Photoacoustic Imaging to Track Magnetic-manipulated Micro-Robots in Deep Tissue
Abstract
:1. Introduction
2. Materials and Methods
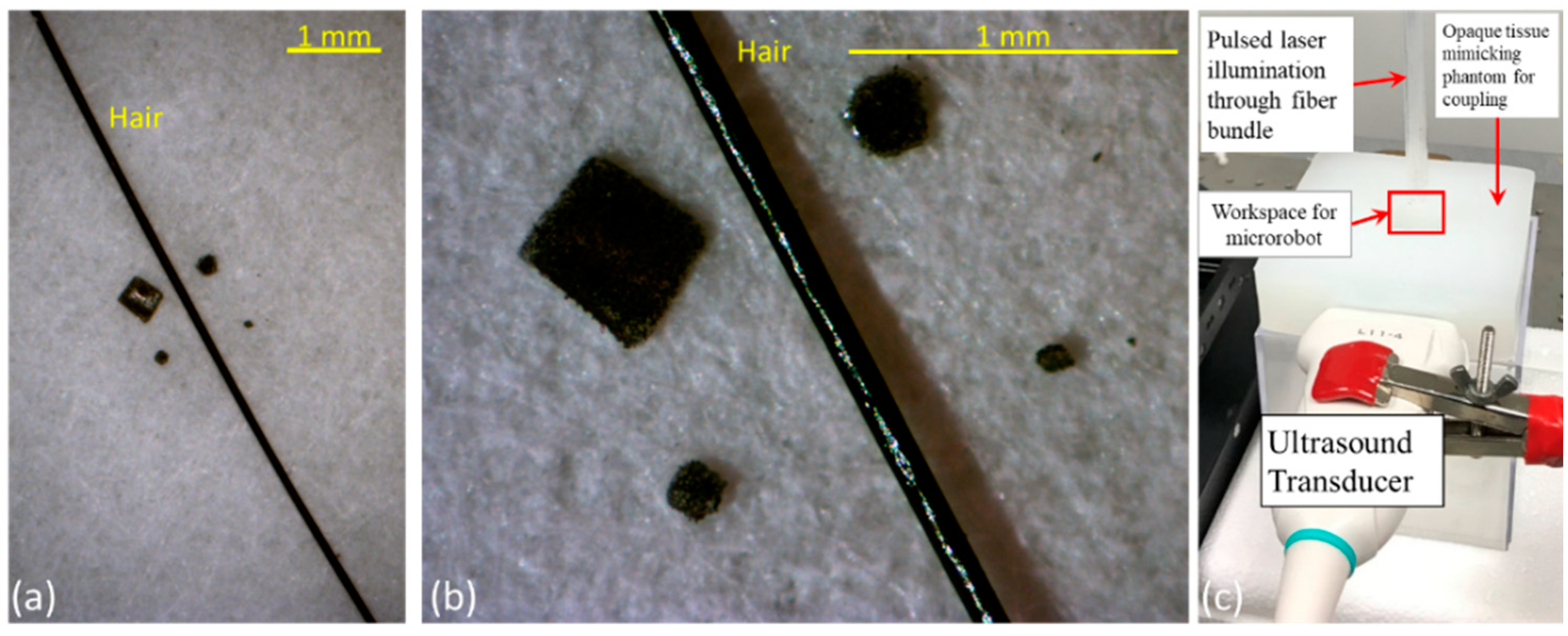
2.1. Design and Development of PA-Sensitive Magnetic Microrobots
2.2. Integrated Ultrasound and Photoacoustic (USPA) Imaging System
2.3. USPA Imaging for Detecting Microrobots during Static and Variable Motion
2.4. USPA Imaging of Microrobots through Biological Tissue and Inside Human Blood
3. Results and Discussion
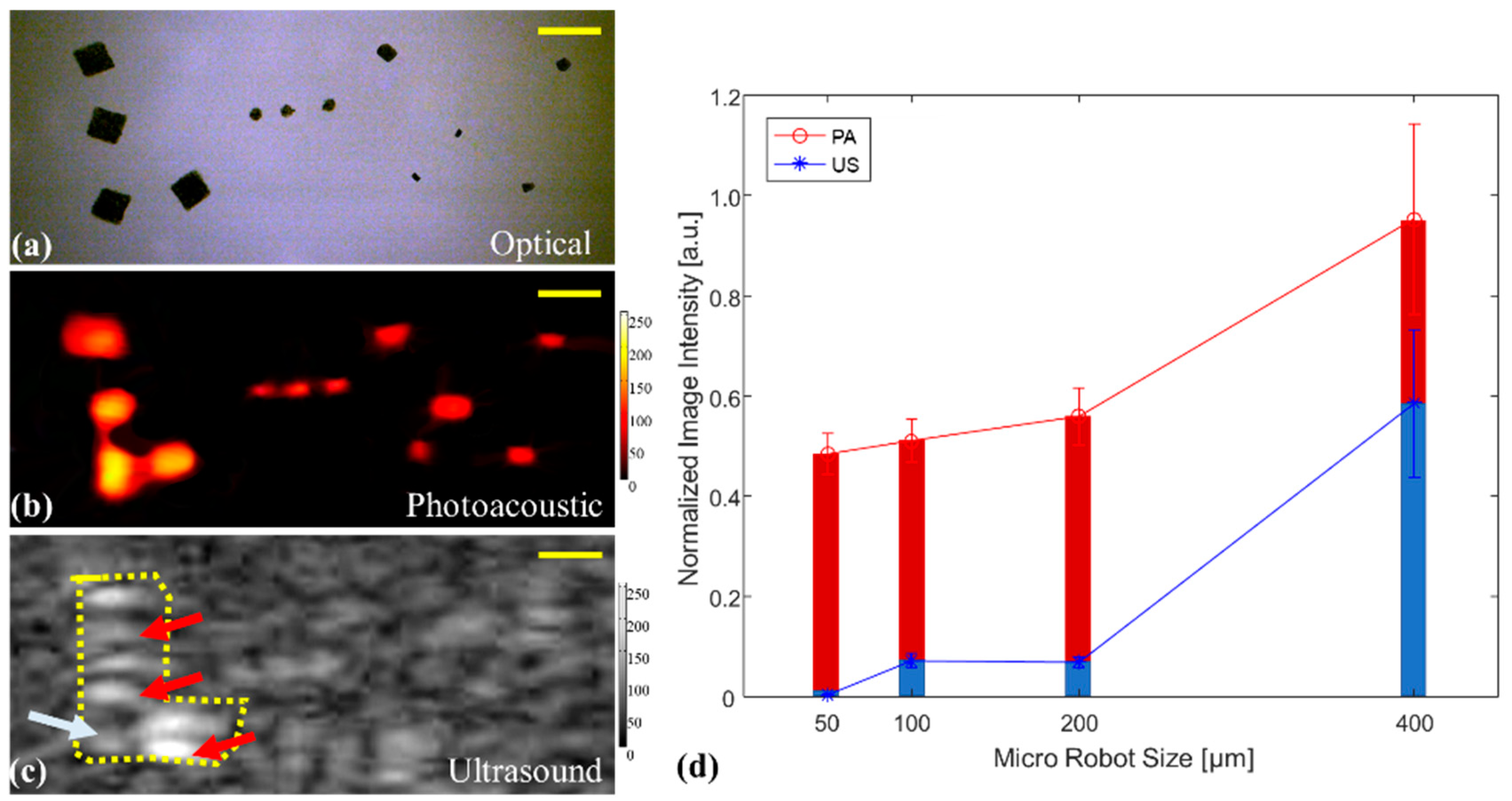
3.1. USPA Imaging for Detecting Stationary and Moving Microrobots
3.2. PA Imaging of Microrobots through Opaque Tissue and in Human Blood
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palagi, S.; Fischer, P. Bioinspired microrobots. Nat. Rev. Mater. 2018, 3, 113. [Google Scholar] [CrossRef]
- Chautems, C.; Zeydan, B.; Charreyron, S.; Chatzipirpiridis, G.; Pane, S.; Nelson, B.J. Magnetically powered microrobots: A medical revolution underway? Eur. J. Cardio-Thorac. Surg. 2017, 51, 405–407. [Google Scholar] [CrossRef] [Green Version]
- Sitti, M.; Ceylan, H.; Hu, W.; Giltinan, J.; Turan, M.; Yim, S.; Diller, E.D. Biomedical Applications of Untethered Mobile Milli/Microrobots. Proc. IEEE 2015, 103, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, L.; Yu, J.; Vong, C.-I.; Chiu, P.W.Y.; Zhang, L. Magnetic Navigation of a Rotating Colloidal Swarm Using Ultrasound Images. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 5380–5385. [Google Scholar]
- Cappelleri, D.; Efthymiou, D.; Goswami, A.; Vitoroulis, N.; Zavlanos, M. Towards mobile microrobot swarms for additive micromanufacturing. Int. J. Adv. Robot. Syst. 2014, 11, 150. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Salehizadeh, M.; Onaizah, O.; Diller, E. Millimeter-scale flexible robots with programmable three-dimensional magnetization and motions. Sci. Robot. 2019, 4, eaav4494. [Google Scholar] [CrossRef]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Edelman, S.K. Understanding Ultrasound Physics; Baker & Taylor: Charlotte, NC, USA, 2003. [Google Scholar]
- Sánchez, A.; Magdanz, V.; Schmidt, O.G.; Misra, S. Magnetic control of self-propelled microjets under ultrasound image guidance. In Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Sao Paulo, Brazil, 12–15 August 2014; pp. 169–174. [Google Scholar]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef] [Green Version]
- Mehrmohammadi, M.; Joon Yoon, S.; Yeager, D.; Emelianov, S.Y. Photoacoustic imaging for cancer detection and staging. Curr. Mol. Imaging 2013, 2, 89–105. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Gomez-Lopez, N.; Basij, M.; Shahvari, A.V.; Vadillo-Ortega, F.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; MehrMohammadi, M. Photoacoustic imaging of the uterine cervix to assess collagen and water content changes in murine pregnancy. Biomed. Opt. Express 2019, 10, 4643–4655. [Google Scholar] [CrossRef]
- Alshahrani, S.S.; Yan, Y.; Alijabbari, N.; Pattyn, A.; Avrutsky, I.; Malyarenko, E.; Poudel, J.; Anastasio, M.; Mehrmohammadi, M. All-reflective ring illumination system for photoacoustic tomography. J. Biomed. Opt. 2019, 24, 046004. [Google Scholar]
- Basij, M.; Yan, Y.; Alshahrani, S.S.; Helmi, H.; Burton, T.K.; Burmeister, J.W.; Dominello, M.M.; Winer, I.S.; Mehrmohammadi, M. Miniaturized phased-array ultrasound and photoacoustic endoscopic imaging system. Photoacoustics 2019, 15, 100139. [Google Scholar] [CrossRef] [PubMed]
- Mehrmohammadi, M.; Yan, Y.; Hernandez-Andrade, E. OP01. 03: Development of an endocavity ultrasound and photoacoustic imaging device for non-invasive assessment of blood oxygenation and perfusion in fetuses and neonates. Ultrasound Obstet. Gynecol. 2019, 54, 84–85. [Google Scholar] [CrossRef] [Green Version]
- Alijabbari, N.; Alshahrani, S.S.; Pattyn, A.; Mehrmohammadi, M. Photoacoustic Tomography with a Ring Ultrasound Transducer: A Comparison of Different Illumination Strategies. Appl. Sci. 2019, 9, 3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Basij, M.; Wang, Z.; Siddiqui, A.; Dong, J.; Alijabbari, N.; Hernandez-Andrade, E.; Gomez-Lopez, N.; Hassan, S.; Mehrmohammadi, M. Multi-parametric acoustic imaging of cervix for more accurate detection of patients at risk of preterm birth. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar]
- Basij, M.; Yan, Y.; Alshahrani, S.; Winer, I.; Burmeister, J.; Dominello, M.; Mehrmohammadi, M. Development of an Ultrasound and Photoacoustic Endoscopy System for Imaging of Gynecological Disorders. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar]
- Mehrmohammadi, M.; Hernandez-Andrade, E.; Gelovani, J.G.; Hassan, S.S.; Yan, Y. Ultrasound and Photoacoustic Systems and Methods for Fetal Brain Assessment During Delivery. Google Patents US 2018 / 0214119 A1, 2 August 2018. [Google Scholar]
- Basij, M.; Yan, Y.; Alshahrani, S.S.; Sau, S.; Iyer, A.; Seward, S.S.; Burmeister, J.W.; Dominello, M.; Mehrmohammadi, M. Combined phased-array ultrasound and photoacoustic endoscope for gynecologic cancer imaging applications. In Proceedings of the Medical Imaging 2018: Ultrasonic Imaging and Tomography, Houston, TX, USA, 13–15 February 2018; p. 105800. [Google Scholar]
- Emelianov, S.Y.; Li, P.-C.; O’Donnell, M. Photoacoustics for molecular imaging and therapy. Phys. Today 2009, 62, 34. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.G. Upon the production and reproduction of sound by light. J. Soc. Telegr. Eng. 1880, 9, 404–426. [Google Scholar] [CrossRef]
- Bell, M.A.L.; Shubert, J. Photoacoustic-based visual servoing of a needle tip. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Su, J.L.; Bouchard, R.R.; Karpiouk, A.B.; Hazle, J.D.; Emelianov, S.Y. Photoacoustic imaging of prostate brachytherapy seeds. Biomed. Opt. Express 2011, 2, 2243–2254. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Li, L.; Yang, Y.; Hu, P.; Li, Y.; Yang, S.-Y.; Wang, L.V.; Gao, W. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 2019, 4, eaax0613. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, Y.-S.; Luke, G.P.; Mehrmohammadi, M.; Cook, J.R.; Emelianov, S.Y. Ultrasound and photoacoustic image-guided photothermal therapy using silica-coated gold nanorods: In-vivo study. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; pp. 233–236. [Google Scholar]
- Chen, Y.-S.; Frey, W.; Kim, S.; Kruizinga, P.; Homan, K.; Emelianov, S. Silica-coated gold nanorods as photoacoustic signal nanoamplifiers. Nano Lett. 2011, 11, 348–354. [Google Scholar] [CrossRef]
- Sivasubramanian, K.; Pramanik, M. High frame rate photoacoustic imaging at 7000 frames per second using clinical ultrasound system. Biomed. Opt. Express 2016, 7, 312–323. [Google Scholar] [CrossRef] [Green Version]
- John, S.; Yan, Y.; Forta, S.Y.; Kabbani, L.; Mehrmohammadi, M. Integrated Ultrasound and Photoacoustic Imaging for Effective Endovenous Laser Ablation: A Characterization Study. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 122–125. [Google Scholar]
- Prahl, S. Optical Absorption of Hemoglobin. 1999. Available online: http://omlc. ogi. edu/spectra/hemoglobin (accessed on 28 April 2020).
- Xiong, D.-L.; Li, Y.; Li, J.-H.; Zheng, H.-H.; Yao, G.-X.; Chen, Y.-J.; Shu, Q. Simultaneous determination of iron, copper, chrome, nickel by multi-wavelength spectrophotometry. Nonferrous Met. Sci. Eng. 2013, 6, 3. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Jing, W.; Mehrmohammadi, M. Photoacoustic Imaging to Track Magnetic-manipulated Micro-Robots in Deep Tissue. Sensors 2020, 20, 2816. https://doi.org/10.3390/s20102816
Yan Y, Jing W, Mehrmohammadi M. Photoacoustic Imaging to Track Magnetic-manipulated Micro-Robots in Deep Tissue. Sensors. 2020; 20(10):2816. https://doi.org/10.3390/s20102816
Chicago/Turabian StyleYan, Yan, Wuming Jing, and Mohammad Mehrmohammadi. 2020. "Photoacoustic Imaging to Track Magnetic-manipulated Micro-Robots in Deep Tissue" Sensors 20, no. 10: 2816. https://doi.org/10.3390/s20102816
APA StyleYan, Y., Jing, W., & Mehrmohammadi, M. (2020). Photoacoustic Imaging to Track Magnetic-manipulated Micro-Robots in Deep Tissue. Sensors, 20(10), 2816. https://doi.org/10.3390/s20102816




