Application of Carbon-Microsphere-Modified Electrodes for Electrochemistry of Hemoglobin and Electrocatalytic Sensing of Trichloroacetic Acid
Abstract
:1. Introduction
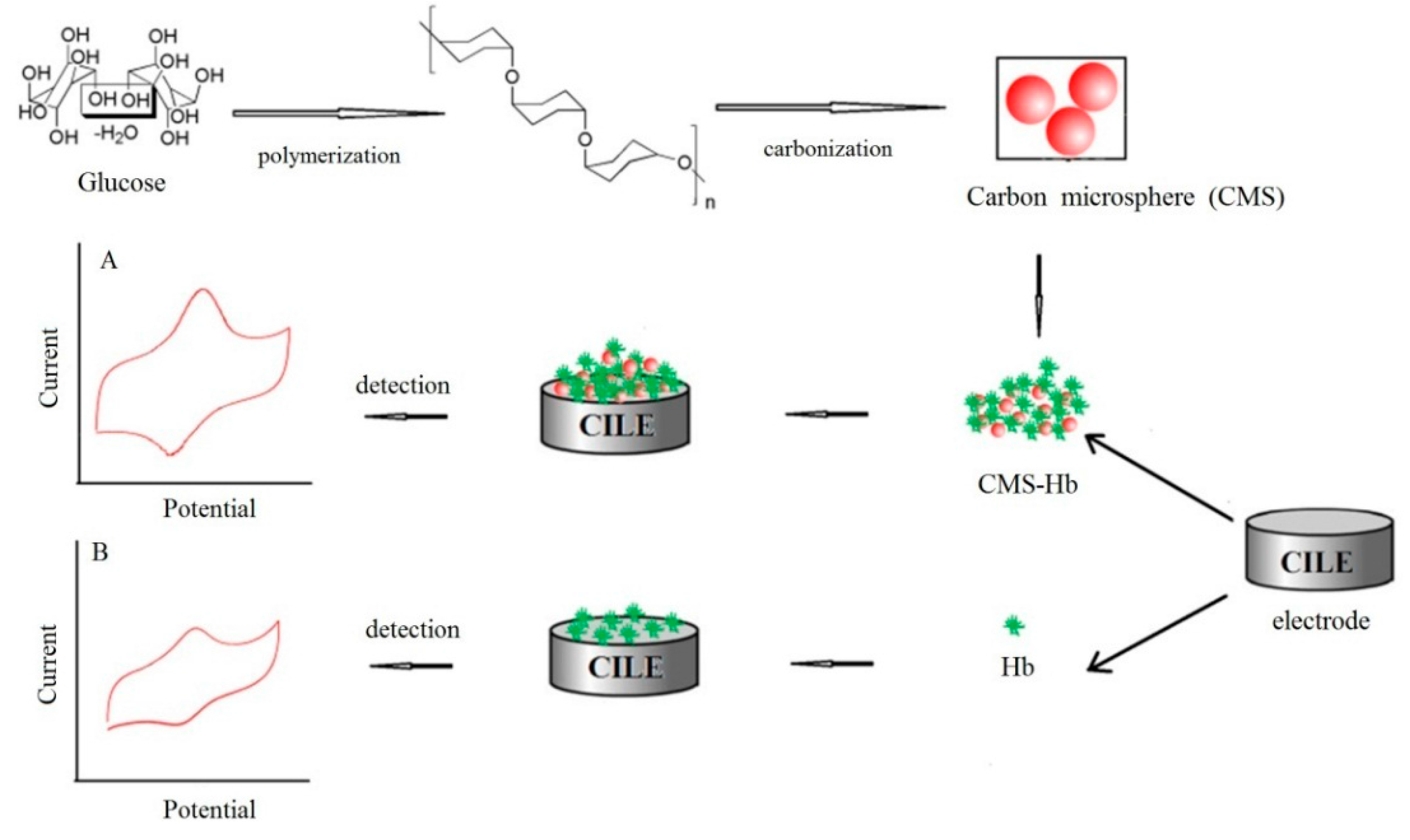
2. Experimental
2.1. Reagents
2.2. Apparatus
2.3. Synthesis of CMS
2.4. Electrode Fabrication
3. Results and Discussion
3.1. Morphological and Structural Characterization

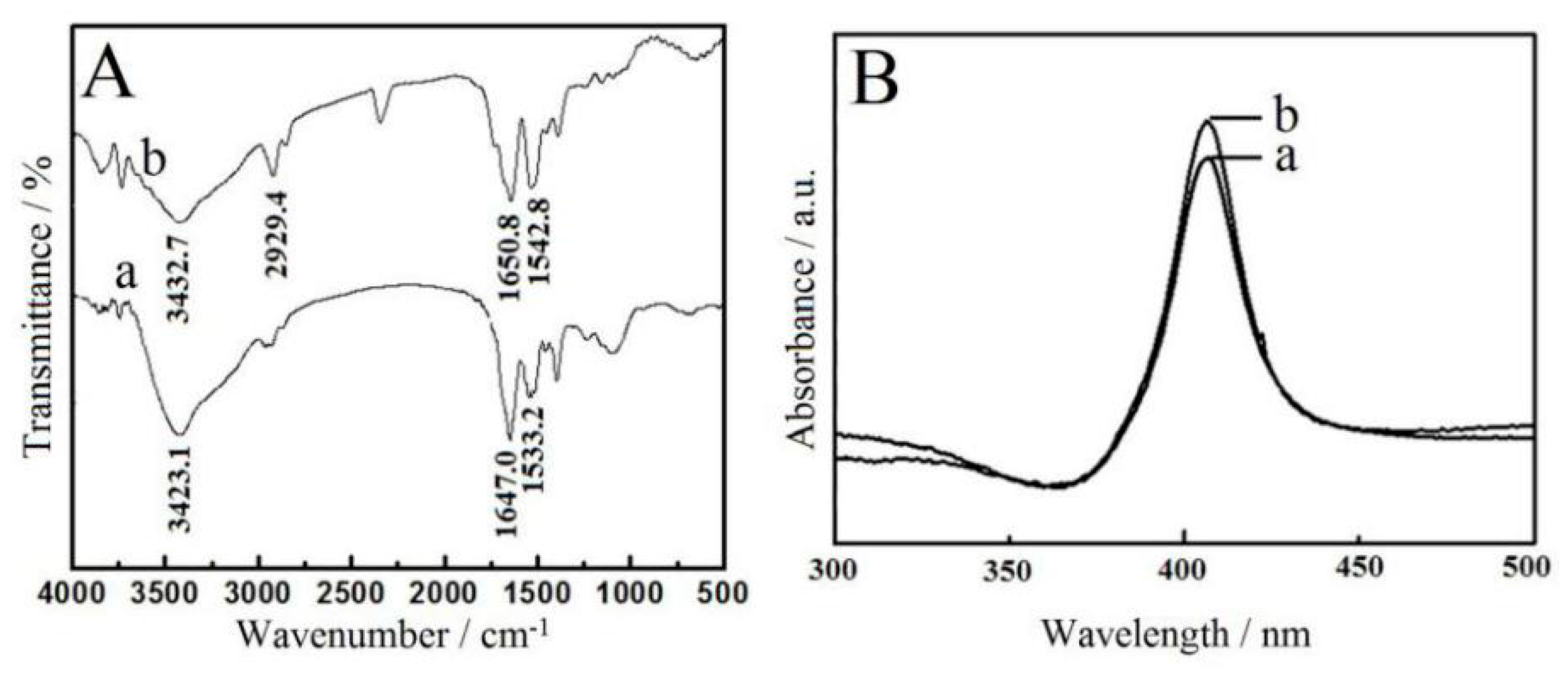
3.2. Spectroscopic Results
3.3. Electrochemical Characterization
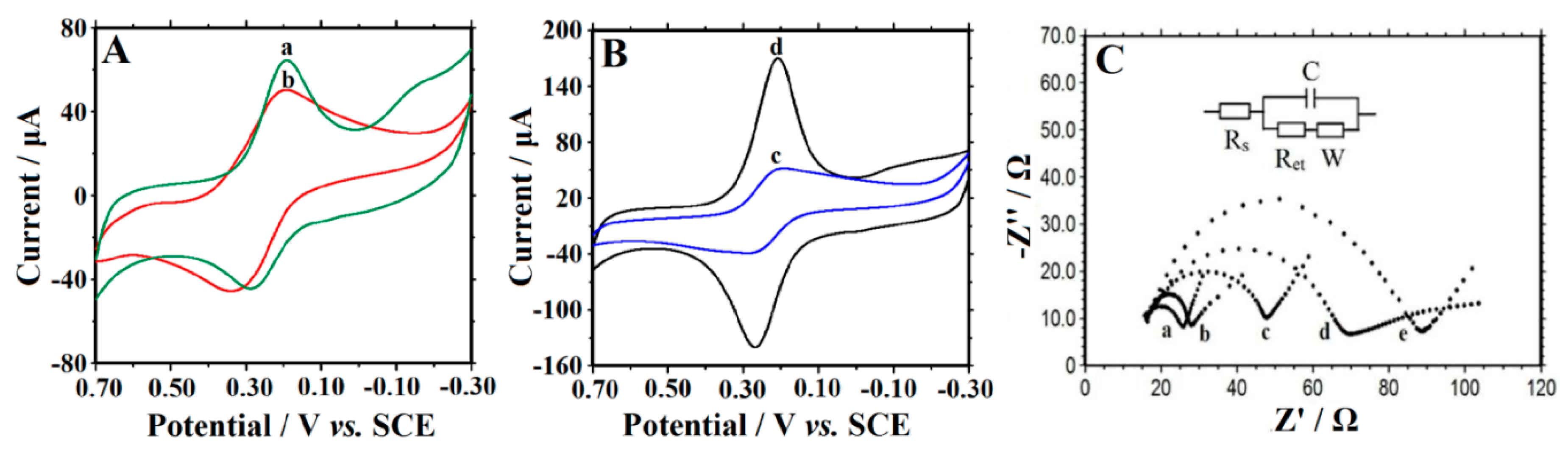
3.4. Direct Electrochemistry of Hb
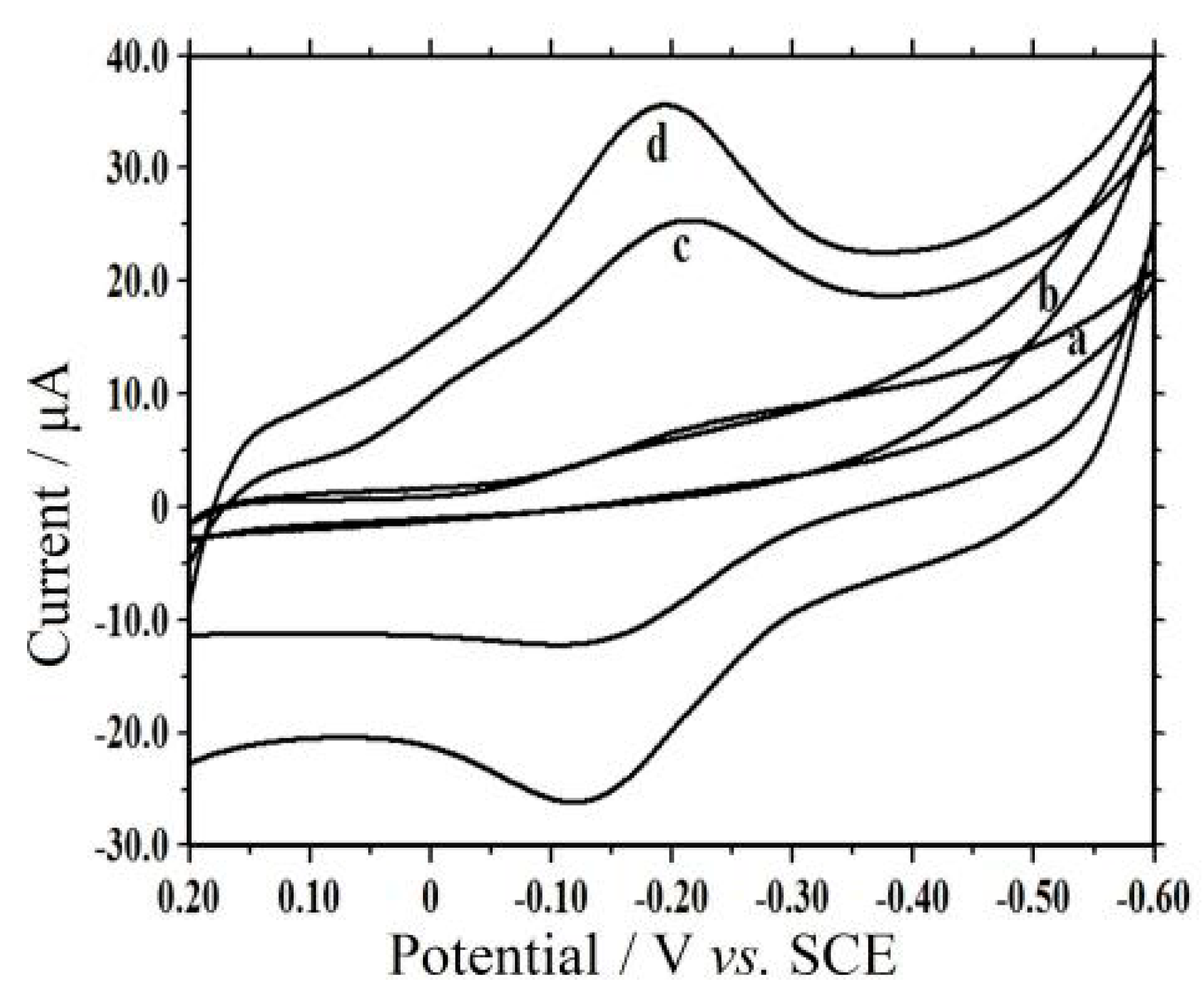
3.5. Electrochemical Investigation
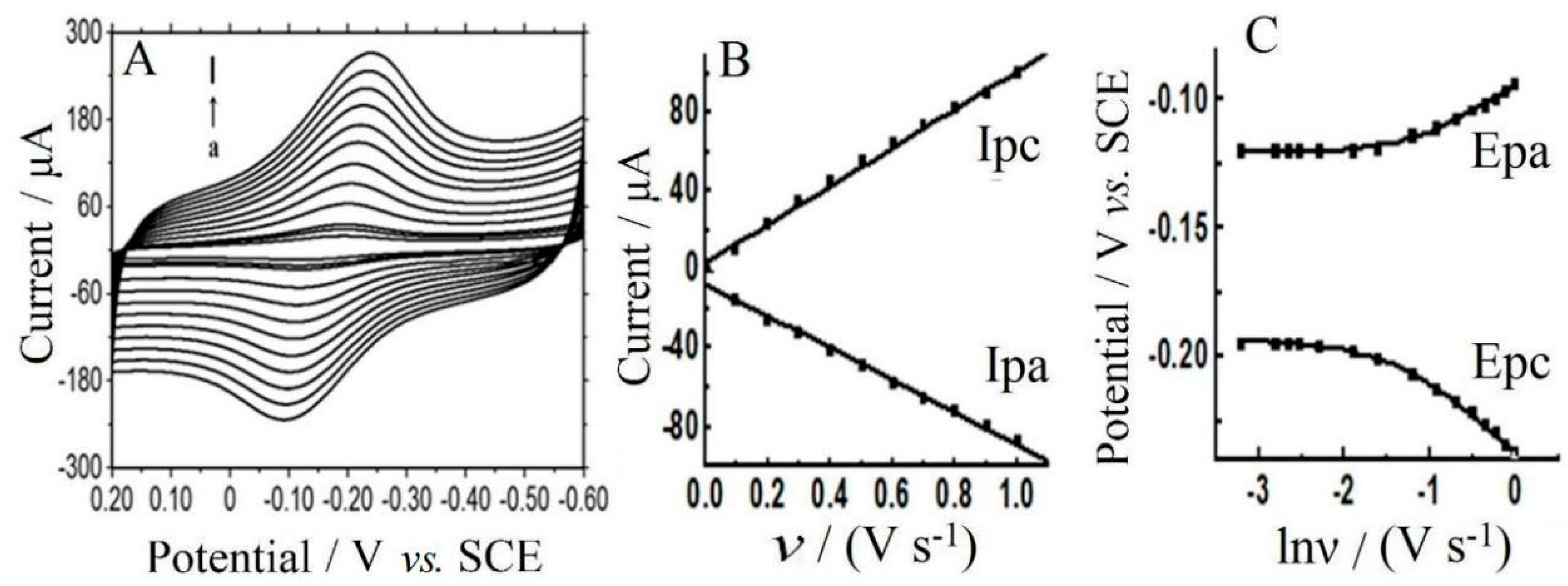
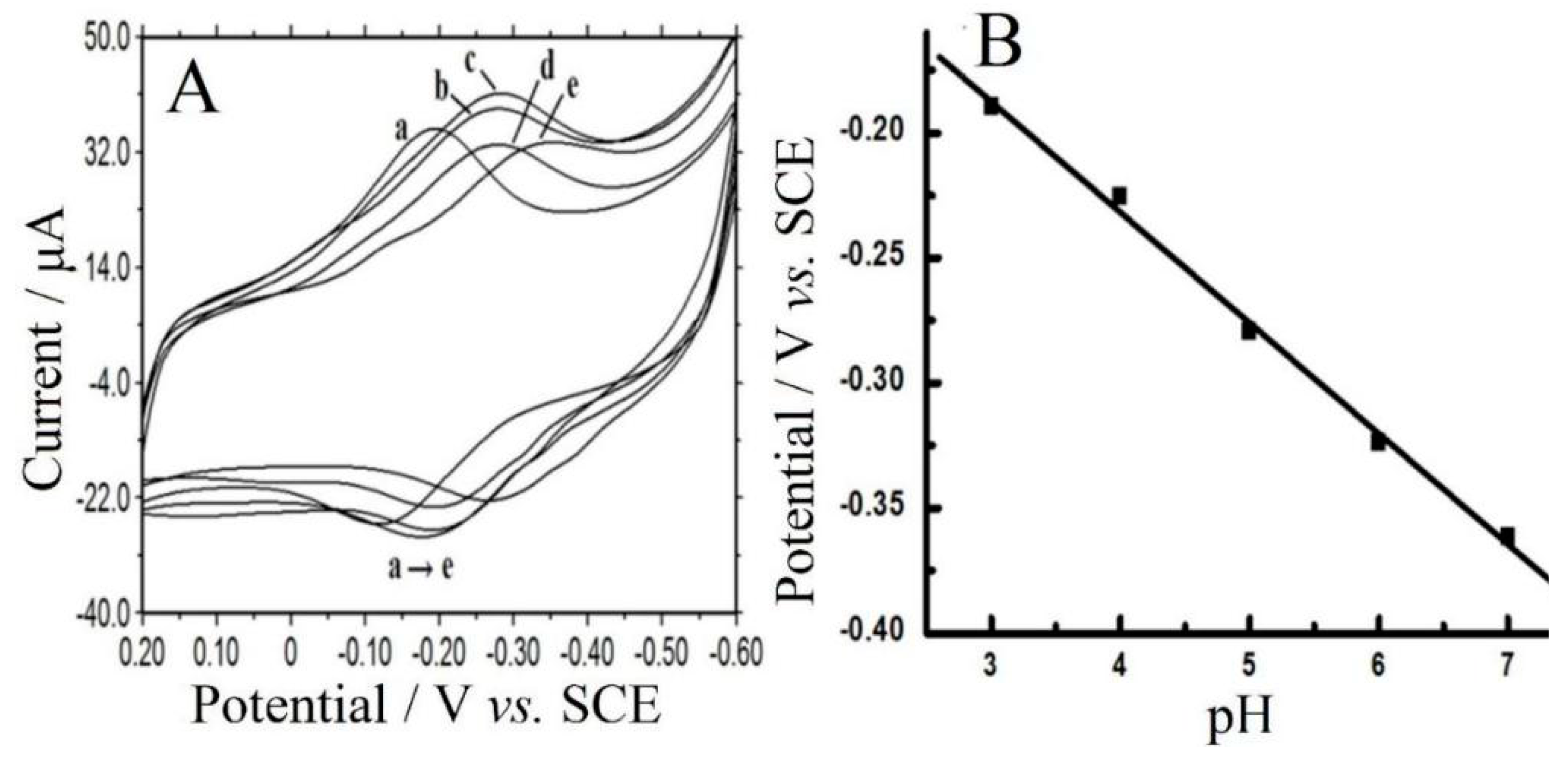
3.6. Electrocatalysis
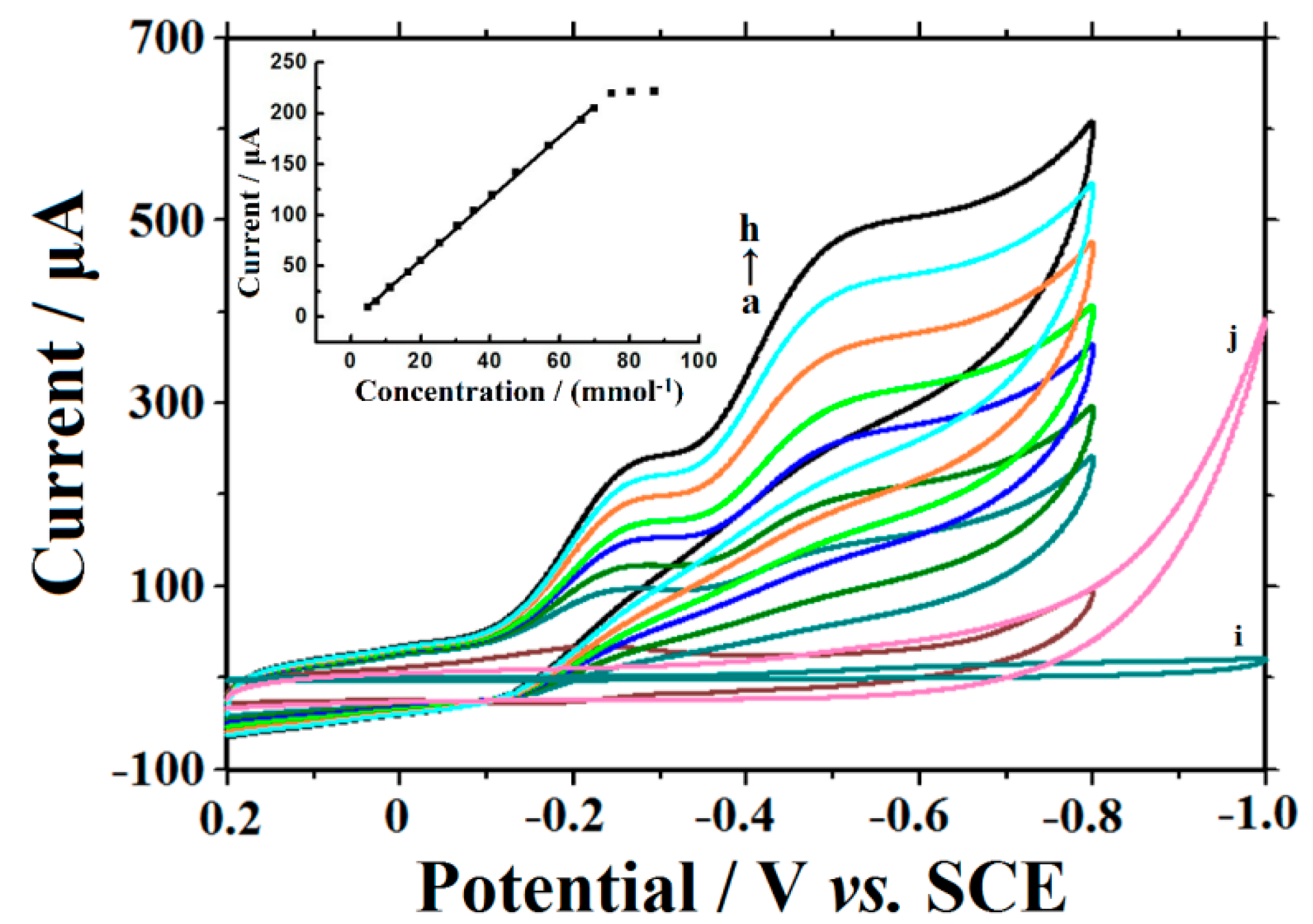
3.7. Analytical Application
| Specimen | Found (mmol·L−1) | Added (mmol·L−1) | Total (mmol·L−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| water | 0 | 2.00 | 2.08 | 104.00 | 3.06 |
| 4.00 | 3.88 | 97.00 | 3.10 | ||
| 6.00 | 6.12 | 102.00 | 2.98 |
3.8. Stability and Reproducibility
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Sigillum | Full Caption | Sigillum | Full Caption |
| BPPF6 | 1-butylpyridinium hexafluorophosphate | HOAC | acetic acid |
| CILE | carbon ionic liquid electrode | IL | ionic liquid |
| CPE | carbon paste electrode | Ipa | anodic peak current |
| CMS | carbon microsphere | Ipc | cathodic peak current |
| CTS | chitosan | KMapp | apparent michaelis-menten constant |
| CV | cyclic voltammograms | MWCNT | multi-walled carbon nanotube |
| EIS | electrochemical impedance spectroscopy | PBS | phosphate buffer solutions |
| E0′ | formal peak potential | Ret | the electron transfer resistance |
| Epa | anodic peak potential | RSD | the relative standard deviation |
| Epc | cathodic peak potential | SEM | scanning electron microscopy |
| GOD | glucose oxidase | TCA | trichloroacetic acid |
| GR | graphene | TEM | transmission electron microscopy |
| GCE | glass carbon electrode | XRD | X-ray diffraction |
| Hb | hemoglobin | Γ* | surface coverage |
References
- Armstrong, F.A.; Hill, H.A.O.; Walton, N.J. Direct electrochemistry of redox proteins. Acc. Chem. Res. 1988, 21, 407–413. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ramanaviciene, A. Hemoproteins in design of biofuel cells. Fuel Cells 2009, 1, 25–36. [Google Scholar] [CrossRef]
- Armstrong, F.A.; Wilson, G.S. Recent developments in faradaic bioelectrochemistry. Electrochim. Acta 2000, 45, 2623–2645. [Google Scholar] [CrossRef]
- Prakash, P.A.; Yogeswaran, U.; Chen, S.M. A review on direct electrochemistry of catalase for electrochemical sensors. Sensors 2009, 9, 1821–1844. [Google Scholar] [CrossRef] [PubMed]
- Blanford, C.F. The birth of protein electrochemistry. Chem. Commun. 2013, 49, 11130–11132. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devadas, B.; Chen, S.M.; Li, Y. Immobilization of enzymes and redox proteins and their electrochemical biosensor applications. ECS Trans. 2013, 50, 35–41. [Google Scholar] [CrossRef]
- Sun, W.; Guo, C.X.; Zhu, Z.H.; Li, C.M. Ionic liquid/mesoporous carbon/protein composite microelectrode and its biosensing application. Electrochem. Commun. 2009, 11, 2105–2108. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Direct electrochemistry of cytochrome c at a glassy carbon electrode modified with single-wall carbon nanotubes. Anal. Chem. 2002, 74, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Li, M.G.; Xu, S.D.; Tang, M.; Liu, L.; Gao, F.; Wang, Y.L. Direct electrochemistry of horseradish peroxidase on graphene-modified electrode for electrocatalytic reduction towards H2O2. Electrochim. Acta 2011, 56, 1144–1149. [Google Scholar] [CrossRef]
- Kang, X.H.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y.H. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Wang, L.Q.; Bae, I.T.; Arey, B.W.; Exarhos, G.J. Hydrothermal syntheses of colloidal carbon spheres from cyclodextrins. J. Phys. Chem. C 2008, 112, 14236–14240. [Google Scholar] [CrossRef]
- Sun, X.M.; Li, Y.D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xia, N.N.; Zhou, T.X.; Tan, S.X.; Jiang, F.P.; Yuan, D.S. Mesoporous carbon spheres: Synthesis, characterization and supercapacitance. Int. J. Electrochem. Sci. 2009, 4, 1063–1073. [Google Scholar]
- Jin, Y.Z.; Gao, C.; Hsu, W.K.; Zhu, Y.Q.; Huczko, A.; Bystrzejewski, M.; Roe, M.; Lee, C.Y.; Acquah, S.; Kroto, H.; et al. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 43, 1944–1953. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Wang, Y.; Su, F.; Wood, C.D.; Lee, J.Y.; Zhao, X.S. Preparation and characterization of carbon nanospheres as anode materials in lithium-ion secondary batteries. Ind. Eng. Chem. Res. 2008, 47, 2294–2300. [Google Scholar] [CrossRef]
- Yan, X.B.; Xu, T.; Xu, S.; Chen, G.; Liu, H.W.; Yang, S.R. Fabrication of carbon spheres on a C: H films by heat-treatment of a polymer precursor. Carbon 2004, 42, 2769–2771. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Torriero, A.A.J. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gao, R.F.; Jiao, K. Research and application of ionic liquids in analytical chemistry. Chin. J. Anal. Chem. 2007, 35, 1813–1819. [Google Scholar]
- Sheng, M.L.; Gao, Y.; Sun, J.Y.; Gao, F. Carbon nanodots–chitosan composite film: a platform for protein immobilization, direct electrochemistry and bioelectrocatalysis. Biosens. Bioelectron. 2014, 58, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, M.X.; Jiao, K. Electrocatalytic oxidation of dopamine at an ionic liquid modified carbon paste electrode and its analytical application. Anal. Bioanal. Chem. 2007, 389, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, B.X.; Zheng, W.; Xia, W.; Guang, H. Photo-responsive behaviors and structural evolution of carbon-nanotube-supported energetic materials under a photoflash. Mater. Lett. 2012, 88, 27–29. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, J.K.; Moffat, D.J.; Mantsch, H.H.; Cameron, D.G. Fourier self-deconvolution: a method for resolving intrinsically overlapped ban. Appl. Spectrosc. 1981, 35, 271–276. [Google Scholar] [CrossRef]
- Rusling, J.F.; Nassar, A.E.F. Enhanced electron transfer for myoglobin in surfactant films on electrodes. J. Am. Chem. Soc. 1993, 115, 11891–11897. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. An integrated approach to electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 7360–7366. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, T.; Zhang, Z.L.; Shen, X.C.; Lu, Z.X.; Pang, D.W.; Wong, K.Y. Direct electrochemistry and electrocatalysis of heme proteins entrapped in agarose hydrogel films in room-temperature ionic liquids. Langmuir 2005, 21, 9260–9266. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Sun, W.; Gong, S.X.; Shi, F.; Cao, L.L.; Ling, L.Y.; Zheng, W.Z.; Wang, W.C. Direct electrochemistry and electrocatalysis of hemoglobin in graphene oxide and ionic liquid composite film. Mater. Sci. Eng. C 2014, 40, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhang, H.J.; Yao, D.; Pu, J.J.; Zhang, Y.; Gao, X.R.; Sun, Y.M. Direct electrochemistry of hemoglobin on graphene/Fe3O4 nanocomposite-modified glass carbon electrode and its sensitive detection for hydrogen peroxide. J. Solid State Electrochem. 2013, 17, 881–887. [Google Scholar] [CrossRef]
- Sun, W.; Guo, Y.Q.; Ju, X.M.; Zhang, Y.Y.; Wang, X.Z.; Sun, Z.F. Direct electrochemistry of hemoglobin on graphene and titanium dioxide nanorods composite modified electrode and its electrocatalysis. Biosens. Bioelectron. 2013, 42, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jin, H.H.; Zhao, G.C. A reagentless nitrite biosensor based on direct electron transfer of hemoglobin on a room temperature ionic liquid/carbon nanotube-modified electrode. Microchim. Acta 2009, 164, 167–171. [Google Scholar] [CrossRef]
- Yamazaki, I.; Araiso, T.; Hayashi, Y.; Yamada, H.; Makino, R. Analysis of acid-base properties of peroxidase and myoglobin. Adv. Biophys. 1978, 11, 249–281. [Google Scholar] [PubMed]
- Liu, H.H.; Tian, Z.Q.; Lu, Z.X.; Zhang, Z.L.; Zhang, M.; Pang, D.W. Direct electrochemistry and electrocatalysis of heme-proteins entrapped in agarose hydrogel films. Biosens. Bioelectron. 2004, 20, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Zhuang, Y.; Li, G.X.; Zhu, J.Q.; Zhu, D.X. Direct electrochemistry and enhanced catalytic activity for hemoglobin in a sodium montmorillonite film. Electroanal 2000, 12, 1156–1158. [Google Scholar] [CrossRef]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.Q.; Wang, Y.; Zhao, R.J.; Jiao, K. Electrochemistry and electrocatalysis of hemoglobin on multi-walled carbon nanotubes modified carbon ionic liquid electrode with hydrophilic EMIMBF4 as modifier. Electrochim. Acta 2009, 54, 4141–4148. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wu, J.; Sun, W.; Jiao, K. Direct electrochemistry of hemoglobin and its electrocatalysis based on a carbon nanotube paste electrode. J. Chin. Chem. Soc. 2009, 56, 561–567. [Google Scholar]
- Sun, W.; Guo, Y.Q.; Lu, Y.P.; Hu, A.H.; Shi, F.; Li, T.T.; Sun, Z.F. Electrochemical biosensor based on graphene, Mg2Al layered double hydroxide and hemoglobin composite. Electrochim. Acta 2013, 91, 130–136. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-C.; Yan, L.-J.; Shi, F.; Niu, X.-L.; Huang, G.-L.; Zheng, C.-J.; Sun, W. Application of Carbon-Microsphere-Modified Electrodes for Electrochemistry of Hemoglobin and Electrocatalytic Sensing of Trichloroacetic Acid. Sensors 2016, 16, 6. https://doi.org/10.3390/s16010006
Wang W-C, Yan L-J, Shi F, Niu X-L, Huang G-L, Zheng C-J, Sun W. Application of Carbon-Microsphere-Modified Electrodes for Electrochemistry of Hemoglobin and Electrocatalytic Sensing of Trichloroacetic Acid. Sensors. 2016; 16(1):6. https://doi.org/10.3390/s16010006
Chicago/Turabian StyleWang, Wen-Cheng, Li-Jun Yan, Fan Shi, Xue-Liang Niu, Guo-Lei Huang, Cai-Juan Zheng, and Wei Sun. 2016. "Application of Carbon-Microsphere-Modified Electrodes for Electrochemistry of Hemoglobin and Electrocatalytic Sensing of Trichloroacetic Acid" Sensors 16, no. 1: 6. https://doi.org/10.3390/s16010006
APA StyleWang, W.-C., Yan, L.-J., Shi, F., Niu, X.-L., Huang, G.-L., Zheng, C.-J., & Sun, W. (2016). Application of Carbon-Microsphere-Modified Electrodes for Electrochemistry of Hemoglobin and Electrocatalytic Sensing of Trichloroacetic Acid. Sensors, 16(1), 6. https://doi.org/10.3390/s16010006






