Evaluation of Commercial Self-Monitoring Devices for Clinical Purposes: Results from the Future Patient Trial, Phase I
Abstract
:1. Introduction
2. Materials and Methods
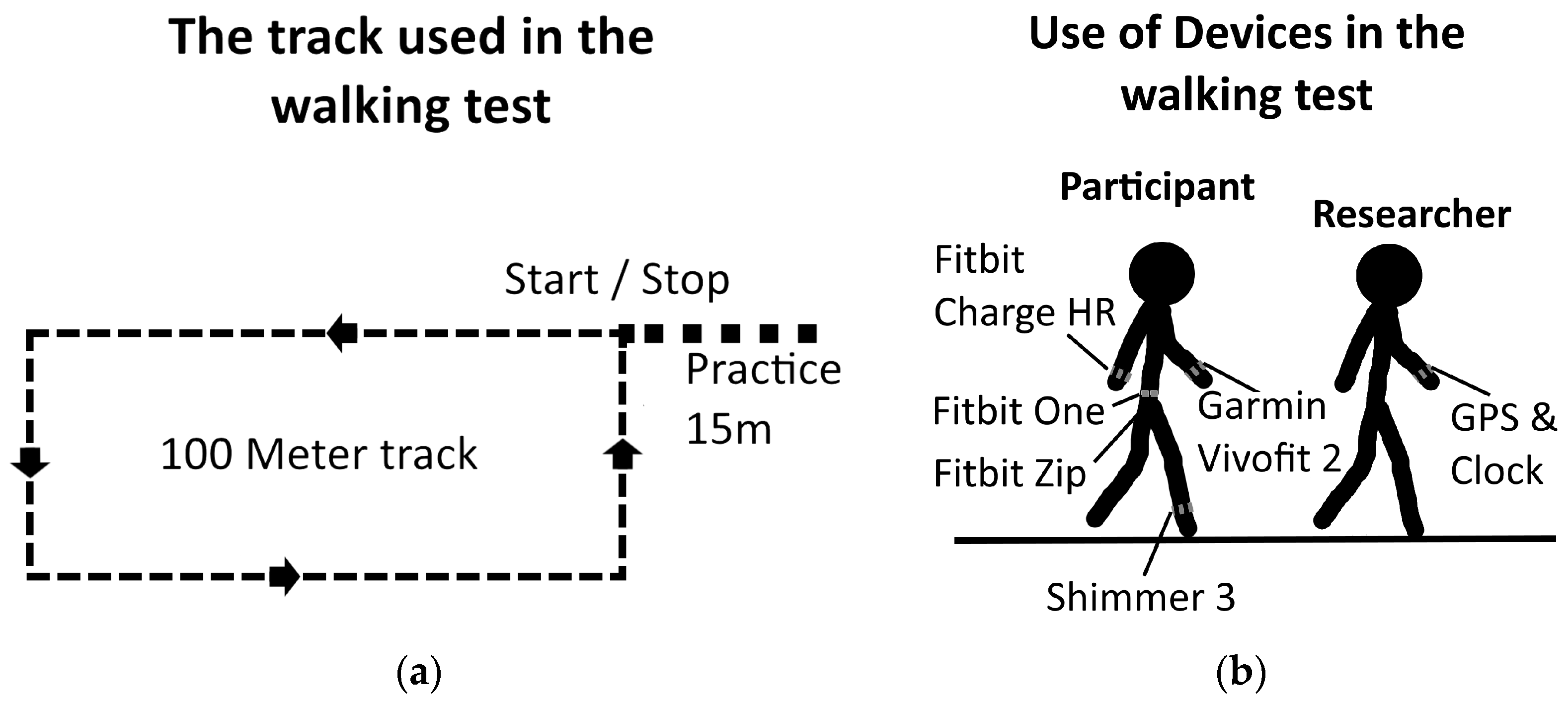
2.1. Standardized Walking Tests
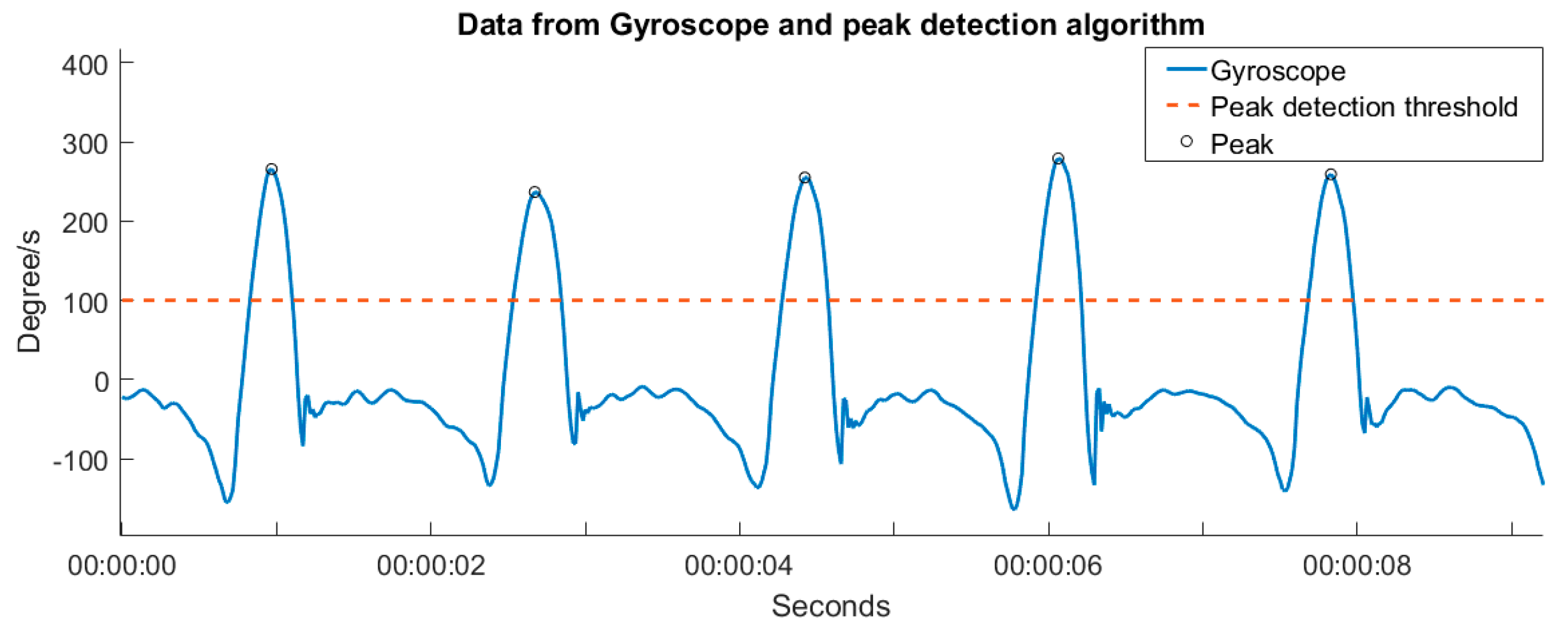
2.2. Calculating the Number of Steps Measured by the Shimmer 3
2.3. Heart Rate Validity
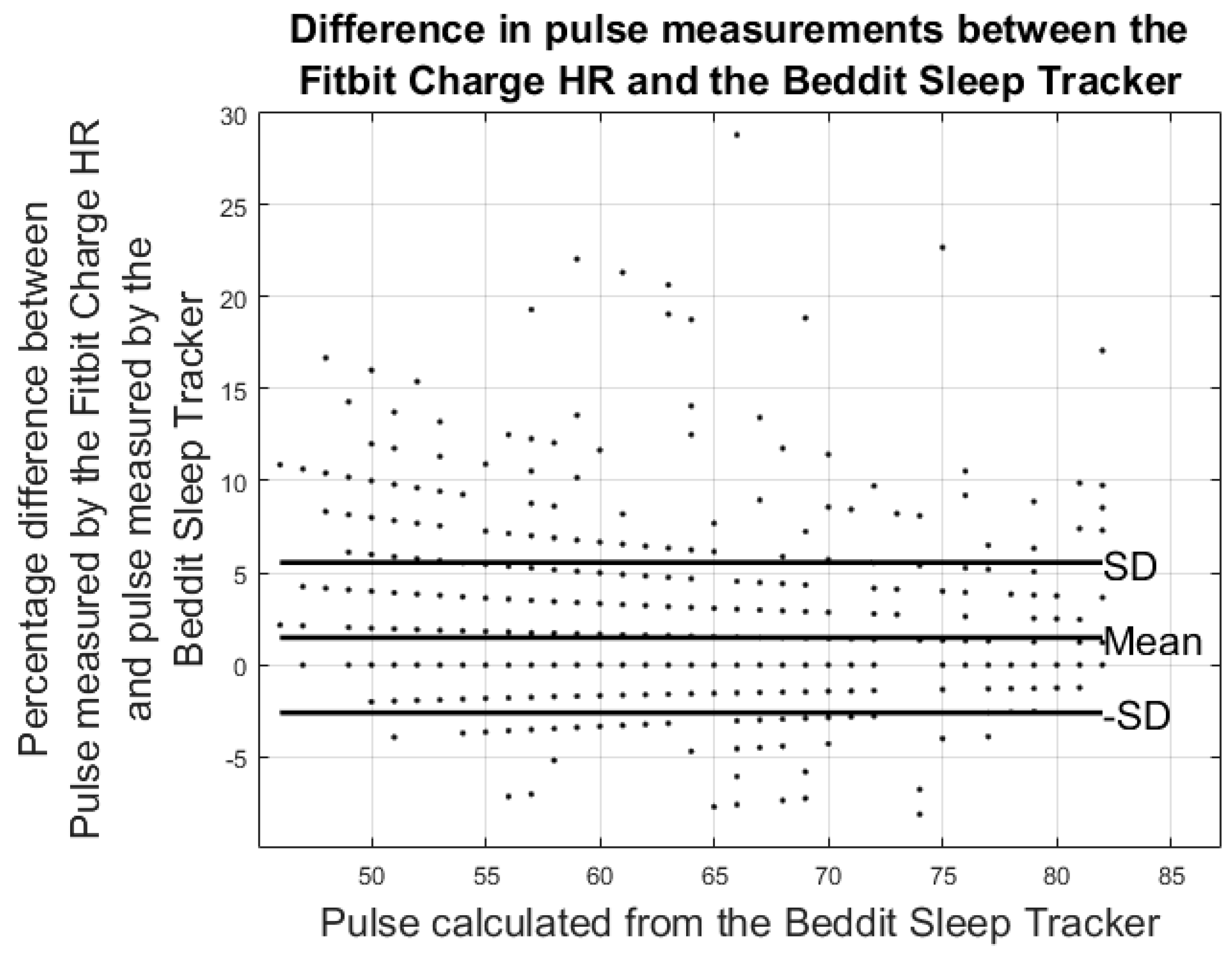
3. Results
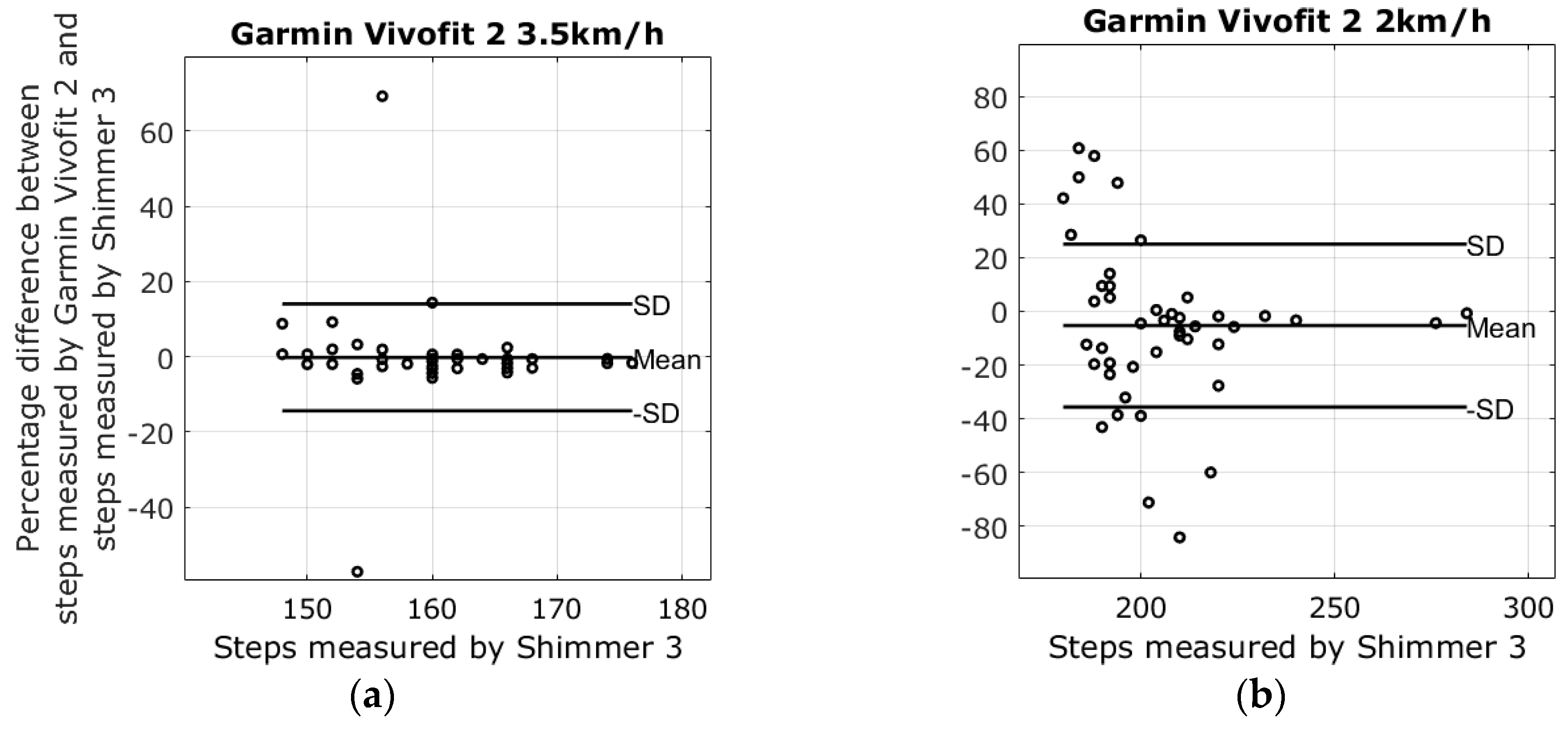
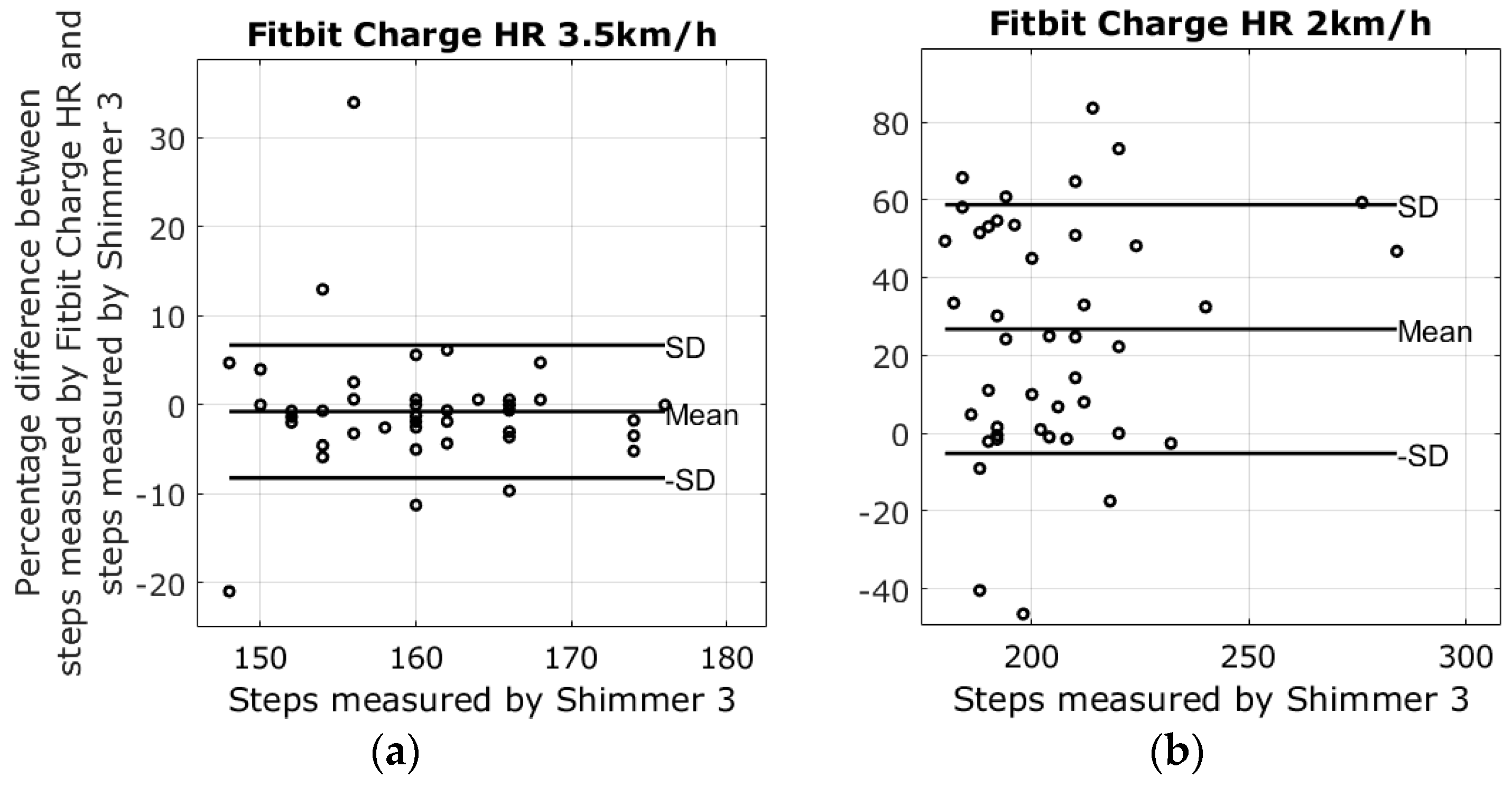
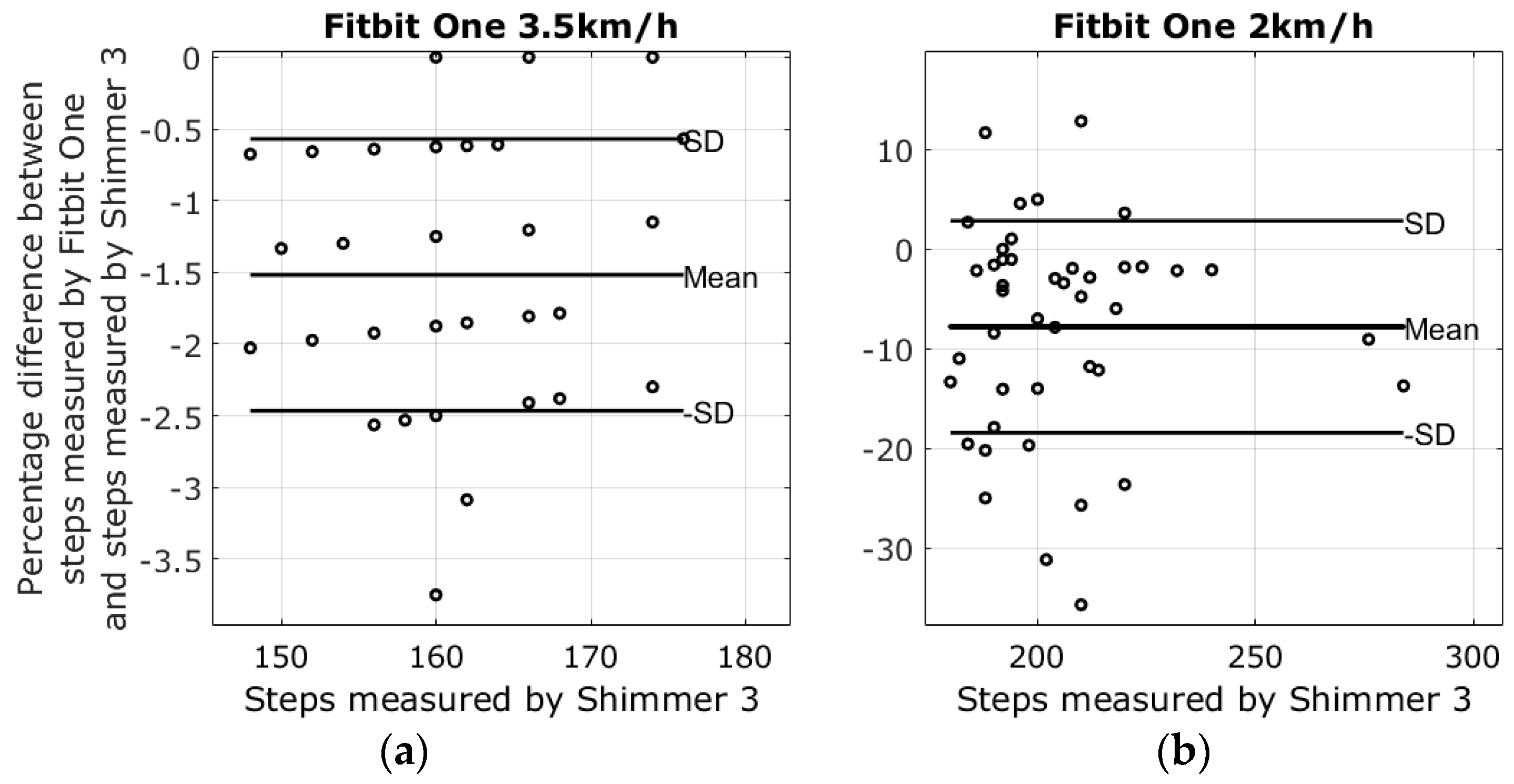
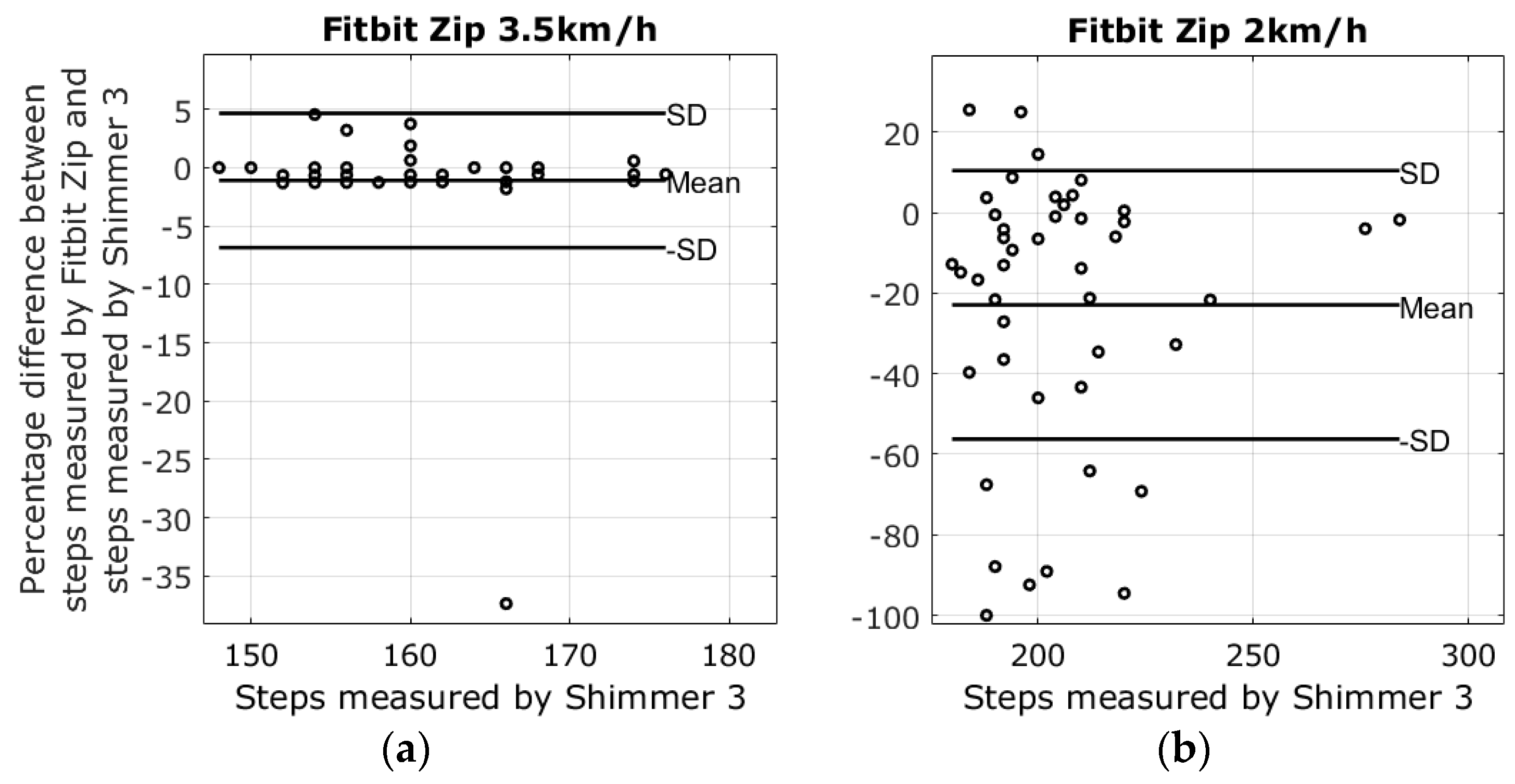
3.1. Results from Walking Tests
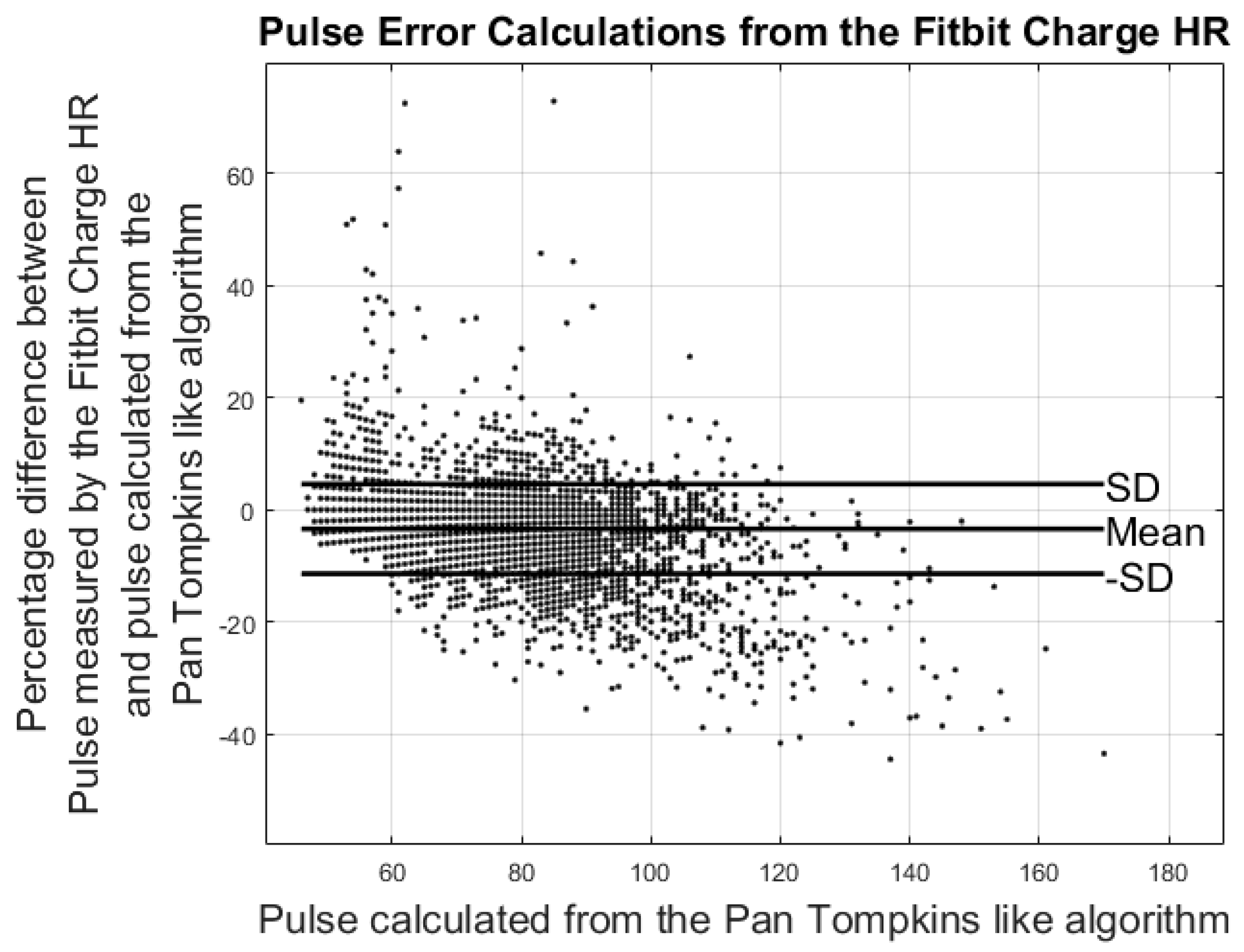
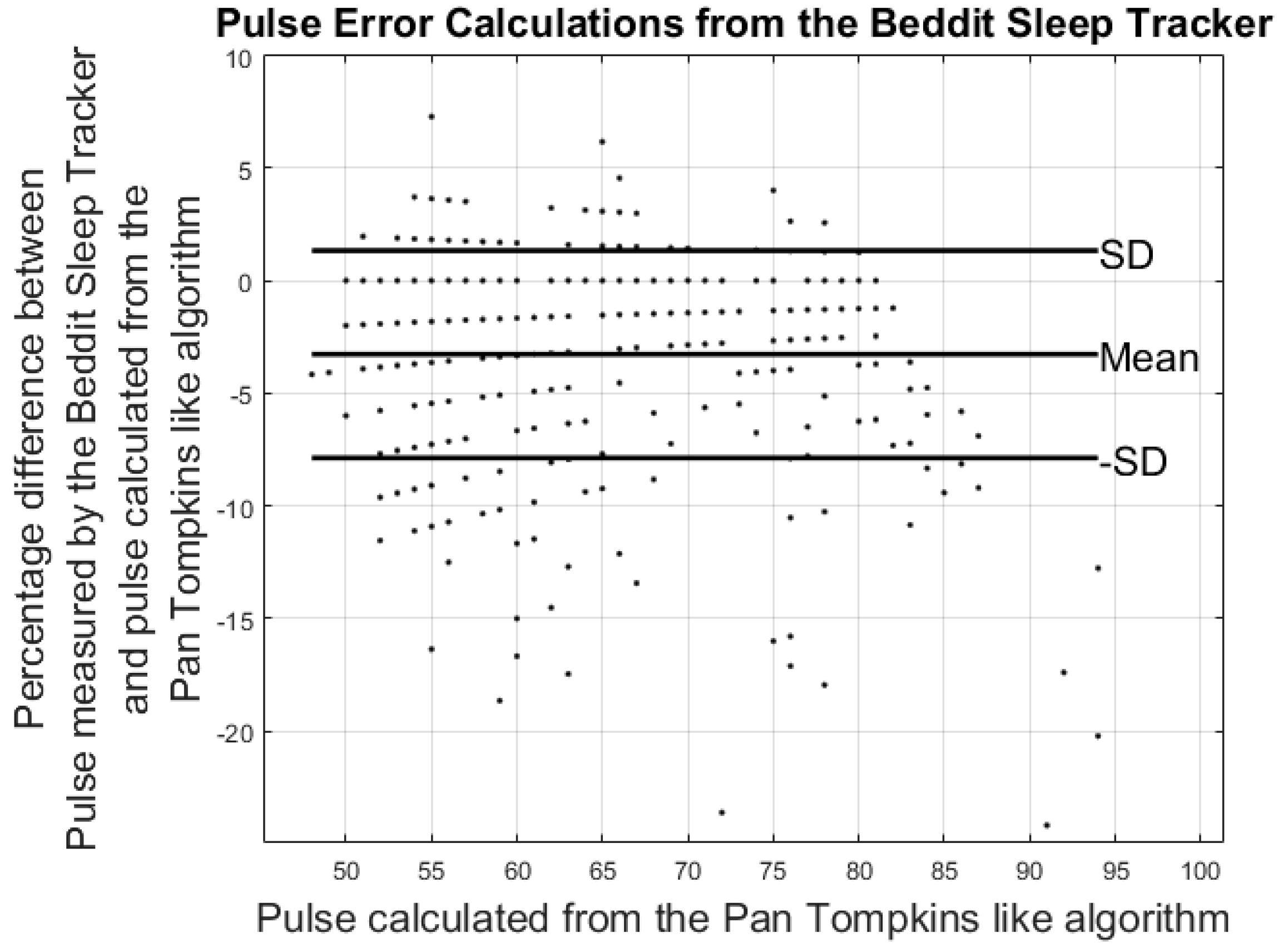
3.2. Results from Heart Rate Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report; US Department of Health and Human Services: Washington, DC, USA, 2008; pp. 155–212.
- Thorup, C.; Hansen, J.; Grønkjær, M.; Andreasen, J.J.; Nielsen, G.; Sørensen, E.E.; Dinesen, B.I. Cardiac Patients’ Walking Activity Determined by a Step Counter in Cardiac Telerehabilitation: Data from the Intervention Arm of a Randomized Controlled Trial. J. Med. Internet Res. 2016, 18, e69. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic Review of the Validity and Reliability of Consumer-Wearable Activity Trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Honko, H.; Andalibi, V.; Aaltonen, T.; Parak, J.; Saaranen, M.; Viik, J.; Korhonen, I. W2E-Wellness Warehouse Engine for Semantic Interoperability of Consumer Health Data. IEEE J. Biomed. Health Inform. 2015, 20, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.P.; Watanabe, S.; Oka, K.; Hiraki, K. Usefulness of Step Counts to Predict Mortality in Japanese Patients With Heart Failure. Am. J. Cardiol. 2013, 111, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.P.; Watanabe, S.; Oka, K.; Hiraki, K.; Morio, Y.; Kasahara, Y.; Osada, N.; Omiya, K.; Shimizu, H. Association between Mental Health and Physical Activity in Patients with Chronic Heart Failure. Disabil. Rehabil. 2014, 36, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Wyatt, H.R.; Thompson, H.; Peters, J.C.; James, O. Pedometer-Measured Physical Activity and Health Behaviors in United States Adults. Med. Sci. Sport Exerc. 2011, 42, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Andalibi, V.; Honko, H.; Christophe, F.; Viik, J. Data Correction for Seven Activity Trackers Based on Regression Models. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 15, 1592–1595. [Google Scholar]
- Storm, F.A.; Heller, B.W.; Mazzà, C. Step Detection and Activity Recognition Accuracy of Seven Physical Activity Monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, J.; Muñoz, A.; Luis Sevillano, J.; Civit, A. The Usefulness of Activity Trackers in Elderly with Reduced Mobility: A Case Study. Stud. Health Technol. Inform. 2013, 192, 759–762. [Google Scholar] [PubMed]
- Harrison, S.L.; Horton, E.J.; Smith, R.; Sandland, C.J.; Steiner, M.C.; Morgan, M.D.L.; Singh, S.J. Physical Activity Monitoring: Addressing the Difficulties of Accurately Detecting Slow Walking Speeds. Heart Lung J. Acute Crit. Care 2013, 42, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Van Remoortel, H.; Giavedoni, S.; Raste, Y.; Burtin, C.; Louvaris, Z.; Gimeno-Santos, E.; Langer, D.; Glendenning, A.; Hopkinson, N.S.; Vogiatzis, I.; et al. Validity of Activity Monitors in Health and Chronic Disease: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Mercer, K.; Giangregorio, L.; Schneider, E.; Chilana, P.; Li, M.; Grindrod, K. Acceptance of Commercially Available Wearable Activity Trackers among Adults Aged over 50 and with Chronic Illness: A Mixed-Methods Evaluation. JMIR mHealth uHealth 2016, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.A.; Mcbride, C.; Heron, L.; Hunter, R.F. The Validation of Fibit Zip™ Physical Activity Monitor as a Measure of Free-living Physical Activity. BMC Res. Notes 2014, 7, 952. [Google Scholar] [CrossRef] [PubMed]
- An, H.-S.; Jones, G.C.; Kang, S.-K.; Welk, G.J.; Lee, J.-M. How Valid are Wearable Physical Activity Trackers for Measuring Steps? Eur. J. Sport Sci. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leicht, A.S.; Crowther, R.G. Pedometer Accuracy During Walking over Different Surfaces. Med. Sci. Sports Exerc. 2007, 39, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, J.; Yu, B.; Shull, P.B. Validity of FitBit, Jawbone UP, Nike+ and other Wearable Devices for Level and Stair Walking. Gait Posture 2016, 48, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and Validation of an Integrated Diagnostic Algorithm Derived from Prameters Monitored in Implantable Devices for Identifying Patients at Risk for Heart Failure Hospitalization in an Ambulatory Setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Photoplethysmography-based Heart Rate Monitoring in Physical Sctivities via Joint Sparse Spectrum Reconstruction. IEEE Trans. Biomed. Eng. 2015, 62, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.E.; An, H.-S.; Dinkel, D.M.; Noble, J.M.; Lee, J.-M. How Accurate are the Wrist-based Heart Rate Monitors during Walking and Running Activities? Are they Accurate Enough? BMJ Open Sport Exerc. Med. 2016, 2, e000106. [Google Scholar] [CrossRef] [PubMed]
- Inglis, S.C. Telemonitoring in Heart Failure: Fact, Fiction, and Controversy. Dove Press J. Smart Homecare Technol. Telehealth 2015, 3, 129–137. [Google Scholar] [CrossRef]
- Hickey, A.M.; Freedson, P.S. Utility of Consumer Physical Activity Trackers as an Intervention Tool in Cardiovascular Disease Prevention and Treatment. Prog. Cardiovasc. Dis. 2016, 58, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Pulignano, G.; Del Sindaco, D.; Di Lenarda, A.; Alunni, G.; Senni, M.; Tarantini, L.; Cioffi, G.; Tinti, M.D.; Barbati, G.; Minardi, G.; et al. Incremental Value of Gait Speed in Predicting Prognosis of Older Adults With Heart Failure. Insights From the IMAGE-HF Study. JACC Heart Fail. 2015, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.A.; Eng, J.J.; Klassen, T.D.; Lim, S.B.; Louie, D.R.; Parappilly, B.; Sakakibara, B.M.; Zbogar, D. Capturing Step Counts at Slow Walking Speeds in Older Adults: Comparison of Ankle and Waist Placement of Measuring Device. J. Rehabil. Med. 2015, 47, 830–835. [Google Scholar] [CrossRef]
- Ferguson, T.; Rowlands, A.V.; Olds, T.; Maher, C. The Validity of Consumer-level, Activity Monitors in Healthy Adults Worn in Free-Living Conditions: A Cross-Sectional Study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Kooiman, T.J.M.; Dontje, M.L.; Sprenger, S.R.; Krijnen, W.P.; van der Schans, C.P.; de Groot, M. Reliability and Validity of Ten Consumer Activity Trackers. BMC Sports Sci. Med. Rehabil. 2015, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Beevi, F.H.A.; Miranda, J.; Pedersen, C.F.; Wagner, S. An Evaluation of Commercial Pedometers for Monitoring Slow Walking Speed Populations. Telemed. e-Health 2015, 22, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Lewis, K.; Directo, D.; Kim, M.J.Y.; Dolezal, B.A. Validation of Biofeedback Wearables for Photoplethysmographic Heart Rate Tracking. J. Sport. Sci. Med. 2016, 15, 540–547. [Google Scholar]
- Albert, N.M.; Dinesen, B.; Spindler, H.; Southard, J.; Bena, J.F.; Catz, S.; Kim, T.Y.; Nielsen, G.; Tong, K.; Nesbitt, T.S. Factors Associated with Telemonitoring use Among Patients with Chronic Heart Failure. J. Telemed. Telecare 2016, 1–9. [Google Scholar] [CrossRef]
| Garmin Vivofit 2 | Fitbit Charge HR | Fitbit One | Fitbit Zip | Beddit Sleep Tracker | |
|---|---|---|---|---|---|
| Firmware | 3.30 | 122 | 60 | 86 | 2.0.1(103) |
| Device validated on step detection | ✔ | ✔ | ✔ | ✔ | |
| Devices validated on pulse | ✔ | ✔ | |||
| Placement | Wrist | Wrist | Hip | Hip | In Bed |
| Garmin Vivofit 2 | Fitbit Charge HR | Fitbit One | Fitbit Zip | |
|---|---|---|---|---|
| 2 km walking speed | −5.3% ± 30.4% | 26.8% ± 32.0% | −7.8% ± 10.5% | −22.9% ± 33.3% |
| 3.5 km walking speed | −0.2% ± 14.2% | −0.7% ± 7.5% | −1.5% ± 0.9% | −1.1% ± 5.8% |
| Fitbit Charge HR vs. ECG-Pulse | Beddit vs. ECG-Pulse | Fitbit Charge HR vs. Beddit (Nighttime) | |
|---|---|---|---|
| Pulse Error%: | −3.42% ± 7.99% | −3.27% ± 4.60% | 1.49% ± 4.08% |
| Hours of data analyzed | 88 h | 36 h | 42 h |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leth, S.; Hansen, J.; Nielsen, O.W.; Dinesen, B. Evaluation of Commercial Self-Monitoring Devices for Clinical Purposes: Results from the Future Patient Trial, Phase I. Sensors 2017, 17, 211. https://doi.org/10.3390/s17010211
Leth S, Hansen J, Nielsen OW, Dinesen B. Evaluation of Commercial Self-Monitoring Devices for Clinical Purposes: Results from the Future Patient Trial, Phase I. Sensors. 2017; 17(1):211. https://doi.org/10.3390/s17010211
Chicago/Turabian StyleLeth, Soren, John Hansen, Olav W. Nielsen, and Birthe Dinesen. 2017. "Evaluation of Commercial Self-Monitoring Devices for Clinical Purposes: Results from the Future Patient Trial, Phase I" Sensors 17, no. 1: 211. https://doi.org/10.3390/s17010211
APA StyleLeth, S., Hansen, J., Nielsen, O. W., & Dinesen, B. (2017). Evaluation of Commercial Self-Monitoring Devices for Clinical Purposes: Results from the Future Patient Trial, Phase I. Sensors, 17(1), 211. https://doi.org/10.3390/s17010211






