Gold Nanoplates for a Localized Surface Plasmon Resonance-Based Boric Acid Sensor
Abstract
:1. Introduction
2. Materials and Methods
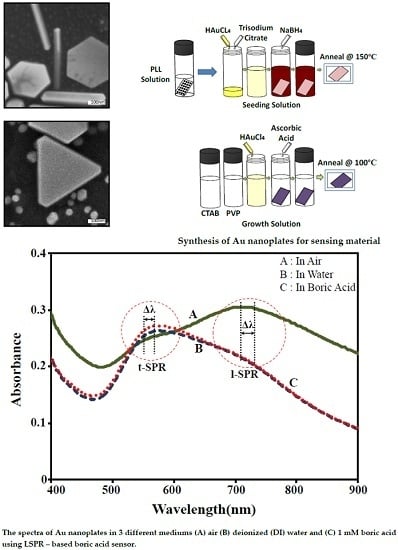
2.1. Preparation of Gold Nanoplates
2.2. Optical Sensor System Setup
3. Results and Discussion
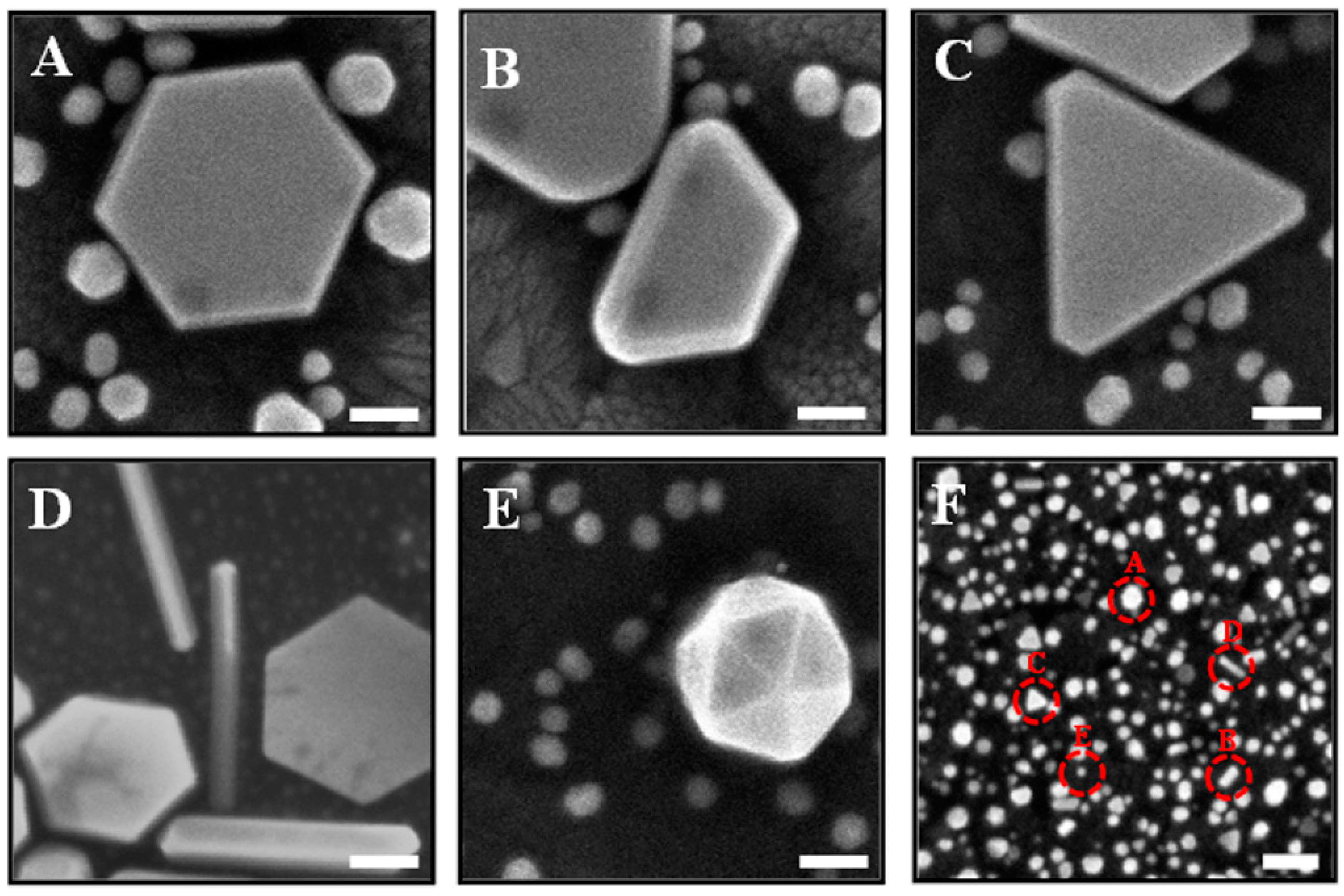
3.1. Gold Nanoplates Characterization
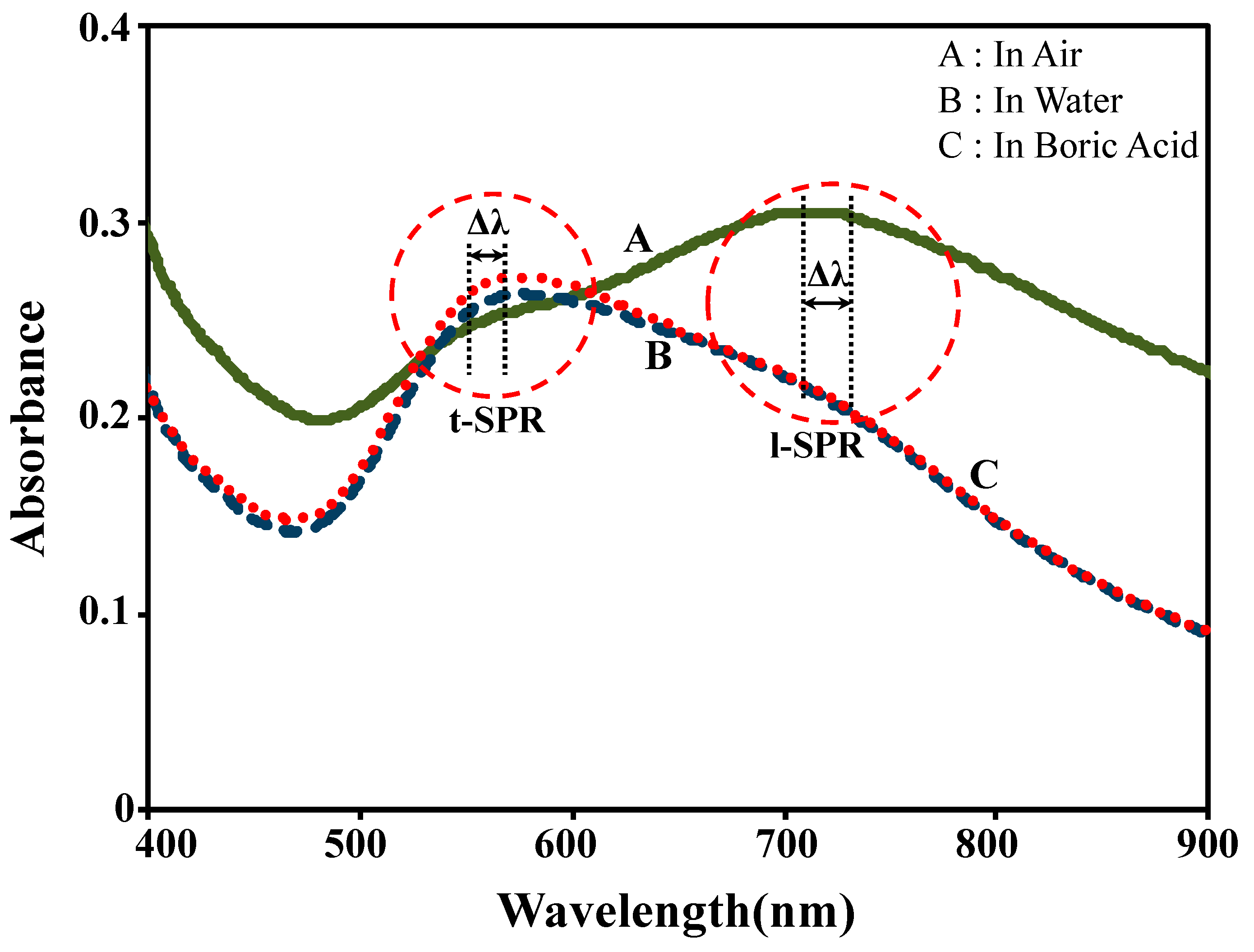
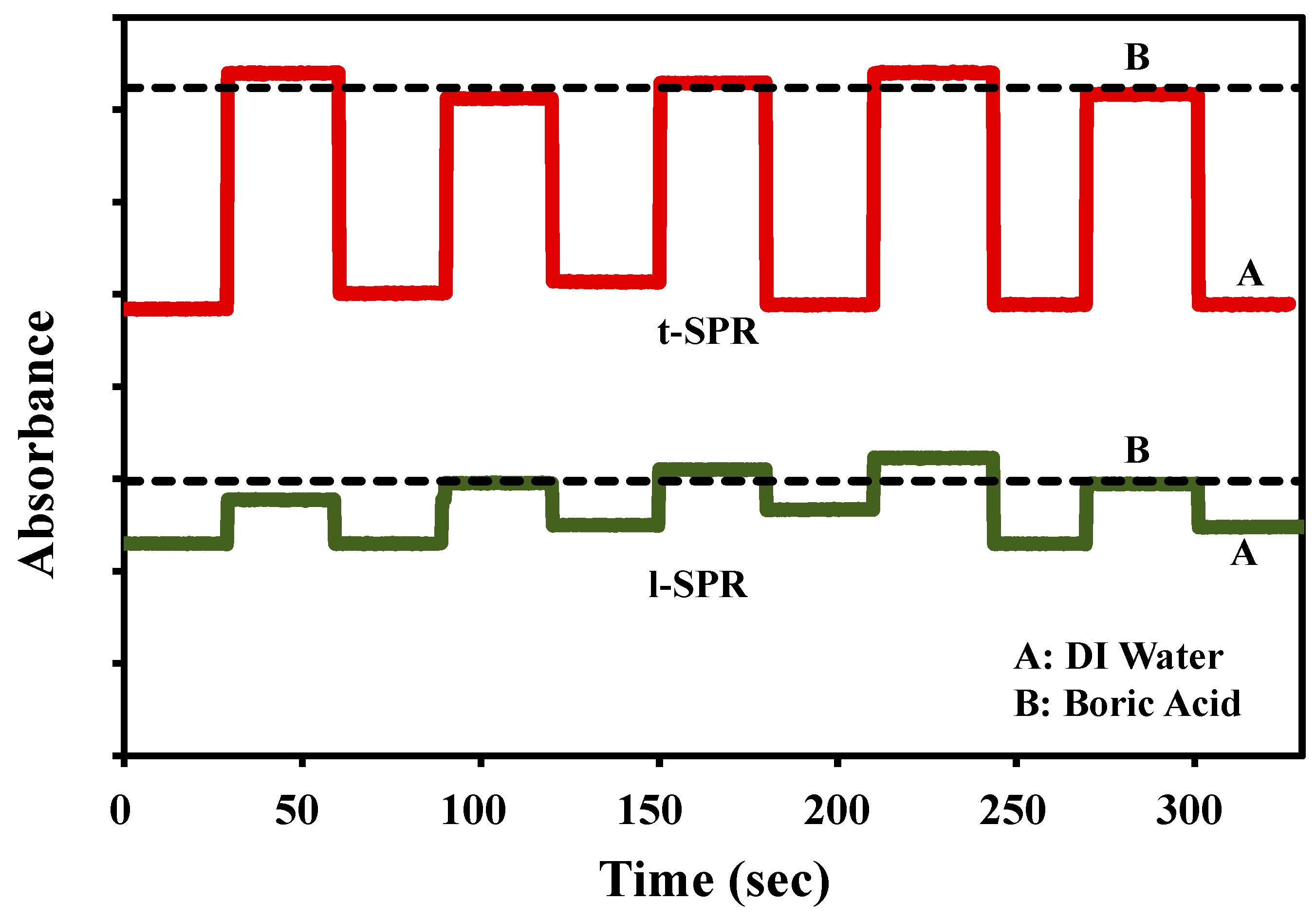
3.2. Plasmonic Sensing Response
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.H.; Yuan, H.; Choi, W.Y.; Eum, N.S.; Kang, S.W. Development of Localized Surface Plasmon Resonance Based Biosensor Using Au Deposited Nano-Porous Aluminum Anodic Oxide Chip. Sens. Lett. 2011, 1, 90–96. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, F.; Lu, J.; Zhao, J. Optimization of the refractive index plasmonic sensing of gold nanorods by non-uniform silver coating. Sens. Actuators B Chem. 2013, 183, 556–564. [Google Scholar] [CrossRef]
- Umar, A.A.; Oyama, M.; Salleh, M.M.; Majlis, B.Y. Silver nanocombs and branched nanowires formation in aqueous binary surfactants solution. J. Nanopart. Res. 2012, 14, 1001. [Google Scholar] [CrossRef]
- Balouch, A.; Umar, A.A.; Tan, S.T.; Nafisah, S.; Saad, S.K.M.; Salleh, M.M.; Oyama, M. Fibrous, ultra-small nanorod-constructed platinum nanocubes directly grown on the ITO substrate and their heterogeneous catalysis application. RSC Adv. 2013, 3, 19789–19792. [Google Scholar] [CrossRef]
- Watt, J.; Cheong, S.; Tilley, R.D. How to control the shape of metal nanostructures in organic solution phase synthesis for plasmonics and catalysis. Nano Today 2013, 8, 198–215. [Google Scholar] [CrossRef]
- Umar, A.A.; Oyama, M. Synthesis of Palladium Nanobricks with Atomic-Step Defects. Cryst. Growth Des. 2008, 8, 1808–1811. [Google Scholar] [CrossRef]
- Balouch, A.; Umar, A.A.; Shah, A.A.; Salleh, M.M.; Oyama, M. Efficient Heterogeneous Catalytic Hydrogenation of Acetone to Isopropanol on Semihollow and Porous Palladium Nanocatalyst. ACS Appl. Mater. Interf. 2013, 5, 9843–9849. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensor. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; Van Duyne, R.P. Localized surface plasmon resonance biosensors. Nanomedicine 2008, 1, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mejri, M.B.; Baccar, H.; Ktari, T.; Aouni, M.; Abdelghani, A. Detection of E. coli Bacteria Using Impedance Spectroscopy and Surface Plasmon Resonance Imaging Based Biosensor. Sens. Lett. 2011, 9, 2130–2132. [Google Scholar] [CrossRef]
- Sekhon, J.S.; Malik, H.K.; Verma, S.S. Tailoring Surface Plasmon Resonance Wavelengths and Sensoric Potential of Core–Shell Metal Nanoparticles. Sens. Lett. 2013, 11, 512–518. [Google Scholar] [CrossRef]
- Hamamoto, K.; Micheletto, R.; Oyama, M.; Umar, A.A.; Kawai, S.; Kawakami, Y. An original planar multireflection system for sensing using the local surface plasmon resonance of gold nanospheres. J. Opt. A Pure Appl. Opt. 2006, 8, 268. [Google Scholar] [CrossRef]
- Chaoming, W.; Haining, W.; Shengli, Z.; Liyuan, M. Polyelectrolyte Multilayers Stabilized Plasmonic Nanosensors. Sens. Lett. 2013, 11, 519–525. [Google Scholar]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sannomiya, T.; Voros, J. Single plasmonic nanoparticles for biosensing. Trends Biotechnol. Rev. 2011, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Green, M.A. Plasmonics for photovoltaic applications. Sol. Energy Mat. Sol Cells 2010, 94, 1481–1486. [Google Scholar] [CrossRef]
- Veres, Á.; Rica, T.; Janovák, L.; Dömök, M.; Buzás, N.; Zöllmer, V.; Seemann, T.; Richardt, A.; Dékány, I. Silver and gold modified plasmonic TiO2 hybrid films for photocatalytic decomposition of ethanol under visible light. Catal. Today 2012, 181, 156–162. [Google Scholar] [CrossRef]
- Su, H.; Ma, Q.; Shang, K.; Liu, T.; Yin, H.; Ai, S. Gold nanoparticles as colorimetric sensor: A case study on E. coli O157:H7 as a model for Gram-negative bacteria. Sens. Actuators B Chem. 2012, 161, 298–303. [Google Scholar] [CrossRef]
- Nengsih, S.; Umar, A.A.; Salleh, M.M.; Oyama, M. Detection of Formaldehyde in Water: A Shape-Effect on the Plasmonic Sensing Properties of the Gold Nanoparticles. Sensors 2012, 12, 10309–10325. [Google Scholar] [CrossRef] [PubMed]
- Morsin, M.; Salleh, M.M.; Umar, A.A. Detection of Boric Acid using Localized Surface Plasmon Resonance Sensor of Gold Nanoparticles. In Proceedings of the 14th International Meeting on Chemical Sensor, Nuremberg, Germany, 20–23 May 2012. [Google Scholar]
- Morsin, M.; Umar, A.A.; Salleh, M.M.; Majlis, B.Y. High sensitivity localized surface plasmon resonance sensor of gold nanoparticles: Surface density effect for detection of boric acid. In Proceedings of the 10th IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 19–21 September 2012. [Google Scholar]
- Cao, J.; Sun, T.; Grattan, K.T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.A.; Iwantono, I.; Abdullah, A.; Salleh, M.M.; Oyama, M. Gold nanonetwork film on the ITO surface exhibiting one-dimensional optical properties. Nanoscale Res. Lett. 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.A.; Oyama, M.; Salleh, M.M.; Majlis, B.Y. Formation of High-Yield Gold Nanoplates on the Surface: Effective Two-Dimensional Crystal Growth of Nanoseed in the Presence of Poly(vinylpyrrolidone) and Cetyltrimethylammonium Bromide. Cryst. Growth Des. 2009, 9, 2835–2840. [Google Scholar] [CrossRef]
- Umar, A.A.; Oyama, M.; Salleh, M.M.; Majlis, B.Y. Formation of Highly Thin, Electron-Transparent Gold Nanoplates from Nanoseeds in Ternary Mixtures of Cetyltrimethylammonium Bromide, Poly(vinyl pyrrolidone), and Poly(ethylene glycol). Cryst. Growth Des. 2010, 10, 3694–3698. [Google Scholar] [CrossRef]
- Goyal, R.N.; Akrajas, A.A.; Oyama, M. Comparison of spherical nanogold particles and nanogold plates for the oxidation of dopamine and ascorbic acid. J. Electroanal. Chem. 2009, 631, 58–61. [Google Scholar] [CrossRef]
- Litovitz, T.L.; Klein-Schwartz, W.; Oderda, G.M.; Schmitz, V. Clinical manifestations of toxicity in a series of 784 boric acid ingestions. Am. J. Emerg. Med. 1988, 6, 209–213. [Google Scholar] [CrossRef]
- See, A.S.; Salleh, A.B.; Bakar, F.A.; Yusof, N.; Abdulamir, A.S.; Heng, L.Y. Risk and Health Effect of Boric Acid. Am. J. Appl. Sci. 2010, 7, 620–627. [Google Scholar] [CrossRef]
- Tréguer-Delapierre, M.; Majimel, J.; Mornet, S.; Duguet, E.; Ravaine, S. Synthesis of non-spherical gold nanoparticles. Gold Bull. 2008, 41, 195–207. [Google Scholar] [CrossRef]
- Morsin, M.; Salleh, M.M.; Umar, A.A.; Yahaya, M. Localized Surface Plasmon Resonance Sensor of Gold Nanoplates for Detection of Boric Acid. Key Eng. Mat. 2014, 605, 356–359. [Google Scholar] [CrossRef]
- Morsin, M.; Mat Salleh, M.; Sahdan, M.Z.; Muji, M.; Zarina, S. Development of plasmonic sensor for detection of toxic materials. ARPN J. Eng. Appl. Sci. 2015, 10, 9083–9087. [Google Scholar]
- Purcell, E.M.; Pennypacker, C.R. Scattering and Absorption of Light by Nonspherical Dielectric Grains. Astrophys. J. 1973, 186, 705–714. [Google Scholar] [CrossRef]
- Draine, B.T.; Flatau, P.J. Discrete-dipole approximation for scattering calculations. J. Opt. Soc. Am. A 1994, 11, 1491–1499. [Google Scholar] [CrossRef]
- Draine, B.T. The discrete dipole approximation for light scattering by irregular targets. In Light Scattering by Nonspherical Particles, Theory, Measurements, and Applications; Mishchenko, M.I., Hovenier, J.W., Travis, L.D., Eds.; Academic Press: New York, NY, USA, 2000; pp. 131–145. [Google Scholar]
- Stuart, D.A.; Haes, A.J.; Yonzon, C.R.; Hicks, E.M.; Van Duyne, R.P. Biological applications of localised surface plasmonic phenomenae. IEE Proc. Nanobiotechnol. 2005, 152, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Barbillon, G.; Bijeon, J.; Plain, J.; Chapelle, M.L.; Adam, P.M.; Royer, P. Biological and chemical gold nanosensors based on localized surface plasmon resonance. Gold Bull. 2007, 40, 240–244. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Duyne, R.P.V. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, D.S. Refractive Index, Molar Refraction and Comparative Refractive Index Study of Propylene Carbonate Binary Liquid Mixtures. Acta Chim. Slov. 2012, 59, 258–263. [Google Scholar] [PubMed]
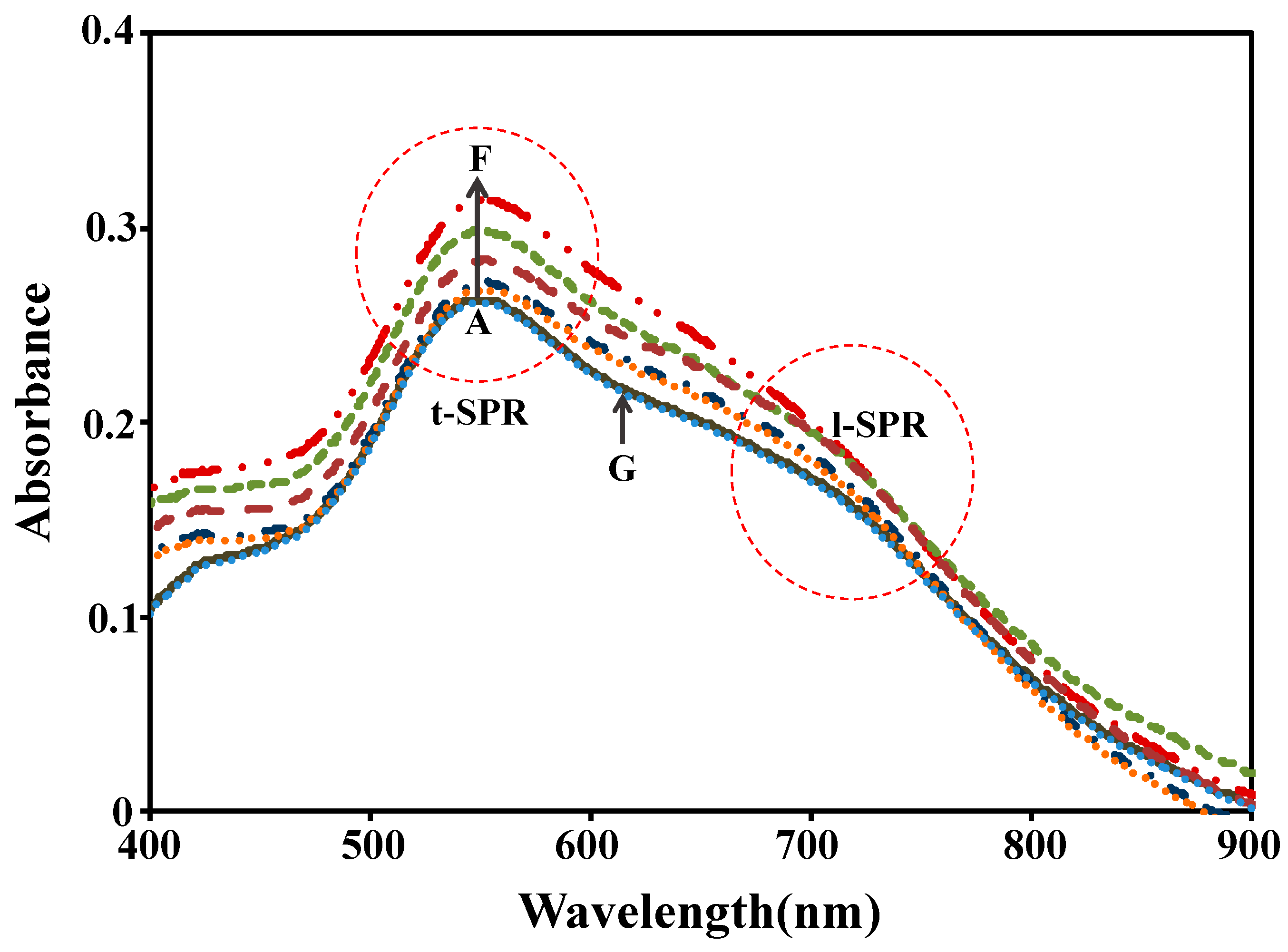
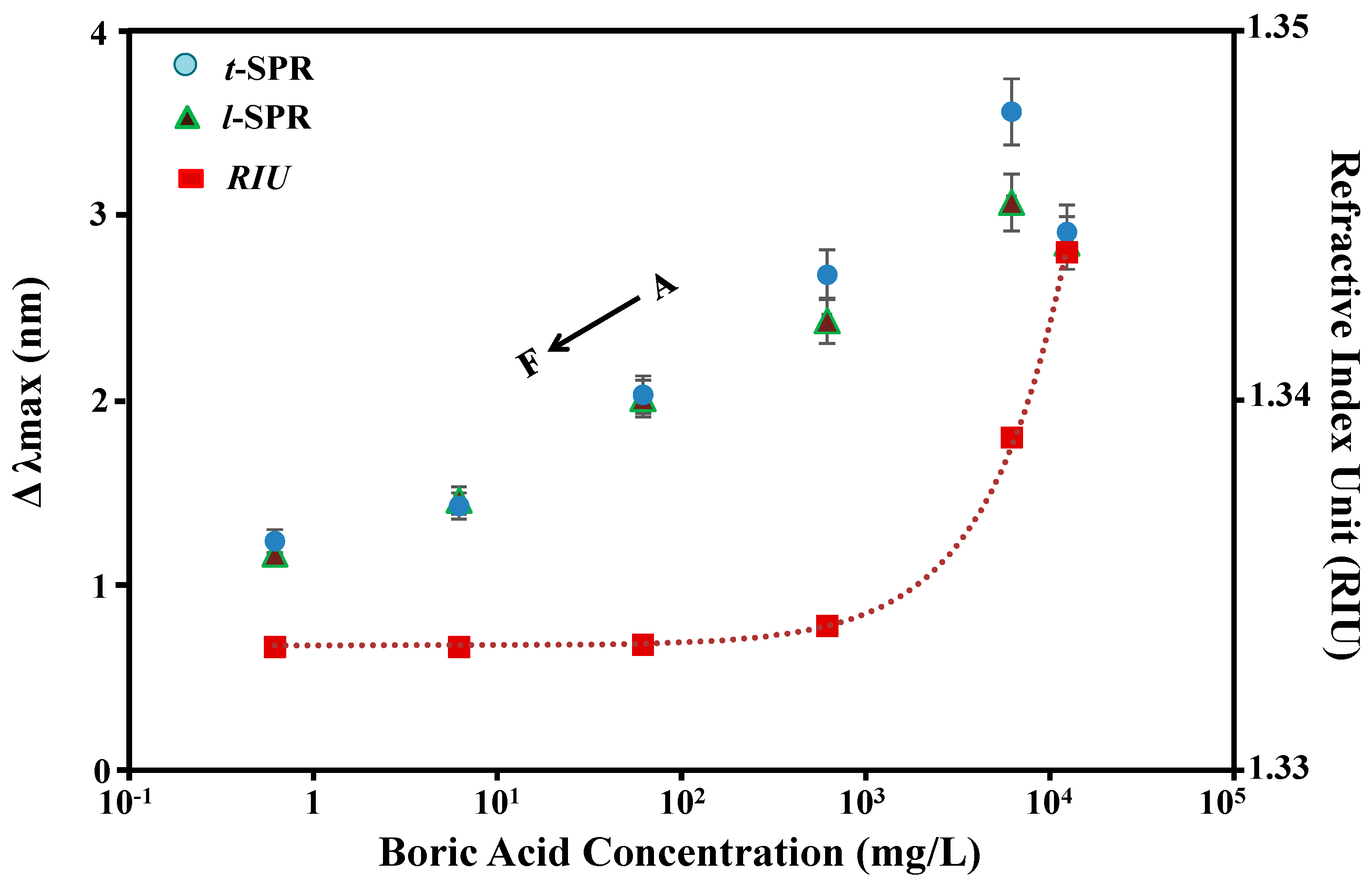
| Parameter | ∆Intensity (10−3) | ∆Peak Position (nm) | ||
|---|---|---|---|---|
| Concentration (mM) | t-SPR | l-SPR | t-SPR | l-SPR |
| 0.01 | 6.67 | 6.25 | 1.24 | 1.17 |
| 0.1 | 10.00 | 9.75 | 1.43 | 1.46 |
| 1.0 | 15.75 | 15.30 | 2.03 | 2.01 |
| 10 | 20.00 | 20.00 | 2.68 | 2.41 |
| 100 | 31.25 | 31.33 | 3.56 | 3.07 |
| 200 | 22.33 | 29.00 | 2.91 | 2.85 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsin, M.; Mat Salleh, M.; Ali Umar, A.; Sahdan, M.Z. Gold Nanoplates for a Localized Surface Plasmon Resonance-Based Boric Acid Sensor. Sensors 2017, 17, 947. https://doi.org/10.3390/s17050947
Morsin M, Mat Salleh M, Ali Umar A, Sahdan MZ. Gold Nanoplates for a Localized Surface Plasmon Resonance-Based Boric Acid Sensor. Sensors. 2017; 17(5):947. https://doi.org/10.3390/s17050947
Chicago/Turabian StyleMorsin, Marlia, Muhamad Mat Salleh, Akrajas Ali Umar, and Mohd Zainizan Sahdan. 2017. "Gold Nanoplates for a Localized Surface Plasmon Resonance-Based Boric Acid Sensor" Sensors 17, no. 5: 947. https://doi.org/10.3390/s17050947
APA StyleMorsin, M., Mat Salleh, M., Ali Umar, A., & Sahdan, M. Z. (2017). Gold Nanoplates for a Localized Surface Plasmon Resonance-Based Boric Acid Sensor. Sensors, 17(5), 947. https://doi.org/10.3390/s17050947