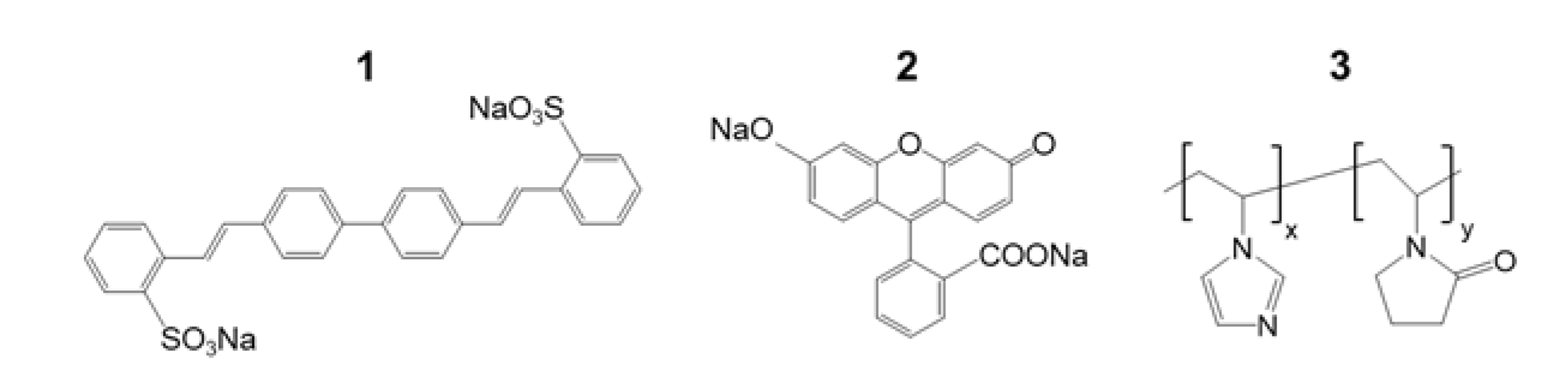
3.1. Sensing Mechanism
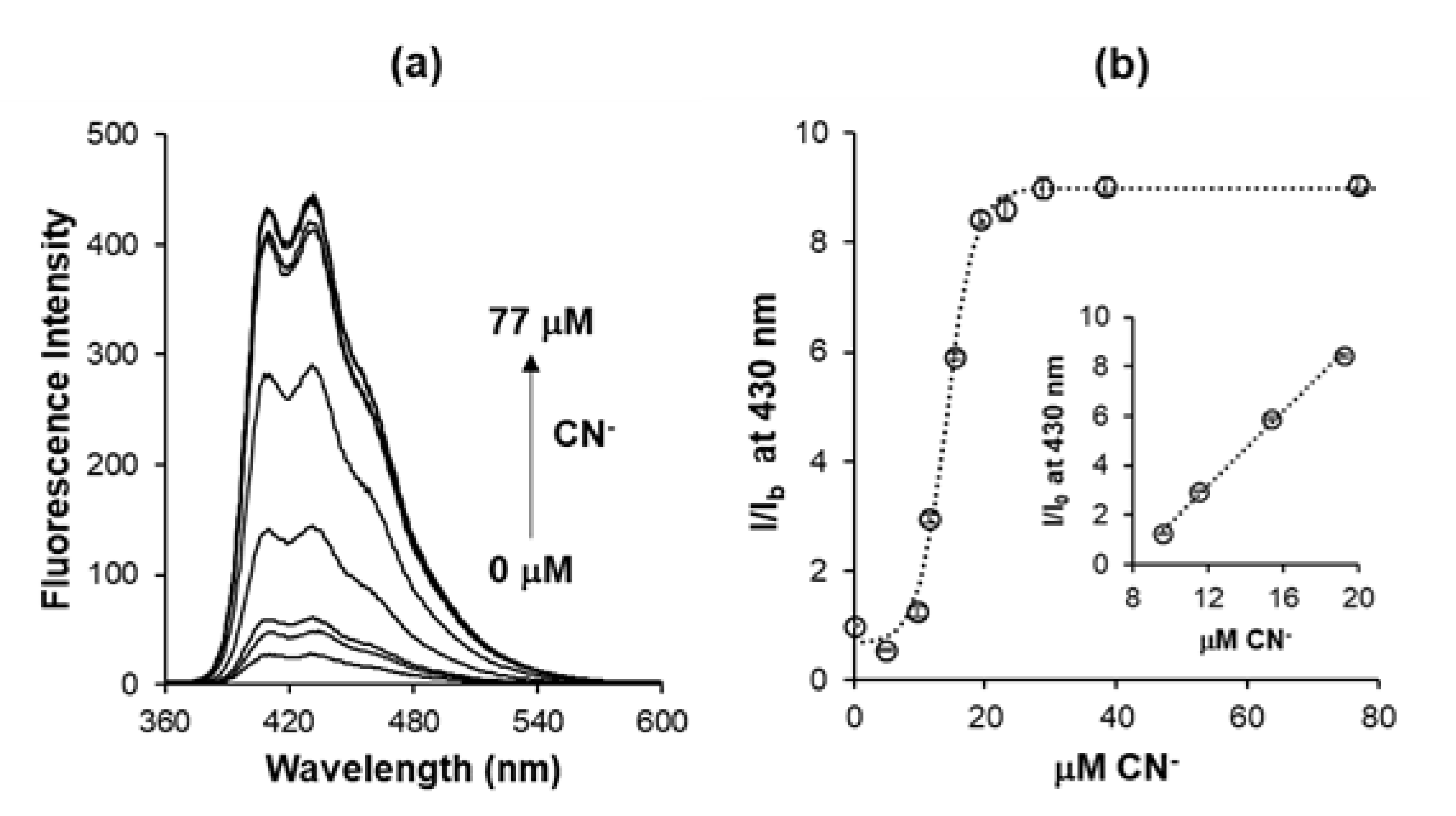
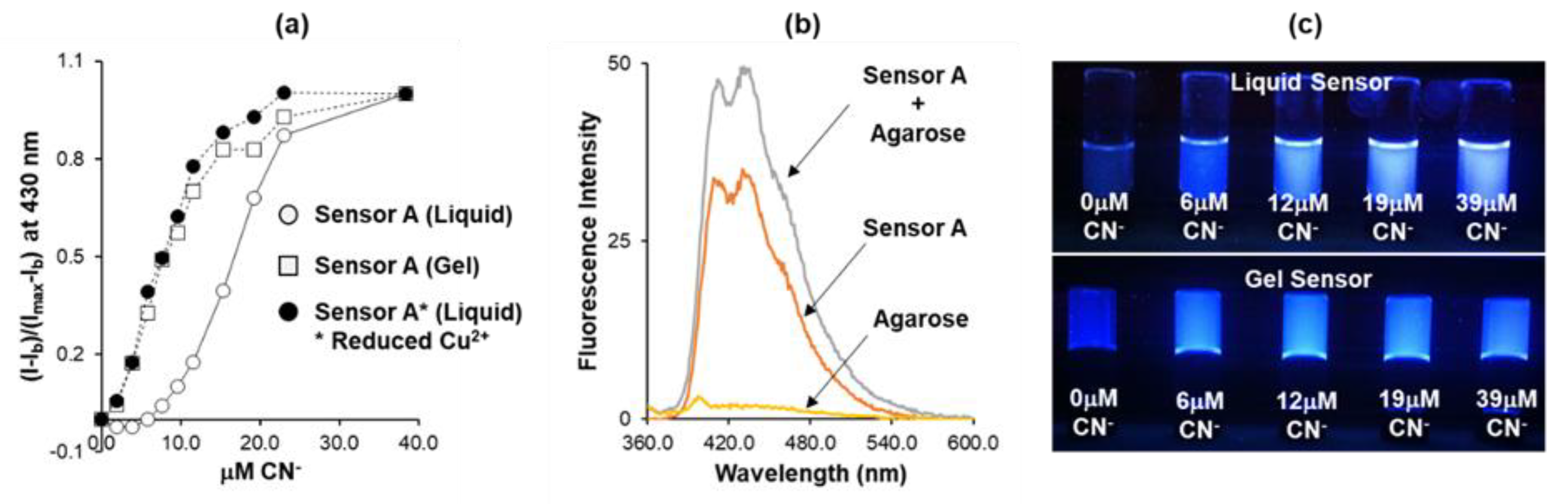
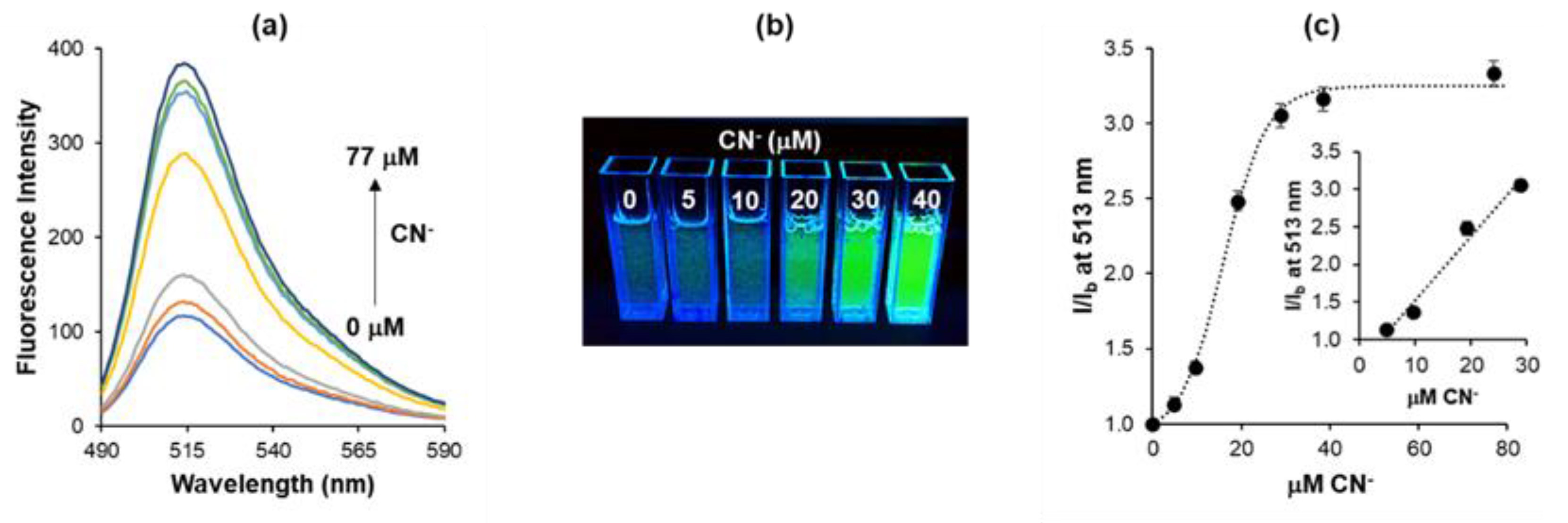
3.2. Sensing Performance and Sensitivity Optimization
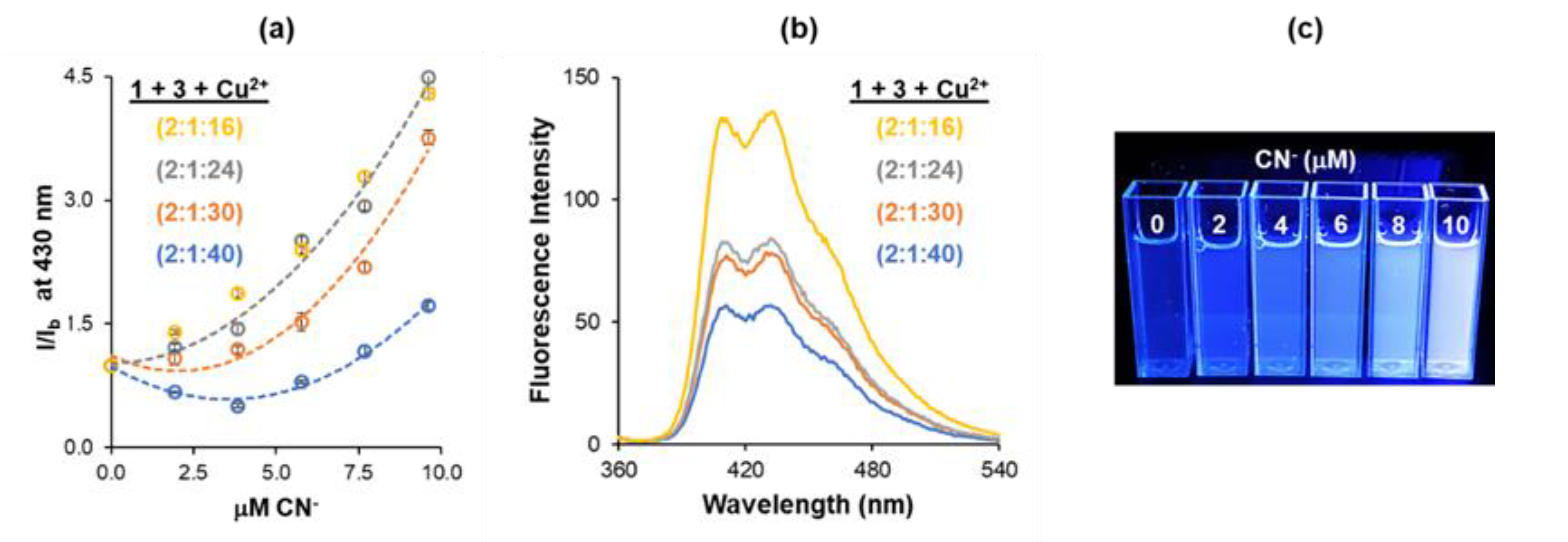
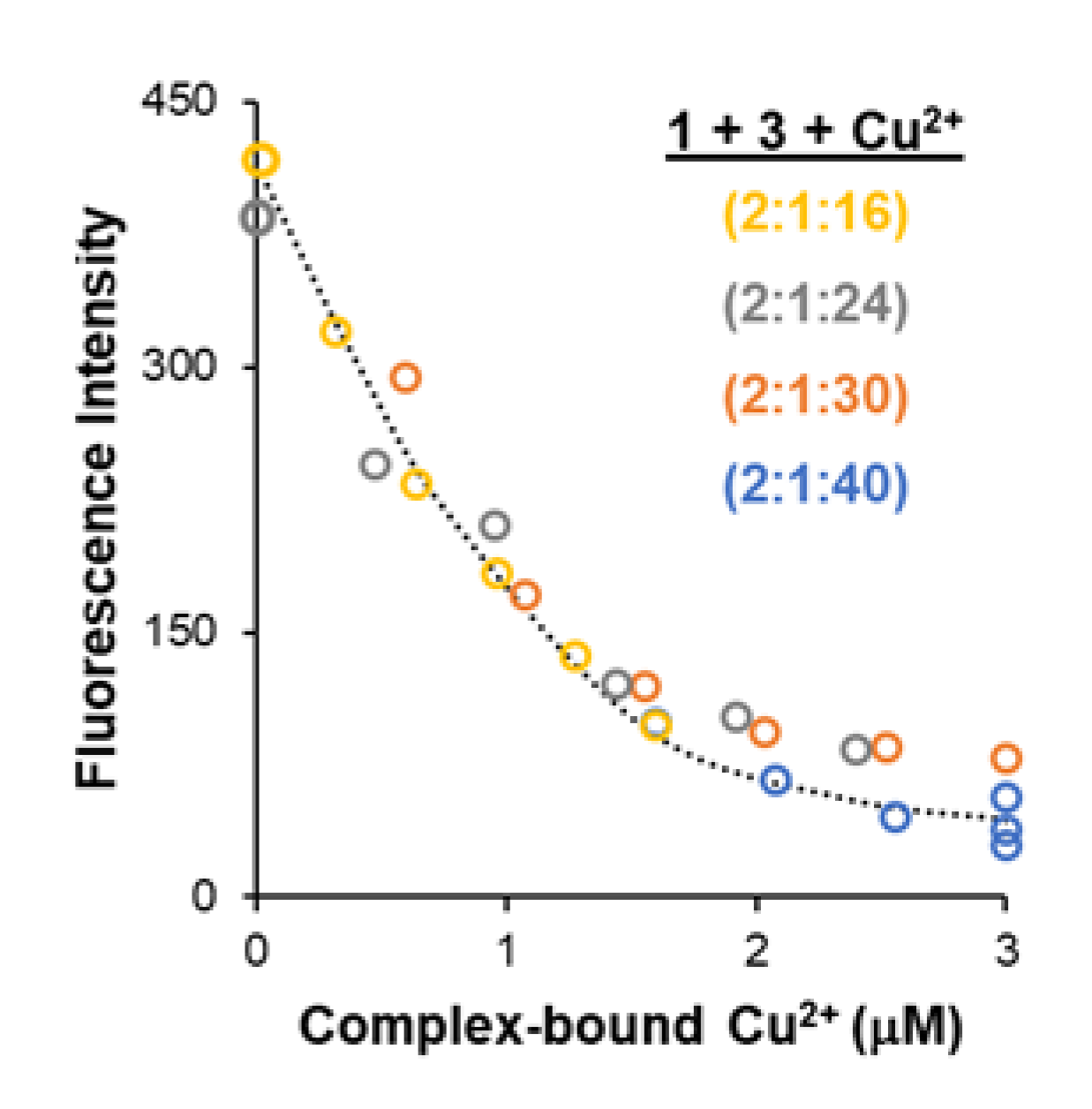
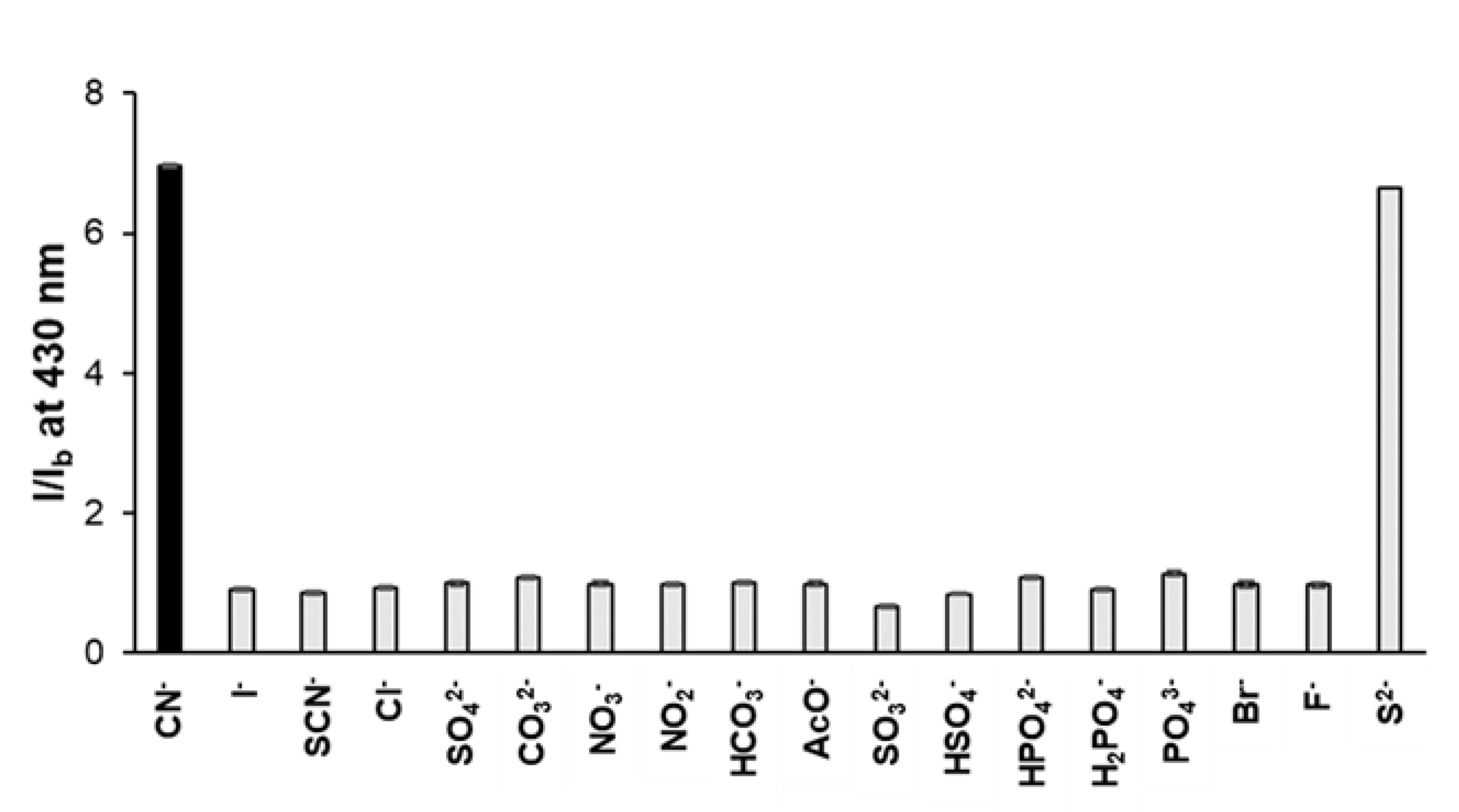
3.3. Overcoming Interference
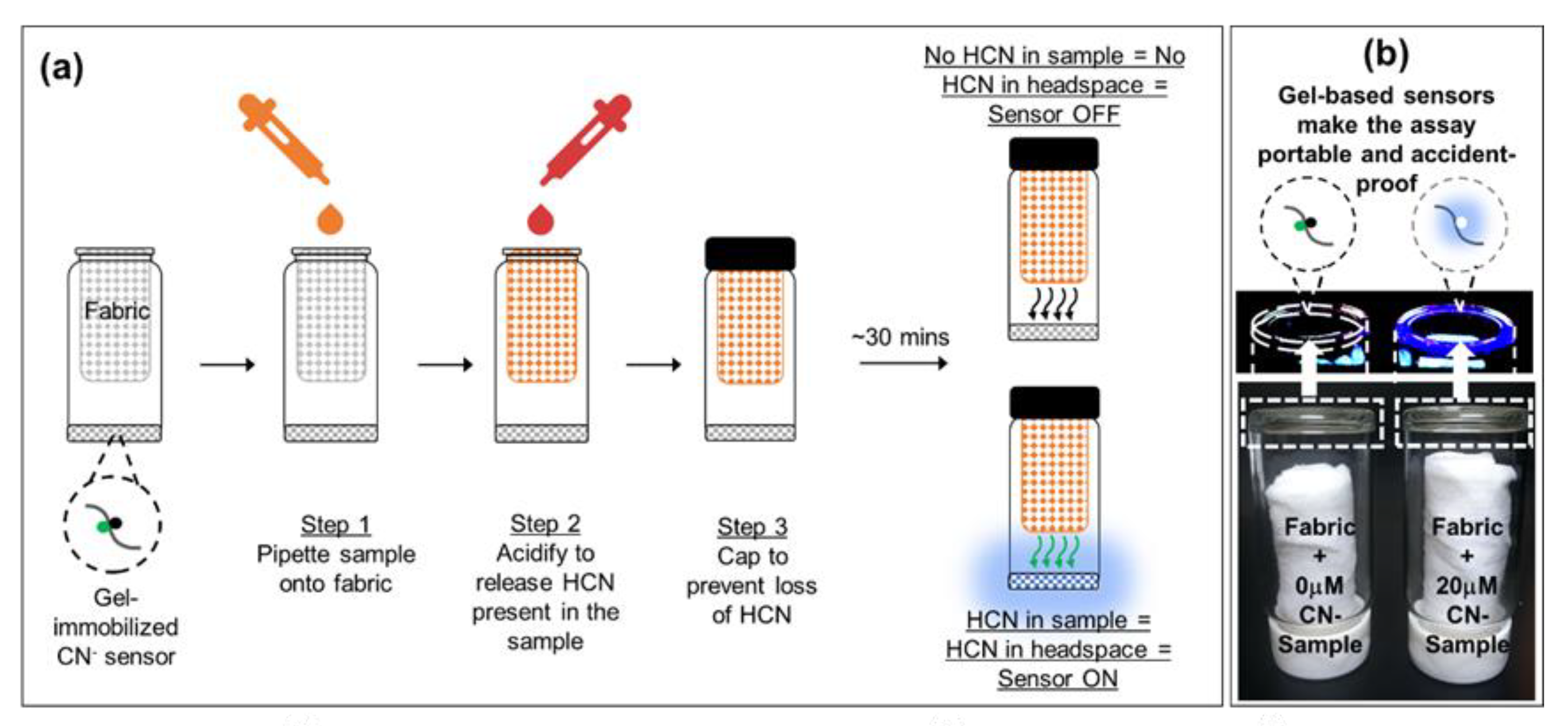
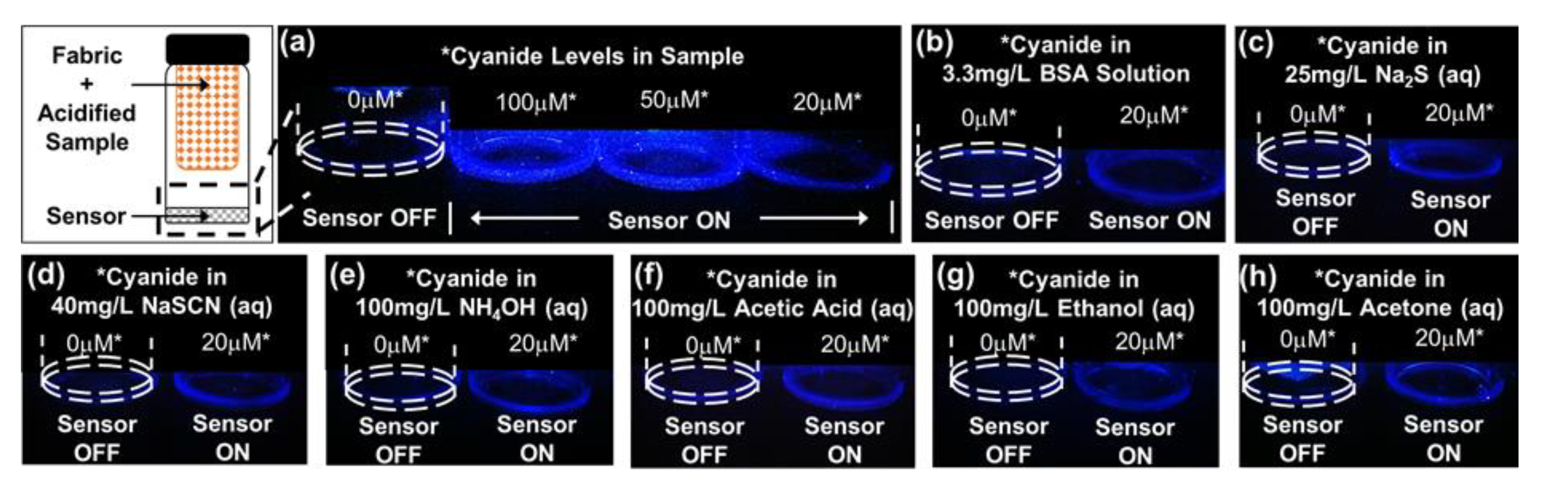
3.4. Cyanide Sensing Hydrogels
3.5. Emission Tuning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mudder, T.I.; Botz, M.M. Cyanide and Society: A Critical Review. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 62–74. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cyanide; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2006. [Google Scholar]
- Alarie, Y. Toxicity of fire smoke. Crit. Rev. Toxicol. 2002, 32, 259–289. [Google Scholar] [CrossRef] [PubMed]
- Lawson-Smith, P.; Jansen, E.C.; Hyldegaard, O. Cyanide intoxication as part of smoke inhalation—A review on diagnosis and treatment from the emergency perspective. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Logue, B.A. A review of rapid and field-portable analytical techniques for the diagnosis of cyanide exposure. Anal. Chim. Acta 2017, 960, 18–39. [Google Scholar] [CrossRef]
- Stamyr, K.; Thelander, G.; Ernstgård, L.; Ahlner, J.; Johanson, G. Swedish forensic data 1992–2009 suggest hydrogen cyanide as an important cause of death in fire victims. Inhal. Toxicol. 2012, 24, 194–199. [Google Scholar] [CrossRef]
- Yeoh, M.J.; Braitberg, G. Carbon monoxide and cyanide poisoning in fire related deaths in Victoria, Australia. J. Toxicol. Clin. Toxicol. 2004, 42, 855–863. [Google Scholar] [CrossRef]
- Ryall, B.; Davies, J.C.; Wilson, R.; Shoemark, A.; Williams, H.D. Pseudomonas aeruginosa, cyanide accumulation and lung function in CF and non-CF bronchiectasis patients. Eur. Respir. J. 2008, 32, 740–747. [Google Scholar] [CrossRef]
- Ryall, B.; Lee, X.Y.; Zlosnik, J.E.A.; Hoshino, S.; Williams, H.D. Bacteria of the Burkholderia cepacia complex are cyanogenic under biofilm and colonial growth conditions. BMC Microbiol. 2008, 8. [Google Scholar] [CrossRef]
- Anderson, R.D.; Roddam, L.F.; Bettiol, S.; Sanderson, K.; Reid, D.W. Biosignificance of bacterial cyanogenesis in the CF lung. J. Cyst. Fibros. 2010, 9, 158–164. [Google Scholar] [CrossRef][Green Version]
- Gilchrist, F.J.; Alcock, A.; Belcher, J.; Brady, M.; Jones, A.; Smith, D.; Španĕl, P.; Webb, K.; Lenney, W. Variation in hydrogen cyanide production between different strains of Pseudomonas aeruginosa. Eur. Respir. J. 2011, 38, 409–414. [Google Scholar] [CrossRef]
- Neerincx, A.H.; Linders, Y.A.M.; Vermeulen, L.; Belderbos, R.A.; Mandon, J.; van Mastrigt, E.; Pijnenburg, M.W.; van Ingen, J.; Mouton, J.W.; Kluijtmans, L.A.J.; et al. Hydrogen cyanide emission in the lung by Staphylococcus aureus. Eur. Respir. J. 2016, 48, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.D.; Jolliffe, K.A.; Pfeffer, F.M. Luminescent probes for the bioimaging of small anionic species in vitro and in vivo. Chem. Soc. Rev. 2015, 44, 4547–4595. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.D.; Ou, D.X.; Li, Q.Q.; Li, Z. An indirect approach for anion detection: The displacement strategy and its application. Chem. Commun. 2012, 48, 8462–8477. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, X.Q.; Yoon, J.Y. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef]
- Li, Z.; Lou, X.; Yu, H.; Li, Z.; Qin, J. An Imidazole-Functionalized Polyfluorene Derivative as Sensitive Fluorescent Probe for Metal Ions and Cyanide. Macromolecules 2008, 41, 7433–7439. [Google Scholar] [CrossRef]
- Zeng, Q.; Cai, P.; Li, Z.; Qin, J.; Tang, B.Z. An imidazole-functionalized polyacetylene: Convenient synthesis and selective chemosensor for metal ions and cyanide. Chem. Commun. 2008, 1094–1096. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nam, S.W.; Kim, G.H.; Song, N.; Jeong, Y.; Shin, I.; Kim, S.K.; Kim, J.; Park, S.; Yoon, J. A near-infrared fluorescent sensor for detection of cyanide in aqueous solution and its application for bioimaging. Chem. Commun. 2010, 46, 8953–8955. [Google Scholar] [CrossRef]
- Nam, S.W.; Chen, X.; Lim, J.; Kim, S.H.; Kim, S.T.; Cho, Y.H.; Yoon, J.; Park, S. In Vivo Fluorescence Imaging of Bacteriogenic Cyanide in the Lungs of Live Mice Infected with Cystic Fibrosis Pathogens. PLoS ONE 2011, 6, e21387. [Google Scholar] [CrossRef]
- Divya, K.P.; Sreejith, S.; Balakrishna, B.; Jayamurthy, P.; Anees, P.; Ajayaghosh, A. A Zn2+-specific fluorescent molecular probe for the selective detection of endogenous cyanide in biorelevant samples. Chem. Commun. 2010, 46, 6069–6071. [Google Scholar] [CrossRef]
- Lou, X.D.; Zhang, L.Y.; Qin, J.G.; Li, Z. An alternative approach to develop a highly sensitive and selective chemosensor for the colorimetric sensing of cyanide in water. Chem. Commun. 2008, 5848–5850. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, A.R.; Shin, I.S.; Kim, H.J.; Hong, J.I. Fluorescence Turn-On Sensor for Cyanide Based on a Cobalt(II)-Coumarinylsalen Complex. Org. Lett. 2010, 12, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Lv, X.; Zhao, Y.; Liu, J.; Sun, Y.Q.; Wang, P.; Guo, W. A Cu(II)-based chemosensing ensemble bearing rhodamine B fluorophore for fluorescence turn-on detection of cyanide. J. Mater. Chem. 2012, 22, 1747–1750. [Google Scholar] [CrossRef]
- Wu, J.; Kwon, B.; Liu, W.; Anslyn, E.V.; Wang, P.; Kim, J.S. Chromogenic/Fluorogenic Ensemble Chemosensing Systems. Chem. Rev. 2015, 115, 7893–7943. [Google Scholar] [CrossRef] [PubMed]
- Baud, F.J.; Barriot, P.; Toffis, V.; Riou, B.; Vicaut, E.; Lecarpentier, Y.; Bourdon, R.; Astier, A.; Bismuth, C. Elevated Blood Cyanide Concentrations in Victims of Smoke Inhalation. N. Engl. J. Med. 1991, 325, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- 2018 Drinking Water Standards and Advisory Tables: United States Environmental Protection Agency. Available online: https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf (accessed on 1 July 2019).
- McAnalley, B.H.; Lowry, W.T.; Oliver, R.D.; Garriott, J.C. Determination of Inorganic Sulfide and Cyanide in Blood Using Specific Ion Electrodes: Application to the Investigation of Hydrogen Sulfide and Cyanide Poisoning. J. Anal. Toxicol. 1979, 3, 111–114. [Google Scholar] [CrossRef]
- Blackledge, W.C.; Blackledge, C.W.; Griesel, A.; Mahon, S.B.; Brenner, M.; Pilz, R.B.; Boss, G.R. New Facile Method to Measure Cyanide in Blood. Anal. Chem. 2010, 82, 4216–4221. [Google Scholar] [CrossRef]
- Fernandes, G.E.; Ugwu, C. Cu2+ sensing via noncovalent complexes of fluorescent whitening agents and imidazole-based polymeric dye transfer inhibitors. J. Appl. Polym. Sci. 2020, 137, 48915. [Google Scholar] [CrossRef]
- Wicholas, M.; Wolford, T. Stable cyanide complexes of copper(II). Inorg. Chem. 1974, 13, 316–318. [Google Scholar] [CrossRef]
- Kavallieratos, K.; Rosenberg, J.M.; Chen, W.-Z.; Ren, T. Fluorescent Sensing and Selective Pb(II) Extraction by a Dansylamide Ion-Exchanger. J. Am. Chem. Soc. 2005, 127, 6514–6515. [Google Scholar] [CrossRef]
- Zheng, Y.; Orbulescu, J.; Ji, X.; Andreopoulos, F.M.; Pham, S.M.; Leblanc, R.M. Development of Fluorescent Film Sensors for the Detection of Divalent Copper. J. Am. Chem. Soc. 2003, 125, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Lippert, J.L.; Robertson, J.A.; Havens, J.R.; Tan, J.S. Structural studies of poly(N-vinylimidazole) complexes by infrared and Raman spectroscopy. Macromolecules 1985, 18, 63–67. [Google Scholar] [CrossRef]
- Trojer, M.A.; Mårtensson, A.; Nydén, M. Replacement of H-bonded bridged water by transition metal ions in poly(1-vinylimidazole-co-methylmethacrylate) copolymers: A vibrational spectroscopy study using mid-FTIR, far-FTIR and ab initio calculations. Vib. Spectrosc. 2012, 61, 38–42. [Google Scholar] [CrossRef]
- Andersson, M.; Hansson, Ö.; Öhrström, L.; Idström, A.; Nydén, M. Vinylimidazole copolymers: Coordination chemistry, solubility, and cross-linking as function of Cu2+ and Zn2+ complexation. Colloid Polym. Sci. 2011, 289, 1361–1372. [Google Scholar] [CrossRef]
- Crerar, D.A.; Barnes, H.L. Ore solution chemistry; V, Solubilities of chalcopyrite and chalcocite assemblages in hydrothermal solution at 200 degrees to 350 degrees C. Econ. Geol. 1976, 71, 772–794. [Google Scholar] [CrossRef]
- Young, C.A.; Dahlgren, E.J.; Robins, R.G. The solubility of copper sulfides under reducing conditions. Hydrometallurgy 2003, 68, 23–31. [Google Scholar] [CrossRef]
- Jha, S.; Calvert, J.W.; Duranski, M.R.; Ramachandran, A.; Lefer, D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H801–H806. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: From brain to gut. Antioxid Redox Signal 2010, 12, 1111–1123. [Google Scholar] [CrossRef]
- Cutting, K. Wound Exudate: Composition and Functions. Br. J. community Nurs. 2003, 8. [Google Scholar] [CrossRef]
- Neilands, J.B. Siderophores: Structure and Function of Microbial Iron Transport Compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Vinnakota, C.V.; Peetha, N.S.; Perrizo, M.G.; Ferris, D.G.; Oda, R.P.; Rockwood, G.A.; Logue, B.A. Comparison of cyanide exposure markers in the biofluids of smokers and non-smokers. Biomarkers 2012, 17, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Kataoka, M.; Seto, Y. Cyanide and Thiocyanate Levels in Blood and Saliva of Healthy Adult Volunteers. J. Health Sci. 2000, 46, 343–350. [Google Scholar] [CrossRef]
- Jackson, R.; Oda, R.P.; Bhandari, R.K.; Mahon, S.B.; Brenner, M.; Rockwood, G.A.; Logue, B.A. Development of a Fluorescence-Based Sensor for Rapid Diagnosis of Cyanide Exposure. Anal. Chem. 2014, 86, 1845–1852. [Google Scholar] [CrossRef]
- David, S.; Patrik, Š.; Francis, J.G.; Warren, L. Hydrogen cyanide, a volatile biomarker of Pseudomonas aeruginosa infection. J. Breath Res. 2013, 7, 044001. [Google Scholar]
- Mohammed, A.; Matt, B.; Mohamed, B.; Teresa, A.R.; Riina, R.R.; Ardeshir, B. Volatile organic compound detection as a potential means of diagnosing cutaneous wound infections. Wound Repair Regen. 2017, 25, 574–590. [Google Scholar] [CrossRef]
- Yin, K.; Wu, Y.; Wang, S.; Chen, L. A sensitive fluorescent biosensor for the detection of copper ion inspired by biological recognition element pyoverdine. Sens. Actuators B Chem. 2016, 232, 257–263. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, X.J.; Fang, D.C.; Jin, L.P. A highly selective and sensitive Zn(II) complex-based chemosensor for sequential recognition of Cu(II) and cyanide dagger. Dalton Trans. 2014, 43, 7298–7303. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Tang, X.L.; Hou, F.P.; Wu, J.; Dou, W.; Qin, W.W.; Ru, J.X.; Zhang, G.L.; Liu, W.S.; Yao, X.J. A reversible fluorescent chemosensor for cyanide in 100% aqueous solution. Sens. Actuators B Chem. 2013, 181, 202–208. [Google Scholar] [CrossRef]
- ASTM D2036-09. Standard Test Methods for Cyanides in Water; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Pourmand, N.; Sanagi, M.M.; Naim, A.A.; Wan Ibrahim, W.A.; Baig, U. Dispersive micro-solid phase extraction method using newly prepared poly(methyl methacrylate) grafted agarose combined with ICP-MS for the simultaneous determination of Cd, Ni, Cu and Zn in vegetable and natural water samples. Anal. Methods 2015, 7, 3215–3223. [Google Scholar] [CrossRef]
- Aksu, Z.; Eğretli, G.; Kutsal, T. A comparative study of copper(II) biosorption on Ca-alginate, agarose and immobilized C. vulgaris in a packed-bed column. Process Biochem. 1998, 33, 393–400. [Google Scholar] [CrossRef]
- Cerchiaro, G.; Sant’Ana, A.C.; Temperini, M.L.; da Costa Ferreira, A.M. Investigations of different carbohydrate anomers in copper(II) complexes with D-glucose, D-fructose, and D-galactose by Raman and EPR spectroscopy. Carbohydr. Res. 2005, 340, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Rendleman, J.A. Metal-polysaccharide complexes—Part I. Food Chem. 1978, 3, 47–79. [Google Scholar] [CrossRef]
- Rendleman, J.A. Metal-polysaccharide complexes—Part II. Food Chem. 1978, 3, 127–162. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J. Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef]
- Samiey, B.; Ashoori, F. Adsorptive removal of methylene blue by agar: Effects of NaCl and ethanol. Chem. Cent. J. 2012, 6, 14. [Google Scholar] [CrossRef]
- Seow, W.Y.; Hauser, C.A.E. Freeze–dried agarose gels: A cheap, simple and recyclable adsorbent for the purification of methylene blue from industrial wastewater. J. Environ. Chem. Eng. 2016, 4, 1714–1721. [Google Scholar] [CrossRef]
- Kshitij, T.; Gaurav, S.; Kumar, A.R. Adsorption removal of malachite green dye from aqueous solution. Rev. Chem. Eng. 2018, 34, 427–453. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Gold, D.H.; Gregor, H.P. Metal-Polyelectrolyte Complexes. VIII. The Poly-N-Vinylimidazole-Copper(II) Complex. J. Phys. Chem. 1960, 64, 1464–1467. [Google Scholar] [CrossRef]
- Bauman, J.E.; Wang, J.C. Imidazole Complexes of Nickel(II), Copper(II), Zinc(II), and Silver(I). Inorg. Chem. 1964, 3, 368–373. [Google Scholar] [CrossRef]
- Trojer, M.A.; Movahedi, A.; Blanck, H.; Nydén, M. Imidazole and Triazole Coordination Chemistry for Antifouling Coatings. J. Chem. 2013, 2013, 946739. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, G.E.; Chang, Y.-W.; Sharma, A.; Tutt, S. One-Step Assembly of Fluorescence-Based Cyanide Sensors from Inexpensive, Off-The-Shelf Materials. Sensors 2020, 20, 4488. https://doi.org/10.3390/s20164488
Fernandes GE, Chang Y-W, Sharma A, Tutt S. One-Step Assembly of Fluorescence-Based Cyanide Sensors from Inexpensive, Off-The-Shelf Materials. Sensors. 2020; 20(16):4488. https://doi.org/10.3390/s20164488
Chicago/Turabian StyleFernandes, Gregory E., Ya-Wen Chang, Akash Sharma, and Sarah Tutt. 2020. "One-Step Assembly of Fluorescence-Based Cyanide Sensors from Inexpensive, Off-The-Shelf Materials" Sensors 20, no. 16: 4488. https://doi.org/10.3390/s20164488
APA StyleFernandes, G. E., Chang, Y.-W., Sharma, A., & Tutt, S. (2020). One-Step Assembly of Fluorescence-Based Cyanide Sensors from Inexpensive, Off-The-Shelf Materials. Sensors, 20(16), 4488. https://doi.org/10.3390/s20164488




