Sensitive Hg2+ Sensing via Quenching the Fluorescence of the Complex between Polythymine and 5,10,15,20-tetrakis(N-methyl-4-pyridyl) Porphyrin (TMPyP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
2.3. Procedure
3. Results and Discussion
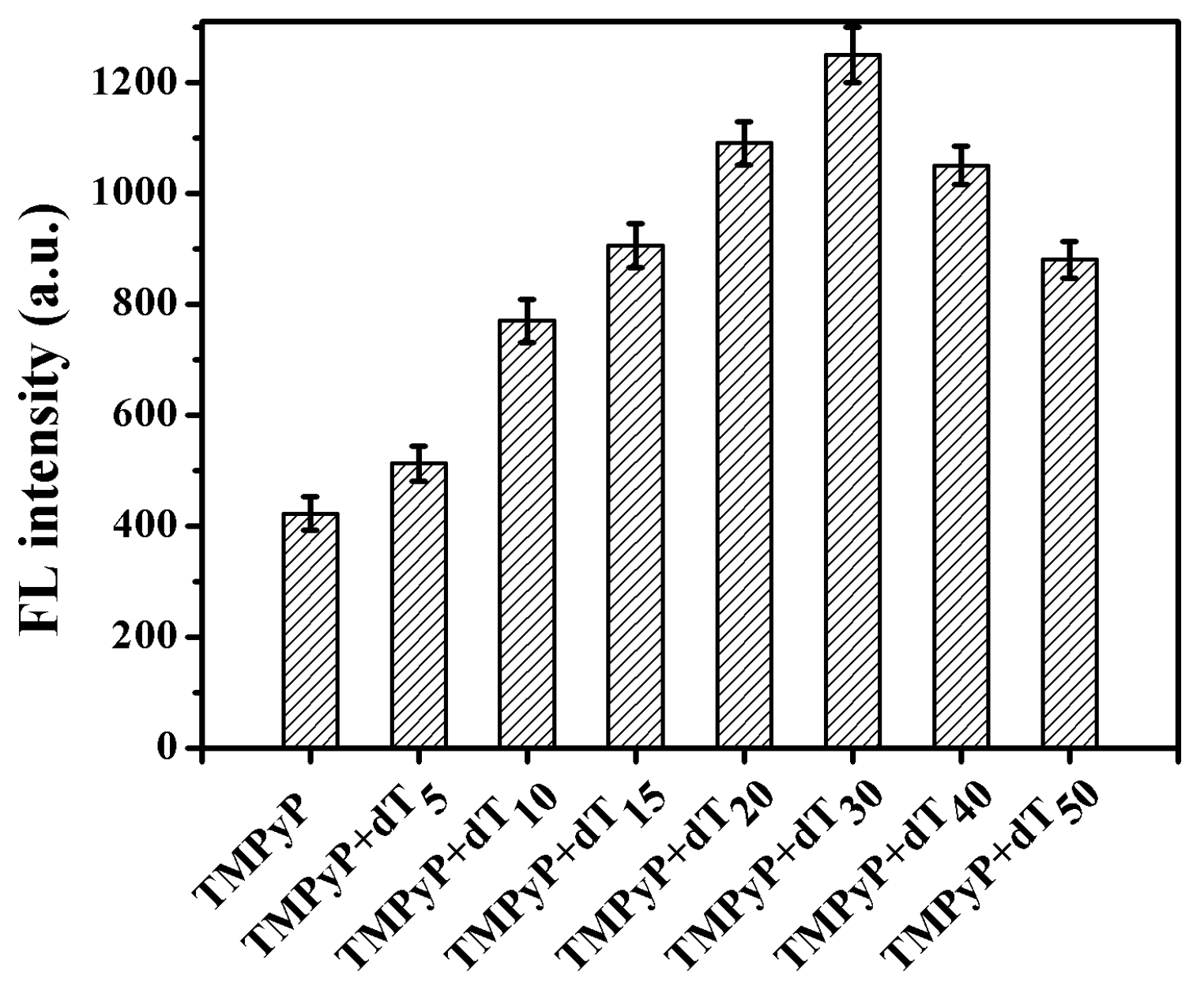
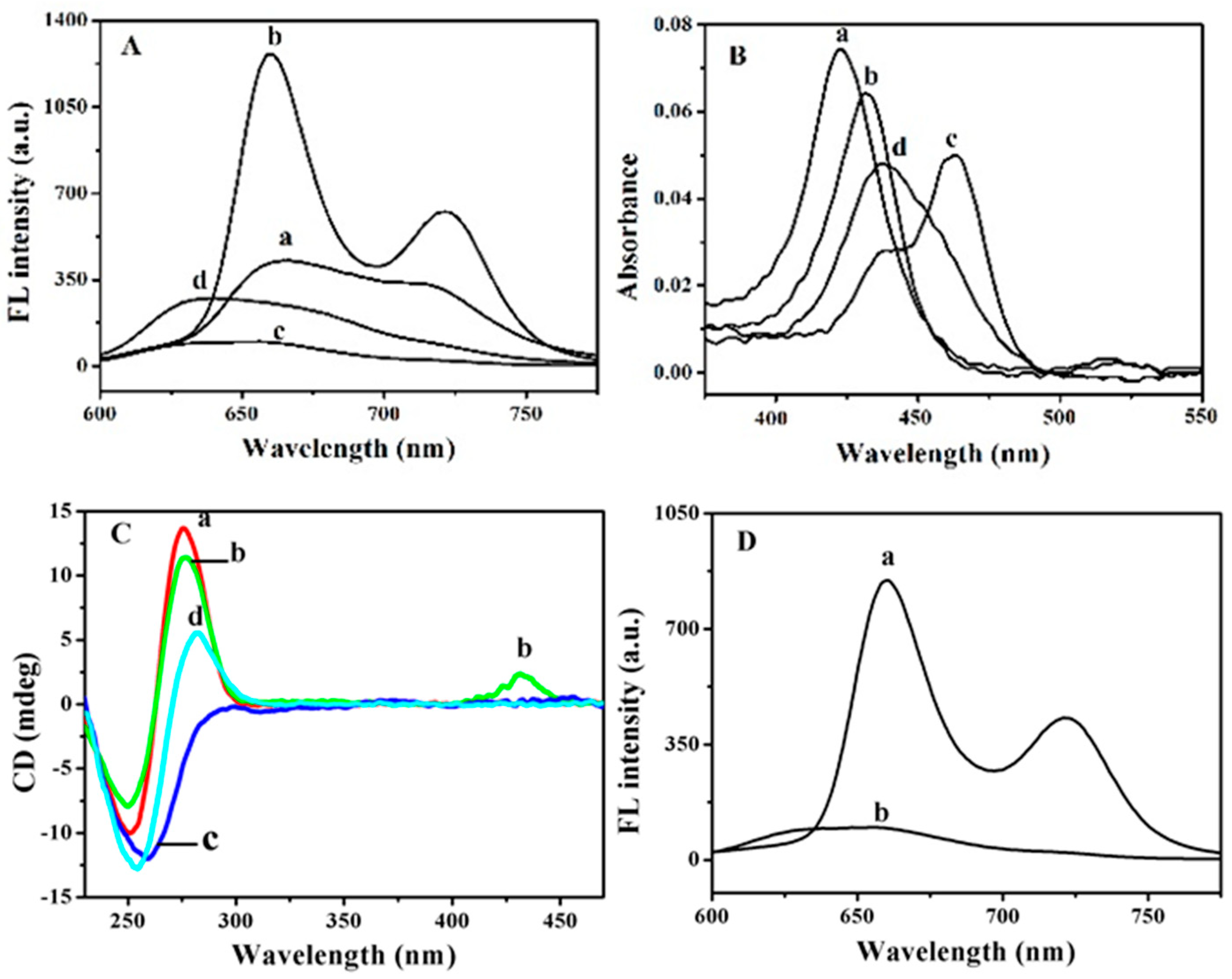
3.1. Interaction between ODNs and TMPyP
3.2. Fluorescence Quenching of TMPyP-dT30 Complex by Hg2+
3.3. Calibration Curve of The Hg2+ Assay
3.4. Selectivity of the Assay
3.5. Practical Samples for the Hg2+ Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biesaga, M.; Pyrzynska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, J.; Ma, F.; Ling, P.; Liu, J.; Ju, H. A porphyrin photosensitized metal-organic framework for cancer cell apoptosis and caspase responsive theranostics. Chem. Commun. 2015, 51, 10831–10834. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Gibbs, E.J.; Villafranca, J.J. Interactions of porphyrins with nucleic acids. Biochemistry 1983, 22, 5409–5417. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Gibbs, E.J.; Gaudemer, A.; Antebi, A.; Bassner, S.; De Poy, L.; Turner, D.H.; Williams, A.; Laplace, F.; Lansard, M.H.; et al. Molecular complexes of nucleosides and nucleotides with a monomeric cationic porphyrin and some of its metal derivatives. J. Am. Chem. Soc. 1985, 107, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Tanaka, H. DNA-porphyrin interactions probed by induced CD spectroscopy. J. Chem. Soc. Chem. Commun. 1994, 13, 1575–1576. [Google Scholar] [CrossRef]
- Gaier, A.J.; Ghimire, S.; Fix, S.E.; McMillin, D.R. Internal versus external binding of cationic porphyrins to single-stranded DNA. Inorg. Chem. 2014, 53, 5467–5473. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Fukuda, K.; Wakami, H.; Nishiyabu, R.; Pasternack, R.F. Factors influencing self-aggregation tendencies of cationic porphyrins in aqueous solution. J. Am. Chem. Soc. 2000, 122, 7494–7502. [Google Scholar] [CrossRef]
- Kubát, P.; Lang, K.; Anzenbacher, P., Jr.; Jursíková, K.; Král, V.; Ehrenberg, B. Interaction of novel cationic meso-tetraphenylporphyrins in the ground and excited states with DNA and nucleotides. J. Chem. Soc. Perkin. Trans. 2000, 6, 933–941. [Google Scholar] [CrossRef]
- Guliaev, A.B.; Leontis, N.B. Cationic 5,10,15,20-tetrakis(N-methylpyridinium-4-yl)porphyrin fully intercalates at 5′-CG-3′ steps of duplex DNA in solution. Biochemistry 1999, 38, 15425–15437. [Google Scholar] [CrossRef] [PubMed]
- Novy, J.; Urbanova, M. Vibrational and electronic circular dichroism study of the interactions of cationic porphyrins with (dG-dC)10 and (dA-dT)10. Biopolymers 2007, 85, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Mita, H.; Yamamoto, Y. Binding of 5,10,15,20-tetrakis(N-methylpyridinium-4-yl)-21H,23H-porphyrin to an AT-rich region of a duplex DNA. Biophys. Chem. 2005, 113, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, J.; Cho, T.; Song, R.; Kim, S.K. Binding of meso-Tetrakis(-methylpyridium-4-yl)porphyrin to triplex oligonucleotides: Evidence for the porphyrin stacking in the major groove. J. Am. Chem. Soc. 2003, 125, 8106–8107. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, I.; Borovok, N.; Kotlyar, A. Interaction of monomolecular G4-DNA nanowires with TMPyP: Evidence for intercalation. Biochemistry 2007, 46, 12925–12929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, J.; Ai, J.; Zhou, Z.; Jia, X.; Wang, E. Label-free G-quadruplex-specific fluorescent probe for sensitive detection. Biosens. Bioelectron. 2013, 39, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Gaier, A.J.; McMillin, D.R. Binding studies of G-quadruplex DNA and porphyrins: Cu(T4) vs sterically friendly Cu(tD4). Inorg. Chem. 2015, 54, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Chan, M.Y.; Huang, P.J.; Smith, B.D.; Liu, J. Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in Water. J. Am. Chem. Soc. 2010, 132, 12668–12673. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.S. Spin-orbit interaction in aromatic molecules. J. Chem. Phys. 1952, 20, 682–686. [Google Scholar] [CrossRef]
- Wu, G.W.; He, S.B.; Peng, H.P.; Deng, H.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. Citrate-capped platinum nanoparticle as a smart probe for ultrasensitive mercury sensing. Anal. Chem. 2014, 86, 10955–10960. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Subramanian, K.; Murugan, K.; Dinakaran, K. Sensitive fluorescence detection of mercury(II) in aqueous solution by the fluorescence quenching effect of MoS2 with DNA functionalized carbon dots. Analyst 2016, 141, 6344–6352. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, R.; Jameso, D.M.; Nishijo, C.K.; Larsen, R.W. Singlet excited state dynamics of tetrakis(4-N-methylpyridyl)porphine associated with DNA nucleotides. J. Phys. Chem. B 1997, 101, 1444–1450. [Google Scholar] [CrossRef]
- Zhu, K.; Hu, X.; Ge, Q.; Sun, Q. Fluorescent recognition of deoxyribonucleic acids by a quantum dot/meso-tetrakis(N-methylpyridinium-4-yl)porphyrin complex based on a photo induced electron-transfer mechanism. Anal. Chim. Acta 2014, 812, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Paulo, P.R.; Costa, S.B. Interactions in noncovalent PAMAM/TMPyP systems studied by fluorescence spectroscopy. J. Phys. Chem. B 2005, 109, 13928–13940. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, K.; Wang, K.; Zhao, F.; Li, N.; Liu, F. Porphyrin assembly on β-cyclodextrin for selective sensing and detection of a zinc ion based on the dual emission fluorescence ratio. Anal. Chem. 2003, 75, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.G. (Ed.) Molecular Luminescence Spectroscopy. Methods and Applications, Part 1; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Choi, J.K.; Sargsyan, G.; Olive, A.M.; Balaz, M. Highly sensitive and selective spectroscopic detection of mercury(II) in water by using pyridylporphyrin-DNA conjugates. Chem. Eur. J. 2013, 19, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Delmarre, D.; Méallet, R.; Bied-Charreton, C.; Pansu, R.B. Heavy metal ions detection in solution, in Sol-gel and with grafted porphyrin monolayers. J. Photochem. Photobiol. A 1999, 124, 23–28. [Google Scholar] [CrossRef]
- Xu, J.; Wu, J.; Zong, C.; Ju, H.; Yan, F. Manganese porphyrin-dsDNA complex: A mimicking enzyme for highly efficient bioanalysis. Anal. Chem. 2013, 85, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Nový, J.; Urbanová, M.; Volka, K. Vibrational and electronic circular dichroism and absorption spectral study of the DNA-5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphyrin interaction. J. Mol. Struct. 2005, 748, 17–25. [Google Scholar] [CrossRef]
- Guo, L.; Hu, H.; Sun, R.; Chen, G. Highly sensitive fluorescent sensor for mercury ion based on photoinduced charge transfer between fluorophore and π-stacked T–Hg(II)-T base pairs. Talanta 2009, 79, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, H.; Ono, A.; Kozasa, T. HgII ion specifically binds with T:T mismatched base pair in duplex DNA. Chem. Eur. J. 2010, 16, 13218–13225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, F.; Zhang, Y.; Peng, F.; Huang, Z.; Zhang, W.; Zhao, W. A dual-mode turn-on fluorescent BODIPY-based probe for visualization of mercury ions in living cells. Analyst 2016, 141, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Wang, Y.; Zhang, Y.; Liu, Y.; Han, X.X.; Zhao, B.; Yang, J. Mercury species induced frequency-shift of molecular orientational transformation based on SERS. Analyst 2016, 141, 4782–4788. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Chen, W.Y.; Yen, Y.C.; Wang, C.W.; Chang, H.T.; Chen, C.F. Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Steve, F.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a Smart-Phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, Y. Ratiometry, wavelength, and intensity: Triple signal readout for colorimetric sensing of mercury ions by plasmonic Cu2-xSe nanoparticles. ACS Sens. 2016, 1, 384–391. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, J.; He, Y.; Zhao, Y.; Zhao, S. A K+-mediated G-quadruplex formation enhancement fluorescence polarization system based on quantum dots for detection of Hg2+ and biothiols. Chem. Commun. 2014, 50, 2049–2051. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.; Wang, F.; Liu, J. Cleavable molecular beacon for Hg2+ detection based on phosphorothioate RNA modifications. Anal. Chem. 2015, 87, 6890–6895. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Lemon, C.M.; Brothers, P.J.; Boitrel, B. Porphyrin complexes of the period 6 main group and late transition metals. Dalton Trans. 2011, 40, 6591–6609. [Google Scholar] [CrossRef] [PubMed]




| Method | Tool | Linear Range | LOD | Time | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| colorimetric | platinum nanoparticles | 0.01–4 nM | 0.0085 nM | 10 min | tap water | [18] |
| colorimetric | gold nanoparticles | 25–750 nM | 50 nM | 40 min | pond and river water | [33] |
| colorimetric | Cu2-xSe nanoparticles | 0–800 nM | 2.7 nM | 10 min | tap, pond and river water | [35] |
| colorimetric | gold nanoparticles and aptamer | 10–1000 nM | 17.3 nM | 20 min | tap, rivers, lakes and ocean water | [34] |
| Raman spectroscopy | silver nanoparticles | 1–1000 μM | 10 nM | 30 min | drinking mineral water | [32] |
| fluorescence | Phosphorothioate RNA Modifications | 0–50 nM | 1.7 nM | 20 min | lake water | [37] |
| fluorescence | MoS2 nanosheet/DNA/ carbon dot | 0–10 nM | 1.02 nM | 15 min | tap and lake water | [19] |
| fluorescence | fluorescent 8-amino BODIPY-based probe | 0.5–5 μM | 49 nM | 50 min | SMMC-7721 cells | [31] |
| fluorescence polarization | CdTe/CdS QDs | 10–800 nM | 8.6 nM | 2.0 h | lake and spiked lake water | [36] |
| fluorescence | polyT-TMPyP | 5–100 nM | 1.3 nM | 60 s | Tap and river water | this work |
| Sample | Added (nM) | Measured (nM) a | Recovery (%) |
|---|---|---|---|
| tap water | 2.0 | 2.1 ± 0.17 | 105 ± 0.085 |
| 5.0 | 4.9 ± 0.32 | 98 ± 0.064 | |
| 10.0 | 9.6 ± 0.62 | 96 ± 0.062 | |
| river water | 2.0 | 2.1 ± 0.11 | 105 ± 0.055 |
| 5.0 | 5.1 ± 0.43 | 102 ± 0.086 | |
| 10.0 | 9.7 ± 0.65 | 97 ± 0.065 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Huang, Y.; Hu, S.; Yi, X.; Wang, J. Sensitive Hg2+ Sensing via Quenching the Fluorescence of the Complex between Polythymine and 5,10,15,20-tetrakis(N-methyl-4-pyridyl) Porphyrin (TMPyP). Sensors 2018, 18, 3998. https://doi.org/10.3390/s18113998
Wu D, Huang Y, Hu S, Yi X, Wang J. Sensitive Hg2+ Sensing via Quenching the Fluorescence of the Complex between Polythymine and 5,10,15,20-tetrakis(N-methyl-4-pyridyl) Porphyrin (TMPyP). Sensors. 2018; 18(11):3998. https://doi.org/10.3390/s18113998
Chicago/Turabian StyleWu, Daohong, Yaliang Huang, Shengqiang Hu, Xinyao Yi, and Jianxiu Wang. 2018. "Sensitive Hg2+ Sensing via Quenching the Fluorescence of the Complex between Polythymine and 5,10,15,20-tetrakis(N-methyl-4-pyridyl) Porphyrin (TMPyP)" Sensors 18, no. 11: 3998. https://doi.org/10.3390/s18113998
APA StyleWu, D., Huang, Y., Hu, S., Yi, X., & Wang, J. (2018). Sensitive Hg2+ Sensing via Quenching the Fluorescence of the Complex between Polythymine and 5,10,15,20-tetrakis(N-methyl-4-pyridyl) Porphyrin (TMPyP). Sensors, 18(11), 3998. https://doi.org/10.3390/s18113998





