Evaluation of Eutrophication in Small Reservoirs in Northern Agricultural Areas of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Identification
2.3. Water Sample Collection and Determination of Environmental Factors
2.4. Data Statistical Analysis
3. Results
3.1. Analysis of Environmental Factors
3.2. Composition of Zooplankton Species
3.3. Standing Stock of Zooplankton
3.4. Structure Characteristics of Zooplankton Communities
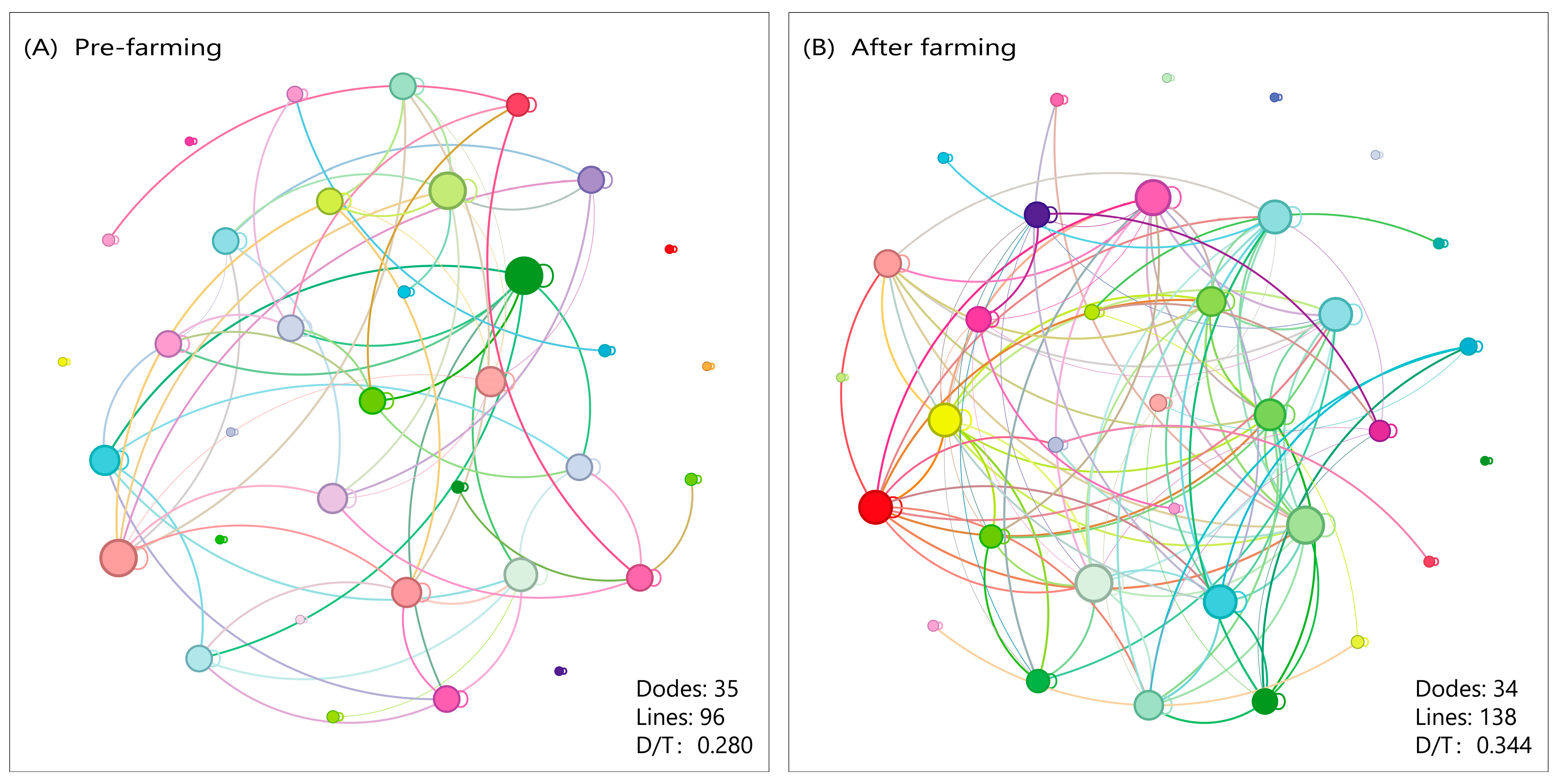
3.5. Stability of Zooplankton Community
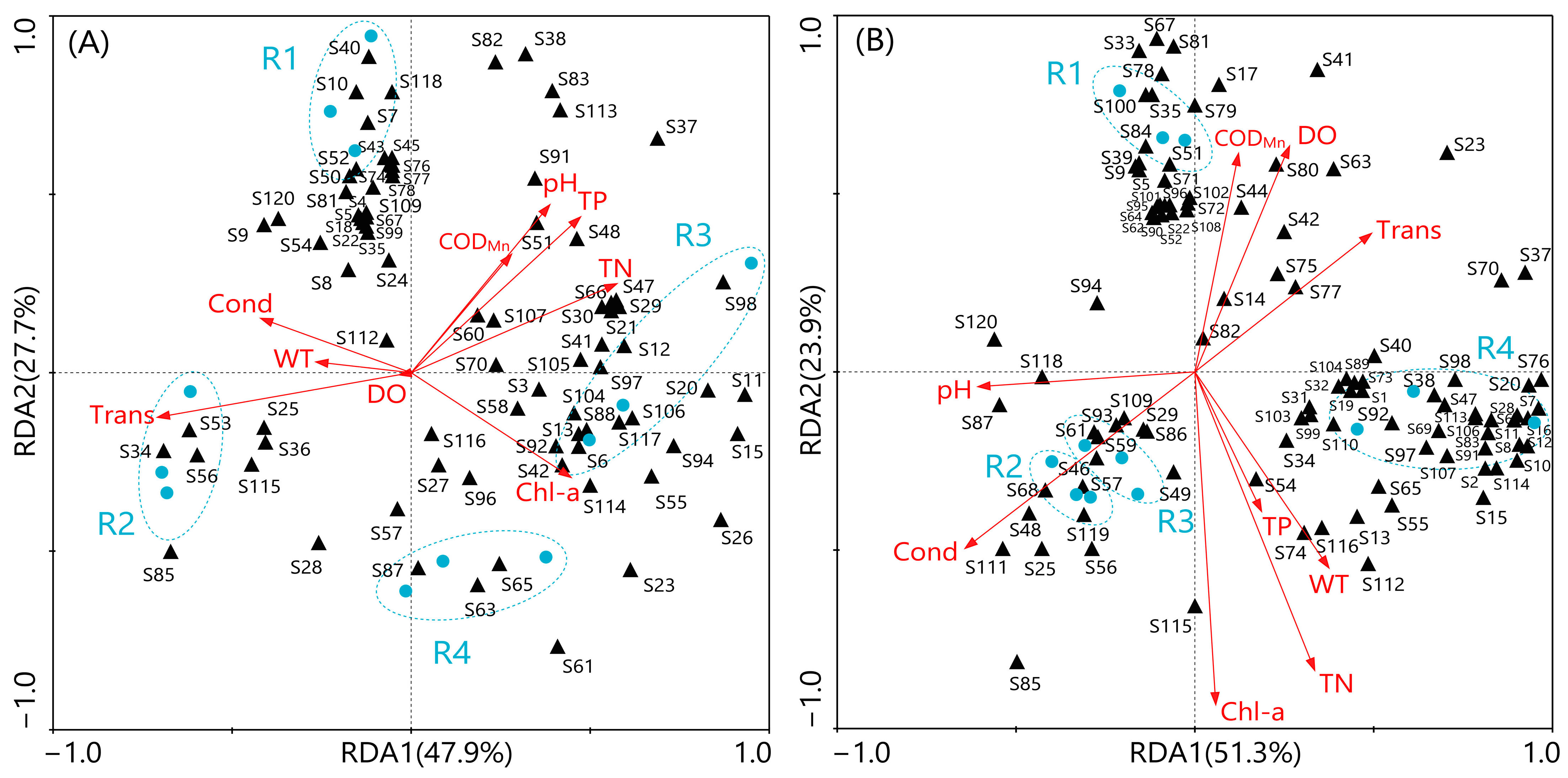
3.6. Relationship Between Zooplankton and Environmental Factors
3.7. Evaluation of Water Body Eutrophication
4. Discussion
4.1. The Structure and Stability of the Zooplankton Community
4.2. Relationship Between Zooplankton Community Structure and Environmental Factors
4.3. Analysis of Eutrophication of Small Reservoir Water Bodies and the Driving Factors
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, L.G. Research on Innovative Strategies for Water Resource Management and Protection in Reservoir Construction and Operation. Water Sci. Technol. 2024, 7, 22–24. [Google Scholar]
- Liao, C.W.; Chen, X.D. Modern Management Framework for Small Reservoirs. China Sci. Tech. Inf. 2022, 02, 112–114. [Google Scholar]
- Makri, S.; Lami, A.; Lods-Crozet, B.; Loizeau, J.L. Reconstruction of trophic state shifts over the past 90 years in a eutrophicated lake in western Switzerland, inferred from the sedimentary record of photosynthetic pigments. J. Paleolimnol. 2019, 61, 129–145. [Google Scholar] [CrossRef]
- Wildemeersch, M.; Tang, S.H.; Ermolieva, T.; Ermoliev, Y.; Rovenskaya, E.; Obersteiner, M. Containing the Risk of Phosphorus Pollution in Agricultural Watersheds. Sustainability 2022, 14, 1717. [Google Scholar] [CrossRef]
- Lawniczak-Malińska, A.; Nowak, B.; Pajewski, K. Agricultural pressures on the quality of ground and surface waters in catchments of artificial reservoirs. Water 2023, 15, 661. [Google Scholar] [CrossRef]
- Allaf, M.M.; Erratt, K.J. Navigating aquaculture losses: Tackling fish-killing phytoflagellates in a changing global landscape. Rev. Aquac. 2024, 16, 2023–2033. [Google Scholar] [CrossRef]
- Guo, J.Y.; Wang, C.F.; Lin, L.; Dong, L.; Gao, Y.; Pan, X.; Guo, Z.W.; Wang, Z.R. Distribution Patterns and Influencing Factors of Nitrogen and Phosphorus Nutrients in the Danjiangkou Reservoir. Environ. Sci. Technol. 2025. Available online: http://kns.cnki.net/kcms/detail/42.1245.X.20250515.1431.002.html (accessed on 10 July 2025).
- Guan, Y.Q. Effects of eutrophication and socioeconomic development on water quality in Taizihe River of Liaoyang City. Water Resour. Hydropower Northeast China 2023, 41, 26–30+65. [Google Scholar]
- Lukhele, T.; Msagati, T.A.M. Eutrophication of Inland Surface Waters in South Africa: An Overview. Int. J. Environ. Res. 2024, 18, 27. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Qi, Y.T.; Cao, X.; Cao, R.S.; Cao, M.J.; Yan, A.L.; Li, E.; Xu, D. Research on the Analysis of and Countermeasures for the Eutrophication of Water Bodies: Waihu Reservoir as a Case Study. Processes 2024, 12, 796. [Google Scholar] [CrossRef]
- Xiao, X.C.; Xie, D.T.; Ni, J.P. Coupling state of agricultural eco-economic system under emission mitigation and sink enhancement of non-point source pollution—A case study of Zhong County in the Three Gorges Reservoir Region. Chin. J. Eco. Agric. 2014, 22, 111–119. [Google Scholar] [CrossRef]
- Tomczyk, P.; Wierzchowski, P.S.; Dobrzynski, J.; Kulkova, I.; Wróbel, B.; Wiatkowski, M.; Kuriqi, A.; Skorulski, W.; Kabat, T.; Prycik, M.; et al. Effective Microorganism Water Treatment Method for Rapid Eutrophic Reservoir Restoration. Environ. Sci. Pollut. Res. 2024, 31, 2377–2393. [Google Scholar] [CrossRef]
- Wang, Z.Z.; He, S.; Chen, L. Characteristics of Planktonic Flora and Fauna and Eutrophication Evaluation of Xiaolangdi Reservoir. Henan Water Resour. South-North Water Divers. 2023, 52, 139–140. [Google Scholar]
- Liu, X.; Huang, W.; Qi, Z.X.; Li, C.B.; Cheng, G.H.; Cai, Y. Seasonal water storage of large reservoirs exacerbates eutrophication risk in the fluctuating backwater zone: A case study of Three Gorges Reservoir, China. Ecol. Indic. 2025, 171, 113100. [Google Scholar] [CrossRef]
- Xu, Y.B.; Guo, A.H.; He, W.F.; Yuan, J.L.; Li, M. Zooplankton community structure in relation to environmental factors in the Shanghetang reservoir. Hubei Agric. Sci. 2020, 59, 69–74. [Google Scholar]
- Chen, M.J.; Chen, F.Z. Water quality evaluation and eutrophication analysis of small reservoirs in Nanjing. Environ. Prot. Sci. 2020, 46, 87–91. [Google Scholar]
- Gu, X.H.; Mao, Z.G.; Ding, H.P.; Wang, Y.P.; Zeng, Q.F.; Wang, L.L. Progress and prospect of lake fishery. J. Lake Sci. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Burkholder, J.A.M.; Kinder, C.A.; Allen, E.H. Watershed development and eutrophying potable source-water reservoirs in a warming temperate/subtropical region. Water 2023, 15, 4007. [Google Scholar] [CrossRef]
- Chi, S.Y.; Wei, C.Z.; Hu, J.; Wang, R.; Zhou, L.F.; Hu, J.X. Correlation between macroinvertebrates and plankton in two deep eutrophic reservoirs. J. Lake Sci. 2020, 32, 1060–1075. [Google Scholar] [CrossRef]
- Wang, J.J. Fauna of Freshwater Rotifers in China; Science Press: Beijing, China, 1961. [Google Scholar]
- Li, H.R.; Gu, Y.; Cai, Q.H.; Dong, X.W.; Ye, L. Zooplankton Size Structure in Relation to Environmental Factors in the Xiangxi Bay of Three Gorges Reservoir, China. Front. Ecol. Evol. 2022, 10, 800025. [Google Scholar] [CrossRef]
- Liang, Y.D.; Kuang, Z.; Sun, N.Z.; Gu, J.L.; Xu, D.P. Effects of different ecological floating bed plant assemblages on water purification and zooplankton community structure in shallow eutrophic lakes. J. Dalian Ocean Univ. 2023, 38, 302–310. [Google Scholar]
- Yang, W.; Sun, Y.C.; Zhang, T.T.; Liu, Q.; Huang, Y.; Ge, Q.; Deng, D.G. Impact of eutrophication on the community structure and species diversity of crustacean zooplankton in small lakes. Acta Ecol. Sin. 2020, 40, 4874–4882. [Google Scholar]
- Wang, L.; Chen, J.; Su, H.J.; Ma, X.F.; Wu, Z.X.; Shen, H.; Yu, J.; Liu, J.R.; Wu, Y.; Ding, G.Y.; et al. Is zooplankton body size an indicator of water quality in (sub) tropical reservoirs in China? Ecosystems 2021, 25, 308–319. [Google Scholar] [CrossRef]
- Wei, N.; Xiang, M.; Wang, Q.Y.; Guo, Z.B.; Wu, Z.H.; Lin, G.; Yang, D.G.; Li, X.M. Characteristics of plankton community structures and impact factors in Geheyan Reservoir in autumn. Chin. J. Fish. 2025, 38, 74–81. [Google Scholar]
- Braun, L.M.; Brucet, S.; Mehner, T. Top-down and bottom-up effects on zooplankton size distribution in a deep stratified lake. Aquat. Ecol. 2021, 55, 527–543. [Google Scholar] [CrossRef]
- Tao, J.; Bu, Y.Q.; Shao, X.D.; Cai, K.; Li, Z.; Lü, X.Y.; Ye, W.R.; Ruan, X.J. Seasonal variations in the community structure of post larval zooplankton in the waters surrounding Xishan Island, Taihu Lake. Environ. Monit. Forewarn. 2025, 17, 22–28. [Google Scholar]
- Li, Y.; Chen, F.Z. Are zooplankton useful indicators of water quality in subtropical lakes with high human impacts? Ecol. Indic. 2020, 113, 106167. [Google Scholar] [CrossRef]
- Hu, H.; Wei, X.Y.; Liu, L.; Wang, Y.B.; Bu, L.K.; Jia, H.J.; Pei, D.S. Biogeographic patterns of meio-and micro-eukaryotic communities in dam-induced river-reservoir systems. Appl. Microbiol. Biotechnol. 2024, 108, 130. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, R.R.; Wang, W.; Yin, Y.M.; Liu, Z.P.; Dai, Z.; Li, M.H.; Yan, Q.L. Study on the changes in the community structure of marine zooplankton in Dalian Bay from 2013 to 2022 and its relationship with environmental factors. Mar. Environ. Sci. 2025, 44, 247–256. [Google Scholar]
- Wang, W.X.; Chen, F.Z.; Gu, X.H. Community structures of zooplankton and its relation to environmental factors in five medium reservoirs in Nanjing City. J. Lake Sci. 2017, 29, 216–223. [Google Scholar] [CrossRef]
- D’Alelio, D.; Libralato, S.; Wyatt, T.; d’Alcalà, M.R. Ecological-network models link diversity, structure and function in the plankton food-web. Sci. Rep. 2016, 6, 21806. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Pang, W.T.; Chen, A.; Luo, J.; Qin, H.; Qian, Z.P.; Wang, Q.X. The indicator effect of rotifers for different nutrient states in water bodies: A case study in waters of Shanghai, China. J. Lake Sci. 2024, 36, 1706–1726. [Google Scholar] [CrossRef]
- Mineeva, N.M.; Lazareva, V.I.; Poddubnyi, S.A.; Zakonnova, A.V.; Kopylov, A.I.; Kosolapov, D.B.; Korneva, L.G.; Sokolova, E.A.; Pyrina, I.L.; Mitropol’skaya, I.V. Structure and functioning of zooplankton communities in the Rybinsk Reservoir under the conditions of climate change. Inland Water Biol. 2024, 17, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Huang, X.F. Freshwater Plankton Research Methods; Science Press: Beijing, China, 1991. [Google Scholar]
- Crustacean Research Group, Institute of Zoology, Chinese Academy of Sciences. Fauna Sinica: Arthropoda: Crustacea: Freshwater Copepoda; Science Press: Beijing, China, 1979. [Google Scholar]
- Jiang, X.Z.; Du, N.S. Fauna Sinica: Arthropoda: Crustacea: Freshwater Cladocera; Science Press: Beijing, China, 1979. [Google Scholar]
- Nie, X.; Hu, X.R.; Liu, G.H.; Jin, B.S.; Qin, H.M. Effects of water level on zooplankton community during ‘plate-shaped lake enclosed in autumn’ in a sub-lake of the Poyang Lake. Acta Hydrobiol. Sin. 2019, 43, 402–414. [Google Scholar]
- Hu, Y.; Zhang, Y.Z.; Jiang, X.Y.; Shao, K.Q.; Tang, X.M.; Gao, G. Seasonal characteristics of nestedness pattern and interaction of plankton assemblages in East Lake Taihu. J. Lake Sci. 2022, 34, 1620–1629. [Google Scholar] [CrossRef]
- GB 11893-89; Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1990.
- HJ 199-2023; Water Quality—Determination of Total Nitrogen—Gas-Phase Molecular Absorption Spectrometry. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2024.
- HJ 897-2017; Water Quality—Determination of Chlorophyll a—Spectrophotometric Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2018.
- GB 11892-89; Water Quality—Determination of Permanganate Index—Acidic Potassium Permanganate method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1990.
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons Inc.: New York, NY, USA, 1975. [Google Scholar]
- Yang, G.M.; He, D.H.; Wang, C.S. Ecology characteristics in the island waters off Zhejiang I. Species dominance of the zooplankton and ecology character of waters. Mar. Environ. Sci. 1998, 17, 49–54. [Google Scholar]
- Xu, D.H.; Sun, X.M.; Chen, B.J.; Xia, B.; Cui, Z.G.; Zhao, J.; Jiang, T.; Liu, C.X.; Qu, K.M. The ecological characteristics of zooplankton in the central Bohai Sea. Prog. Fish. Sci. 2016, 37, 7–18. [Google Scholar]
- Wang, M.C.; Liu, X.Q.; Zhang, J.H. Evaluate method and classification standard on lake eutrophication. Environ. Monit. China 2002, 18, 47–49. [Google Scholar] [CrossRef]
- Wu, X.M.; Hao, R.J.; Pan, H.B.; Wei, H.; Wang, L.Q. Community structure of zooplankton and its relationship with environmental factors in Huangpu River. Ecol. Environ. Sci. 2018, 27, 1128–1137. [Google Scholar]
- Liu, X.; Lei, M.J.; Huang, X.L.; Wang, M.X.; Lei, Q. Diversity of metazoan zooplankton and its relationship with water environmental factors in Da’ao Reservoir. J. Yangtze River Sci. Res. Inst. 2024, 41, 53–59. [Google Scholar]
- Zheng, Z.X.; Zhu, X.G.; Ye, B.B.; Yuan, Z.; Yang, W.T.; Liu, Y.N. Structural characteristics and influencing factors of zooplankton community in the Xuancheng section of the Shuiyang River. J. Biol. 2025, 42, 75–83. [Google Scholar]
- Xiang, H.; Lin, F.; Wu, Y.P.; Tian, B.; Cai, Z.Y.; Wu, Y.P.; Huang, P.J.; Liu, Q.F.; Zhou, Z.G. Correlation analysis between zooplankton community structure characteristics and environmental factors in the Yichang section in middle reaches of the Yangtze River. Heilongjiang Fish. 2022, 5, 3–12. [Google Scholar]
- Lei, Q.; Bao, Y.F.; Tan, Q.J.; Xu, Y.Z.; Gong, B.; Jian, Y.Z.; Jin, L.; Xiong, D.N.; Li, T.C. Zooplankton community structure and its relationship with environmental factors in cascade reservoirs in the lower reaches of the Lancang River. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2023, 51, 128–138. [Google Scholar]
- Zhang, J.M.; Zhou, X.X.; Li, Q.H.; Zhang, Y.F.; Shen, Y.J. Status of Plankton Community Structure, Diversity and Water Quality in the Lower Reaches of the Fu River. J. China West Norm. Univ. (Nat. Sci. Ed.) 2025. Available online: http://kns.cnki.net/kcms/detail/51.1699.N.20241226.1556.002.html (accessed on 10 July 2025).
- Zhang, Q.; Xu, X.M.; Chen, Q. Seasonal variation characteristics of zooplankton seasonal variation and drivers of zooplankton community in reservoirs of Chaobai River Basin. Acta Sci. Nat. Univ. Pekin. 2023, 59, 290–300. [Google Scholar]
- Wei, C.J.; Qiu, P.F.; Wang, F.C.; Qiao, Z.Y.; Zhan, X.L.; Feng, W.S.; Li, R.H.; Gong, Y.C. Zooplankton community dynamics and its relationship with the growth of Aphanizomenon sp. in Yuqiao Reservoir, Tianjin. J. Hydroecol. 2024, 45, 134–144. [Google Scholar]
- Li, J.; Liang, Y.Y.; Tang, X.X.; Han, Q.; Yin, F.; Guo, N.C.; Lu, W.X. Metazooplankton Community Structure and Trophic State Assessment of Chaohu Lake. J. Aquat. Ecol. 2023, 44, 73–81. [Google Scholar]
- Brito, S.L.; Maia-Barbosa, P.M.; Pinto-Coelho, R.M. Zooplankton as an indicator of trophic conditions in two large reservoirs in Brazil. Lakes Reserv. Res. Manag. 2011, 16, 253–264. [Google Scholar] [CrossRef]
- Dai, D.C.; Ma, X.Z.; Zhang, W.B.; Zhang, Y.; Zhou, Z. Metazooplankton community structure and ecological evaluation of water quality of Eriocheir sinesis ecological culture ponds. J. Ecol. Rural Environ. 2021, 37, 208–216. [Google Scholar]
- Yang, Y.F.; Huang, X.F. The influence of silver carp and bighead on the zooplankton community structure. J. Lake Sci. 1992, 4, 78–86. [Google Scholar] [CrossRef]
- Chen, X.L.; Shang, J.W.; Wang, Y.W.; Sun, M.F.; Han, J.M.; Zhang, S.Y.; Li, W.Q.; Qin, H.M. Seasonal dynamic changes and driving factors of zooplankton community in a silver carp and bighead carp stocked reservoir. J. Lake Sci. 2025, 37, 566–579. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhao, K.; Cao, Y.; Wu, B.; Pang, W.T.; You, Q.M.; Wang, Q.X. Zooplankton community structure and its relationship with environmental factors in Poyang Lake. Acta Ecol. Sin. 2020, 40, 6644–6658. [Google Scholar]
- Wang, Y.W.; Zhang, S.Y.; Sun, M.F.; Han, J.M.; Wang, Z.Y.; Chen, X.L.; Chen, Z.F.; Qin, H.M. Spatial-temporal pattern and stability analysis of zooplankton community structure in the Lower Yellow River in China. Diversity 2025, 17, 162. [Google Scholar] [CrossRef]
- La, H.S.; Park, K.; Wåhlin, A.; Arrigo, K.R.; Kim, D.S.; Yang, E.J.; Atkinson, A.; Fielding, S.; Im, J.; Kim, T.W.; et al. Zooplankton and micronekton respond to climate fluctuations in the Amundsen Sea polynya, Antarctica. Sci. Rep. 2019, 9, 10087. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Q.; Fu, M.; Jiang, T.; Wang, Y.M.; Wang, D.Y. Structural characteristics of zooplankton and phytoplankton communities and its relationship with environmental factors in a typical tributary reservoir in the Three Gorges Reservoir region. Environ. Sci. 2021, 42, 2303–2312. [Google Scholar]
- Wang, L.J.; Du, Y.Y.; Zhang, S.H.; Zhang, Y.P.; Wang, T. Plankton community structure and its relationship with environmental factors in the Qingtu Lake wetland. Wetl. Sci. 2025, 23, 341–351. [Google Scholar]
- He, X.J.; Wang, W.X. Allocation and stoichiometric regulation of phosphorus in a freshwater zooplankton under limited conditions: Implication for nutrient cycling. Sci. Total Environ. 2020, 728, 138795. [Google Scholar] [CrossRef]
- Liao, X.L. Study on monitoring of permanganate index in water environment. China Resour. Compr. Util. 2022, 40, 136–138. [Google Scholar]
- Yang, H.Y. Relationship Between Phytoplankton Succession History and Human Activities in Watershed: A Case Study of Qilu Lake; Central China Normal University: Wuhan, China, 2020. [Google Scholar]
- Li, Y.J.; Cheng, D.Y.; Adam, N.A.; Mirzalevens, S.; Zhang, G.W. Exploring the nexus between coastal tourism growth and eutrophication: Challenges for environmental management. Mar. Pollut. Bull. 2025, 216, 117922. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.P.; Xi, Y.L.; Wen, X.L.; Huang, L. Eutrophication impact on community structure and species diversity of rotifers in five lakes of Anhui. Biodivers. Sci. 2007, 15, 344–355. [Google Scholar] [CrossRef]
- Yang, G.L.; Lv, G.H.; Zhu, J.Q.; Xu, Z.; Jin, C.H. Characteristics of zooplankton community in Hengshan Reservoir and water quality assessment. Acta Hydrobiol. Sin. 2014, 38, 720–728. [Google Scholar]
- Li, L.M.; Ye, J.Q.; Zhou, L.C.; Huang, M. Investigation and evaluation of eutrophication in a river-type reservoir of Dazhou. J. Green Sci. Technol. 2024, 26, 129–132+137. [Google Scholar]
- Zhang, X.Y.; Fan, X.P.; Liu, D.Y.; Xu, F.S.; Gan, X.Y.; Fan, X.Y.; Dong, W.Z. Investigation and evaluation on agricultural non-point source pollution in Danjiangkou Reservoir area of Hubei Province. Hubei Agric. Sci. 2012, 51, 3460–3464. [Google Scholar]
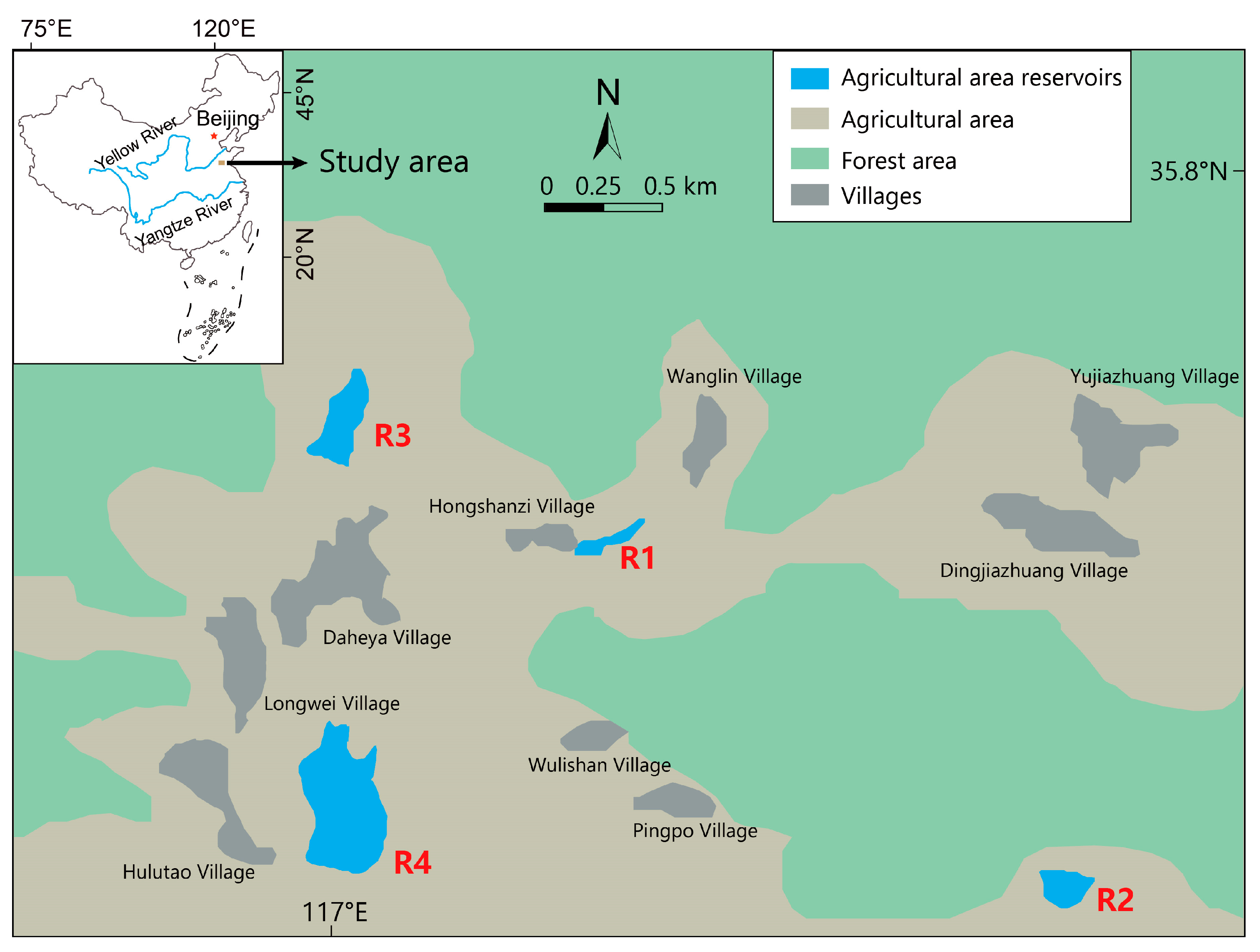
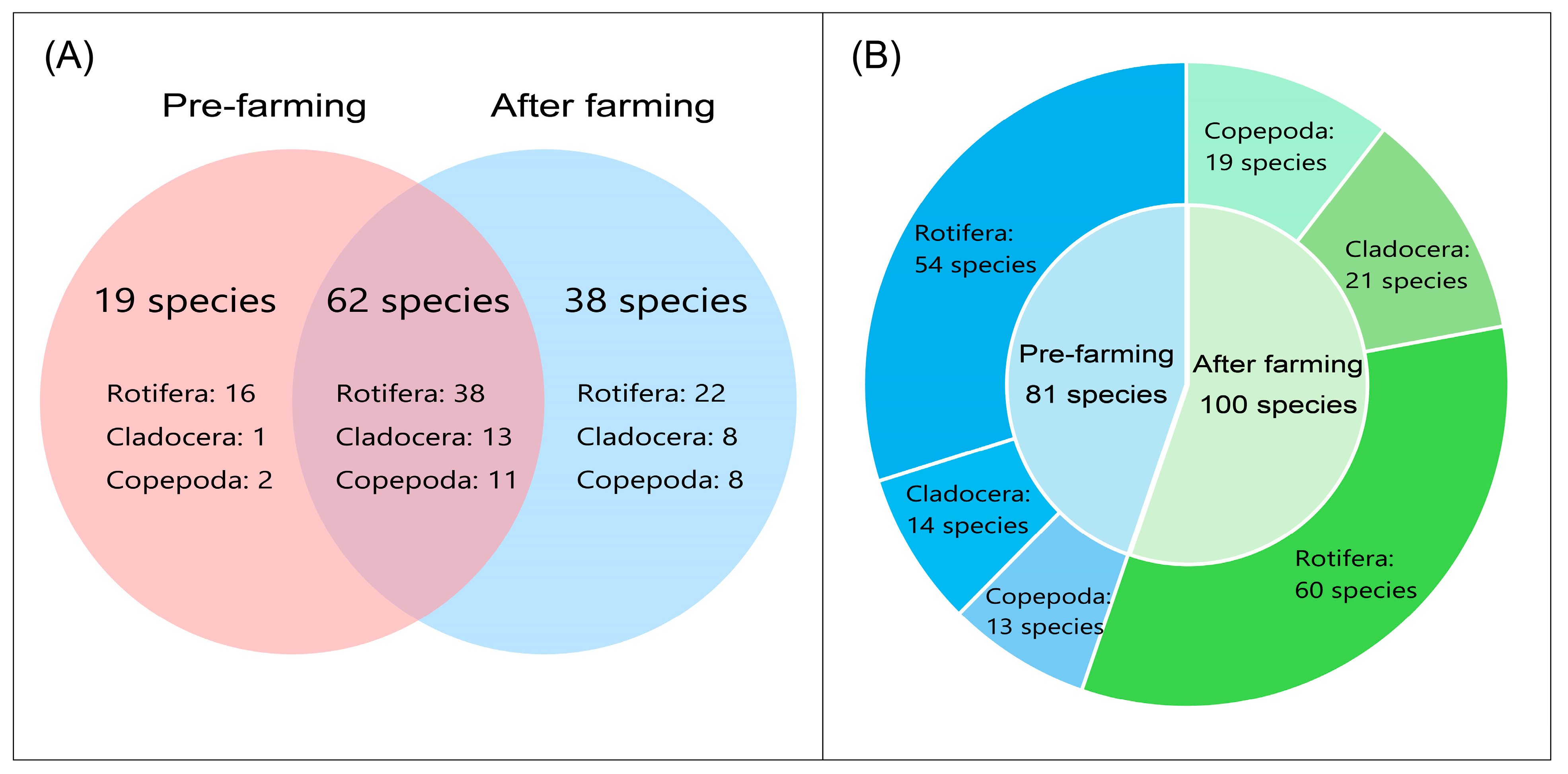

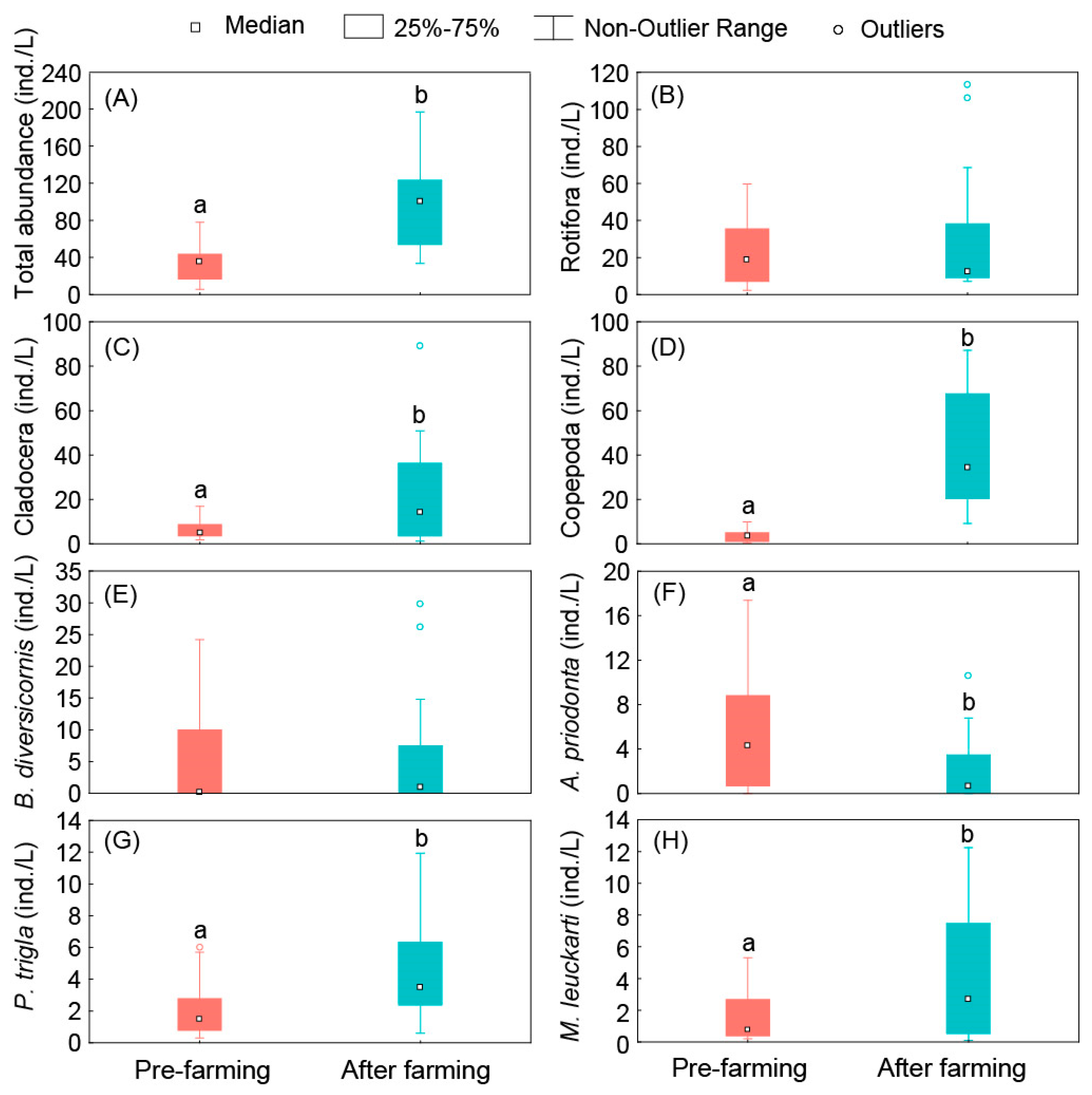
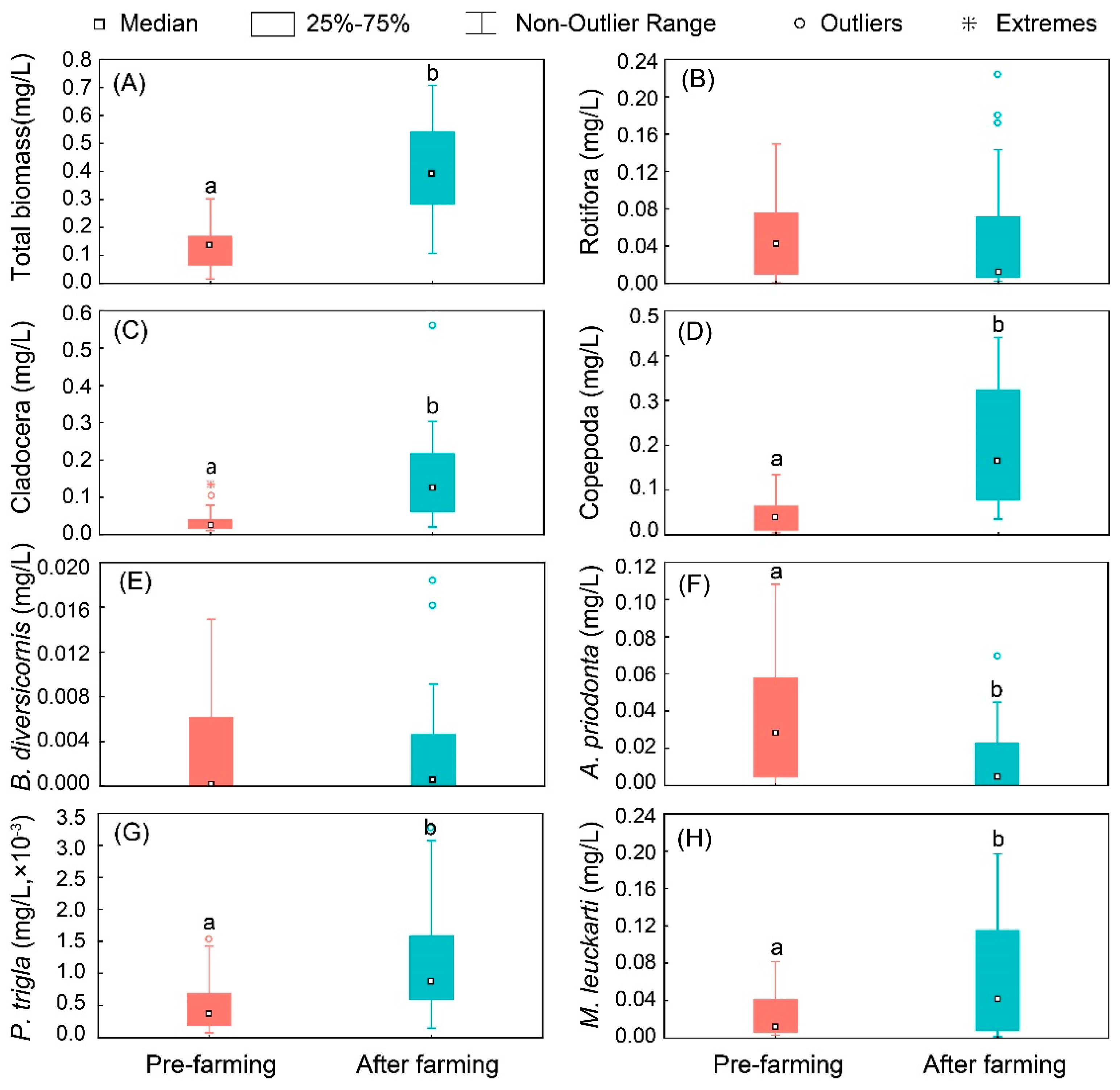

| Reservoir Number | Elevation | Maximum Depth | Reservoir Surrounding Environment |
|---|---|---|---|
| R1 | 161.28 m | 1.68 m | The breeding of silver carp and bighead carp is carried out. The downstream is adjacent to the artificial forest area, and there is a small area of farmland upstream. The domestic sewage from the surrounding residential areas is directly discharged into the reservoir. There are many human activities. |
| R2 | 152.65 m | 1.47 m | The left side and the upper reaches of the reservoir are surrounded by secondary forests. On the right side, there are residential areas distributed, but they are relatively far away, and there are fewer human activities. |
| R3 | 141.52 m | 2.35 m | Adjacent to the village, there are a large number of artificial forests on both sides of the upstream river where the reservoir flows into. There are many human activities, and the water body is yellowish-brown with high transparency. |
| R4 | 129.95 m | 4.12 m | The rivers flowing into the reservoir have many branches, and there are large areas of residential areas distributed upstream of each branch. There are artificial forests on both sides of the reservoir, and there are many human activities. |
| Research Period | Reservoir | Cond | pH | DO | Trans | TN | TP | Chl-a | CODMn |
|---|---|---|---|---|---|---|---|---|---|
| (us/cm) | (mg/L) | (cm) | (mg/L) | (mg/L) | (mg/m3) | (mg/L) | |||
| Pre-farming | R1 | 281.50 ± 3.33 a | 8.50 ± 0.14 a | 4.03 ± 0.36 a | 36.50 ± 2.52 | 2.07 ± 0.06 | 0.16 ± 0.02 | 8.92 ± 0.52 a | 6.97 ± 0.19 a |
| R2 | 330.33 ± 3.68 a | 8.41 ± 0.03 a | 4.36 ± 0.35 | 50.33 ± 4.42 a | 1.50 ± 0.10 a | 0.09 ± 0.00 a | 5.56 ± 0.95 a | 5.52 ± 0.21 a | |
| R3 | 300.33 ± 5.95 a | 8.99 ± 0.01 a | 4.07 ± 0.42 | 34.83 ± 1.59 a | 1.94 ± 0.03 a | 0.13 ± 0.02 a | 8.70 ± 0.54 a | 6.07 ± 0.01 a | |
| R4 | 242.33 ± 4.92 a | 7.87 ± 0.12 a | 4.19 ± 0.27 a | 34.67 ± 2.17 | 2.08 ± 0.06 a | 0.15 ± 0.00 a | 12.99 ± 0.40 a | 6.79 ± 0.23 a | |
| After farming | R1 | 313.83 ± 0.93 b | 7.05 ± 0.18 b | 5.71 ± 0.02 b | 30.17 ± 0.83 | 2.07 ± 0.02 | 0.20 ± 0.00 | 31.64 ± 0.96 b | 8.38 ± 0.11 b |
| R2 | 458.50 ± 5.57 b | 6.98 ± 0.15 | 5.36 ± 0.12 | 26.17 ± 2.74 b | 2.91 ± 0.03 b | 0.19 ± 0.00 b | 53.67 ± 0.23 b | 7.63 ± 0.25 b | |
| R3 | 347.83 ± 0.44 b | 7.43 ± 0.18 b | 4.92 ± 0.19 | 24.67 ± 2.73 b | 2.52 ± 0.11 b | 0.25 ± 0.01 b | 45.57 ± 0.95 b | 8.16 ± 0.02 b | |
| R4 | 298.17 ± 13.15 b | 6.64 ± 0.11 b | 5.47 ± 0.09 b | 31.00 ± 1.50 | 2.89 ± 0.08 b | 0.22 ± 0.00 b | 47.81 ± 1.91 b | 8.06 ± 0.05 b |
| Dominant Species | Dominance(Y) | Species Serial Number | |
|---|---|---|---|
| Pre-Farming | After Farming | ||
| Rotifera | |||
| Brachionus calyciflorus | * | 0.059 | S8 |
| Brachionus quadridentatus | 0.034 | * | S11 |
| Brachionus diversicornis | 0.170 | 0.041 | S15 |
| Keratella cochlearis | 0.052 | * | S23 |
| Argonotholca foliacea | 0.061 | - | S26 |
| Asplanchna priodonta | 0.160 | 0.022 | S37 |
| Asplanchna girodi | 0.023 | * | S38 |
| Polyarthra trigla | 0.063 | 0.050 | S61 |
| Cladocera | |||
| Ceriodaphnia quadrangula | 0.076 | * | S82 |
| Scapholeberis mucronata | 0.029 | * | S83 |
| Bosmina longirostris | * | 0.157 | S85 |
| Chydorus ovalis | 0.020 | * | S98 |
| Copepoda | |||
| Microcyclops varicans | * | 0.159 | S112 |
| Mesocyclops leuckarti | 0.051 | 0.047 | S116 |
| Reservoir | TLI Comprehensive Nutrient Status Index and Eutrophication Evaluation Results | |
|---|---|---|
| Before Farming | After Farming | |
| R1 | 53.12 (Mild eutrophication) | 65.36 (Moderate eutrophication) |
| R2 | 56.74 (Mild eutrophication) | 65.27 (Moderate eutrophication) |
| R3 | 48.61 (mesotrophic level) | 68.87 (Moderate eutrophication) |
| R4 | 55.33 (Mild eutrophication) | 66.50 (Moderate eutrophication) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Q.; Shao, Y.; Bian, X.; Sun, M.; Chen, Z.; Han, J.; Zhang, S.; Han, S.; Qin, H. Evaluation of Eutrophication in Small Reservoirs in Northern Agricultural Areas of China. Diversity 2025, 17, 520. https://doi.org/10.3390/d17080520
Jing Q, Shao Y, Bian X, Sun M, Chen Z, Han J, Zhang S, Han S, Qin H. Evaluation of Eutrophication in Small Reservoirs in Northern Agricultural Areas of China. Diversity. 2025; 17(8):520. https://doi.org/10.3390/d17080520
Chicago/Turabian StyleJing, Qianyu, Yang Shao, Xiyuan Bian, Minfang Sun, Zengfei Chen, Jiamin Han, Song Zhang, Shusheng Han, and Haiming Qin. 2025. "Evaluation of Eutrophication in Small Reservoirs in Northern Agricultural Areas of China" Diversity 17, no. 8: 520. https://doi.org/10.3390/d17080520
APA StyleJing, Q., Shao, Y., Bian, X., Sun, M., Chen, Z., Han, J., Zhang, S., Han, S., & Qin, H. (2025). Evaluation of Eutrophication in Small Reservoirs in Northern Agricultural Areas of China. Diversity, 17(8), 520. https://doi.org/10.3390/d17080520




