Abstract
Renicolid digeneans parasitise aquatic birds. In molecular trees, they are divided into three clades, one of which, the ‘duck clade’, parasitises anatids. Renicola mollissima, a member of this clade, parasitises sea ducks, mainly eiders. Its life cycle remains unknown. We verified the diagnosis of R. mollissima using integrated morphological and molecular data and provided the first information on its life cycle in northern Palaearctic. We proved that intramolluscan stages of R. mollissima, previously known as Cercaria pacifica 2, develop in intertidal snails Littorina squalida and L. saxatilis. We provided a detailed morphological description of cercariae and adults of R. mollissima and a discriminative analysis with closely related species. Molecular data demonstrated an amphiboreal distribution of R. mollissima and the existence of a single population in Europe and the North Pacific. Using molecular methods, we also found metacercariae of an unknown renicolid species from the ‘duck clade’, designated as Cercaria cf. nordica I, in subtidal mussels of the Barents Sea. All individuals of C. cf. nordica I examined in our study were represented by the same haplotype. We discuss possible ways of formation of this phylogeographic structure, the composition of the ‘duck clade’ and the evolutionary pathways of the family Renicolidae.
1. Introduction
The family Renicolidae (Plagiorchioidea) comprises species parasitising at the stage of sexual adults (maritae) the kidneys of aquatic birds. In the trixenous life cycles of renicolids, the role of the first intermediate host (FIH) is played mainly by marine and estuarine molluscs. Sporocysts forming in the molluscs reproduce parthenogenetically and produce free-swimming larvae, cercariae. They emerge into the water and, for further implementation of the life cycle, must infect second intermediate hosts (SIH) represented mostly by molluscs (usually bivalves) or fish. The larvae developing in SIH (metacercariae) reach infectivity for definitive hosts (DH), birds, which become infected by consuming prey with metacercariae.
Despite repeated attempts to develop the classification of the family Renicolidae, it remains confusing [1,2]. Only two genera are currently recognised as valid: Renicola Cohn, 1904 and Nephromonorcha Leonov, 1958. Their adults differ in the number of testes: two separate testes in the former and one testis (resulting from merging of two) in the latter genus [1,3]. Progress in our understanding of the classification and evolution of renicolids has been achieved owing to the integrative taxonomy, which combines molecular methods with traditional morphological ones [4,5]. Three clades are clearly distinguished in the phylograms of the Renicolidae based on nuclear and mitochondrial DNA sequences of the species studied to date [6,7,8]. Clade I (‘Parvicaudata’ group) contains species with styleted cercariae with a simple tail (Xiphidiocercaria group) using coastal caenogastropods as FIHs. Their SIHs are molluscs (presumably bivalves) and, less often, polychaetes, while DHs are gulls and, probably, waders. Clade II consists of species with large non-styleted cercariae with tail fins (Rhodometopa group) or non-styleted larvae of the transitional morphotype from typical xiphidiocercariae to cercariae of Rhodometopa group. Their FIHs are sublittoral caenogastropods, SIHs are fishes, and DHs are various groups of seabirds such as auks, gulls, petrels, shags, penguins, etc.
Clade III, which we provisionally refer to as the ‘duck clade’, includes Renicola mediovitellata Bychovskaja-Pavlovskaja, 1950 and R. somateriae Belopolskaja, 1952. They are parasites of marine anatids and/or anatids periodically visiting the coasts. A detailed description of these two species and their life cycles has been provided in our previous study [7]. Kulachkova [9] described one more species, R. mollissima Kulachkova, 1957, from common eiders of the White Sea. Later, R. mollissima was recorded in Chukotka [10] and in the north of the Sea of Okhotsk [11]. Molecular data on this species have been absent, and its life cycle has remained unknown.
We collected adults of R. mollissima during our parasitological surveys at the White Sea and studied them with the use of molecular and morphological methods. Moreover, using molecular markers, we managed to identify sporocysts and cercariae from littoral molluscs Littorina spp. from the White Sea, the Sea of Okhotsk and the Sea of Japan as intramolluscan stages of R. mollissima. In our phylogenetic trees based on molecular markers, R. mollissima was placed in the ‘duck clade’. In addition, we found metacercariae of some Renicola sp. in sublittoral mussels in the material collected during voyages in the southeastern part of the Barents Sea (SE Barents Sea). Molecular data showed that it also belonged to the ‘duck clade’ but differed from its other members.
Here we present the results of these studies. We also discuss the probable species composition, distribution and phylogeography of renicolids parasitising anseriforms in northern Holarctic and hypothesise about the evolution of the Renicolidae.
2. Materials and Methods
2.1. Material Collection and Treatment
The material for this study was collected in the White Sea, the Sea of Okhotsk and the Sea of Japan during the warm seasons of 2005–2024. Adults corresponding to the morphological and morphometric characteristics of R. mollissima described by Kulachkova [9] were isolated from the kidneys of two common eiders obtained in May and August 2024 at the White Sea in accordance with the local regulations. The adults were located in renal tubules in pairs, which is characteristic of renicolids. One individual from each pair was fixed with 96% ethanol for the molecular analysis. The other individual was fixed with 70% ethanol on a glass slide under light pressure of a cover slip. Worms flattened in this way were stained with aluminous carmine, cleared in isobutyl alcohol and xylene, and mounted in Canada balsam. These whole mounts were used for morphological studies. They were drawn and photographed with the help of a Leica DM2500 compound microscope (Leica Microsystems, Wetzlar, Germany) equipped with a TrueChrome 4KPro digital camera (Tucsen, Fuzhou, China) in the Zoological Institute of the Russian Academy of Science (further abbreviated as ZISP) (St. Petersburg, Russia). We also examined syntypes of R. mollissima preserved in the Collection of Helminths, section Trematoda, of ZISP.
Intramolluscan stages of Cercaria pacifica 2 Pois, Tsimbaljuk & Ardasheva, 1974, identified on the basis of the description in Pois et al. [12], were found in intertidal snails Littorina squalida Broderip & G. B. Sowerby I, 1829 from the Sea of Okhotsk and the Sea of Japan (Table 1) and in one snail L. mandshurica Schrenk, 1861 in the Sea of Japan (Vostok Bay). At the White Sea, the intramolluscan stages of this species were found in littoral snails Littorina saxatilis (Olivi, 1792) collected on the Kem’-Ludy archipelago of the Kandalaksha Bay (Table 1). The molluscs were dissected under a stereomicroscope and checked for infection with renicolid intramolluscan stages. Some sporocysts with cercariae isolated from infected molluscs were fixed with 96% ethanol for molecular studies. Live sporocysts and cercariae were observed, measured and photographed using an Olympus CH40 compound microscope (Olympus Optical Co. Ltd., Tokyo, Japan) equipped with an Olympus XC-30 digital camera (Olympus Optical Co. Ltd.) at the “Kartesh” White Sea Biological Station of ZISP. During fieldwork at the coasts of the Sea of Okhotsk, renicolid intramolluscan stages were studied in vivo using an Amplival microscope (Carl Zeiss Jena, Oberkochen, Germany) with an amateur Sony Cyber-shot DSC-W100 camera (Sony Corporation, Tokyo, Japan) connected to the eyepiece. Only fully-formed cercariae were used for morphometric studies and scanning electron microscopy (SEM). Cercariae to be measured were fixed by heating in a drop of seawater on the object slide (until the water started to evaporate), and then gently pressed with a coverslip. Sporocysts and encysted metacercariae were measured in vivo. For SEM examination, cercariae were fixed and treated as described in Galaktionov et al. [13] and viewed under a FEI Quanta 250 (FEI, Eindhoven, The Netherlands) scanning electron microscope in the “Taxon” Research Resource Centre (http://www.ckp-rf.ru/ckp/3038/, last accessed on 20 June 2025) of ZISP. All measurements presented in the paper are in micrometres, with the mean given in parentheses. The drawings were made with the aid of a camera lucida. In this study, the prevalence of renicolid intramolluscan stages is defined as the percentage of infected molluscs within the total sample.

Table 1.
List of samples of renicolids used in this study and their GenBank accession numbers.
Blue mussels Mytilus edulis Linnaeus, 1758 infected with renicolid metacercariae were collected by dredging at depths of 9–15 m in the Khaipudyr Bay and the Chosha Bay (southeastern Barents Sea—SE Barents Sea) during expeditions onboard RV Vladimir Kuznetsov in July–August 2022 and 2024. The mussels were dissected under the stereomicroscope in the onboard laboratory, and encysted renicolid metacercariae were extracted and fixed with 96% ethanol for further molecular studies. In addition, the sequences of molecular markers were also obtained for the adult of R. mediovitellata from the kidney of common eider S. mollissima shot in the northern part of the Sea of Okhotsk (Shelikhov Bay, Cape Taygonos) in 2008 (see [7] for details).
2.2. DNA Extraction, Amplification and Sequencing
We sequenced fragments of 28S ribosomal RNA (rRNA), cox1 and 12S rRNA genes for sporocysts, cercariae and adults of Renicola spp. from infected snails and birds (Table 1). Genomic DNA was extracted from ethanol-fixed isolates using cetrimonium bromide (CTAB) detergent according to the published protocol with modifications [14]. Fixed samples were rinsed in 1× phosphate-buffered saline for 15 min prior to extraction.
PCR reactions and purification of products were performed according to published protocols [6,15]. We used primers ZX-1 (5′-ACCCGCTGAATTTAAGCATAT-3′) and 1500R (5′-GCTATCCTGAGGGAAACTTCG-3′) for 28S rRNA D1-D3 fragment [16,17], JB3 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and JB4-5 (5′-TAAAGAAAGAACATAATGAAAATG-3′) for cox1 [18] and Tre12S-F (5′-GTGCCAGCADYYGCGGTTA-3′) and Tre12S-R (5′-AGCAGCAYATHGACCTG-3′) for 12S rRNA gene [15]. DNA sequencing was performed at the Resource Centre for Development of Molecular and Cellular Technologies, St. Petersburg State University. All sequences obtained in this study were deposited in GenBank (Table 1).
2.3. Alignments and Phylogenetic Analyses
Newly generated sequences as well as partial sequences of 28S rRNA gene and cox1 genes retrieved from GenBank were aligned, trimmed and analysed in Geneious 7.1.4 (http://www.geneious.com) [19]. Phylogenetic relationships were reconstructed using Bayesian inference (BI) in MrBayes v. 3.2.6 [20] and maximum likelihood (ML) in MEGA 11 [21]. The most suitable evolutionary models were determined using the corrected Akaike information criterion in PartitionFinder (https://github.com/brettc/partitionfinder, last accessed on 20 June 2025). The Hasegawa–Kishino–Yano model with estimates of gamma-distributed among-site rate variation (HKY + G, the corresponding model setting for BI was nst = 2 rates = gamma) was chosen as best fitted for 28S rRNA gene, while HKY + G + I (the corresponding model setting for BI was nst = 2 rates = invgamma) was chosen as best fitted for cox1 and 12SrRNA genes. The following parameters were used for the ML analysis: 1000 bootstrap replications and a moderate branch filter during tree reconstruction. During BI, the MCMC analysis was run for 500,000 generations with sampling every 100 generations. Convergence diagnostics were calculated every 1000 generations, and the standard deviation of split frequencies was monitored to assess run convergence. The first 25% of samples were discarded as burn-in. Genetic divergences between taxa were calculated as uncorrected p-distances with 1000 bootstrap iterations for each gene region using MEGA 11 [21]. A median joining haplotype network was reconstructed with PopART 1.7 [22].
3. Results
Our preliminary conjecture that intramolluscan stages of Cercaria pacifica 2 from periwinkles L. squalida of the Sea of Japan and the Sea of Okhotsk were identical with those from L. saxatilis of the White Sea, which was based on the analysis of cercarial morphology, was confirmed by the analysis of molecular markers. Moreover, molecular data indicated that sporocysts and larvae of C. pacifica 2 were life cycle stages of R. mollissima (See Section 3.2 Molecular Results).
3.1. Description
- Family Renicolidae Dollfus, 1939
- Renicola mollissima Kulachkova, 1957
- [syn. Cercaria pacifica 2 Pois, Tsimbaljuk & Ardasheva, 1974]
- Type host (definitive): common eider Somateria mollissima (Linnaeus, 1758)
- Other hosts (definitive): King eider Somateria spectabilis (Linnaeus, 1758), long-tailed duck Clangula hyemalis (Linnaeus, 1758), common goldeneye Bucephala clangula (Linnaeus, 1758)
- Site of infection in definitive host: kidney tubules
- Type locality: White Sea
- Other localities (in definitive host): Chukotka, Sea of Okhotsk
- Type material: 3 syntypes on the slide of V. G. Kulachkova # 3750 deposited ZISP.
- Representative slides: slides of V.G. Kulachkova ## 3751-1–3751-2 deposited in ZISP. Slides ## 3752-1–3752-2 (paragenophores) and 3752-3 deposited in ZISP.
- First intermediate host: Littorina squalida Broderip & G. B. Sowerby I, 1829, L. saxatilis
- (Olivi, 1792), L. mandshurica (Schrenck, 1861) (under question—see Section 3. Results for details) (Caenogastropoda: Littorinimorpha: Littorinidae) (natural)
- Site of infection in first intermediate host: gonad
- Localities (in first intermediate host): White Sea, Sea of Okhotsk, Sea of Japan
- Second intermediate host: Littorina squalida, L. saxatilis, unknown bivalves
- Representative DNA sequences: cox1 (PV644169–PV644171, PV644173–PV644183), 28S (PV639403–PV639404), 12S rRNA (PV642434–PV642437) (according to Table 1).
Five adult worms obtained from the White Sea common eiders were identified as R. mollissima based on the description of Kulachkova [9] and the comparison with the syntypes deposited in ZISP. The description in Kulachkova [9] is fairly detailed; however, it is published in a poorly accessible source. Below we give a brief morphological characterisation of R. mollissima and supply some details missing in the original description.
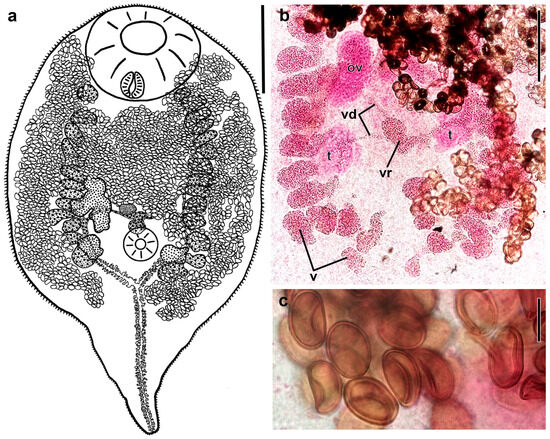
Figure 1.
Adult of Renicola mollissima (common eider Somateria mollissima, White Sea) (ventral view) (a). Microphotographs showing vitellaria and vitelline ducts in the central part of the body (b) and eggs in the uterus (c) (from slide # 3750). Abbreviations: ov: ovary; t: testis; v: vitellaria; vd: vitelline duct; and vr: vitelline reservoir. Scale bars: (a) 300 μm, (b) 200 μm, and (c) 20 μm.
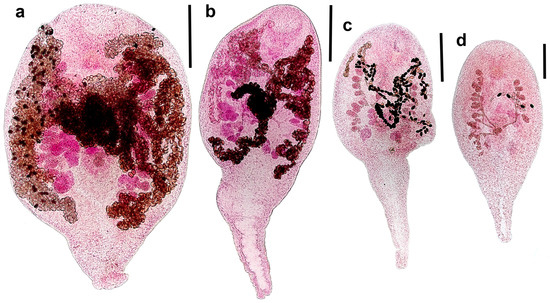
Figure 2.
Representative microphotographs of adults of Renicola mollissima (ventral view). Syntype from slide 3750 filled with eggs made by Kulachkova (common eider Somateria mollissima, White Sea) (a). Specimen from slide 3752-1 (common eider S. mollissima shot in September 2024) (b). Young specimen with few eggs in the uterus from slide 3750 (c). Young specimen with first eggs in the uterus from slide 3751-2 (d). Scale bars: (a–c) 300 μm, and (d) 200 μm.
Body ovoid, rounded anteriorly and attenuated posteriorly to form caudal process, its length reaching half of body length in young worms (Figure 1a and Figure 2b,c). Entire body covered with spines, 20–30 long and 1.8–3.4 wide at base on forebody, gradually decreasing in size posteriorly from ventral sucker. Size of worms varies greatly depending on number of eggs in uterus (Figure 2). Oral sucker subterminal to terminal, transversely elongated-oval. Ventral sucker small. Prepharynx absent, pharynx small, oesophagus short, two caeca, extending to base of caudal process. Testes oval, lobbed or smooth-edged, lying in posterior third of body, opposite to each other. Left testis somewhat larger than right testis. Seminal vesicle lies approximately at the level of the posterior to middle part of ovary, median or slightly dextral of body midline. Ovary dextral, pretesticular, larger than testes, trilobed. Vitellarium follicular; follicles in two fields passing along dorsal body side at considerable distance from body edges and extending from level of oral sucker to posterior edges of testes or slightly further but not reaching base of caudal process. Follicles are scattered, contacting each other only in worms with numerous eggs in a distended uterus. Posterior 4–6 follicles on each body side often displaced medially (Figure 1b and Figure 2).
Vitelline follicles of each row connected with ducts forming 2–4 common ducts, which pass medially and merge to form a large vitelline reservoir in the area of ventral sucker dorsally (Figure 1b and Figure 2b–d). In more mature worms, ducts from each row of follicles merge to form a common vitelline duct which then connects to vitelline reservoir (Figure 1a and Figure 2a). Uterine loops pass laterally and centrally. We did not find any worms in which a distended uterus with densely packed eggs filled the entire body, either in our material or on the slides of Kulachkova preserved in ZISP. Eggs numerous, elongated-oval, operculate, with thin eggshell (Figure 1c). Excretory bladder Y-shaped, with numerous lateral diverticula, bifurcating approximately at level of base of caudal process, with arms extending into forebody up to level of oral sucker.

Table 2.
Morphometric parameters (in µm) of Renicola spp. adults.
Table 2.
Morphometric parameters (in µm) of Renicola spp. adults.
| R. mollissima from Somateria mollissima, White Sea (OUR Measurements 1: N = 8) | R. mollissima from Somateria mollissima, White Sea (Our Data, N = 5) | R. somateriae from Somateria mollissima, Sea of Okhotsk (After Galaktionov et al. [7]) | R. mediovitellata from Somateria mollissim, Sea of Okhotsk (After Galaktionov et al. [7]) | R. ovocallosa from Clangula hyemalis, Baltic Sea (After Reimer [23]) | |
|---|---|---|---|---|---|
| Body length | 1181–2220 (1742 ± 118) | 1285–1940 (1723 ± 162) | 1102–1305 (1208 ± 45) | 1174–2422 (1798 ± 160) | 900–1300 |
| Body width | 833–1283 (1053 ± 62) | 576–1305 (908 ± 134) | 705–930 (850 ± 43) | 551–1285 (938 ± 89) | 860–1250 |
| Oral sucker length | 181–414 (304 ± 27) | 135–423 (285 ± 46) | 210–338 (271 ± 27) | 238–382 (312 ± 21) | 330–475 * |
| Oral sucker width | 316–507 (415 ± 24) | 238–493 (368 ± 48) | 278–406 (329 ± 26) | 144–202 (176 ± 9) | – |
| Pharynx length | 81–127 (104 ± 6) | 65–108 (92 ± 8) | 79–95 (86 ± 3) | 58–90 (73 ± 4) | 80–87 |
| Pharynx width | 78–130 (99 ± 6) | 60–112 (91 ± 10) | 51–68 (62 ± 3) | 54–72 (68 ± 2) | 75–77 |
| Esophagus length | – | – | 160–170 (166 ± 3) | – | – |
| Ventral sucker length | 62–145 (116 ± 11) | 95–164 (121 ± 14) | 59–68 (63 ± 1) | 41–81 (69 ± 6) | 25–48 * |
| Ventral sucker width | 60–175 (127 ± 14) | 102–160 (120 ± 12) | 52–76 (63 ± 4) | 41–77 (66 ± 5) | – |
| Left testes length | 95–154 (120 ± 7) | 67–154 (110 ± 21) | 71–92 (84 ± 5) | 54–97 (82 ± 10) | – |
| Left testes width | 69–140 (92 ± 9) | 54–117 (86 ± 18) | 51–80 (64 ± 7) | 36–54 (45 ± 9) | – |
| Right testes length | 85–153 (110 ± 9) | 67–140 (107 ± 17) | 71–83 (76 ± 3) | 61–97 (77 ± 11) | – |
| Right testes width | 63–100 (78 ± 4) | 43–112 (70 ± 16) | 52–75 (62 ± 5) | 50–58 (54 ± 4) | – |
| Seminal vesicle length | 46–71 (55 ± 3) | 58–68 (64 ± 3) | 26–57 (46 ± 8) | 58–65 (61 ± 2) | – |
| Seminal vesicle width | 40–68 (54 ± 3) | 54–76 (68 ± 7) | 26–58 (46 ± 8) | 29–54 (40 ± 7) | – |
| Ovary length | 150–276 (223 ± 15) | 122–320 (217 ± 38) | 133–185 (167 ± 13) | 108–245 (172 ± 40) | – |
| Ovary width | 82–204 (148 ± 13) | 82–146 (111 ± 13) | 111–134 (122 ± 5) | 76–162 (121 ± 25) | – |
| Egg length (EL) | 25–36 (30 ± 0.5) | 26–35 (31 ± 0.5) | 28–38 (34 ± 0.4) | 25–36 (31 ± 0.6) | 32–35 |
| Egg width (EW) | 15–21 (18 ± 0.3) | 17–22 (19 ± 0.3) | 23–32 (29 ± 0.3) | 14–22 (19 ± 0.3) | 25–27 |
| EL/EW | 1.3–2 (1.7 ± 0.04) | 1.4–2 (1.6 ± 0.03) | 1.1–1.4 (1.2 ± 0.02) | 1.4–2 (1.7 ± 0.04) | – |
| Egg shell sickness | 1.5–2.4 (1.9 ± 0.1) | 1.3–2.1 (1.8 ± 0.05) | 3.6–5.6 (4.4 ± 0.11) | 1.4–3.2 (2.3 ± 0.13) | 4.5 |
N—number of measured individuals. * Diameter of organs. 1 Measurements from the slides # 3750 (syntypes) and 3751-1, 3751-2 of Kulachkova deposited in ZISP.
Sporocysts occupy molluscan gonad tissue, forming a tumour-like structure. Sporocysts elongate oval, 400–2100 × 150–660 (Figure 3). Cercariae of xiphidiocercaria type. Small, body oval, highly contractile, body slightly more than ca. 1.5 times longer than tail (Figure 4a,d). Cercariae from molluscs of different species differ in size: those from L. squalida are larger than those from L. saxatilis (Table 3).
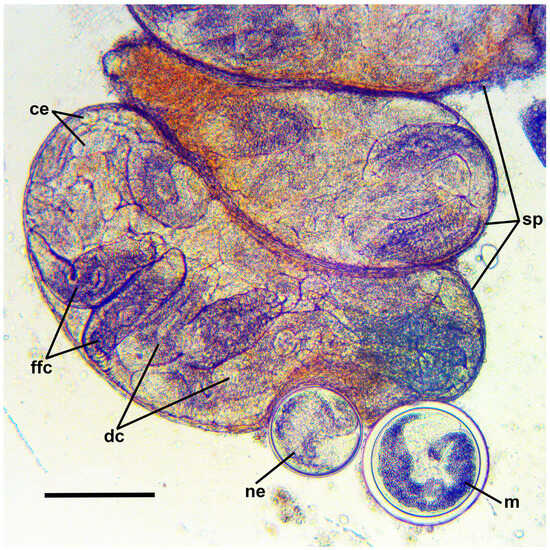
Figure 3.
Microphotograph of daughter sporocysts and encysted metacercariae of Renicola mollissima from the gonad of Littorina saxatilis (White Sea). Abbreviations: ce: cercarial embryos; dc: developing cercariae; ffc: fully-formed cercariae; m: fully-formed encysted metacercaria; ne: newly encysted metacercaria; and sp: sporocysts. Scale bar: 200 μm.
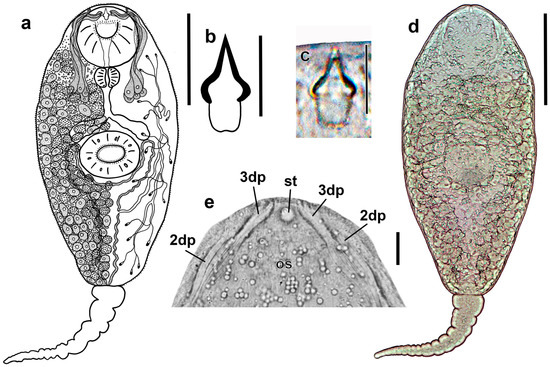
Figure 4.
Cercaria of Renicola mollissima. Drawing of the cercaria (ventral view) (a). Drawing (b) and microphotograph (c) of the stylet (ventral view). Microphotograph of the cercaria (ventral view) (d) and phase contrast view of the anterior end of a flattened, live cercaria (ventral view), showing stylet and distal parts of ducts of penetration gland cells (e). Abbreviations: 3dp: 3 ducts of penetration gland cells open medially and ventrally near stylet; and 2dp: 2 narrower ducts of penetration gland cells open posteriorly and dorsolaterally to the former; os: oral sucker; and st: stylet (pointing upwards in the microphotograph). Scale bars: (a,d) 100 μm, and (b,c,e) 10 μm.

Table 3.
Morphometric parameters (in µm) of cercariae of Renicola spp.
Table 3.
Morphometric parameters (in µm) of cercariae of Renicola spp.
| Renicola mollissima from Littorina saxatilis, White Sea (Our Data: N = 35) | Cercaria pacifica 2 from Littorina mandshurica Sea of Japan (Our Data: N = 9) | Cercaria pacifica 2 from Littorina squalida, Sea of Okhotsk (Our Data: N = 13) | Cercaria pacifica 2 from Littorina squalida, Sea of Japan (After Pois et al. [12]) | Cercaria nordica 1 from Neptunea borealis, Barents Sea (After Marasaev [24]) | Renicola somateria from Buccinum undatum, Barents Sea (After Galaktionov et al. [7]) | Renicola mediovitellata from Nucella lapillus, Barents Sea (After Galaktionov et al. [7]) | |
|---|---|---|---|---|---|---|---|
| Body length (BL) | 270–308 (299 ± 3.3) | 260–297 (283 ± 4) | 342–409 (364 ± 7.1) | 229–260 | 355–380 | 312–406 (345 ± 6.4) | 281–312 (300 ± 2.6) |
| Body width | 94–125 (113 ± 1.9) | 94–117 (107 ± 2.2) | 104–183 (130 ± 6.2) | 92–98 | 100–120 | 99–130 (110 ± 3.4) | 78–109 (97 ± 1.9) |
| Tail length (TL) | 172–229 (192 ± 2.4) | 152–196 (162 ± 5.1) | 183–268 (221 ± 6.1) | 140–182 | 220–265 | 192–250 (218 ± 3.6) | 177–203 (190 ± 2) |
| Tail width | 21–34 (25 ± 0.6) | 21–24 (23 ± 0.4) | 24–31 (29 ±1.1) | 16–22 | – | 21–26 (25 ± 0.6) | 16–26 (22 ± 0.6) |
| Oral sucker length (OSL) | 48–66 (55 ± 0.9) | 42–54 (47 ± 1.5) | 54–73 * (65 ±1.8) | 39–44 * | 50–55 * | 55–65 (61 ± 0.8) | 43–58 (50 ± 0.8) |
| Oral sucker width (OSW) | 40–56 (47 ± 0.7) | 40–45 (43 ± 0.5) | – | – | – | 48–60 (54 ± 1.1) | 40–50 (45 ± 06) |
| Pharynx length | 14–25 (18 ± 1.2) | 14–21 (17 ± 0.7) | 15–25 (19 ± 0.9) | – | – | 18–28 (23 ± 0.9) | 15–23 (18 ± 0.5) |
| Pharynx width | 13–22 (17 ± 0.8) | 14–20 (17 ± 0.6) | 18–20 (18 ± 0.3) | – | – | 15–25 (20 ± 0.8) | 15–25 (18 ± 0.5) |
| Ventral sucker length (VSL) | 45–60 (52 ± 0.7) | 42–51 (46 ± 0.9) | 60–70 * (67 ± 1.5) | 39–47 * | 60–65 * | 40–68 (56 ± 2.1) | 45–53 (50 ± 0.6) |
| Ventral sucker width (VSW) | 45–60 (52 ± 0.7) | 43–53 (47 ± 1.1) | – | – | – | 45–60 (53 ± 1.4) | 43–53 (48 ± 0.6) |
| Stylet length | 10–15 (12 ± 0.2) | 11–15 (13 ± 0.4) | 10–20 (14 ± 0.9) | 12 | 11 | 9–11 (10 ± 0.4) | 8–10 (9 ± 0.2) |
| Stylet width (of the handle) | 7–10 (7 ± 0.1) | 8–9 (8 ± 0.2) | 5–8 (7 ± 0.3) | 4 | – | 5 | 3–4 (3 ± 0.2) |
| BL/TL | 1.3–1.8 (1.6 ± 0.05) | 1.5–1.9 (1.7 ± 0.04) | 1.4–2 (1.7 ± 0.04) | – | – | 1.3–1.8 (1.6 ± 0.04) | 1.5–1.7 (1.6 ± 0.02) |
| OSL/WSL | 1–1.3 (1 ± 0.02) | 0.9–1 (1 ± 0.03) | 0.8–1.1 * (1 ± 0.03) | – | – | 0.9–1.5 (1.1 ± 0.04) | 0.9–1.2 (1 ± 0.02) |
| OSW/VSW | 0.7–1.1 (0.9 ± 0.01) | 0.8–1 (0.9 ± 0.02) | – | – | – | 0.8–1.2 (1 ± 0.04) | 0.9–1.1 (0.9 ± 0.02) |
N—number of measured individuals; *—diameter of organs.
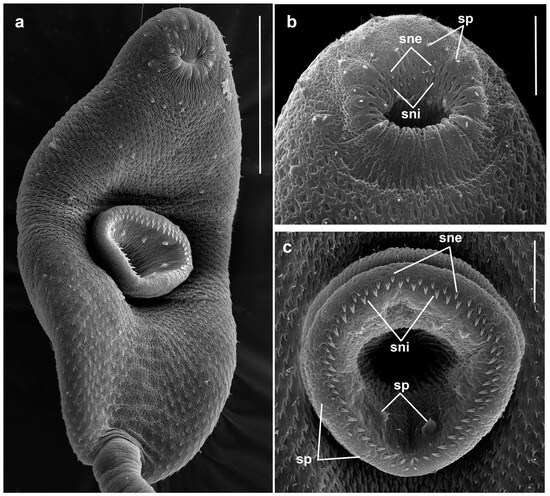
Figure 5.
Cercaria of Renicola mollissima. SEM microphotographs showing the cercaria (a) and spines in the oral (b) and the ventral sucker (c). Abbreviations: sne: spines of external row; sni: spines of internal row; and sp: sensory papillae. Scale bars: (a) 50 μm, (b,c) 10 μm.
However, molecular data indicate that they are conspecific (see Section 3.2 Molecular Results). Entire body covered with spines 1.1–1.3 long and 0.4–0.5 wide at base. Spines reduced along midline from level of posterior edge of ventral sucker to tail base. This area looks like an imprint of the tail (Figure 5a). Oral sucker ventro-subterminal, muscular, approximately the same size as ventral sucker. Oral sucker armed with two rows of spines that are somewhat larger (1.5–2.0 × 0.5–0.7) than those elsewhere on the body: outer row complete (30–35 spines); inner row incomplete, comprising 11–15 spines along anterior margin of oral sucker (Figure 5b). Stylet small, its anterior part, making up about 2/3 of length, with light-refracting cutting edges, pointed tip and bulbous base. Stylet handle rounded (Figure 4b,c).
Ventral sucker equatorial, armed with 2 alternating rows of large spines (1.7–2.1 × 0.6–1.0) of 40–42 (Figure 5c). Anteriorly to external row of spines, ventral sucker bears six characteristic short sensory papillae (two anterior and four posterior) surrounded by convex tegumental collars. Deeper, after posterior row of spines, eight sensory papillae with short cilium and high tegumental collar arranged along circumference of sucker (Figure 5c). Penetration gland cells numbering five pairs. Their nucleated bodies are arranged symmetrically on either side of the oesophagus, approximately at the level of its middle and posteriorly. Their ducts pass forward skirting oral sucker dorsolaterally; three wider ducts open medially and ventrally near external opening of stylet pocket, while two more narrow ones open posteriorly and dorsolaterally to the former (Figure 4a,e). Contents of penetration gland cells are finely granular. Entire body of larva densely packed with tegumental cystogenous gland cells. Two types of these cells are distinctly seen: cells with coarsely granular contents and cells with granular unstaining contents. Cells of first type with distinct nuclei, nuclei in cells of second type indistinguishable.
At final stages of larva formation, gland cells apparently discharge some of their contents into tegument, granular material being visible throughout body and not only in cells. Prepharynx absent, pharynx rounded, intestine short, bifurcating anteriorly of ventral sucker. Excretory bladder Y-shaped, its arms skirting ventral sucker posteriorly. Main collecting tubes open at either side into the unpaired part of the bladder close to its bifurcation. Excretory formula 2 [(3 + 3 + 3) + (3 + 3 + 3)] = 36.
Infection of L. squalida with intramolluscan stages of R. mollissima is common along the coast of the Sea of Okhotsk, but its local variations are considerable (Table 4). In the coastal areas surveyed in our study, the highest prevalence, reaching almost 40%, was recorded in the Astronomicheskaya Bay. At the White Sea, infection of L. saxatilis with intramolluscan stages of R. mollissima was found so far only at the Kem’-Ludy archipelago. Our attribution of the cercariae from the mollusc L. mandshurica at the Sea of Japan (Russkiy island, Peter the Great Bay) to R. mollissima should be considered provisional because it was based only on morphological criteria.

Table 4.
Prevalence of Littorina squalida with intramolluscan stages of Renicola mollissima on the coasts of the Sea of Okhotsk.
- Metacercaria
Encysted metacercariae are always found in periwinkles infected with sporocysts with fully-formed cercariae (Figure 3). Cysts spherical, 190–220 in diameter. In Astronomicheskaya Bay, where a high prevalence of intramolluscan stages of R. mollissima was noted in L. squalida during the study period (Table 4), the prevalence of renicolid metacercariae in these snails reaches 80%. Apparently, the role of SIH can also be played by bivalves, as in closely related species.
- Cercaria cf. nordica I
Metacercariae tentatively identified as C. cf. nordica I (see Section 3.3 Remarks for our reasons for this identification) were revealed only based on molecular data (see Section 3.2 Molecular Results). They were found in sublittoral blue mussels M. edulis in the Chosha Bay and the Khaipudyr Bay of the SE Barents Sea. Localised in the hepatopancreas, they could not be distinguished during dissection from metacercariae of R. somateriae, which are localised in the same organ. The latter species was identified based on the comparison of the cox1 gene sequences with the corresponding sequences of the adults obtained in our previous study and with those of the intramolluscan stages of R. somateriae available in GenBank [7]. Total prevalence of metacercariae of these two species in mussels made up 5–50% in the Chosha Bay and reached 40% in the Khaipudyr Bay.
3.2. Molecular Results
We generated two new sequences of the partial 28S rRNA gene (1159 bp), 14 cox1 sequences (318 bp), and four 12S rRNA sequences (334 bp) for R. mollissima (Table 1). In addition, four new cox1 sequences were obtained for R. somateriae (metacercariae from the blue mussels obtained in the Chosha Bay and the Khaipudyr Bay) and one for R. mediovitellata (adult from common eider S. mollissima obtained in Shelikhov Bay, Sea of Okhotsk). The same three renicolid clades—Clade I + (Clade II + Clade III)—that had been identified in our earlier study [7] can be seen in the newly constructed phylograms (Figure 6 and Figure 7).
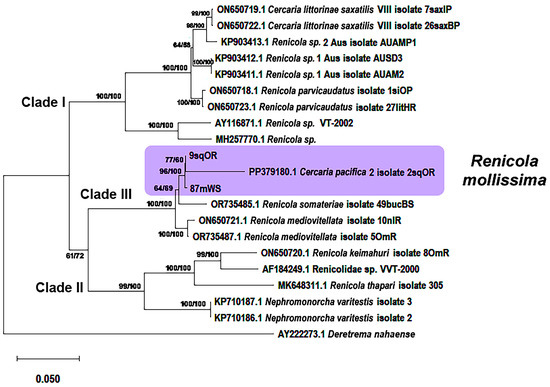
Figure 6.
Phylogenetic relationships between Renicola spp. based on maximum-likelihood and Bayesian inference (BI) analyses of the D1–D3 fragment of the 28S rRNA genes dataset: phylogenetic tree reconstructed with D1–D3 fragments of the 28S rRNA genes. Maximum-likelihood bootstrap support values inferred from 1000 replicates are followed by posterior probabilities from BI analysis. Magenta block indicates R. mollissima clade.
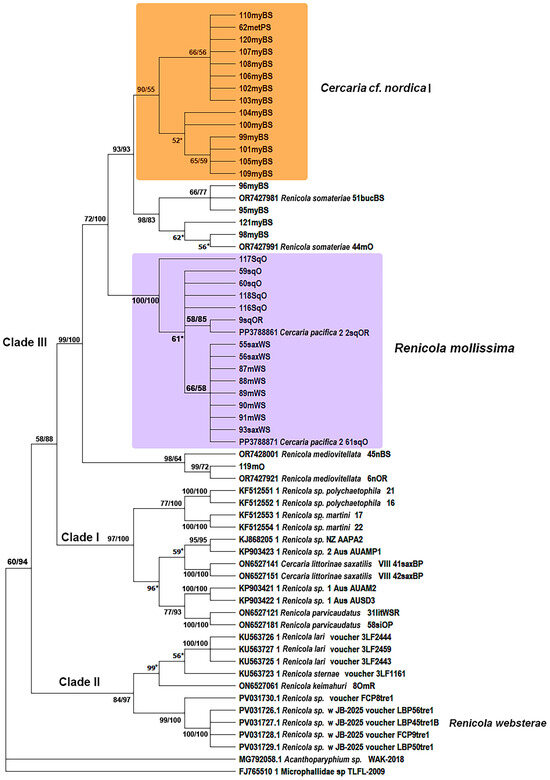
Figure 7.
Phylogenetic relationships between Renicola spp. based on maximum-likelihood and Bayesian inference (BI) analyses of the cox1 gene dataset. Maximum-likelihood bootstrap support values inferred from 1000 replicates are followed by posterior probabilities from BI analysis; only values ≥ 50 are shown. Asterisks indicate only bootstrap values. Magenta block indicates R. mollissima clade.
Phylogenetic analyses based on two mitochondrial markers and the D1–D3 fragments of 28S rRNA gene showed that the adults from the common eider at the White Sea (Chupa inlet, 88mWS–91mWS), which we identified as R. mollissima based on morphological characters, matched those of the intramolluscan stages of C. pacifica 2 isolated from L. squalida in the Sea of Okhotsk and the Sea of Japan as well as those from L. saxatilis in the White Sea (Figure 6, Figure 7 and Figure 8). This means that they are life cycle stages of the same species, R. mollissima. In our previous study [7], this species was referred to as C. pacifica 2 (isolate 2sqOR, GenBank accession numbers PP379180 and PP378886) (Table 1).
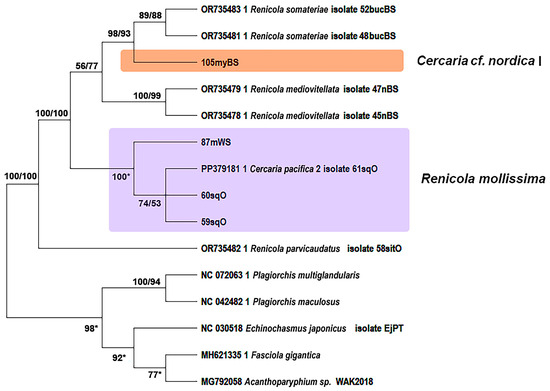
Figure 8.
Phylogenetic relationships between Renicola spp. based on maximum likelihood and Bayesian inference (BI) analyses of the mitochondrial 12S rRNA partial gene dataset. Maximum likelihood bootstrap support values inferred from 1000 replicates are followed by posterior probabilities from BI analysis. Asterisks indicate only bootstrap values. Magenta block indicates R. mollissima clade.
According to all three markers, sequences of R. mollissima form sister branches with those of R. somateriae. Their proximity is confirmed by p-distance values. Genetic distances between R. mollissima and other species correspond to interspecific levels: 0.041 ± 0.005 to 0.156 ± 0.010 for 28S rRNA, 0.077 ± 0.014 to 0.271 ± 0.025 for cox1, and 0.062 ± 0.012 to 0.464 ± 0.032 for 12S rRNA (Tables S1–S3 in Supplementary Materials). The intragroup distances support the conspecificity: 0.031 ± 0.004 (28S rRNA), 0.060 ± 0.002 (cox1), and 0.050 ± 0.010 (12S rRNA). The closest relative of R. mollissima is R. somateriae, with pairwise distances of 0.077 ± 0.014 (cox1) and 0.056 ± 0.013 (12S rRNA) (Tables S2 and S3 in Supplementary Materials). The Pacific isolates (2sqOR and 9sqOR) form a separate branch from the White Sea sample (87mWS) according to the 28S rRNA phylogeny (Figure 6), and a similar pattern can be seen for the 12S marker (Figure 8). However, in the tree based on the cox1 gene, the support for the branches within the R. mollissima clade is rather low for most of the Pacific samples (59–60sqO, 116sqO, 118sqO) except 117sqO; in a further twist, isolate 61sqO from the Sea of Okhotsk clusters with the isolates from the White Sea (Figure 7).
Metacercariae of renicolids from the blue mussels of Chosha Bay and Khaipudyr Bay (SE Barents Sea) were distinctly divided into two species on the basis of cox1 fragments (Figure 7). Most of the samples probably belong to C. cf. nordica I. The cox1 gene sequences of the remaining samples (Figure 7: 95myBS, 96myBS, 98myBS, 121myBS) matched the sequences of R. somateriae obtained in our previous study [7] and represented in GenBank (OR742798.1 and OR742799.1). Similarly to R. mollissima, C. cf. nordica I belongs to a clade closely related to R. somateriae: the p-distance values between them were only 0.03 ± 0.009 (cox1) and 0.028 ± 0.009 (12S rRNA) (Tables S2 and S3 in Supplementary Materials).
We identified six distinct haplotypes of R. mollissima based on cox1 sequences (Figure 9). All isolates from the White Sea and one from the Sea of Japan clustered into a single haplotype, suggesting a lack of local differentiation. Another isolate from the Sea of Japan represented a separate haplotype, differing by only one nucleotide substitution from one of the haplotypes from the Sea of Okhotsk. Notably, one haplotype from the Sea of Okhotsk differed from its closest relative by eight substitutions, indicating a higher level of divergence within this region. The negative value of Tajima’s D (−2.971, p = 1) suggests an excess of low-frequency polymorphisms. Although the limited sample size precludes definitive conclusions about the population structure, our data are consistent with the scenario of a recent demographic expansion.
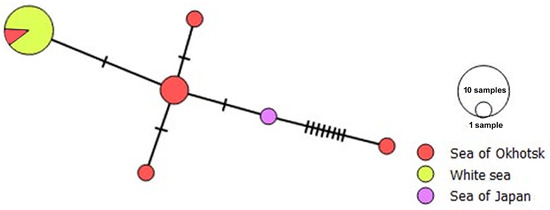
Figure 9.
A median joining haplotype network for Renicola mollissima based on partial cox1 gene sequences. The colours in the haplotype network indicate sampling regions, and the circle size is proportional to the sample size. Hatchmarks represent nucleotide substitutions.
In contrast, only one haplotype was found after an analysis of 14 C. cf. nordica I specimens, with an intragroup p-distance of 0.005 ± 0.002. Tajima’s D value was close to zero, indicating the pattern consistent with neutral evolution.
3.3. Remarks
Our molecular results provide convincing evidence that adults obtained in our study from the common eiders at the White Sea and intramolluscan stages of C. pacifica 2 from L. squalida from the Sea of Okhotsk and the Sea of Japan, and L. saxatilis from the White Sea are different life cycle stages of the same species, R. mollissima. Its adults can be easily distinguished from related species of the ‘duck clade’ by the characteristic arrangement of vitellaria. In R. mediovitellata and R. somateriae, two rows of vitelline follicles run dorsally along the intestinal caeca or intracaecally from the level of the pharynx to the base of the caudal process [7,25,26], while in R. mollissima these rows do not reach the base of the caudal process, ending approximately at the level of the testes. In immature adults of R. mollissima, vitelline follicles at each side of the body are arranged more loosely than in individuals of R. mediovitellata and R. somateriae of approximately the same age and do not form clear parallel rows. In contrast to the latter two species, vitelline follicles in R. mollissima contact each other only in old worms with a strongly distended uterus packed with eggs.
In the duck parasite R. ovocallosa Reimer, 1971, vitellaria form a medio-lateral field in the equatorial part of the body [23]. Though the eggs of R. mollissima are similar to those of R. mediovitellata in shape and size, they lack the small pointed knob on the egg pole opposite to the operculum; besides, the eggs of R. somateriae are rounded to oval with a very thick eggshell (3.5–5.8) [7]. The eggs of R. ovocallosa also have a thick eggshell (4.5) [23] and are similar in shape and size to the eggs of R. somateriae.
A characteristic feature of R. mollissima adults is that the uterus with eggs does not fill the entire body of the mature worm up to the base of the caudal process. Uterine loops in R. mollissima are concentrated in the central part of the body anterior of the ventral sucker and on the sides, where they extend from the anterior end almost to the base of the caudal process. This feature is also noted in the original description by Kulachkova [9].
Our molecular data made it possible to identify the cercaria of R. mollissima as C. pacifica 2 from snails L. squalida of the Sea of Japan. C. pacifica 2 in Pois et al. [12] has a somewhat smaller size than the larvae examined in our study, probably because Pois et al. [12] measured ethanol-fixed, and thus contracted, larvae. Cercaria pacifica 2 is identical to cercariae from L. squalida and L. saxatilis examined in this study in respect of morphology, including the shape and size of the stylet. In our material, cercariae obtained from L. squalida were larger than those from L. saxatilis and L. mandshurica (Table 3). This is probably associated with the fact that snails of the former species are much larger than snails of the latter two species, which are similarly sized. Cercariae produced by larger snails are known to be larger than those produced by smaller ones (e.g., [27,28,29,30]), probably because larger snails have more resources that can be channelled to cercarial production [28]. Cercariae of R. mollissima appear to be a case in point.
In morphology, the cercaria of R. mollissima is most similar to the cercaria of R. somateriae from Buccinum undatum Linnaeus, 1758 and to Cercaria nordica I Marasaev, 1988 from Neptunea communis (Middendorff, 1849). The larvae of R. mollissima from L. saxatilis are smaller than the cercariae of R. somateriae, but those from the larger snails L. squalida, which are closer in size to B. undatum, are almost identical with cercariae of R. somateriae in respect of morphometry. However, they differ in the shape of the stylet, which has a distinct bulbous base in the larvae of R. mollissima and no such structure in the larvae of R. somateriae. Another difference concerns the number of large spines in the suckers: they are more numerous in the larvae of R. somateriae. The stylet shape in C. nordica I is similar to that of R. mollissima larvae, but, judging from the figure in Marasaev (Figure 1b, p. 255 in [24]), the bulbous base is not expressed in them. The number and position of the ducts of penetration glands are also different: in C. nordica I, 2 or 3 ducts open at the tip of the stylet on each side and one duct opens dorsolaterally from them [24].
Renicolid xiphidiocercaria Cercaria emasculans Pelseneer, 1906, C. brevicauda Pelseneer, 1906 and Cercaria littorinae VI Sannia & James, 1977 from L. saxatilis of the Atlantic coast of France, UK (Wales) and Iceland differ considerably from the larvae of R. mollissima. In C. emasculans, the stylet is larger (17–25) than in the larvae of R. mollissima, while in C. brevicauda it is much smaller (6–8) [31]. The bulbous base of the stylet is not expressed in either of these species. Besides, C. emasculans has three pairs of penetration glans, C. brevicauda has two pairs, while C. littorinae VI has only one pair [31,32]. Xiphidiocercariae are also characteristic of the group of species included in Clade I (‘Parvicaudata’ group) based on molecular data (for details see: [6]). In addition to the differences in marker DNA sequences, the cercariae of these species, such as Renicola parvicaudatus (Stunkard & Shaw, 1931) Galaktionov et al., 2022, Cercaria littorinae saxatilis VIII Galaktionov et al., 2022, Renicola sp. NZ O’Dwyer et al., 2014 [33], Renicola sp. 1 Aus O’Dwyer et al., 2015, Renicola sp. 2 Aus O’Dwyer et al., 2015 [34], Renicola sp. Martorelli et al., 2008, Renicola sp. “polychaetophila” Hechinger & Miura, 2014 [35] and Renicola sp. “martini” Hechinger & Miura, 2014 [35] differ from those of R. mollissima in morphometry, stylet structure, number and position of the ducts of penetration glands and the number of large spines in oral and ventral suckers [6,33,34,35,36]. As for xiphidiocercariae described by Cable [37], for which molecular data are absent, Cercaria caribbea XXXII Cable, 1956 and C. caribbea XXXIII Cable, 1956 from molluscs Cerithideopsis costata (da Costa, 1778) (Potaminidae) differ by a greater number of penetration gland cells and another arrangement of their ducts as well as the stylet shape (bullet-like in the former and conical in the latter). Cercaria opaca Holliman, 1961 from the snail Littoraria irrorata (Say, 1822) (Littorinidae) (Florida) is distinguished by the conical stylet and numerous penetration glands [38].
We provisionally identified renicolid metacercariae from mussels Mytilus edulis of the Chosha Bay and the Khaipudyr Bay (SE Barents Sea), which were molecularly different from the larvae of R. somateriae from the same bivalves, as C. cf. nordica I, because Marasaev [24] described intramolluscan stages of C. nordica I from benthic molluscs N. communis (syn. N. borealis (R. A. Philippi, 1850)) precisely from these areas. Cercaria nordica I is similar to the larvae of R. somateriae in size and stylet shape, differing only in the number of penetration gland cells [7]. Neptunea communis, FIH of C. nordica I, belongs to the same family (Buccinidae) as B. undatum, FIH of R. somateriae, and these two molluscan species inhabit the same sublittoral biotopes of the Barents Sea.
4. Discussion
Our study confirmed the circumpolar distribution of R. mollissima and clarified its transmission ways in the North Atlantic (NA) and the North Pacific (NP). The main DH of R. mollissima is common eider, from which it was originally described [9]. Common eider is a specialised benthos feeder, and much of its diet consists of molluscs playing the role of SIH for R. mollissima (e.g., [39,40,41,42,43,44,45]). In the Kandalaksha Reserve (White Sea, Kandalaksha Bay), up to 39.4% of ducklings aged more than 2 weeks and 14.7% of adult birds were infected with this parasite in 1950–1952 [9]. According to Kulachkova [9], the birds are infected in summer, and in autumn, all adult worms are mature and their prevalence in birds is the highest. After the eiders arrive from wintering places in ice-free parts of the White Sea in May, the prevalence is 2–3 times lower, but all worms are mature and have numerous eggs. No renicolids are recorded in eiders in June; apparently, the worms die by this time. In July, only young worms are recorded. This means that the life span of R. mollissima is approximately one year, and the eggs are dispersed in the biotope in large numbers in spring-early summer, immediately after the arrival of eiders at the nesting sites. Our data agree with Kulachkova’s conclusion. Only large mature adults filled with eggs were found in a female common eider dissected in May, while adults from the eider examined in early September, although mature, contained relatively few eggs (Figure 2b).
An examination of the collection of R. mollissima whole mounts by Kulachkova stored in ZISP showed that, in addition to common eider, this parasite is also found at the White Sea in long-tailed duck and common goldeneye. In the Asian part of its distribution, adults of R. mollissima were recorded in the Pacific common eider of Chukotka (Anadyr Estuary) (in 5 out of 18 dissected birds, with an infection intensity of 5–77) [10] and in the north of the Sea of Okhotsk [11].
The cercaria of R. mollissima was described as C. pacifica 2 from the molluscs L. squalida in the Sea of Japan [12]. We found intramolluscan stages of this species in L. squalida from the Sea of Okhotsk and the Sea of Japan and also, sporadically, in L. mandshurica from the Sea of Japan. There is no doubt that L. squalida is the main FIH of R. mollissima in the NP. This mollusc is widely distributed in the littoral and the upper sublittoral of NP seas from the north of the Sea of Japan in the south to the coast of Kamchatka and Alaska in the north [46,47,48]. The infection of L. squalida with intramolluscan stages of R. mollissima in some localities in the north of the Sea of Okhotsk can be quite high (Table 4). No infection with R. mollissima was found in Littorina sitkana R.A. Philippi, 1846 from the same localities or in other coastal areas of the Sea of Okhotsk, where parasitological surveys of L. sitkana were conducted in 2003–2013 (KG, personal observations). It is noteworthy that the infection of L. squalida with intramolluscan stages of R. mollissima at the Asian coast extends southwards far beyond the distribution of common eider, which is restricted in the south by the Babushkin Bay (~58° N) [49]. This clearly indicates that the role of DH of R. mollissima there is played by some other duck species.
At the White Sea, intramolluscan stages of R. mollissima have been recorded only in L. saxatilis. They have not been found in Littorina (Littorina) littorea (Linnaeus, 1758), the only representative of the subgenus Littorina in NA. At the same time, L. (L.) squalida and L. (L.) littorea are Pacific-Atlantic sister taxa [50,51]. Their origin was associated with the opening of the Bering Strait, estimated at 3.5–4 Ma BP, and the penetration of Pacific marine fauna into the Atlantic [52,53,54,55]. The subsequent glaciation at 2.4 Ma BP resulted in the closure of the trans-Arctic migration route and the formation of the Pacific-Atlantic sister taxa. These events also resulted in the formation of other Atlantic periwinkles, including L. saxatilis, but their Pacific sister taxa are L. natica D. Reid, 1996 and L. aleutica Dall, 1782, not L. squalida or any other Pacific species of the subgenus Littorina [51]. Intramolluscan stages of renicolids were not found in snails L. aleutica and L. natica from Kresta Bay and the Saint Lawrence Bay (Bering Sea, Chukchi Peninsula) (KG, personal observations). Based on the phylogenetic data, one might expect to find infection with R. mollissima in NA seas in L. littorea, but these snails play the role of FIH for only one renicolid, R. parvicaudatus [6].
We may conjecture that R. mollissima did not get into NA together with the ancestor of L. littorea (“missing the boat”) or, for some reason, could not naturalise there (“drowning on arrival”). This species probably formed in NP in association with L. squalida. The fact that cox1 sequences of isolates from NP and NA coincide or differ, usually, by 1–2 substitutions, indicates that the introduction of R. mollissima into NA from NP is fairly recent. Negative values of Tajima’s D also indicate a recent expansion. It could happen during one of the warm interglacials of the Pleistocene (e.g., in Eemian interglacial, ca. 129–116 thousand years ago) or even after the last glaciation (<11 thousand years ago). Negative values of Tajima’s D also indicate a recent expansion. In this case, the haplotype from NP, which differs from the others by 8–10 substitutions, may reflect the ancestral state. Further studies involving a larger number of isolates are necessary for a deeper understanding of the phylogeography of R. mollissima.
It is noteworthy that the only NA region where R. mollissima has been found so far is the White Sea, where it has been recorded in both definitive and intermediate hosts. It has not been reported from common eiders or other sea ducks elsewhere in NA [40,56,57,58,59,60], though it should be noted that in most of these studies, renicolids were not identified down to the species level. Intramolluscan stages of digeneans in periwinkles from NA have been extensively studied (e.g., [6,31,61,62,63,64,65,66,67,68,69,70,71,72,73,74]), and infection with R. mollissima has not been detected in them anywhere except the White Sea. It is possible that R. mollissima might yet be found in other areas of NA after comprehensive research. At the same time, it cannot be ruled out that the White Sea, which lies on a major migration route of waterfowl along the East-Atlantic Flyway [75,76], was the first place where R. mollissima arrived from NP.
There is no doubt that C. cf. nordica I also parasitises diving ducks at the adult stage. Its metacercariae were found in sublittoral mussels in the Khaipudyr and the Chosha bays, where ducks, especially King eider S. spectabilis, concentrate during moulting and autumn migration [77,78,79], but auks, which are good divers, are absent. Sublittoral mussels, which are inaccessible to gulls, are the basis of the ducks’ diet in this region [80]. Along with the larvae of C. cf. nordica I, metacercariae of R. somateriae, another duck parasite, were identified in the same mussels with the help of molecular methods. This is the first reliable record of the larvae of R. somateriae in mussels, which means that these molluscs should be considered as its natural SIH.
In the phylograms, C. cf. nordica I is very close to R. somateriae, the distances between them only slightly exceeding the level of intra-species variation (Supplementary Materials, Tables S1–S3). Their FIH are closely related buccinid molluscs: N. communis and B. undatum, respectively. C. cf. nordica I and R. somateriae might actually be cryptic species. If metacercariae of C. cf. nordica I indeed belong to the same species as the intramolluscan stages from N. communis, its cercariae are very similar to those of R. somateriae (see: Section 3.3 Remarks). The adults of C. cf. nordica I may be morphologically indistinguishable from those of R. somateriae and could be identified as the latter during parasitological surveys of ducks.
The genetic composition of the metacercariae of C. cf. nordica I examined in our study was strikingly homogeneous, with no variation by the cox1 gene. Infection of N. communis with intramolluscan stages of C. nordica I has been detected so far only in the SE Barents Sea (Pechora Sea and adjacent water areas). Neptunea communis is an Arctic-boreal species with an almost circumpolar-Arctic distribution. It inhabits all Arctic seas from the Barents Sea in the west to the Northern Bering Sea and Alaska in the east [47,48,81]. Considering the distribution of FIH, we can assume that C. nordica I is also an Arctic or Arctic-boreal species transmitted in high latitudes. If the metacercariae under consideration indeed belong to C. nordica I, a high homogeneity of haplotypes of C. cf. nordica I may be the result of the gene flow within the Arctic provided by migrating ducks. Additional data are required to confirm this hypothesis. To note, a low haplotype diversity by the cox1 gene is also characteristic of the sister species of C. cf. nordica I, R. somateriae, whose FIH are trans-Arctic molluscs, Buccinum spp. [7]. It seems that the trans-Arctic range of FIH combined with the possibility of long-term survival of adults in migrating anatids contributes to the smoothing of the genetic structure of populations of these renicolids.
The lack of haplotype variability in C. cf. nordica I found in our study might reflect a recent founder event or introduction of this lineage into the SE Barents Sea, which has not yet generated enough mutations to shift the site-frequency spectrum. It should be noted, however, that Chubrik [72] mentioned the finding of cercariae, which she identified as Cercaria parvicaudata Stunkard & Show, 1931, in the mollusc Neptunea despecta (Linnaeus, 1758) in the western Barents Sea. It is possible that the cercariae in question actually belonged to C. nordica I. In this case, the range of this species may be wider, since N. despecta is distributed in boreal waters of the NA [81].
Our results expanded the composition of Clade III identified by Galaktionov et al. [7], which now includes four molecularly confirmed species: R. mediovitellata, R. somateriae, R. mollissima and C. cf. nordica I. Renicola brantae McIntosh et Farr, 1952, described from Canada goose Branta canadensis (Linnaeus, 1758) in North Carolina, was synonymised with R. mediovitellata in our previous study [7]. All these species use anseriform birds as definitive hosts. Their association on the molecular tree into the monophyletic Clade III indicates the validity of allocating ‘duck’ species into the subgenus Anatirenicola Odening, 1962, proposed by Odening [82]. The adults of this clade/subgenus are characterised by the arrangement of vitelline follicles in two dorsolateral rows along the sides of the body. Testes are separate, lying symmetrically relative to the ventral sucker, ovary lobed. In our opinion, R. ovocallosa, described from long-tailed duck in the North Sea [23], is likely to be another member of Anatirenicola, being morphologically similar to other species of this subgenus, especially R. somateriae. To verify this assumption, molecular data and a more detailed morphological description of R. ovocallosa adults are necessary.
The addition of new isolates to the molecular trees based on cox1 and partial 28S rRNA in this study (Figure 6 and Figure 7) did not change the distribution of renicolid species across the three clades as compared to the trees in [6,7,8]. This means that we may be reasonably sure that these clades, though not comprising the entire species diversity of renicolids, reflect the directions of their evolution fairly well. Species of Clade I and Clade III appear to be closer to the ancestral state, which is characterised by xiphidiocercariae and the use of invertebrates (molluscs and polychaetes) as SIH. These features are characteristic of the superfamily Microphalloidea Ward, 1901 (suborder Xiphidiata), to which the family Renicolidae belongs [17,83,84]. Species of these two clades are specialised to be transmitted in coastal and estuarine biotopes, with members of Clade I parasitising gulls and waders and members of Clade III parasitising benthivorous ducks.
Apparently, it was parasitism in fish-eating larids that was the starting point for the transition from the use of invertebrates to the use of fish as SIH. This fundamental change brought about the cercariae of the Rhodometopa morphotype (through several transitional morphotypes) and the possibility of colonising strictly or predominantly fish-eating birds such as auks, penguins, pelicans, etc. [6,7,85,86,87]. Colonisation of fish-eating birds was, apparently, the main trend in renicolid evolution and the one conducive to their flourishing: most of the renicolid species known to date are associated with fish-eating birds [3]. Owing to parasitism of renicolid metacercariae in mobile fish hosts, the range of definitive hosts could be expanded to include birds of open sea areas. Unfortunately, there are still few or almost none molecular data on renicolids parasitising as adults waders, ardeids, rallids, pelecanids, gaviids, alcids, etc. and little information on their life cycles [3]. It seems probable that parasites of specialised fish-eating birds will be assigned to Clade II. However, it cannot be ruled out that new clades would be identified in the phylogenetic tree of the Renicolidae, refining our hypothesis about the evolutionary directions of this digenean family.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17080512/s1, Table S1: Mean genetic divergence between renicolid species estimated as p-distance for the D1–D3 fragment of 28S rRNA gene. Table S2: Mean genetic divergence between renicolid species estimated as p-distance for the fragment of cox1 mitochondrial gene. Table S3: Mean genetic divergence between Renicola spp. and Fasciola gigantica estimated as p-distance for the fragment of 12S rRNA mitochondrial gene.
Author Contributions
Conceptualization, K.V.G. and A.I.S.; Material collection, K.V.G., K.V.R. and A.I.S.; Methodology, K.V.G., A.I.S. and A.A.M.; software, A.I.S., K.V.G., A.A.M. and A.E.R.; validation, K.V.G., A.I.S., K.V.R., A.A.M. and A.E.R.; writing—original draft preparation, K.V.G. and A.I.S.; writing—review and editing, K.V.G. and A.I.S.; visualization, K.V.G., A.I.S. and A.A.M.; supervision, K.V.G.; and funding acquisition, K.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (23-14-00329). Sampling at the Barents Sea and the White Sea and work at the “Taxon” Research Resource Center were partly financed by the State Academic Program (125012800903-5). The funders had no role in the study design, data collection, analyses, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are available from the authors upon request, and the sequences used in this study are available in the GenBank database: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 20 June 2025), PV644169–PV644201, PV642434–PV642438 and PV639403–PV639404.
Acknowledgments
The authors are grateful to the White Sea Biological Station of the Zoological Institute of the Russian Academy of Sciences (ZIN RAS), Institute of Biological Problems of the North of the Far East Branch of the Russian Academy of Sciences (IBPN RAS) for providing fieldwork infrastructure. We thank the crew of the RV “Professor Vladimir Kuznetsov”, whose professional skills made it possible for us to work in the coastal waters of the SE Barents Sea (including the Pechora Sea). We also thank the members of our research group: Kirill Nikolaev, Anna Vinogradova, Ivan Levakin, Daniil Fedorov, Alina Sokolova, Anastsija Smoljaninova and Georgy Kremnev for their help with sampling and primary treatment of the material. We wish to acknowledge the IEPUI, “Taxon” Research Resource Center (http://www.ckp-rf.ru/ckp/3038/, accessed on 10 June 2024) of ZIN RAS and the research resource Center “Molecular and Cell Technologies” of St. Petersburg State University for granting access to their facilities. We are grateful to Natalia Lentsman for her help with the manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gibson, D. Family Renicolidae Dollfus, 1939. In Keys to Trematoda 3; CABI Publishing: Wallingford, UK, 2008; pp. 591–594. [Google Scholar]
- Kharoo, V.K. A Review of the history and classification of the family Renicolidae Dollfus, 1939 (Trematoda: Digenea). Indian J. Fundam. Appl. Life Sci. 2013, 3, 6–12. [Google Scholar]
- Sudarikov, V.E.; Stenko, R.P. Trematodes of the family Renicolidae. In Helminths of Farming and Hunting Animals; Nauka: Moscow, Russia, 1984; pp. 34–89. (In Russian) [Google Scholar]
- Blasco-Costa, I.; Poulin, R. Parasite life-cycle studies: A plea to resurrect an old parasitological tradition. J. Helminthol. 2017, 91, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Bass, L.G.; Campos-Camacho, J.; Dittel-Meza, F.A.; Fonseca, C.; Huang-Qiu, Y.Y.; Olivares, R.W.I.; Romero-Vega, L.M.; Villegas-Rojas, F.; Solano-Barquero, A. Integrative taxonomy in helminth analysis: Protocols and limitations in the twenty-first century. Parasites Vectors 2025, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Galaktionov, K.V.; Solovyeva, A.I.; Blakeslee, A.M.H.; Skírnisson, K. Overview of Renicolid Digeneans (Digenea, Renicolidae) from marine gulls of Northern Holarctic with remarks on their species statuses, phylogeny and phylogeography. Parasitology 2023, 150, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Galaktionov, K.V.; Solovyeva, A.I.; Miroliubov, A.; Romanovich, A.E.; Skírnisson, K. Untangling the “Renicola somateria” (Digenea, Renicolidae) muddle: Actual number of species and their distribution and transmission in the Holarctic. Diversity 2024, 16, 402. [Google Scholar] [CrossRef]
- Presswell, B.; Bennett, J. Two new species of kidney fluke (Trematoda: Renicolidae) from New Zealand penguins (Spheniscidae), with a description of Renicola websterae n. sp. Syst. Parasitol. 2025, 102, 26. [Google Scholar] [CrossRef] [PubMed]
- Kulachkova, V.G. New Species of renal trematodes Renicola mollissima sp. nov from common eider. Trans. Leningr. Soc. Nat. 1957, 73, 198–203. (In Russian) [Google Scholar]
- Ryzhikov, K.M.; Timofeeva, T.N.; Dudorova, E.N. To cognition of trematodes from the Chukotka eider ducks. Proc. Helminthol. Lab. USSR Acad. Sci. 1966, 17, 157–168. (In Russian) [Google Scholar]
- Atrashkevich, G.I.; Orlovskaja, O.M.; Regel, K.V. The first data about the parasites of common eider’ (Somateria mollissima) population of the Sea of Okhotsk. In Proceedings of the IV Congress of the Russian Society of Parasitologists, St. Petersburg, Russia, 20–25 October 2008; Galaktionov, K.V., Dobrovolskij, A.A., Eds.; Lemma: St Petersburg, Russia, 2008; pp. 35–38. (In Russian). [Google Scholar]
- Pois, N.V.; Tsimbaljuk, A.K.; Ardasheva, N.B. three new species of marine cercariae from the intertidal zone. Parazitologiya 1974, 53, 413–419. (In Russian) [Google Scholar]
- Galaktionov, K.V.; Solovyeva, A.I.; Miroliubov, A. Elucidation of Himasthla leptosoma (Creplin, 1829) Dietz, 1909 (Digenea, Himasthlidae) life cycle with insights into species composition of the North Atlantic Himasthla associated with periwinkles Littorina spp. Parasitol. Res. 2021, 120, 1649–1668. [Google Scholar] [CrossRef] [PubMed]
- Winnepenninckx, B.; Backeljau, T.; De Wachter, R. Extraction of high molecular weight DNA from Molluscs. Trends Genet. 1993, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.E.; Saralamba, N.; Saralamba, S.; Ruangsittichai, J.; Thaenkham, U. The potential use of mitochondrial ribosomal genes (12S and 16S) in DNA barcoding and phylogenetic analysis of Trematodes. BMC Genom. 2022, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Palm, H.W.; Waeschenbach, A.; Olson, P.D.; Littlewood, D.T.J. Molecular phylogeny and evolution of the Trypanorhyncha Diesing, 1863 (Platyhelminthes: Cestoda). Mol. Phylogenet. Evol. 2009, 52, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic Variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-Feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Reimer, L.W. Neue Cerearien Der Ostsee Mit Einer Diskussion Ihrer Möglichen Zuordnung Und Einem Bestimmungsschlüssel. Parasitol. Schriftenr. 1971, 21, 125–149. [Google Scholar]
- Marasaev, S.F. New Renicolid cercaria from the mollusc Neptunea borealis (Prosobranchia, Buccinidae). Parazitologiya 1988, 22, 254–258. (In Russian) [Google Scholar]
- Bychovskaja-Pavlovskaja, I.E. New species of kidney parasites (genus Renicola) from birds. Dokl. Akad. Nauk. SSSR 1950, 71, 415–416. (In Russian) [Google Scholar]
- Belopolskaja, M.M. Parasites of marine waterfowl. Uch Zap. Leningr. Gos. Univ. Ser. Biol. Nauk. 1952, 141, 127–180. (In Russian) [Google Scholar]
- Loker, E.S. A Comparative study of the life-histories of mammalian schistosomes. Parasitology 1983, 87, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Springer, Y.P.; Keeney, D.B.; Poulin, R. Intra- and interclonal phenotypic and genetic variability of the trematode Maritrema novaezealandensis. Biol. J. Linn. Soc. 2011, 103, 106–116. [Google Scholar] [CrossRef]
- McCarthy, H.O.; Fitzpatrick, S.; Irwin, S.W.B. Life history and life cycles: Production and behavior of trematode cercariae in relation to host exploitation and next-host characteristics. J. Parasitol. 2002, 88, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Moné, H.; Théron, A. Schistosoma mansoni—Biomphalaria glabrata: Dynamics of the sporocyst population in relation to the miracidial dose and the host size. Can. J. Zool. 1993, 71, 1880–1885. [Google Scholar] [CrossRef]
- James, B.L. The Digenea of the intertidal prosobranch, Littorina saxatilis (Olivi). J. Zool. Syst. Evol. Res. 1969, 7, 273–316. [Google Scholar] [CrossRef]
- Sannia, A.; James, B.L. The Digenea in marine molluscs from Eyjafjördur, North Iceland. Ophelia 1977, 16, 97–109. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Blasco-Costa, I.; Poulin, R.; Faltýnková, A. Four marine digenean parasites of Austrolittorina spp. (Gastropoda: Littorinidae) in New Zealand: Morphological and Molecular Data. Syst. Parasitol. 2014, 89, 133–152. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, K.; Faltýnková, A.; Georgieva, S.; Kostadinova, A. An Integrative taxonomic investigation of the diversity of digenean parasites infecting the intertidal snail Austrolittorina unifasciata Gray, 1826 (Gastropoda: Littorinidae) in Australia. Parasitol. Res. 2015, 114, 2381–2397. [Google Scholar] [CrossRef] [PubMed]
- Hechinger, Y.R.; Miura, O. Two “new” Renicolid trematodes (Trematoda: Digenea: Renicolidae) from the California horn snail, Cerithidea californica (Haldeman, 1840) (Gastropoda: Potamidida). Zootaxa 2014, 3784, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Martorelli, S.R.; Fredensborg, B.L.; Leung, T.L.F.; Poulin, R. Four Trematode cercariae from the New Zealand Intertidal snail Zeacumantus subcarinatus (Batillariidae). N. Z. J. Zool. 2008, 35, 73–84. [Google Scholar] [CrossRef]
- Cable, R.M. Marine cercariae of Puerto Rico. In Scientific Survey of Porto Rico and the Virgin Islands; New York Academy of Sciences: New York, NY, USA, 1956; pp. 491–577. [Google Scholar]
- Holliman, R.B. Larval trematodes from the Apalachee Bay Area, Florida, with a checklist of known marine cercariae arranged in a key to their super-families. Tulane Stud. Zool. 1961, 9, 1–74. [Google Scholar]
- Merkel, F.R.; Jamieson, S.E.; Falk, K.; Mosbech, A. The diet of common eiders wintering in Nuuk, Southwest Greenland. Polar Biol. 2007, 30, 227–234. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Węsławski, J.M.; Stempniewicz, L. Food chain, parasites and climate changes in the High Arctic: A case study on trophically transmitted parasites of common eider Somateria mollissima at Franz Josef Land. Polar Biol. 2021, 44, 1321–1342. [Google Scholar] [CrossRef]
- Belopol’skij, L.O. Ecology of Colonial Seabirds of the Barents Sea; USSR Academy of Sciences Publishing: Moscow, Russia, 1957. (In Russian) [Google Scholar]
- Pethon, P. Food and feeding habits of the common eider (Somateria mollissima). Nytt Mag. Zool. 1967, 15, 97–111. [Google Scholar]
- Bustnes, J.O.; Erikstad, K.E. The Diets of sympatric wintering populations of common eider Somateria mollissima and King Eider S. spectabilis in Northern Norway. Ornis Fenn. 1988, 65, 163–168. [Google Scholar]
- Krasnov, Y.V.; Shklyarevich, G.A.; Goryaev, Y.I. Feeding habit of the common eider Somateria mollissima in the White Sea. Dokl. Biol. Sci. 2009, 427, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Bianki, V.V.; Boiko, N.S.; Ninburg, E.A.; Shklyarevich, G.A. Feeding of common eider of the White Sea. In Ecology and Morphology of Eiders in the USSR (Ecologia i Morphologia gag v SSSR); Uspensky, S.M., Ed.; Nauka: Moscow, Russia, 1979; pp. 126–170. (In Russian) [Google Scholar]
- Golikov, A.N.; Kussakin, O.G. Shell-Bearing gastropods of the intertidal zone of the seas of the USSR. Opredeliteli Po faune SSSR Izd. Zool. Institutom AN SSSR 1978, 116, 292. (In Russian) [Google Scholar]
- Sirenko, B.I. List of Species of Free-Living Invertebrates of Eurasian Arctic Seas and Adjacent Deep Waters; Russian Academy of Sciences, Zoological Institute: St. Petersburg, Russia, 2001. [Google Scholar]
- Sirenko, B.I. Check-List of Species of Free-Living Invertebrates of the Russian Far Eastern Seas; Russian Academy of Sciences, Zoological Institute: St. Petersburg, Russia, 2013. [Google Scholar]
- Krechmar, A.V.; Kondratyev, A.V. Waterfowl Birds of North-East Asia; NESC FEB RAS: Magadan, Russia, 2006; 458p. (In Russian) [Google Scholar]
- Reid, D.G.; Rumbak, E.; Thomas, R.H. DNA, Morphology and fossils: Phylogeny and evolutionary rates of the gastropod genus Littorina. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1996, 351, 877–895. [Google Scholar] [CrossRef]
- Reid, D.G.; Dyal, P.; Williams, S.T. A Global Molecular phylogeny of 147 periwinkle species (Gastropoda, Littorininae). Zool. Scr. 2012, 41, 125–136. [Google Scholar] [CrossRef]
- Golikov, A.N.; Skarlato, O.A. Evolution of the Arctic ecosystems during the Neogene period. In The Arctic Seas: Climatology, Oceanography, Geology, and Biology; Herman, Y., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1989; pp. 257–279. [Google Scholar]
- Vermeij, G.J. Anatomy of an Invasion: The Trans-Arctic Interchange. Paleobiology 1991, 17, 281–307. [Google Scholar] [CrossRef]
- Briggs, J.C. Global Biogeography; Briggs, J.C., Ed.; Elsvier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Briggs, J.C. Marine centres of origin as evolutionary engines. J. Biogeogr. 2003, 30, 1–18. [Google Scholar] [CrossRef]
- Thieltges, D.W.; Hussel, B.; Baekgaard, H. Endoparasites in common eiders Somateria mollissima from birds killed by an oil spill in the Northern Wadden Sea. J. Sea Res. 2006, 55, 301–308. [Google Scholar] [CrossRef]
- Skirnisson, K. Association of Helminth infections and food consumption in common eiders Somateria mollissima in Iceland. J. Sea Res. 2015, 104, 41–50. [Google Scholar] [CrossRef]
- Garden, E.; Rayski, C.; Thom, V. A Parasitic disease in eider ducks. Bird. Study 1964, 11, 280–287. [Google Scholar] [CrossRef]
- Grytner-Zięcina, B.; Sulgostowska, T. Trematodes of Oidemia fusca (L.), Oidemia nigra (L.) and Somateria mollissima (L.) from the Baltic coast. Acta Parasitol. Pol. 1978, 25, 121–128. [Google Scholar]
- Kuklin, V.V.; Kuklina, M.M. Helminths of Birds of the Barents Sea: Fauna, Ecology and Impact on the Hosts; Kola Science Centre Russian Academy Science Publication: Apatity, Russia, 2005. (In Russian) [Google Scholar]
- Podlipaev, S.A. Trematode parthenitae and larvae in the intertidal molluscs of the Eastern Murman. In Ecological and Experimental Parasitology. Vol. 2.; Poljansky, Y.I., Ed.; Izdatel’stvo Leningradskogo Universiteta: Leningrad, Russia, 1979; pp. 47–101. (In Russian) [Google Scholar]
- Laukner, G. Diseases of Mollusca: Gastropoda. In Diseases of Marine Animals. Vol I.; Kinne, O., Ed.; Wiley: Chichester, UK; New York, NY, USA; Brisbane, Australia; Toronto, ON, Canada, 1980; pp. 311–424. [Google Scholar]
- Irwin, S.W.B. Incidence of trematode parasites in two populations of Littorina saxatilis (Olivi) from the North shore of Belfast Lough. Irish Nat. J. 1983, 21, 26–29. [Google Scholar]
- Matthews, P.M.; Montgomery, W.I.; Hanna, R.E.B. Infestation of Littorinids by larval Digenea around a Small Fishing port. Parasitology 1985, 90, 277–287. [Google Scholar] [CrossRef]
- Newell, C.R. The Marine fauna and flora of the Isles of Scilly: Some marine Digeneans from invertebrate hosts. J. Nat. Hist. 1986, 20, 71–77. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Bustness, J. Distribution patterns of marine bird Digenean larvae in periwinkles along the Southern Barents sea coast. Dis. Aquat. Organ. 1999, 37, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Thieltges, D.W.; Krakau, M.; Andresen, H.; Fottner, S.; Reise, K. Macroparasite community in molluscs of a tidal basin in the Wadden Sea. Helgol. Mar. Res. 2006, 60, 307–316. [Google Scholar] [CrossRef]
- Granovitch, A.; Johannesson, K. Digenetic trematodes in four species of Littorina from the West coast of Sweden. Ophelia 2000, 53, 55–65. [Google Scholar] [CrossRef]
- James, B.L. The Distribution and keys of species in the family Littorinidae and of their Digenean parasites, in the region of Dale, Pembrokeshire. F. Stud. 1968, 2, 615–650. [Google Scholar]
- Werding, B. Morphologie, Entwicklung Und Ökologie Digener Trematoden-Larven Der Strandschnecke Littorina littorea. Mar. Biol. 1969, 3, 306–333. [Google Scholar] [CrossRef]
- Pohley, W.J. Relationships among three species of Littorina and their larval Digenea. Mar. Biol. 1976, 37, 179–186. [Google Scholar] [CrossRef]
- Chubrik, G.K. Fauna and ecology of trematode larvae from the molluscs of Barents and White Seas. In Life Cycles of Parasitic Worms of Northern Seas (Proceedings of the Murmansk Marine Biological Institute of the Kola Branch of the USSR Academy of Sciences 10(14)); Polanski, Y.I., Ed.; Nauka: Moscow-Leningrad, Russia, 1966; pp. 78–159. (In Russian) [Google Scholar]
- Robson, E.M.; Williams, I.C. Relationships of some species of digenea with the marine prosobranch Littorina littorea (L.) II. The effect of larval Digenea on the reproductive biology of L. littorea. J. Helminthol. 1971, 45, 145–159. [Google Scholar] [CrossRef]
- Combescot-Lang, C. Étude Des Trématodes Parasiles de Littorina saxalilis (Olivi) et de Leurs Effets Sur Cet Hôte. Ann. Parasitol. 1976, 51, 27–36. [Google Scholar] [CrossRef]
- Isakov, Y. MAR Project and conservation of waterfowl breeding in the USSR. In Proceedings of the Second European Meeting on Wildfowl Conservation, Noordwijk aan Zee, The Netherlands, 9–14 May 1966; Salverda, Z., Ed.; Ministry of Cultural Affairs, Recreation and Social Welfare: The Hague, The Netherlands, 1967; pp. 125–138. [Google Scholar]
- Scott, D.A.; Rose, P.M. Atlas of Anatidae Populations in Africa and Western Eurasia; Wetlands International: Wageningen, The Netherlands, 1996. [Google Scholar]
- Krasnov, Y.V.; Gavrilo, M.V.; Chernook, V.I. Distribution of Birds over the Pechora Sea: Data of Aerial Surveys. Zool. Z. 2004, 83, 449–458. (In Russian) [Google Scholar]
- Krasnov, Y.V.; Ezhov, A.V.; Galaktionov, K.V.; Shavykin, A.A. The numbers and seasonal distribution of the western population of the king eider (Somateria spectabilis): Monitoring organization in the northern seas of Russia. Biol. Bull. 2021, 48, 1041–1050. [Google Scholar] [CrossRef]
- Krasnov, Y.V.; Goryaev, Y.I.; Shavykin, A.A.; Nikolaeva, N.G.; Gavrilo, M.V.; Chernook, V.I. Atlas of the Pechora Sea Birds: Distribution, Abundance, Dynamics, Problems of Protection; Publishing Company of the Kola Branch of the Russian Academy of Sciences: Apatity, Russia, 2002. (In Russian) [Google Scholar]
- Sukhotin, A.A.; Krasnov, Y.V.; Galaktionov, K.V. Subtidal populations of the blue mussel Mytilus eEdulis as key determinants of waterfowl flocks in the Southeastern Barents Sea. Polar Biol. 2008, 31, 1357–1363. [Google Scholar] [CrossRef]
- Golikov, A.N. Gastropod molluscs of the genus Neptunea Bolten. In Fauna SSSR. Mollyuski. Vol. V, No. 1; USSR Academy of Sciences Publishing: Saint Petersburg, Russia, 1963. (In Russian) [Google Scholar]
- Odening, K. Neue Trematoden Aus Vietnamesischen Vogeln Des Berliner Tierparks (Mit Einer Revision Der Familie Renicolidae). Bijdr. Dierkd. 1962, 32, 49–63. [Google Scholar] [CrossRef]
- Pérez-Ponce De León, G.; Hernández-Mena, D.I. Testing the Higher-Level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the “next-generation” tree of life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Bray, R.A.; Olson, P.D.; Littlewood, D.T.J. Life cycle evolution in the Digenea: A new perspective from phylogeny. Adv. Parasitol. 2003, 54, 197–254. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.A. Probable relationship between the Rhodometopa group of cercariæ and the trematode genus Renicola Cohn. Nature 1953, 171, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.A. Studies on the life-history and ecology of the trematode genus Renicola Cohn, 1904. Proc. Zool. Soc. Lond. 1956, 126, 1–50. [Google Scholar] [CrossRef]
- Cable, R.M. Marine cercariae from Curaçao and Jamaica. Z. Parasitenkd. 1963, 23, 429–469. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).