A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fabrication of the Sensing System
2.3. Detection of OPs
2.4. Multi-Assay of Fruit Juice
3. Results and Discussion
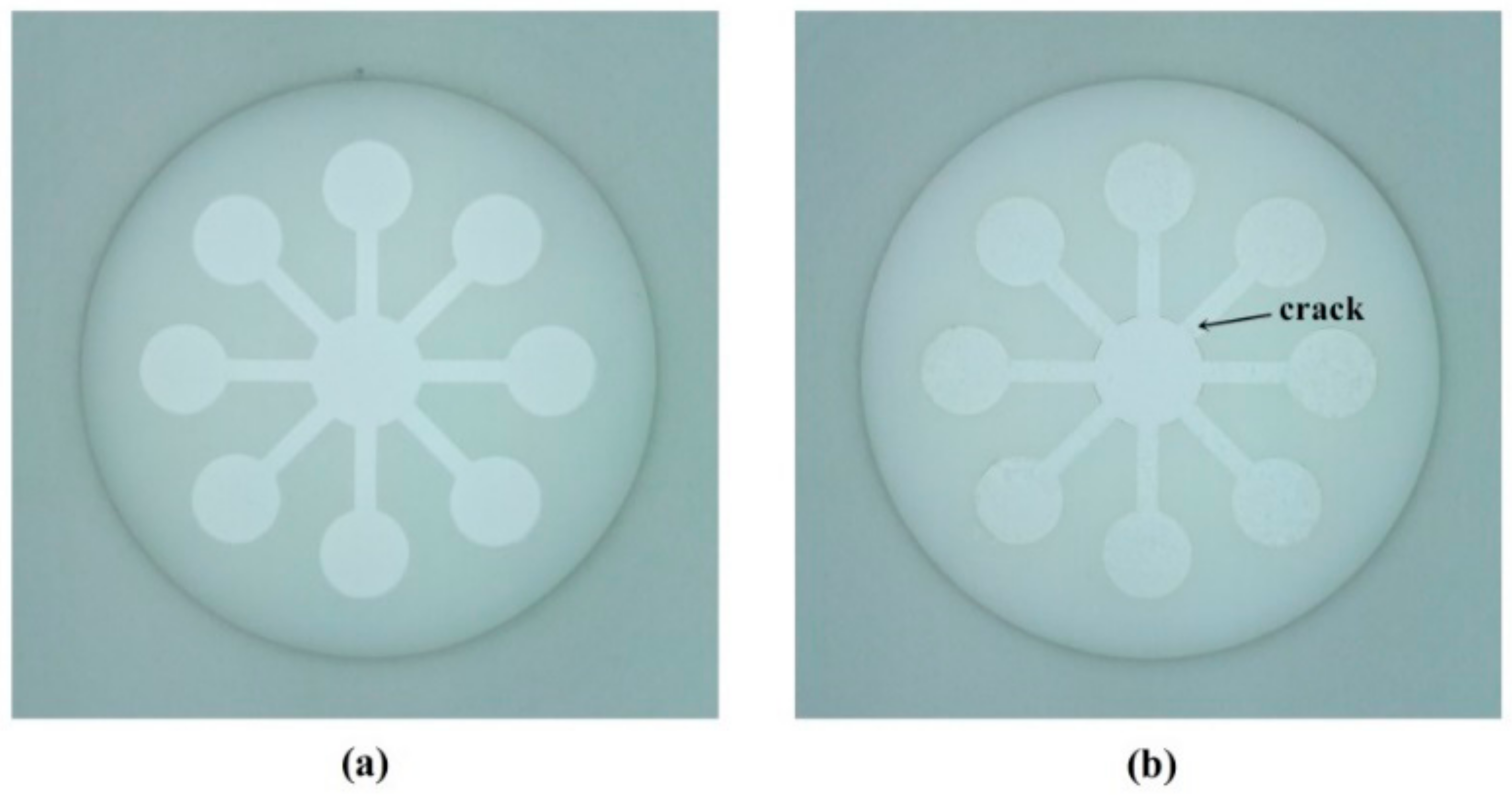
3.1. Construction of the Sensing System
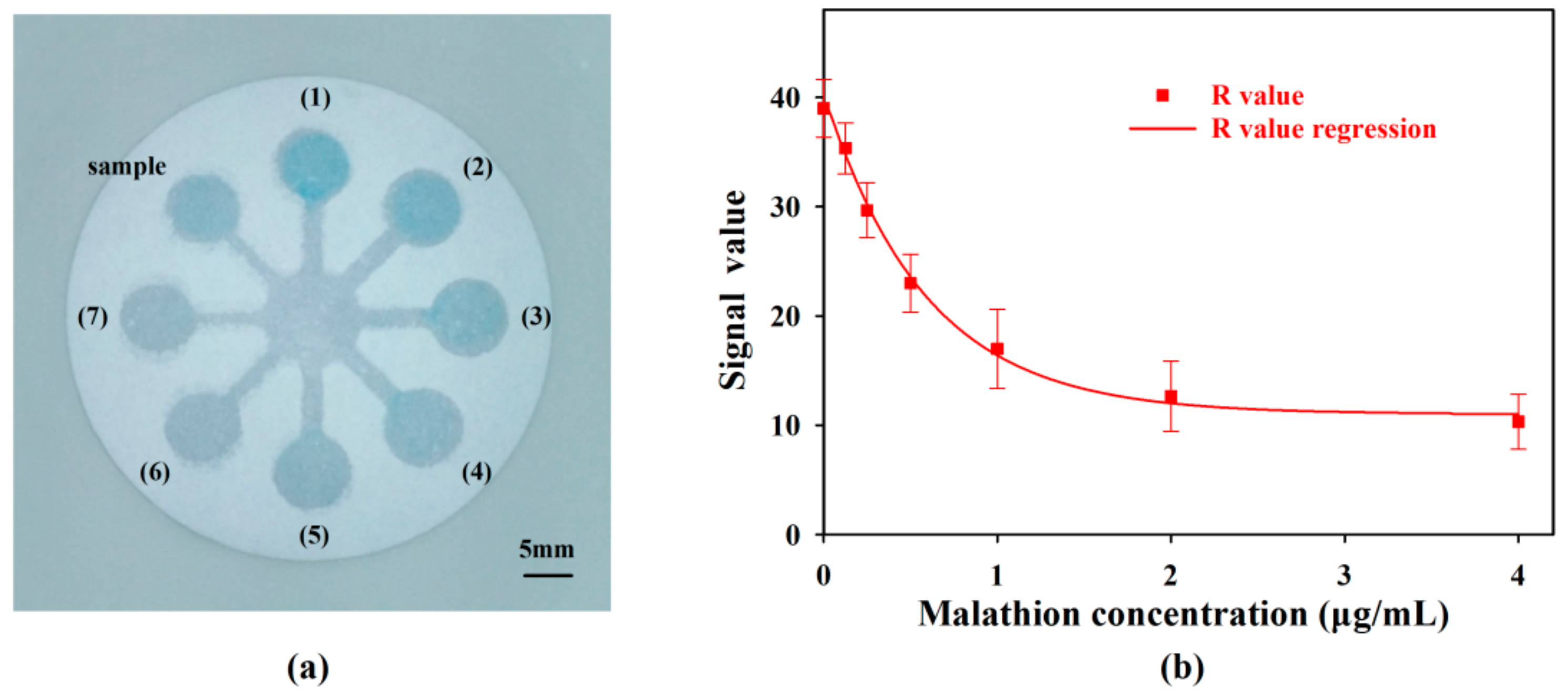
3.2. Detection of OPs
3.2.1. Detection Principle
3.2.2. Condition Optimization
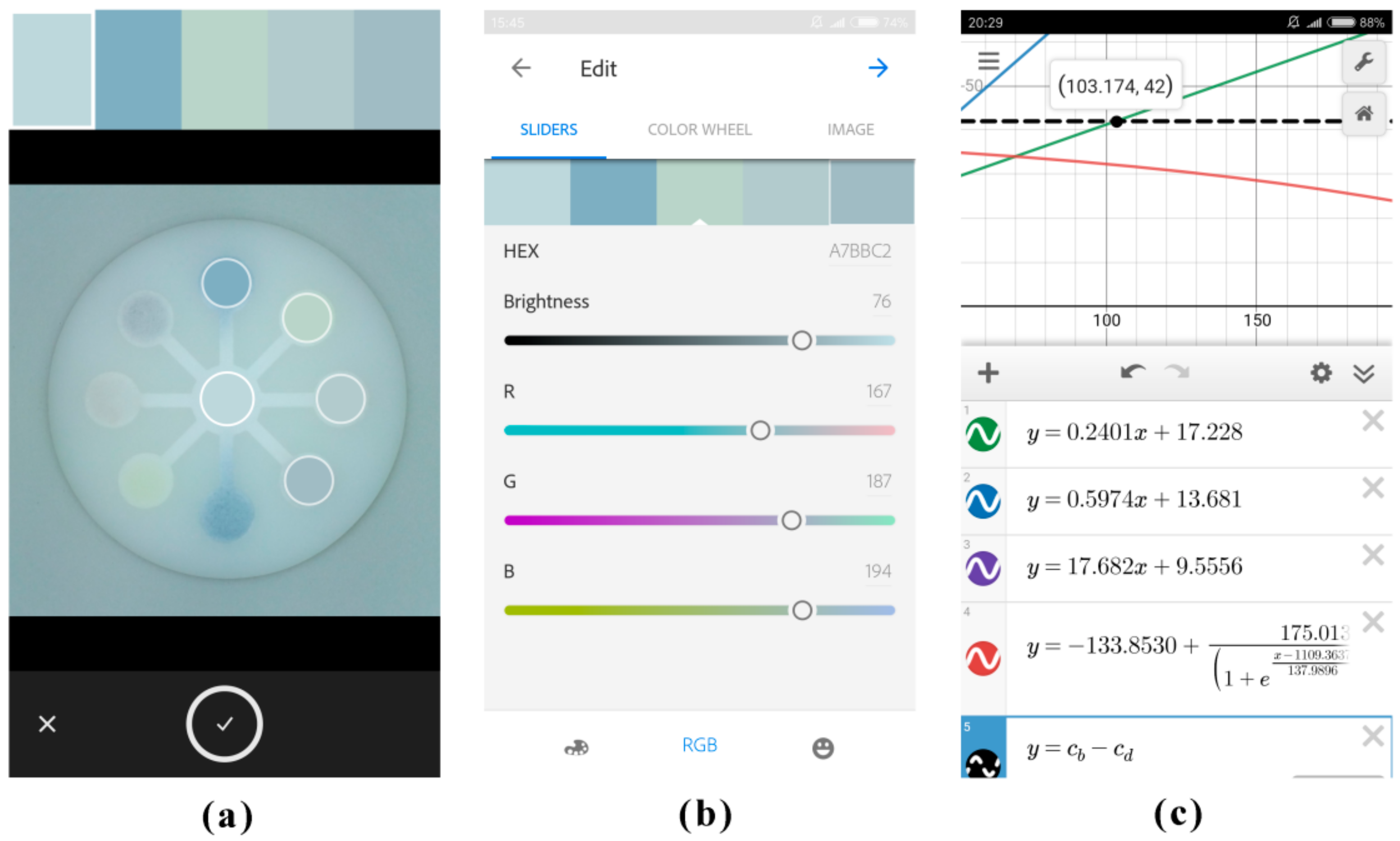
3.2.3. Effect of the Imaging Platform
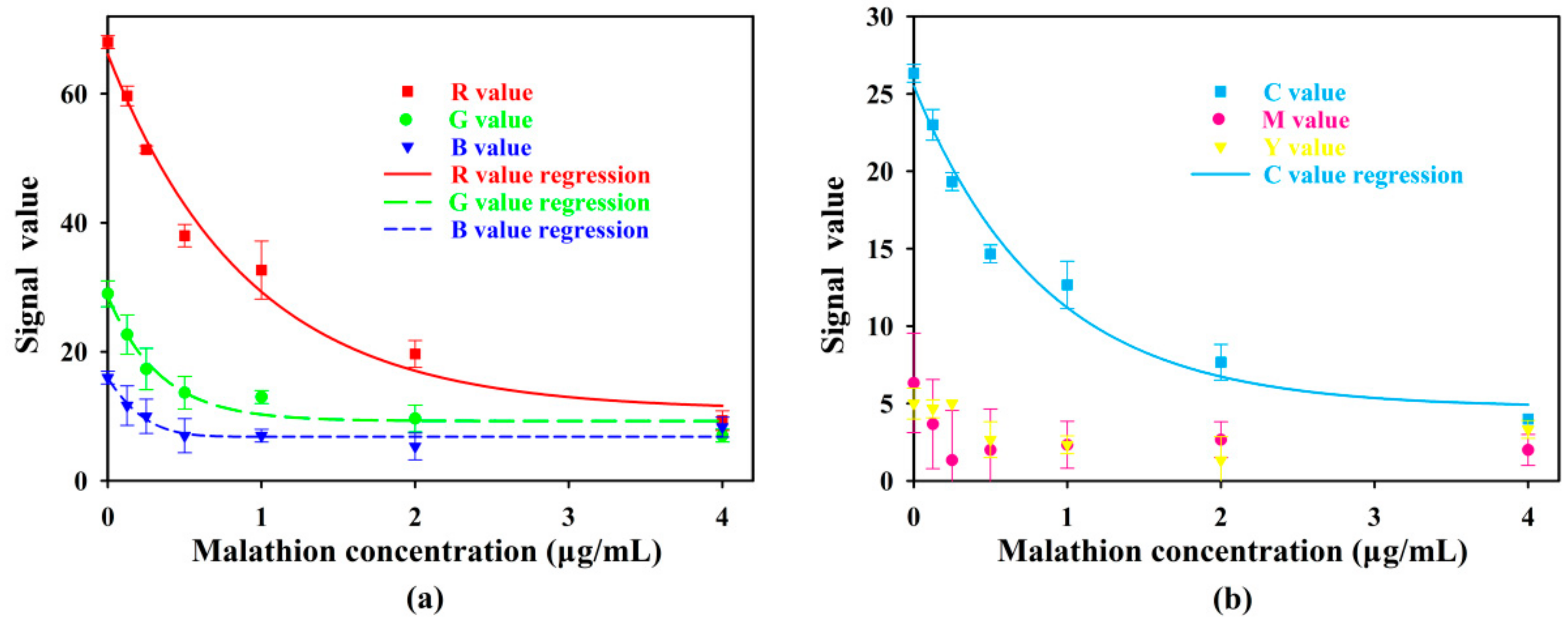
3.2.4. Sensing Performance
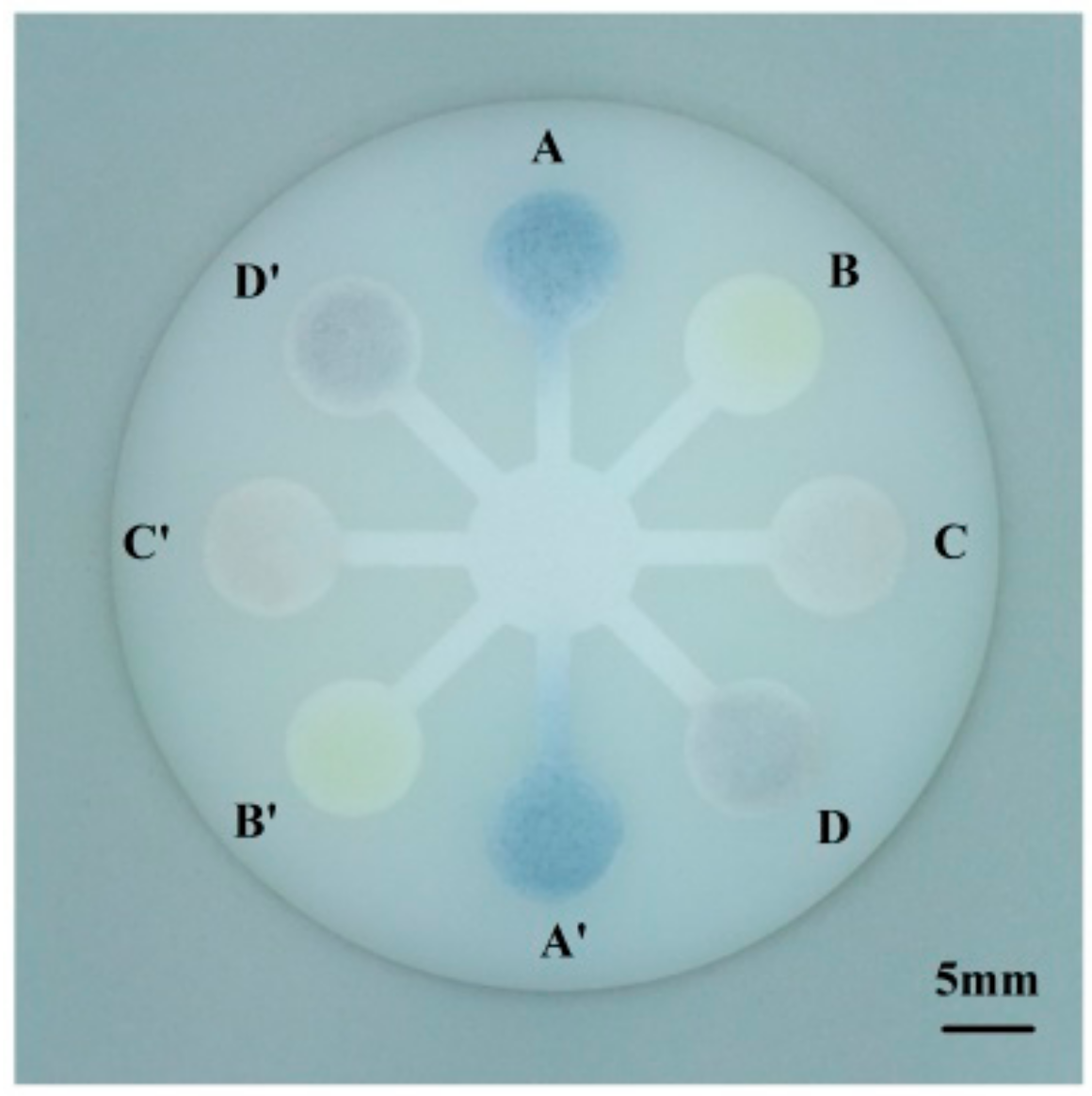
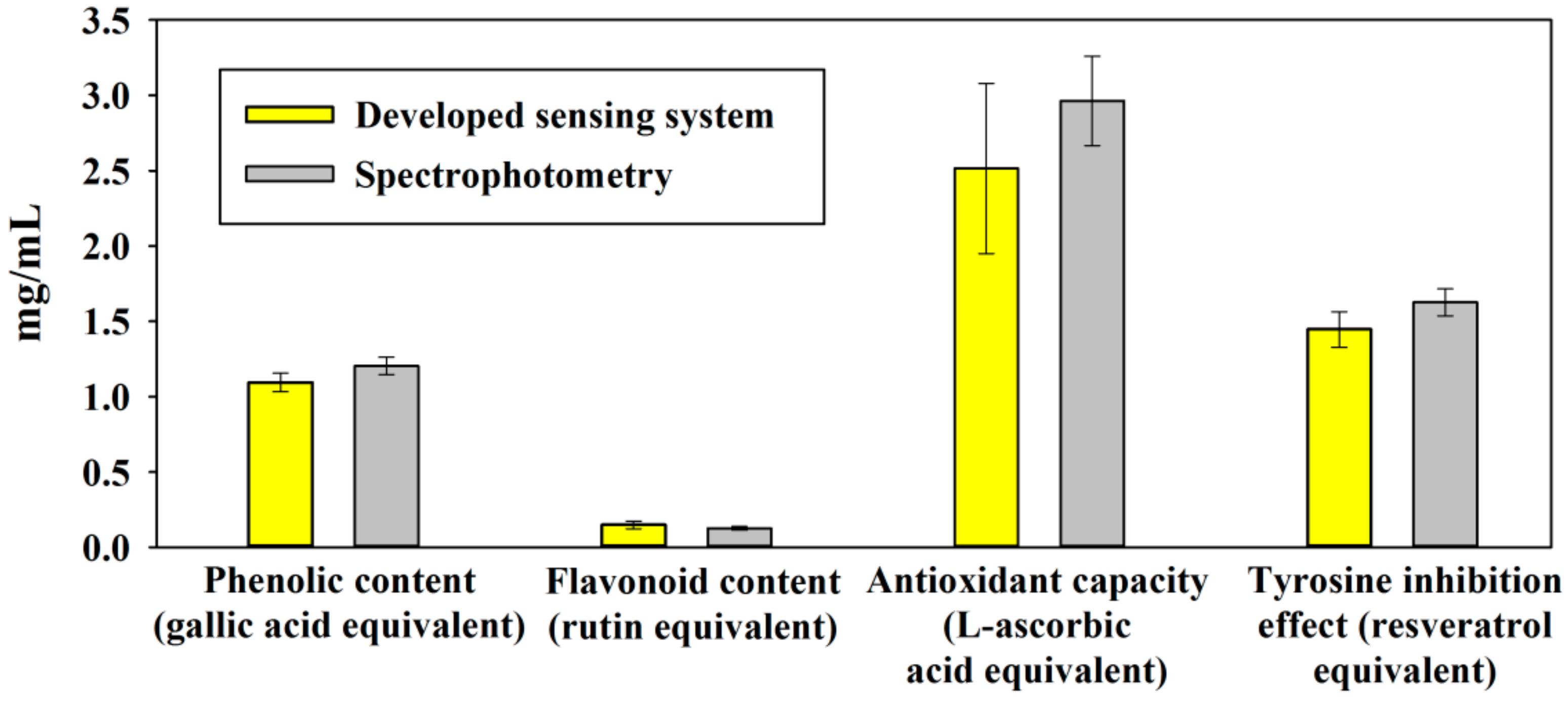
3.3. Multi-Assay of Fruit Juice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Bisetty, K. Smartphone based bioanalytical and diagnosis applications: A review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Su, Y.; Chen, H. Recent progress in optical biosensors based on smartphone platforms. Sensors 2017, 17, 2449. [Google Scholar] [CrossRef] [PubMed]
- Mathaweesansurn, A.; Maneerat, N.; Choengchan, N. A mobile phone-based analyzer for quantitative determination of urinary albumin using self-calibration approach. Sens. Actuators B 2017, 242, 476–483. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Cai, Z.; Liu, K.; Li, J.; Zhang, B.; He, J. Sensitive colorimetric assay for uric acid and glucose detection based on multilayer-modified paper with smartphone as signal readout. Anal. Bioanal. Chem. 2018, 410, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, F.; Wong, J.X.H.; Yu, H. Integrated smartphone-app-chip system for on-Site parts-per-billion-level colorimetric quantitation of Aflatoxins. Anal. Chem. 2017, 89, 8908–8916. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Sengel, T.Y.; Gumus, Z.P.; Arslan, M.; Coskunol, H.; Timur, S.; Yagci, Y. Mobile phone sensing of Cocaine in a lateral flow assay combined with a biomimetic material. Anal. Chem. 2017, 89, 9629–9632. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, R.; Periasamy, A.P.; Roy, P.; Chen, Y.W.; Chang, H.T. Smart app-based on-field colorimetric quantification of mercury via analyte-induced enhancement of the photocatalytic activity of TiO2-Au nanospheres. Anal. Bioanal. Chem. 2018, 410, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.; Yang, J.-S.; Song, J.; Choi, J.-S.; Jung, H.-I. Smart Forensic Phone: Colorimetric analysis of a bloodstain for age estimation using a smartphone. Sens. Actuators B 2017, 243, 221–225. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Oncescu, V.; O’Dell, D.; Erickson, D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 2013, 13, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-Based Microfluidic Devices: Emerging themes and applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, C.A.; Mitchell, H.T.; Martinez, A.W. Paper-based standard addition assays. Anal Methods 2014, 6, 1296–1300. [Google Scholar] [CrossRef]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-cost fabrication of paper-based microfluidic devices by one-step plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef] [PubMed]
- Nuchtavorn, N.; Macka, M. A novel highly flexible, simple, rapid and low-cost fabrication tool for paper-based microfluidic devices (μPADs) using technical drawing pens and in-house formulated aqueous inks. Anal. Chim. Acta 2016, 919, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Santana-Jimenez, L.A.; Marquez-Lucero, A.; Osuna, V.; Estrada-Moreno, I.; Dominguez, R.B. Naked-Eye detection of glucose in saliva with bienzymatic paper-based sensor. Sensors 2018, 18, 1071. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yu, S.; Jiang, Y.; He, Q.; Chen, H. Fabrication of paper-based microfluidic devices by plasma treatment and its application in glucose determination. Acta Chim. Sin. 2014, 72, 1099–1104. [Google Scholar] [CrossRef]
- He, Q.; Ma, C.; Hu, X.; Chen, H. Method for fabrication of paper-based microfluidic devices by alkylsilane self-assembling and UV/O3-patterning. Anal. Chem. 2013, 85, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Q.; Wu, W.; Nie, J.; Fu, J. 3D printed paper-based microfluidic analytical devices. Micromachines 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chang, L.; Wu, H.; Huang, S.; Chu, H.; Huang, M. Antioxidant and antityrosinase activity of aqueous extracts of green asparagus. Food Chem. 2011, 127, 141–146. [Google Scholar] [CrossRef]
- Roy Fukuto, T. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reyes, J.F.; Gilbert-Lopez, B.; Molina-Diaz, A.; Fernandez-Alba, A.R. Determination of Pesticide Residues in Fruit-Based Soft Drinks. Anal. Chem. 2008, 80, 8966–8974. [Google Scholar] [CrossRef] [PubMed]
- Hayward, D.G.; Wong, J.W. Organohalogen and organophosphorous pesticide method for Ginseng Root—A comparison of gas chromatography-single quadrupole mass spectrometry with high resolution time-of-flight mass spectrometry. Anal. Chem. 2009, 81, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Schultz, C.W.; Cui, C.; Li, X.; Yu, H. On-site chip-based colorimetric quantitation of organophosphorus pesticides using an office scanner. Sens. Actuators B 2015, 215, 577–583. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z. Nano-composite ZrO2/Au film electrode for voltammetric detection of parathion. Sens. Actuators B 2008, 133, 607–612. [Google Scholar] [CrossRef]
- Narakathu, B.B.; Guo, W.; Obare, S.O.; Atashbar, M.Z. Novel approach for detection of toxic organophosphorus compounds. Sens. Actuators B 2011, 158, 69–74. [Google Scholar] [CrossRef]
- Standardization Administration of China. Standards for Drinking Water Quality; GB 5749-2006; Standardization Administration of China: Beijing, China, 2006. [Google Scholar]
- Biswas, S.; Tripathi, P.; Kumar, N.; Nara, S. Gold nanorods as peroxidase mimetics and its application for colorimetric biosensing of malathion. Sens. Actuators B 2016, 231, 584–592. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, P.; Kumar, N.; Nara, S. Colorimetric sensing of malathion using palladium-gold bimetallic nanozyme. Biosens. Bioelectron. 2017, 92, 280–286. [Google Scholar] [CrossRef] [PubMed]






| Technique Followed | Limit of Detection (ng/mL) | Ref. |
|---|---|---|
| Spectrophotometry | 1780 | [30] |
| Spectrophotometry | 60 | [31] |
| Scanner-readable plastic microchip | 100 | [26] |
| Smartphone-based sensing system | 51.9 | this study |
| Spiked Domestic Water Sample | Observed Concentration (μg/mL) | Expected Concentration (μg/mL) | Recovery (%) | Relative Standard Deviations (%) |
|---|---|---|---|---|
| I | 0.23 ± 0.02 | 0.20 | 115.0 | 8.70 |
| II | 0.78 ± 0.02 | 0.80 | 97.5 | 2.56 |
| III | 1.29 ± 0.10 | 1.20 | 107.5 | 7.75 |
| IV | 2.55 ± 0.15 | 2.40 | 106.3 | 5.88 |
| Detection Index | Selected Signal | Limit of Detection |
|---|---|---|
| Phenolic content (μg gallic acid equivalent/mL) | G | 11.17 |
| Flavonoid content (μg rutin equivalent/mL) | B | 3.88 |
| Antioxidant capacity (mg L-ascorbic acid equivalent/mL) | G | 0.089 |
| Tyrosine inhibition effect (μg resveratrol equivalent/mL) | G | 34.357 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Wang, M. A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays. Sensors 2018, 18, 4002. https://doi.org/10.3390/s18114002
Wu F, Wang M. A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays. Sensors. 2018; 18(11):4002. https://doi.org/10.3390/s18114002
Chicago/Turabian StyleWu, Feiyi, and Min Wang. 2018. "A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays" Sensors 18, no. 11: 4002. https://doi.org/10.3390/s18114002
APA StyleWu, F., & Wang, M. (2018). A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays. Sensors, 18(11), 4002. https://doi.org/10.3390/s18114002




