Gliding on the Edge: The Impact of Climate Change on the Habitat Dynamics of Two Sympatric Giant Flying Squirrels, Petaurista elegans and Hylopetes phayrei, in South and Southeast Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographic Distribution and Study Area
2.2. Secondary Occurrence Records
2.3. Covariate Selection
2.4. Model Preparation and Evaluation
2.5. Landscape Configuration Assessment
3. Results
3.1. Model Evaluation and Assessment
3.2. Variable Importance in Two Modeling Approaches
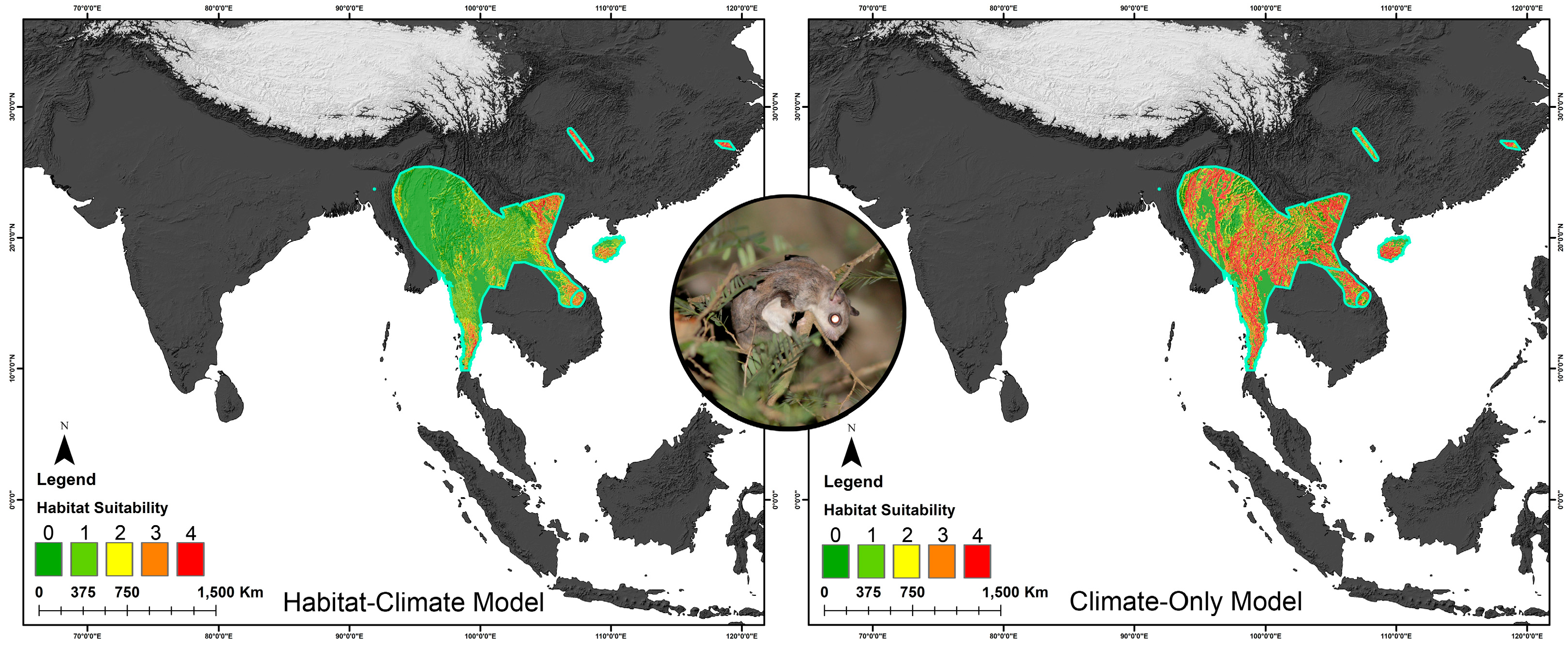
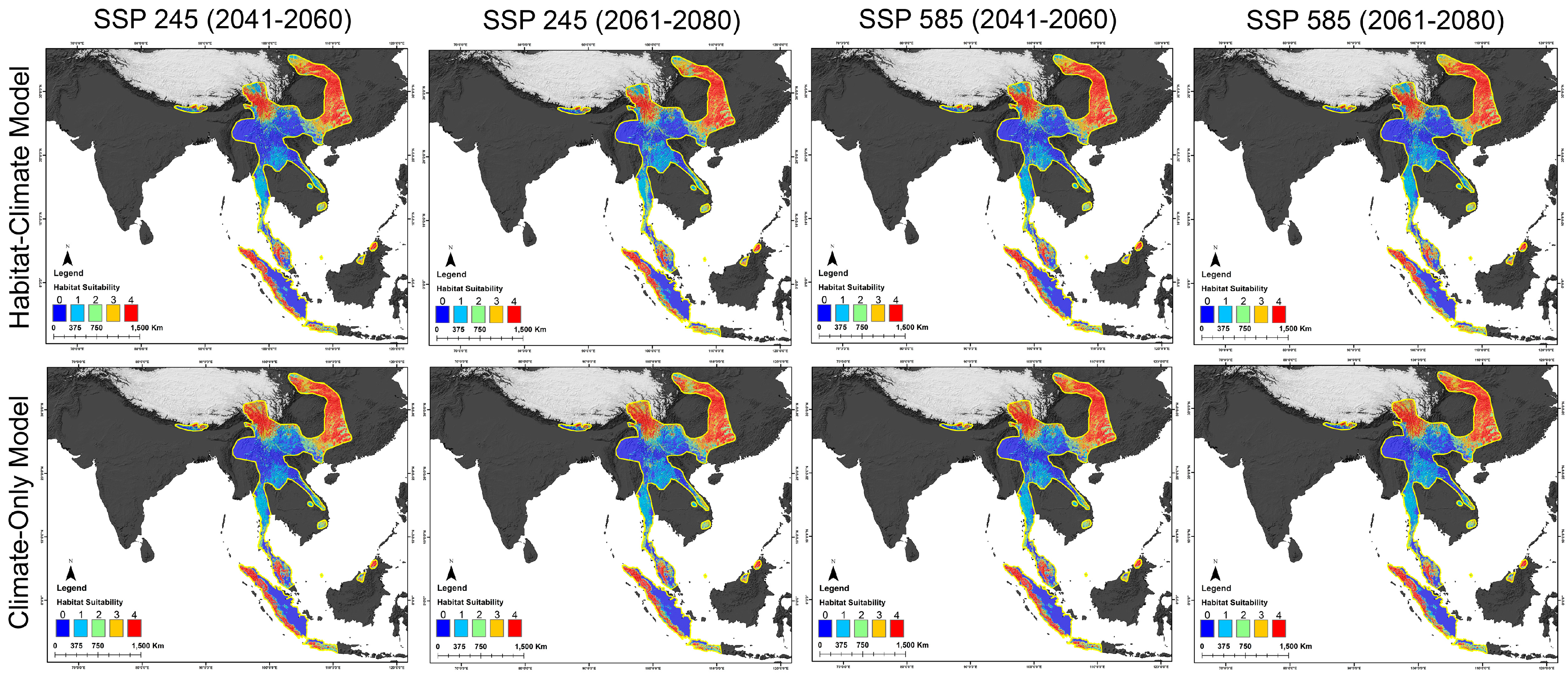
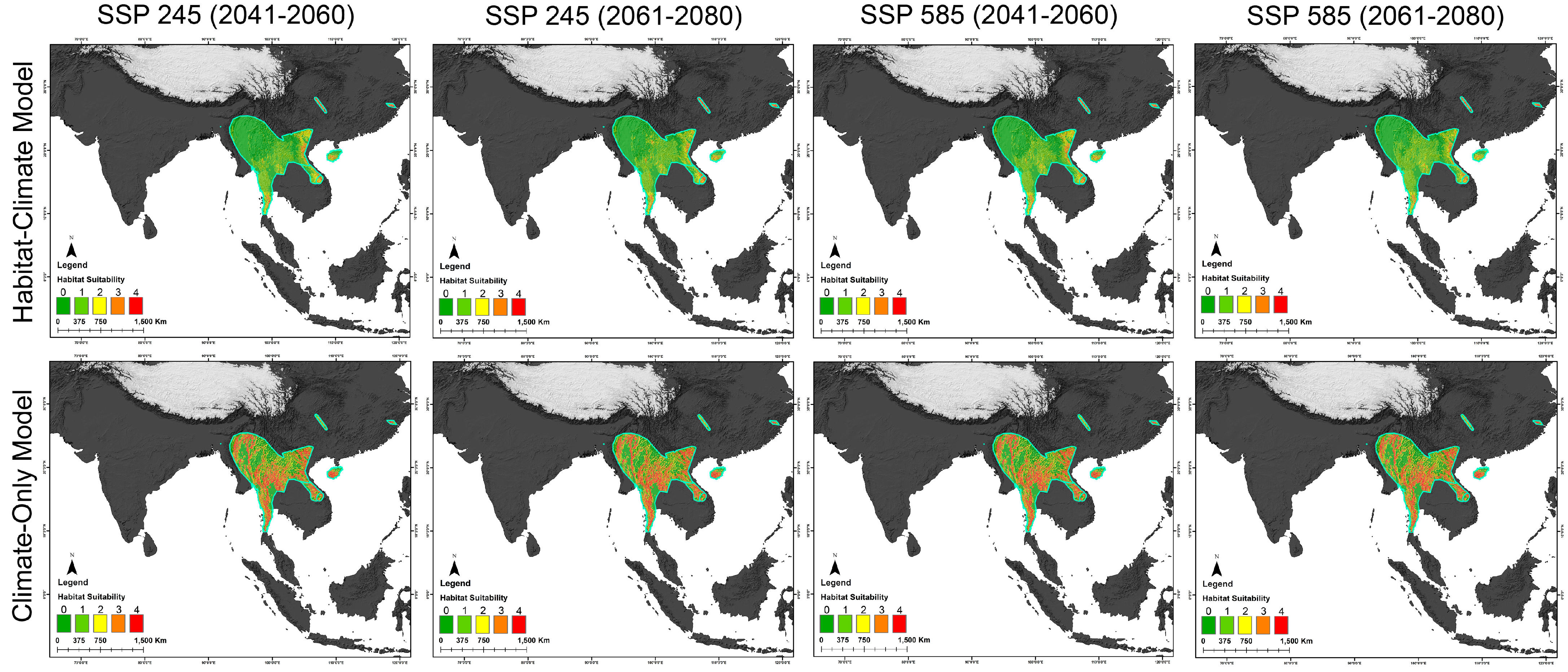
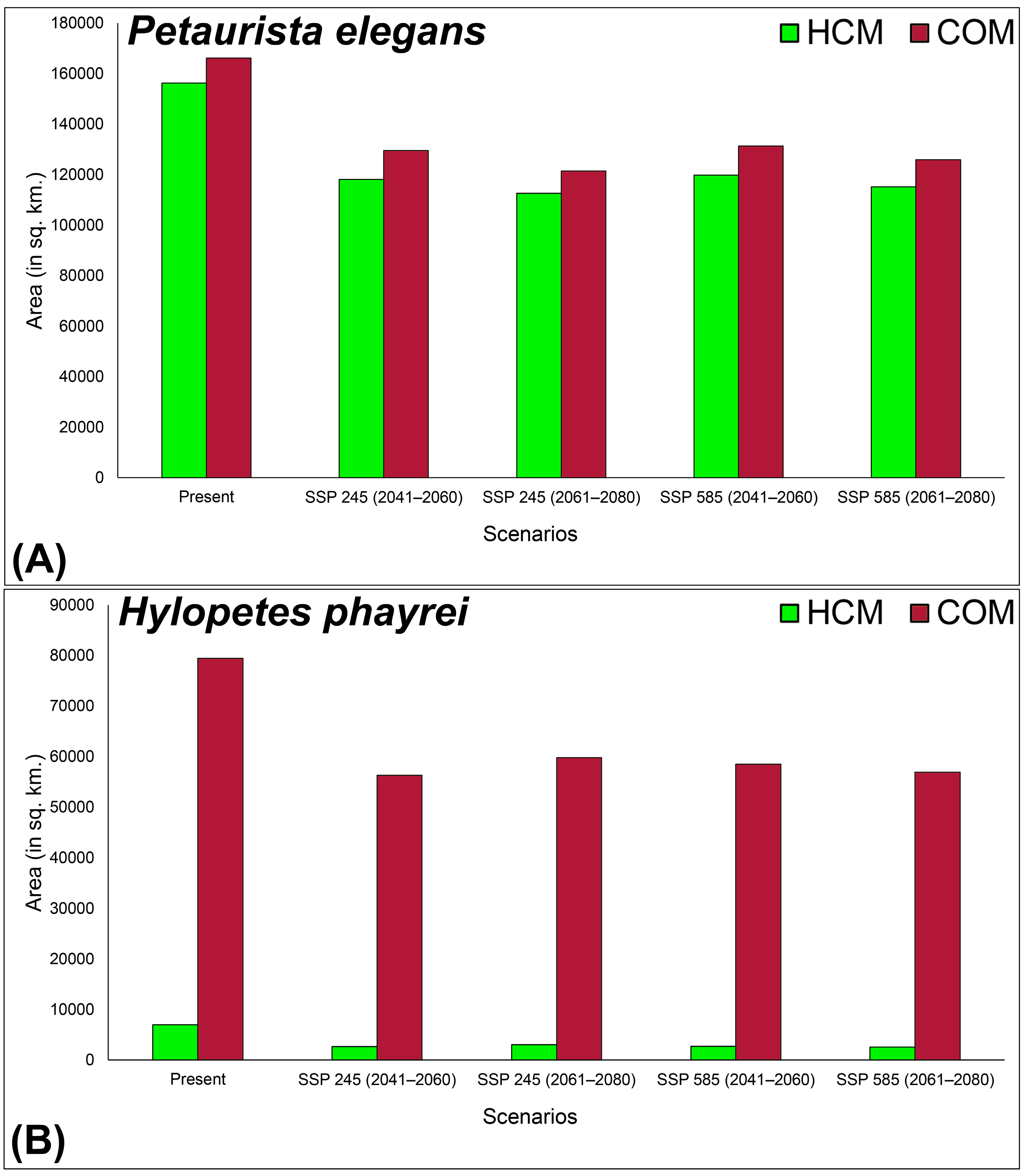
3.3. Habitat Dynamics in Present and Future
3.4. Habitat Shape Geometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stibig, H.-J.; Achard, F.; Carboni, S.; Raši, R.; Miettinen, J. Change in Tropical Forest Cover of Southeast Asia from 1990 to 2010. Biogeosciences 2014, 11, 247–258. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Posa, M.R.C.; Lee, T.M.; Bickford, D.; Koh, L.P.; Brook, B.W. The State and Conservation of Southeast Asian Biodiversity. Biodivers. Conserv. 2010, 19, 317–328. [Google Scholar] [CrossRef]
- Hughes, A.C. Understanding the Drivers of Southeast Asian Biodiversity Loss. Ecosphere 2017, 8, e01624. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Estoque, R.C.; Ooba, M.; Avitabile, V.; Hijioka, Y.; DasGupta, R.; Togawa, T.; Murayama, Y. The Future of Southeast Asia’s Forests. Nat. Commun. 2019, 10, 1829. [Google Scholar] [CrossRef]
- Koli, V.K. Biology and conservation status of flying squirrels (Pteromyini, Sciuridae, Rodentia) in India: An update and review. Proc. Zool. Soc. 2016, 69, 9–21. [Google Scholar] [CrossRef]
- Casanovas-Vilar, I.; Garcia-Porta, J.; Fortuny, J.; Sanisidro, Ó.; Prieto, J.; Querejeta, M.; Llácer, S.; Robles, J.M.; Bernardini, F.; Alba, D.M. Oldest skeleton of a fossil flying squirrel casts new light on the phylogeny of the group. eLife 2018, 7, e39270. [Google Scholar] [CrossRef] [PubMed]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 615. [Google Scholar] [CrossRef]
- Carey, A.B.; Harrington, C.A. Small mammals in young forests: Implications for management for sustainability. For. Ecol. Manag. 2001, 154, 289–309. [Google Scholar] [CrossRef]
- Nandini, R.; Parthasarathy, N. Food habits of the Indian giant flying squirrel (Petaurista philippensis) in a rain forest fragment, Western Ghats. J. Mammal. 2008, 89, 1550–1556. [Google Scholar] [CrossRef]
- Dudley, R.; Byrnes, G.; Yanoviak, S.P.; Borrell, B.; Brown, R.M.; McGuire, J.A. Gliding and the functional origins of flight: Biomechanical novelty or necessity? Annu. Rev. Ecol. Evol. Syst. 2007, 38, 179–201. [Google Scholar] [CrossRef]
- Chaitanya, R.; McGuire, J.A.; Karanth, P.; Meiri, S. Their fates intertwined: Diversification patterns of the Asian gliding vertebrates may have been forged by dipterocarp trees. Proc. R. Soc. B 2023, 290, 20231379. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, G.; Spence, A.J. Ecological and biomechanical insights into the evolution of gliding in mammals. Integr. Comp. Biol. 2011, 51, 991–1001. [Google Scholar] [CrossRef]
- McGuire, J.A.; Dudley, R. The biology of gliding in flying lizards (genus Draco) and their fossil and extant analogs. Integr. Comp. Biol. 2011, 51, 983–990. [Google Scholar] [CrossRef]
- Lin, Y.S.; Progulske, D.R.; Lee, P.F.; Day, Y.T. Bibliography of Petauristinae (Rodentia, Sciuridae). J. Taiwan Mus. 1985, 38, 49–57. [Google Scholar]
- Lee, P.F.; Liao, C.Y. Species Richness and Research Trend of Flying Squirrels. J. Taiwan Mus. 1998, 51, 1–20. [Google Scholar]
- Umapathy, G.; Kumar, A. The Occurrence of Arboreal Mammals in the Rain Forest Fragments in Anamalai Hills, South India. Biol. Conserv. 2000, 92, 311–319. [Google Scholar] [CrossRef]
- Kumara, H.N.; Singh, M. Distribution and Relative Abundance of Giant Squirrels and Flying Squirrels in Karnataka, India. Mammalia 2006, 70, 40–47. [Google Scholar] [CrossRef]
- Puyravaud, J.P.; Davidar, P.; Laurance, W.F. Cryptic Destruction of India’s Native Forests. Conserv. Lett. 2010, 3, 390–394. [Google Scholar] [CrossRef]
- Molur, S. Petaurista elegans. The IUCN Red List of Threatened Species. 2016, e.T16719A22272724. Available online: https://www.iucnredlist.org/species/16719/22272724 (accessed on 17 February 2025). [CrossRef]
- Tizard, R.J. Hylopetes phayrei. The IUCN Red List of Threatened Species. 2016, e.T10605A22244042. Available online: https://www.iucnredlist.org/species/10605/22244042 (accessed on 17 February 2025). [CrossRef]
- Byrnes, G.; Lim, N.T.; Spence, A.J. Take-Off and Landing Kinetics of a Free-Ranging Gliding Mammal, the Malayan Colugo (Galeopterus variegatus). Proc. Biol. Sci. 2008, 275, 1007–1013. [Google Scholar] [CrossRef]
- Thorington, R.W., Jr.; Hoffmann, R.S. Family Sciuridae. In Mammal Species of the World; Wilson, D.E., Reader, D.M., Eds.; The John Hopkins University Press: Baltimore, MD, USA, 2005; pp. 754–818. [Google Scholar]
- Molur, S.; Srinivasulu, C.; Srinivasulu, B.; Walker, S.; Nameer, P.O.; Ravikumar, L. Status of Non-Volant Small Mammals: Conservation Assessment and Management Plan (C.A.M.P) Workshop Report; Zoo Outreach Organisation/CBSG-South Asia: Coimbatore, India, 2005. [Google Scholar]
- Stafford, B.J.; Thorington, R.W.; Kawamichi, T. Gliding Behavior of Japanese Giant Flying Squirrels (Petaurista leucogenys). J. Mammal. 2002, 83, 553–562. [Google Scholar] [CrossRef]
- Jackson, S.M. Glide Angle in the Genus Petaurus and a Review of Gliding in Mammals. Mammal Rev. 2000, 30, 9–30. [Google Scholar] [CrossRef]
- Ortega-Huerta, M.A.; Peterson, A.T. Modelling spatial patterns of biodiversity for conservation prioritization in North-Eastern Mexico. Divers. Distrib. 2004, 10, 39–54. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Netw. Conserv. Educ. Pract. Cent. Biodivers. Conserv. Am. Mus. Nat. Hist. 2010, 3, 54–89. [Google Scholar] [CrossRef]
- Kujala, H.; Moilanen, A.; Araújo, M.B.; Cabeza, M. Conservation planning with uncertain climate change projections. PLoS ONE 2013, 8, e53315. [Google Scholar] [CrossRef]
- Eyre, A.C.; Briscoe, N.J.; Harley, D.K.P.; Lumsden, L.F.; McComb, L.B.; Lentini, P.E. Using species distribution models and decision tools to direct surveys and identify potential translocation sites for a critically endangered species. Divers. Distrib. 2022, 28, 700–711. [Google Scholar] [CrossRef]
- Hu, W.; Onditi, K.O.; Jiang, X.; Wu, H.; Chen, Z. Modeling the potential distribution of two species of shrews (Chodsigoa hypsibia and Anourosorex squamipes) under climate change in China. Diversity 2022, 14, 87. [Google Scholar] [CrossRef]
- Koli, V.K.; Jangid, A.K.; Singh, C.P. Habitat suitability mapping of the Indian giant flying squirrel (Petaurista philippensis Elliot, 1839) in India with ensemble modeling. Acta Ecol. Sin. 2023, 43, 644–652. [Google Scholar] [CrossRef]
- Abedin, I.; Kamalakannan, M.; Mukherjee, T.; Choudhury, A.; Singha, H.; Abedin, J.; Banerjee, D.; Kim, H.-W.; Kundu, S. Fading into obscurity: Impact of climate change on suitable habitats for two lesser-known giant flying squirrels (Sciuridae: Petaurista) in Northeastern India. Biology 2025, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Abedin, I.; Kamalakannan, M.; Mukherjee, T.; Singha, H.; Banerjee, D.; Kim, H.-W.; Kundu, S. Eco-spatial modeling of two giant flying squirrels (Sciuridae: Petaurista): Navigating climate resilience and conservation roadmap in the Eastern Himalaya and Indo-Burma biodiversity hotspots. Life 2025, 15, 589. [Google Scholar] [CrossRef]
- Smith, A.T.; Xie, Y. A Guide to the Mammals of China; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Evans, T.D.; Duckworth, J.W.; Timmins, R.J. Field Observations of Larger Mammals in Laos, 1994–1995. Mammalia 2000, 64, 55–100. [Google Scholar] [CrossRef]
- Thorington, R.W., Jr.; Koprowski, J.L.; Steele, M.A.; Whatton, J.F. Squirrels of the World; The Johns Hopkins University Press: Baltimore, MD, USA, 2012. [Google Scholar]
- Duckworth, J.W.; Salter, R.E.; Khounboline, K. Wildlife in Lao PDR: 1999 Status Report; IUCN: Vientiane, Laos, 1999. [Google Scholar]
- Duckworth, W. Hylopetes phayrei (Blyth, 1859). Available online: http://www.ieaitaly.org/samd (accessed on 17 February 2025).
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic, and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J. Species distribution modeling and ecological niche modeling: Getting the concepts right. Braz. J. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Karra, K.; Kontgis, C.; Statman-Weil, Z.; Mazzariello, J.C.; Mathis, M.; Brumby, S.P. Global Land Use/Land Cover with Sentinel-2 and Deep Learning. In Proceedings of the IGARSS 2021—2021 IEEE International Geoscience and Remote Sensing Symposium, Brussels, Belgium, 11–16 July 2021; IEEE: New York, NY, USA, 2021. [Google Scholar]
- SEDAC. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Footprint Dataset (Geographic). Available online: https://gis.earthdata.nasa.gov/portal/home/item.html?id=e1f0028da5d84d0da724b6dc783f3f23 (accessed on 13 November 2024).
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and workflow management for species habitat modeling. Ecography 2013, 36, 129–135. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Andrews, M.B.; Ridley, J.K.; Wood, R.A.; Andrews, T.; Blockley, E.W.; Booth, B.; Burke, E.; Dittus, A.J.; Florek, P.; Gray, L.J.; et al. Historical simulations with HadGEM3-GC3.1 for CMIP6. J. Adv. Model. Earth Syst. 2020, 12, e2019MS001995. [Google Scholar] [CrossRef]
- Li, L.; Xie, F.; Yuan, N. On the long-term memory characteristic in land surface air temperatures: How well do CMIP6 models perform? Atmos. Ocean. Sci. Lett. 2023, 16, 100291. [Google Scholar] [CrossRef]
- Gautam, S.; Shany, V.J. Navigating climate change in southern India: A study on dynamic dry-wet patterns and urgent policy interventions. Geosyst. Geoenviron. 2024, 3, 100263. [Google Scholar] [CrossRef]
- Allen, B.J.; Hill, D.J.; Burke, A.M.; Clark, M.; Marchant, R.; Stringer, L.C.; Williams, D.R.; Lyon, C. Projected future climatic forcing on the global distribution of vegetation types. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230011. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Miller, J. Species distribution modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails. 2012. Available online: https://pubs.usgs.gov/publication/70118102 (accessed on 13 November 2024).
- Hayes, M.A.; Cryan, P.M.; Wunder, M.B. Seasonally-dynamic presence-only species distribution models for a cryptic migratory bat impacted by wind energy development. PLoS ONE 2015, 10, e0132599. [Google Scholar] [CrossRef] [PubMed]
- Lavazza, L.; Morasca, S.; Rotoloni, G. On the reliability of the area under the ROC curve in empirical software engineering. In Proceedings of the 27th International Conference on Evaluation and Assessment in Software Engineering, Oulu, Finland, 14–16 June 2023; pp. 93–100. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination capacity in species distribution models depends on the representativeness of the environmental domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC plots: Calibrating species distribution models with presence-only data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Gen. Tech. Rep. PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; pp. 122–351. [Google Scholar]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Levins, R. Extinctions. In Some Mathematical Questions in Biology; Lectures on Mathematics in the Life Sciences; American Mathematical Society: Providence, RI, USA, 1970; Volume 2, pp. 77–107. [Google Scholar]
- Geldmann, J.; Manica, A.; Burgess, N.D.; Coad, L.; Balmford, A. A Global-Level Assessment of the Effectiveness of Protected Areas at Resisting Anthropogenic Pressures. Proc. Natl. Acad. Sci. USA 2019, 116, 23209–23215. [Google Scholar] [CrossRef]
- Graham, V.; Geldmann, J.; Adams, V.M.; Negret, P.J.; Sinovas, P.; Chang, H.-C. Southeast Asian Protected Areas Are Effective in Conserving Forest Cover and Forest Carbon Stocks Compared to Unprotected Areas. Sci. Rep. 2021, 11, 23760. [Google Scholar] [CrossRef]
- Thao, N.P.; Eales, J.; Lam, D.M.; Hien, V.T.; Garside, R. What Are the Impacts of Activities Undertaken in UNESCO Biosphere Reserves on Socio-Economic Wellbeing in Southeast Asia? A Systematic Review. Environ. Evid. 2023, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K.L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [CrossRef]
- Sodhi, N.S. Tropical biodiversity loss and people—A brief review. Basic Appl. Ecol. 2008, 9, 93–99. [Google Scholar] [CrossRef]
- Okinen, M.; Hanski, I.; Numminen, E.; Valkama, J.; Selonen, V. Promoting species protection with predictive modelling: Effects of habitat, predators, and climate on the occurrence of the Siberian flying squirrel. Biol. Conserv. 2019, 230, 37–46. [Google Scholar] [CrossRef]
- Selonen, V.; Hongisto, K.; Hänninen, M.; Turkia, T.; Korpimäki, E. Weather and biotic interactions as determinants of seasonal shifts in abundance measured through nest-box occupancy in the Siberian flying squirrel. Sci. Rep. 2020, 10, 14465. [Google Scholar] [CrossRef]
- Bedoya-Canas, L.E.; López-Hernández, F.; Cortés, A.J. Climate change responses of high-elevation Polylepis forests. Forests 2024, 15, 811. [Google Scholar] [CrossRef]
- Vernes, K. Gliding Performance of the Northern Flying Squirrel (Glaucomys sabrinus) in Mature Mixed Forest of Eastern Canada. J. Mammal. 2001, 82, 1026–1033. [Google Scholar] [CrossRef]
- Flaherty, E.A.; Ben-David, M.; Smith, W.P. Quadrupedal Locomotor Performance in Two Species of Arboreal Squirrels: Predicting Energy Savings of Gliding. J. Comp. Physiol. B 2010, 180, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.Z.; Li, Q.Q.; Jin, L.; Chen, J. Integrated Approach to Predicting Habitat Suitability and Evaluating Quality Variations of Notopterygium franchetii under Climate Change. Sci. Rep. 2024, 14, 26927. [Google Scholar] [CrossRef] [PubMed]
- Payne, B.L.; Bro-Jørgensen, J. Disproportionate climate-induced range loss forecast for the most threatened African antelopes. Curr. Biol. 2016, 26, 1200–1205. [Google Scholar] [CrossRef]
- Dubos, N.; Montfort, F.; Grinand, C.; Nourtier, M.; Deso, G.; Probst, J.M.; Razafimanahaka, J.H.; Andriantsimanarilafy, R.R.; Rakotondrasoa, E.F.; Razafindraibe, P.; et al. Are narrow-ranging species doomed to extinction? Projected dramatic decline in future climate suitability of two highly threatened species. Perspect. Ecol. Conserv. 2022, 20, 18–28. [Google Scholar] [CrossRef]
- Costa-Pinto, A.L.; Bovendorp, R.S.; Heming, N.M.; Malhado, A.C.; Ladle, R.J. Where could they go? Potential distribution of small mammals in the Caatinga under climate change scenarios. J. Arid Environ. 2024, 221, 105133. [Google Scholar] [CrossRef]
- Williams, S.E.; Bolitho, E.E.; Fox, S. Climate Change in Australian Tropical Rainforests: An Impending Environmental Catastrophe. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 1887–1892. [Google Scholar] [CrossRef]
- Radchuk, V.; Kramer-Schadt, S.; Fickel, J.; Wilting, A. Distributions of Mammals in Southeast Asia: The Role of the Legacy of Climate and Species Body Mass. J. Biogeogr. 2019, 46, 2350–2362. [Google Scholar] [CrossRef]
- Howard, J.M. Gap Crossing in Flying Squirrels: Mitigating Movement Barriers through Landscape Management and Structural Implementation. Forests 2022, 13, 2027. [Google Scholar] [CrossRef]
- Lindenmayer, D. Small patches make critical contributions to biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 717–719. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef] [PubMed]


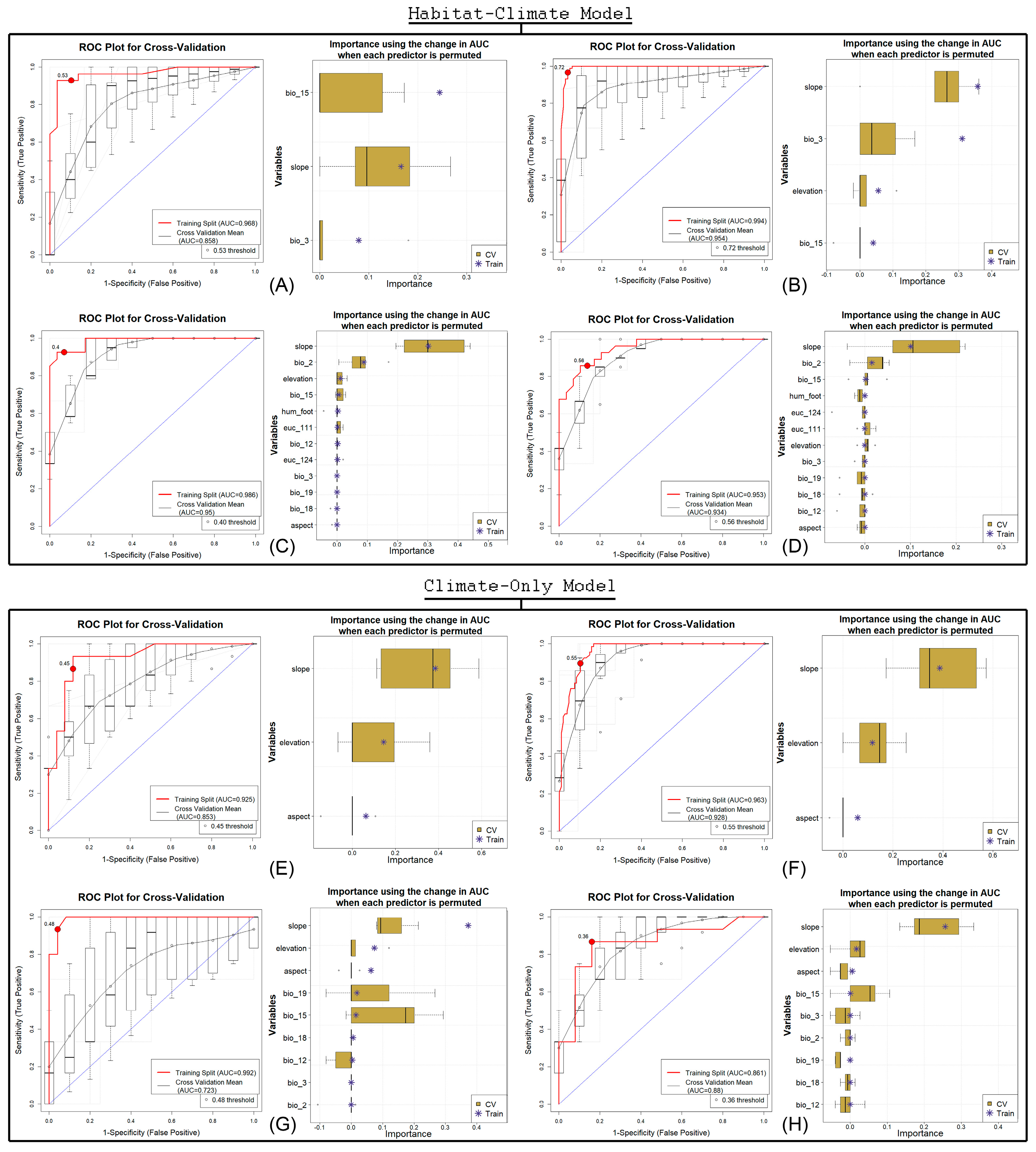

| Species | Modeling Approach | Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|---|
| Petaurista elegans | HCM | GLM | Train | 0.968 | 0.097 | 91.2 | 0.825 | 0.825 | 0.897 | 0.929 |
| CV | 0.871 | 86.4 | 0.727 | 0.727 | 0.867 | 0.86 | ||||
| MARS | Train | 0.927 | 0.028 | 83.5 | 0.671 | 0.671 | 0.837 | 0.833 | ||
| CV | 0.899 | 80.2 | 0.601 | 0.602 | 0.818 | 0.783 | ||||
| MaxEnt | Train | 0.986 | 0.052 | 92.9 | 0.857 | 0.857 | 0.931 | 0.926 | ||
| CV | 0.934 | 79.7 | 0.587 | 0.588 | 0.827 | 0.76 | ||||
| RF | Train | 0.934 | 0.006 | 82.5 | 0.649 | 0.649 | 0.828 | 0.821 | ||
| CV | 0.928 | 83.8 | 0.68 | 0.677 | 0.827 | 0.853 | ||||
| COM | GLM | Train | 0.968 | 0.026 | 91.2 | 0.825 | 0.825 | 0.897 | 0.929 | |
| CV | 0.942 | 88.2 | 0.76 | 0.763 | 0.9 | 0.86 | ||||
| MARS | Train | 0.811 | 0.047 | 77.6 | 0.555 | 0.497 | 0.781 | 0.774 | ||
| CV | 0.764 | 72.3 | 0.437 | 0.387 | 0.717 | 0.721 | ||||
| MaxEnt | Train | 0.982 | 0.041 | 92.9 | 0.857 | 0.857 | 0.931 | 0.926 | ||
| CV | 0.941 | 82.1 | 0.633 | 0.636 | 0.867 | 0.767 | ||||
| RF | Train | 0.956 | 0.012 | 86 | 0.719 | 0.719 | 0.862 | 0.857 | ||
| CV | 0.944 | 87.8 | 0.76 | 0.757 | 0.827 | 0.933 | ||||
| Hylopetes phayrei | HCM | GLM | Train | 0.968 | 0.110 | 91.2 | 0.825 | 0.825 | 0.897 | 0.929 |
| CV | 0.858 | 84.7 | 0.693 | 0.693 | 0.833 | 0.86 | ||||
| MARS | Train | 0.994 | 0.04 | 96.7 | 0.934 | 0.934 | 0.968 | 0.966 | ||
| CV | 0.954 | 91.6 | 0.829 | 0.83 | 0.936 | 0.893 | ||||
| MaxEnt | Train | 0.986 | 0.036 | 92.9 | 0.857 | 0.857 | 0.931 | 0.926 | ||
| CV | 0.95 | 87.8 | 0.76 | 0.756 | 0.86 | 0.9 | ||||
| RF | Train | 0.953 | 0.019 | 86 | 0.719 | 0.719 | 0.862 | 0.857 | ||
| CV | 0.934 | 82.5 | 0.653 | 0.651 | 0.793 | 0.86 | ||||
| COM | GLM | Train | 0.925 | 0.072 | 87.5 | 0.747 | 0.737 | 0.88 | 0.867 | |
| CV | 0.853 | 82.5 | 0.64 | 0.646 | 0.84 | 0.8 | ||||
| MARS | Train | 0.963 | 0.035 | 89.7 | 0.794 | 0.785 | 0.898 | 0.896 | ||
| CV | 0.928 | 84.1 | 0.671 | 0.664 | 0.862 | 0.81 | ||||
| MaxEnt | Train | 0.992 | 0.269 | 94.9 | 0.892 | 0.892 | 0.958 | 0.933 | ||
| CV | 0.723 | 66.4 | 0.777 | 0.782 | 0.896 | 0.689 | ||||
| RF | Train | 0.861 | 0.019 | 85 | 0.707 | 688 | 0.84 | 0.867 | ||
| CV | 0.88 | 85 | 0.653 | 0.669 | 0.92 | 0.733 |
| Species | Approach | Variables | GLM | MARS | MaxEnt | RF | μ (Mean) | μ (Mean) % |
|---|---|---|---|---|---|---|---|---|
| Petaurista elegans | HCM | aspect | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 | 0.11 |
| bio_12 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| bio_15 | 0.258 | 0.040 | 0.051 | 0.035 | 0.096 | 20.12 | ||
| bio_18 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| bio_3 | 0.079 | 0.310 | 0.017 | 0.000 | 0.102 | 21.28 | ||
| elevation | 0.000 | 0.056 | 0.009 | 0.000 | 0.016 | 3.42 | ||
| euc_111 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| euc_112 | 0.000 | 0.000 | 0.080 | 0.000 | 0.020 | 4.19 | ||
| hum_foot | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.01 | ||
| slope | 0.148 | 0.358 | 0.313 | 0.151 | 0.243 | 50.86 | ||
| COM | aspect | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | |
| bio_12 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| bio_15 | 0.312 | 0.040 | 0.042 | 0.010 | 0.101 | 23.37 | ||
| bio_18 | 0.000 | 0.000 | 0.003 | 0.000 | 0.001 | 0.18 | ||
| bio_3 | 0.057 | 0.310 | 0.025 | 0.001 | 0.098 | 22.78 | ||
| elevation | 0.000 | 0.056 | 0.017 | 0.000 | 0.018 | 4.23 | ||
| slope | 0.205 | 0.358 | 0.256 | 0.035 | 0.213 | 49.45 | ||
| Hylopetes phayrei | HCM | aspect | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 |
| bio_12 | 0.228 | 0.000 | 0.002 | 0.000 | 0.058 | 9.81 | ||
| bio_15 | 0.139 | 0.000 | 0.005 | 0.003 | 0.037 | 6.23 | ||
| bio_18 | 0.180 | 0.000 | 0.002 | 0.000 | 0.046 | 7.76 | ||
| bio_19 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| bio_2 | 0.000 | 0.000 | 0.003 | 0.000 | 0.001 | 0.12 | ||
| bio_3 | 0.365 | 0.000 | 0.093 | 0.000 | 0.115 | 19.53 | ||
| elevation | 0.000 | 0.165 | 0.161 | 0.004 | 0.082 | 14.04 | ||
| euc_111 | 0.444 | 0.267 | 0.002 | 0.003 | 0.179 | 30.52 | ||
| euc_124 | 0.045 | 0.000 | 0.000 | 0.000 | 0.011 | 1.91 | ||
| hum_foot | 0.000 | 0.000 | 0.128 | 0.000 | 0.032 | 5.44 | ||
| slope | 0.000 | 0.000 | 0.000 | 0.109 | 0.027 | 4.63 | ||
| COM | aspect | 0.063 | 0.059 | 0.063 | 0.006 | 0.048 | 9.59 | |
| bio_12 | 0.000 | 0.000 | 0.003 | 0.000 | 0.001 | 0.14 | ||
| bio_15 | 0.000 | 0.000 | 0.015 | 0.001 | 0.004 | 0.81 | ||
| bio_18 | 0.000 | 0.000 | 0.006 | 0.000 | 0.001 | 0.28 | ||
| bio_19 | 0.000 | 0.000 | 0.018 | 0.000 | 0.004 | 0.90 | ||
| bio_2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| bio_3 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | ||
| elevation | 0.145 | 0.118 | 0.074 | 0.017 | 0.088 | 17.79 | ||
| slope | 0.386 | 0.387 | 0.372 | 0.257 | 0.350 | 70.49 |
| Species | Approach | Scenario | NP | PD | LPI | TE | LSI | AI |
|---|---|---|---|---|---|---|---|---|
| Petaurista elegans | HCM | Present | 651 | 3,001,949 | 15.2705 | 1144.5 | 36.5282 | 80.8217 |
| SSP 245 (2041–2060) | 399 | 1,840,174 | 10.5029 | 894.936 | 32.7815 | 75.2138 | ||
| SSP 245 (2061–2080) | 449 | 2,070,772 | 10.3516 | 894.684 | 33.5994 | 69.2102 | ||
| SSP 585 (2041–2060) | 403 | 1,858,622 | 11.1277 | 888.384 | 32.3425 | 73.6517 | ||
| SSP 585 (2061–2080) | 397 | 1,830,950 | 11.026 | 871.08 | 32.4063 | 67.2352 | ||
| COM | Present | 599 | 2,762,162 | 16.8868 | 1130.556 | 34.9584 | 82.2072 | |
| SSP 245 (2041–2060) | 342 | 1,577,292 | 10.1311 | 964.656 | 33.7765 | 76.5359 | ||
| SSP 245 (2061–2080) | 396 | 1,826,338 | 9.6707 | 964.236 | 34.8906 | 69.2237 | ||
| SSP 585 (2041–2060) | 365 | 1,683,367 | 10.5038 | 959.952 | 33.4152 | 75.9 | ||
| SSP 585 (2061–2080) | 358 | 1,651,083 | 10.5347 | 947.184 | 33.6597 | 64.3357 | ||
| Hylopetes phayrei | HCM | Present | 106 | 1,103,088 | 0.6076 | 109.956 | 16.3625 | 59.652 |
| SSP 245 (2041–2060) | 52 | 541,226.8 | 0.1744 | 44.688 | 10.8571 | 52.8166 | ||
| SSP 245 (2061–2080) | 47 | 489,185.7 | 0.1763 | 48.384 | 10.8679 | 50.0458 | ||
| SSP 585 (2041–2060) | 57 | 593,267.8 | 0.1744 | 46.62 | 11.1 | 50.1307 | ||
| SSP 585 (2061–2080) | 52 | 541,226.8 | 0.1744 | 43.512 | 10.5714 | 47.8616 | ||
| COM | Present | 565 | 5,879,666 | 20.637 | 1020.096 | 45.6541 | 66.0939 | |
| SSP 245 (2041–2060) | 461 | 4,797,391 | 6.6196 | 803.88 | 42.7232 | 62.3236 | ||
| SSP 245 (2061–2080) | 499 | 5,192,837 | 9.3713 | 839.496 | 43.2641 | 60.9305 | ||
| SSP 585 (2041–2060) | 480 | 4,995,114 | 7.0307 | 826.98 | 42.9913 | 59.6983 | ||
| SSP 585 (2061–2080) | 469 | 4,880,643 | 6.7517 | 813.288 | 43.0311 | 55.2882 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Kamalakannan, M.; Banerjee, D.; Kim, H.-W.; Singha, H.; Kundu, S. Gliding on the Edge: The Impact of Climate Change on the Habitat Dynamics of Two Sympatric Giant Flying Squirrels, Petaurista elegans and Hylopetes phayrei, in South and Southeast Asia. Diversity 2025, 17, 403. https://doi.org/10.3390/d17060403
Abedin I, Kamalakannan M, Banerjee D, Kim H-W, Singha H, Kundu S. Gliding on the Edge: The Impact of Climate Change on the Habitat Dynamics of Two Sympatric Giant Flying Squirrels, Petaurista elegans and Hylopetes phayrei, in South and Southeast Asia. Diversity. 2025; 17(6):403. https://doi.org/10.3390/d17060403
Chicago/Turabian StyleAbedin, Imon, Manokaran Kamalakannan, Dhriti Banerjee, Hyun-Woo Kim, Hilloljyoti Singha, and Shantanu Kundu. 2025. "Gliding on the Edge: The Impact of Climate Change on the Habitat Dynamics of Two Sympatric Giant Flying Squirrels, Petaurista elegans and Hylopetes phayrei, in South and Southeast Asia" Diversity 17, no. 6: 403. https://doi.org/10.3390/d17060403
APA StyleAbedin, I., Kamalakannan, M., Banerjee, D., Kim, H.-W., Singha, H., & Kundu, S. (2025). Gliding on the Edge: The Impact of Climate Change on the Habitat Dynamics of Two Sympatric Giant Flying Squirrels, Petaurista elegans and Hylopetes phayrei, in South and Southeast Asia. Diversity, 17(6), 403. https://doi.org/10.3390/d17060403













