A Wireless ExG Interface for Patch-Type ECG Holter and EMG-Controlled Robot Hand
Abstract
:1. Introduction
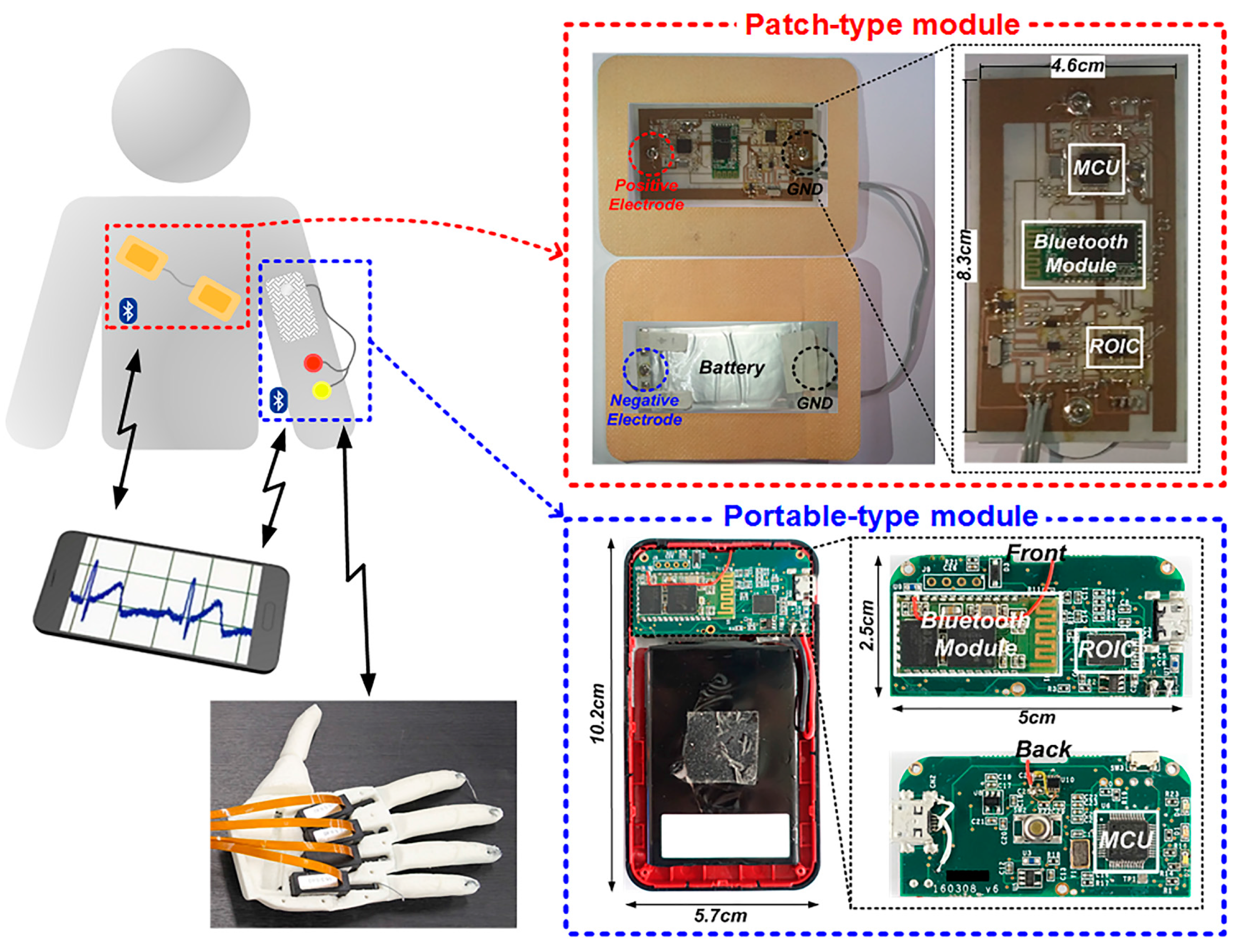
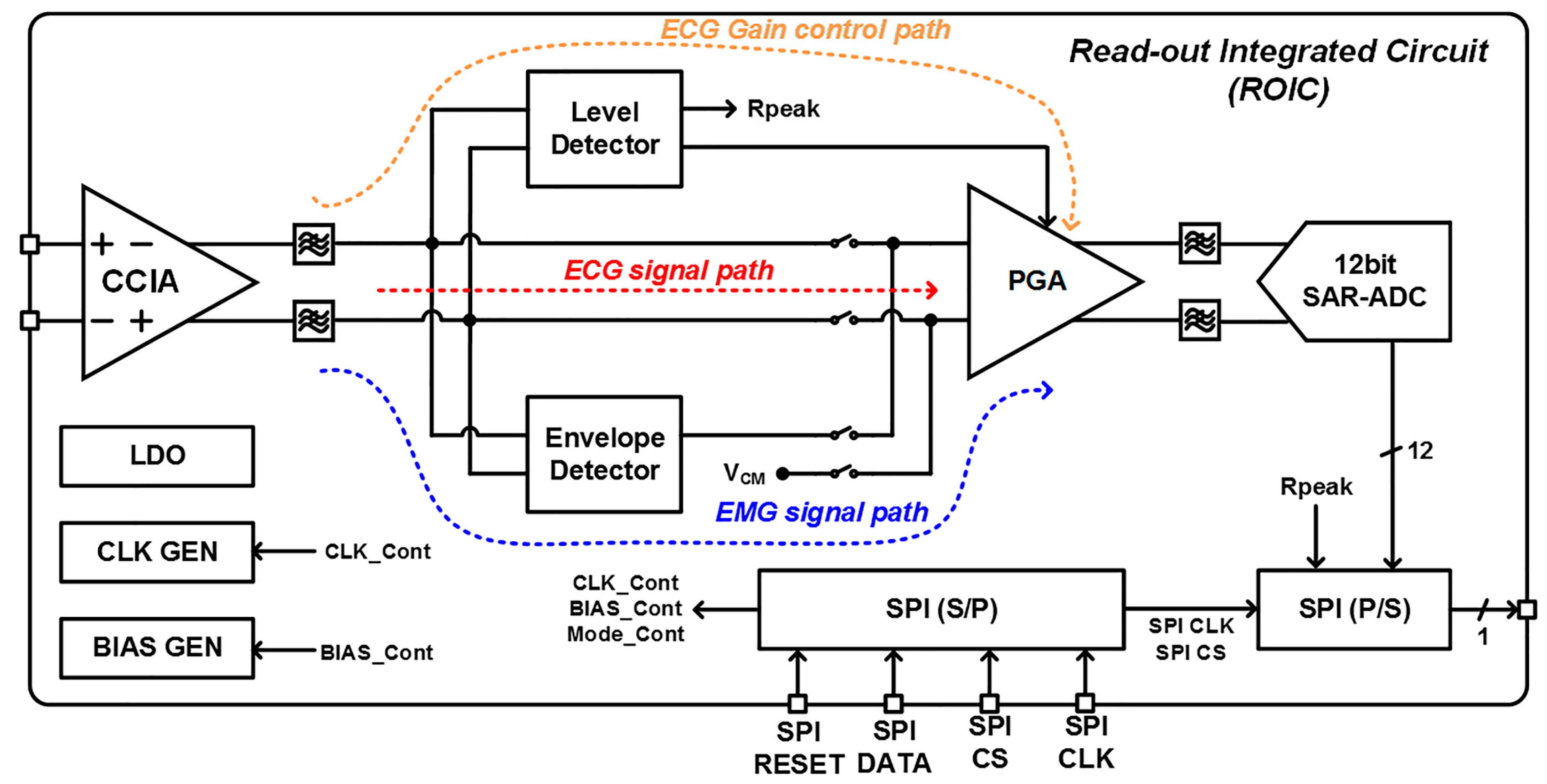
2. ExG Interface Architecture
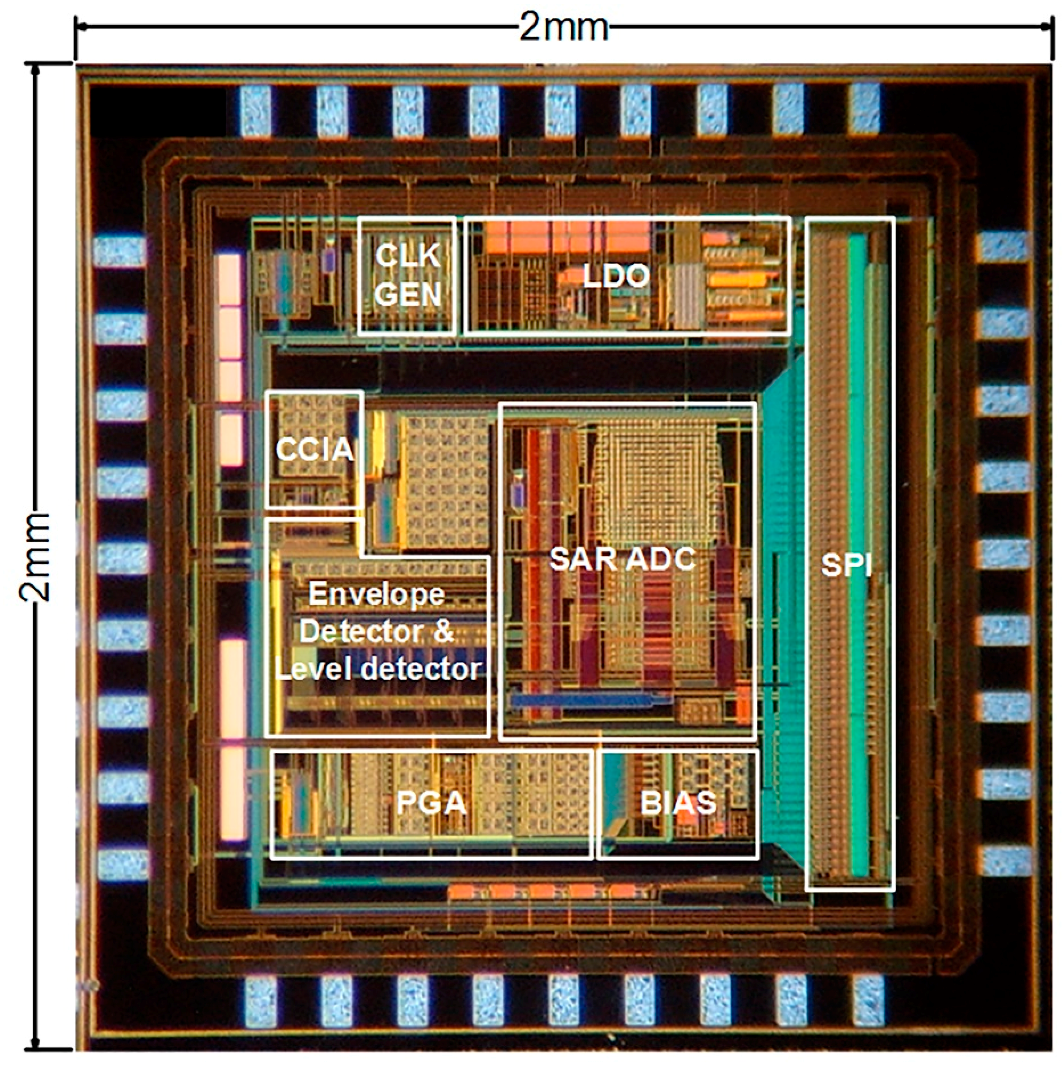
3. ROIC Implementation
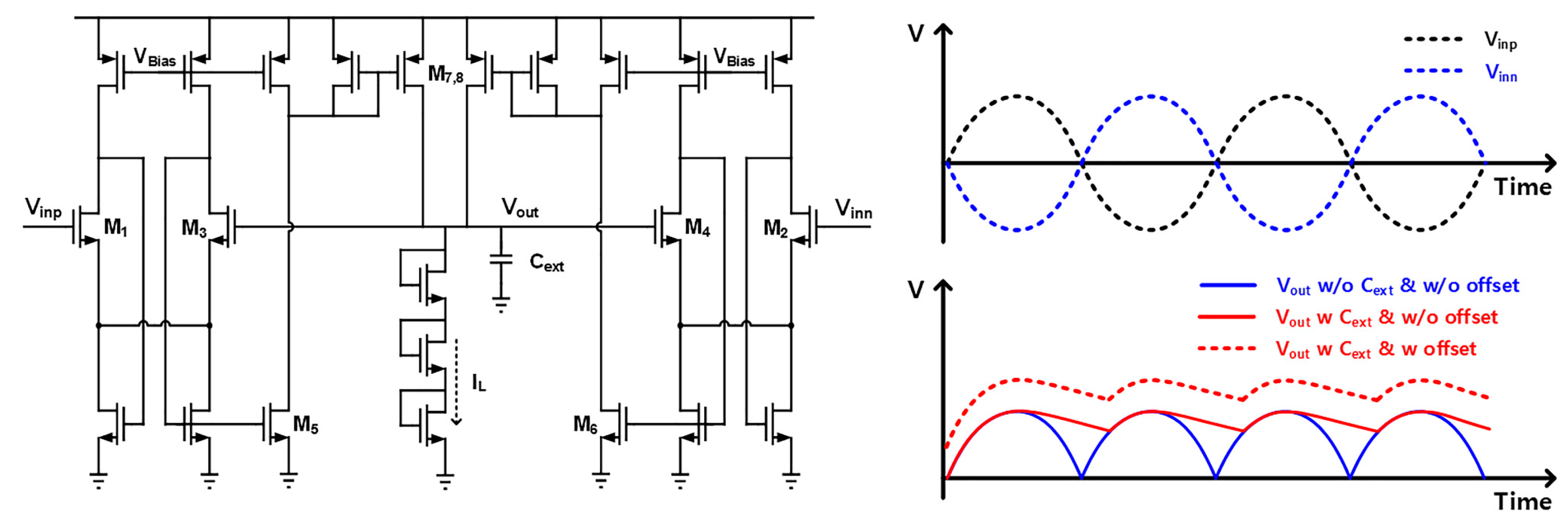
3.1. CCIA and PGA
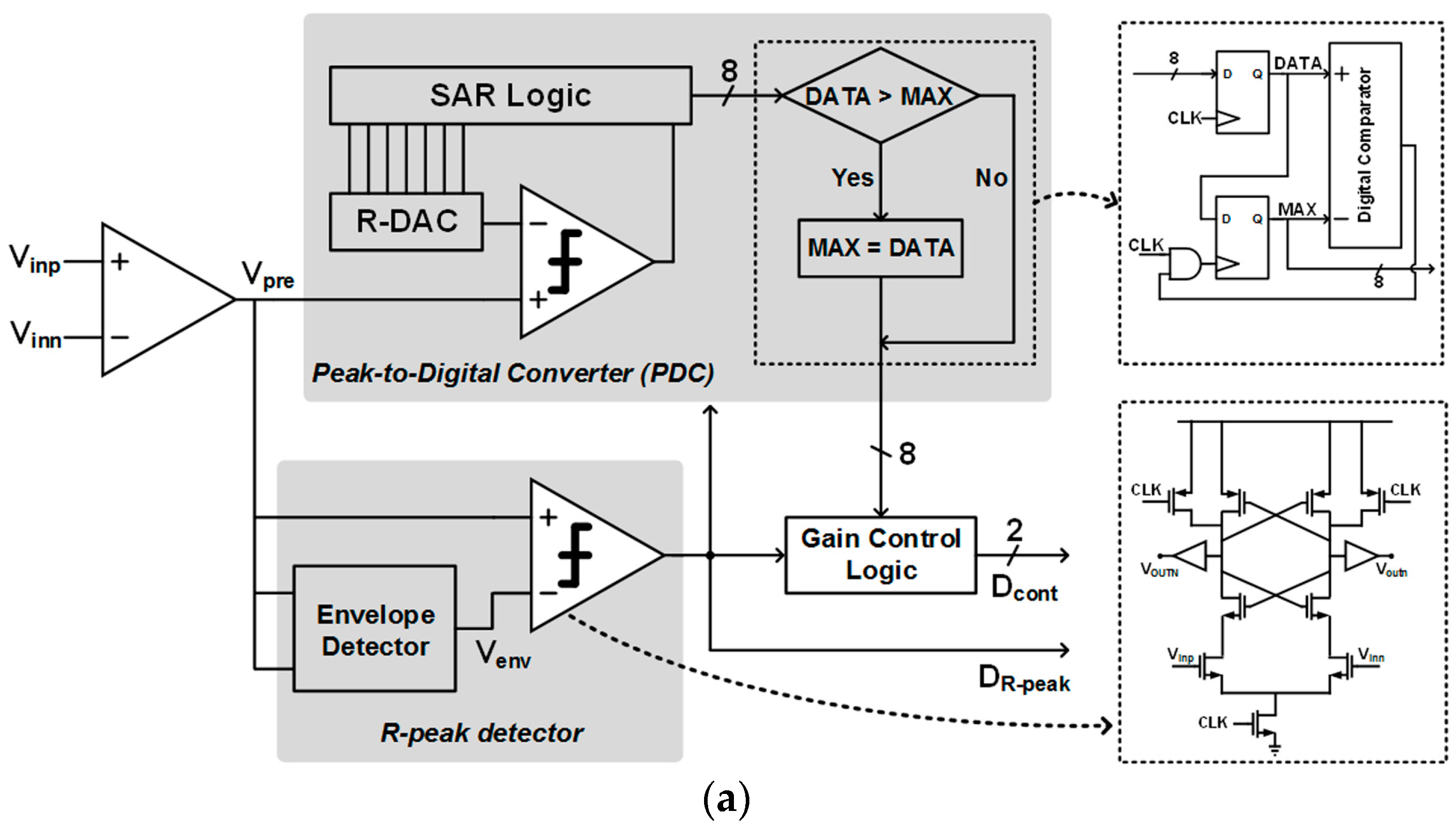
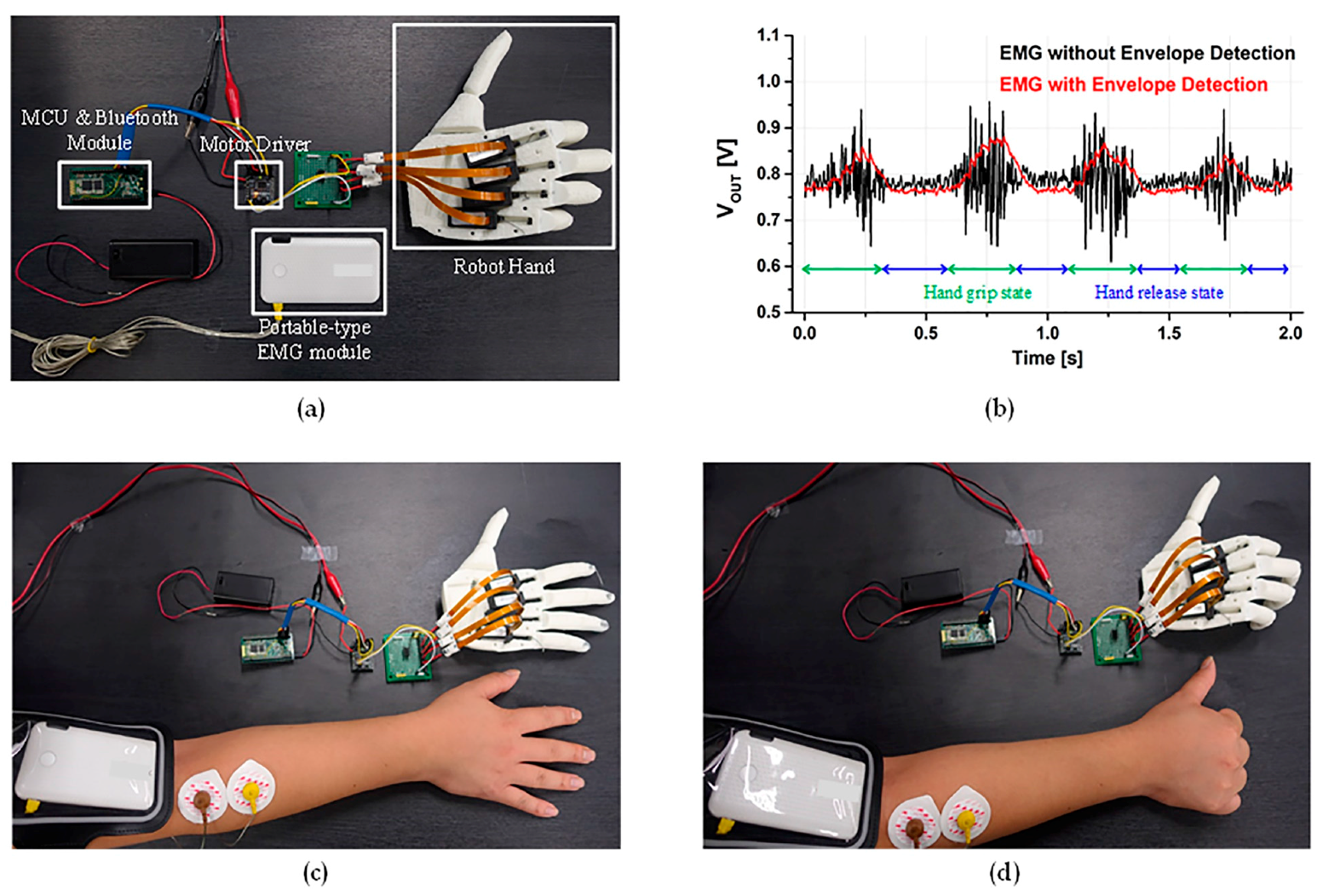
3.2. Envelope and Level Detectors
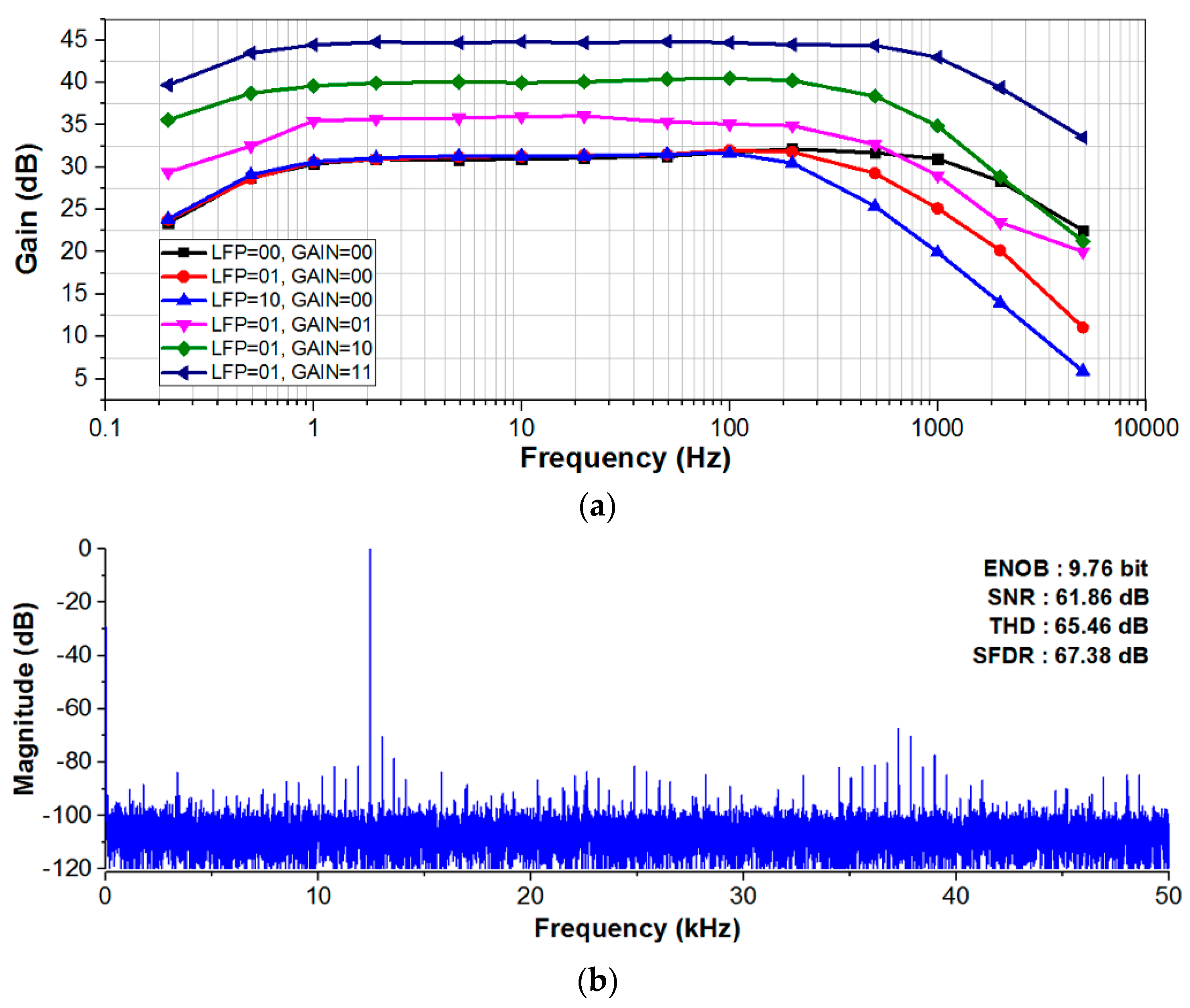
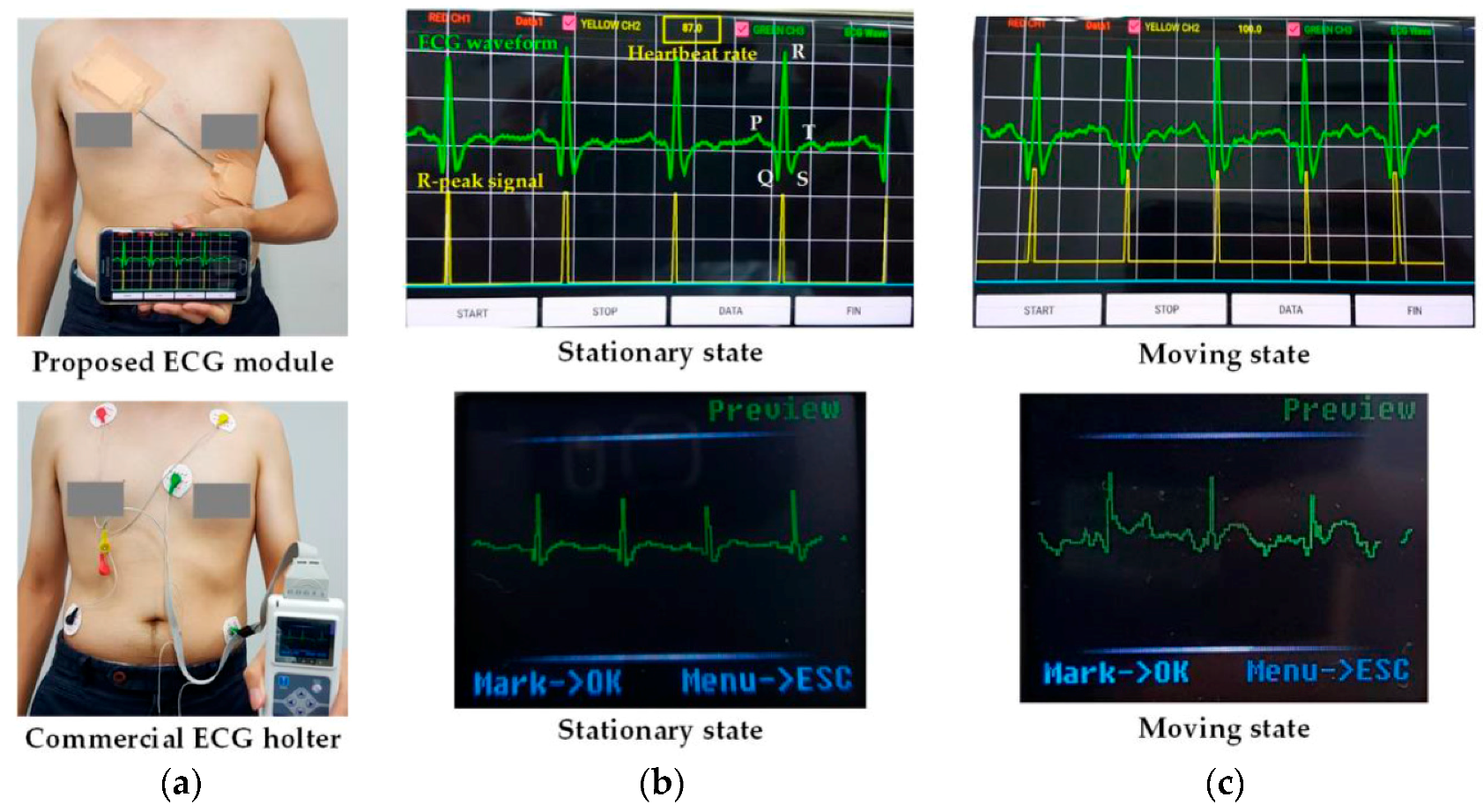
4. Experimental Results
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Konijnenburg, M.; Stanzione, S.; Yan, L.; Jee, D.-W.; Pettine, J.; van Wegberg, R.; Kim, H.; van Liempd, C.; Fish, R.; Schuessler, J.; et al. A Multi(bio)sensor Acquisition System with Integrated Processor, Power Management, 8 × 8 LED Drivers, and Simultaneously Synchronized ECG, BIO-Z, GSR, and Two PPG Readouts. IEEE J. Solid State Circuits 2016, 51, 2584–2595. [Google Scholar] [CrossRef]
- Woo, S.-H.; Choi, Y.-Y.; Kim, D.-J.; Bien, F.; Kim, J.-J. Tissue-Informative Mechanism for Wearable Non-invasive Continuous Blood Pressure Monitoring. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-J.; van Hoof, C. Bio-Medical CMOS ICs, 1st ed.; Springer: New York, NY, USA, 2010; p. 32. ISBN 978-1-4419-6596-7. [Google Scholar]
- Choi, S.; Kim, D.-J.; Choi, Y.-Y.; Park, K.; Kim, S.-W.; Woo, S.-H.; Kim, J.-J. A Multisensor Mobile Interface for Industrial Environment and Healthcare Monitoring. IEEE Trans. Ind. Electron. 2017, 64, 2344–2352. [Google Scholar] [CrossRef]
- Gupta, R.; Mitra, M.; Bera, J. ECG Acquisition and Automated Remote Processing, 1st ed.; Springer: New York, NY, USA, 2014; p. 53. ISBN 978-81-322-1556-1. [Google Scholar]
- Torfs, T.; Chen, Y.-H.; Kim, H.; Yazicioglu, R.-F. Noncontact ECG Recording System with Real Time Capacitance Measurement for Motion Artifact Reduction. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, T.; Marisa, T.; Kohler, L.; Haeberlin, A.; Wildhaber, R.-A.; Abächerli, R.; Goette, J.; Jacomet, M.; Vogel, R. A Baseline Wander Tracking System for Artifact Rejection in Long-Term Electrocardiography. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, S. A Motion-tolerant Heart Rate Detection Method Using Bio-impedance and MUSIC Algorithm. In Proceedings of the 2015 IEEE Sensors, Busan, Korea, 1–4 November 2015; pp. 1–4. [Google Scholar]
- Liu, X.; Zhou, J.; Yang, Y.; Wand, B.; Lan, J.; Wang, C.; Luo, J.; Goh, W.-L.; Kim, T.T.-H.; Je, M. A 457 nW Near-Threshold Cognitive Multi-Functional ECG Processor for Long-Term Cardiac Monitoring. IEEE J. Solid State Circuits 2014, 49, 2422–2434. [Google Scholar] [CrossRef]
- Moy, T.; Huang, L.; Rieutort-Louis, W.; Wu, C.; Cuff, P.; Wagner, S.; Sturm, J.C.; Verma, N. An EEG Acquisition and Biomarker-Extraction System Using Low-Noise-Amplifier and Compressive-Sensing Circuits Based on Flexible, Thin-Film Electronics. IEEE J. Solid State Circuits 2017, 52, 309–321. [Google Scholar] [CrossRef]
- Deepu, C.-J.; Zhang, X.; Liew, W.-S.; Wong, D.L.T.; Lian, Y. An ECG-on-Chip with 535 nW/Channel Integrated Lossless Data Compressor for Wireless Sensors. IEEE J. Solid State Circuits 2014, 49, 2435–2448. [Google Scholar] [CrossRef]
- He, D.-D.; Sodini, C.-G. A 58 nW ECG ASIC With Motion-Tolerant Heartbeat Timing Extraction for Wearable Cardiovascular Monitoring. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, Y.; Kim, H.; Ko, H. Fully Integrated Biopotential Acquisition Analog Front-End IC. Sensors 2015, 15, 25139–25156. [Google Scholar] [CrossRef] [PubMed]
- Enz, C.-C.; Temes, G.-C. Circuit Techniques for Reducing the Effects of Op-Amp Imperfections: Autozeroing, Correlated Double Sampling, and Chopper Stabilization. Proc. IEEE 1996, 84, 1584–1614. [Google Scholar] [CrossRef]
- Fuketa, H.; Yoshioka, K.; Shinozuka, Y.; Ishida, K.; Yokota, Y.; Matsuhisa, N.; Inoue, Y.; Sekino, M.; Sekitani, T.; Takamiya, M.; et al. 1 μm-Thickness Ultra-Flexible and High Electrode-Density Surface Electromyogram Measurement Sheet with 2 V Organic Transistors for Prosthetic Hand Control. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, S.; Eom, W.; Kim, J.-J. A Reconfigurable Readout Integrated Circuit for Heterogeneous Display-based Multi-Sensor Systems. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-C.; Lin, T.-H. Measurement and parameter characterization of pseudo-resistor based CCIA for biomedical applications. In Proceedings of the IEEE International Symposium on Bioelectronics and Bioinformatics (IEEE ISBB), Chung Li, Taiwan, 11–14 April 2014; pp. 1–4. [Google Scholar]
- Park, S.-B.; Wilson, J.-E.; Ismail, M. The CHIP—Peak Detectors for Multistandard Wireless Receivers. IEEE Circuits Devices Mag. 2006, 22, 6–9. [Google Scholar] [CrossRef]


| Parameter | This Work | [7] | [12] | [13] |
|---|---|---|---|---|
| ROIC Application | ECG, EMG, EEG | ECG | ECG | ECG, EEG |
| Module type | Patch-type ECG module, Portable-type EMG module | N.A. | Ear clip-type ECG module | N.A |
| Functionality | R-peak detection, EMG envelope detection | Baseline wander tracking | R-peak detection | N.A. |
| Process | 0.18 μm CMOS | N.A. | 0.18 μm CMOS | 0.18 μm CMOS |
| Chip Area (mm2) | 4 mm2 | N.A. | 3.24 mm2 | 24.01 mm2 |
| Passband (Hz) | 0.6–1500 Hz (programmable) | DC–500 Hz | 0.5–22 Hz | 1–100 Hz (programmable) |
| Gain (dB) | 31.3–44.8 dB (programmable) | 48 dB | 47–88 dB (programmable) | 47.3–71.9 dB (programmable) |
| Supply voltage (V) | 1.8 V (ROIC) 3 V (Module) | 3 V | 0.8 V | 3.3 V |
| Power consumption (μW) | 37.3 μW (ROIC) 0.2 μW (ADC) | N.A. (ROIC) 160 μW (ADC) | 58 nW (ROIC) | 12.5 μW (ROIC) |
| ADC resolution (bit) | 12 bit | 12 bit | N.A. | 12 bit |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Choi, Y.Y.; Kim, D.J.; Chae, H.Y.; Park, K.; Oh, Y.M.; Woo, S.H.; Kim, J.J. A Wireless ExG Interface for Patch-Type ECG Holter and EMG-Controlled Robot Hand. Sensors 2017, 17, 1888. https://doi.org/10.3390/s17081888
Lee K, Choi YY, Kim DJ, Chae HY, Park K, Oh YM, Woo SH, Kim JJ. A Wireless ExG Interface for Patch-Type ECG Holter and EMG-Controlled Robot Hand. Sensors. 2017; 17(8):1888. https://doi.org/10.3390/s17081888
Chicago/Turabian StyleLee, Kwangmuk, Yun Young Choi, Dae Jung Kim, Hee Young Chae, Kyeonghwan Park, Young Min Oh, Sung Hun Woo, and Jae Joon Kim. 2017. "A Wireless ExG Interface for Patch-Type ECG Holter and EMG-Controlled Robot Hand" Sensors 17, no. 8: 1888. https://doi.org/10.3390/s17081888
APA StyleLee, K., Choi, Y. Y., Kim, D. J., Chae, H. Y., Park, K., Oh, Y. M., Woo, S. H., & Kim, J. J. (2017). A Wireless ExG Interface for Patch-Type ECG Holter and EMG-Controlled Robot Hand. Sensors, 17(8), 1888. https://doi.org/10.3390/s17081888





