Abstract
The genus Quercus, including species like pedunculate oak (Quercus robur L.), can play a key role in maintaining climate-resistant mixed forests due to its broad ecological spectrum and drought tolerance. Unfortunately, in some parts of Europe, clearcutting has drastically reduced the oak population. An example of this event is our survey of heritage Oak—Lime forests in European Russia, which were transformed into pure aspen stands. The aim of our study was to provide forecasts and silvicultural recommendations for the passive restoration of these forests. We took a chronosequence approach to assess changes associated with natural succession over 60 years. In our survey of the development of oaks, limes and accompanying tree species (aspen, birches, maples, elms), we used 190 plots ranging across a wide spectrum of forest disturbance due to clearcutting. We demonstrate that aspen reproduce rapidly by root suckers after cutting and occupy more than 60% of the space. But the dominance of aspen decreases continuously from the age of 30, and then the lime trees begin to dominate. Oak does not show successful natural regeneration. Therefore, we recommend planting oak seedlings or sowing acorns, i.e., active restoration, in combination with the natural restoration of lime.
1. Introduction
The pedunculate oak (Quercus robur L.) is predicted to adapt to the expected climatic changes [1,2]. Due to its wide ecological scope and great drought tolerance, oak can become an essential component of climate-resilient mixed forests [3,4]; thus, it is regarded as an available option to meet the challenges of future climate change [5,6,7]. Also, the genus oak, with its great species abundance [8,9], might be suitable for multifunctional forest management, including the production of timber [10,11,12].
The natural range of pedunculate oak covers all of western Europe—with the exception of most of the Iberian Peninsula—Scandinavia, central Europe, Turkey, the Republics of Belarus, Lithuania, Latvia, Estonia, Ukraine and much of the territory of the European part of Russia [13]. Oak forests are also naturally present in the central Volga region of Russia [14,15,16], but the degradation of oak forests in these areas has been progressing over a long period of time. The oak forests in this region have been actively exploited for the past 300 years, and the development of shipbuilding by Peter the Great (1672–1725) depleted large volumes of oak timber [17]. In later years, other anthropogenic factors such as wars, the construction of a large hydroelectric power station and, of course, the lack of forest management reduced the oak population by 1/3 [18]. Another noteworthy reason is the extremely hard winters of the region, such as 1941–1942 and especially 1978–1979, when the temperature dropped down to −50 to −54 °C. As an example, about half of all oaks in the Republic of Tatarstan (a part of the central Volga region [19]) died after the winter of 1978–1979 [1].
As reported by Petrov [20] and Puryaev et al. [18], the oak stands in Tatarstan have been shown to regenerate ‘unsatisfactorily’ with certain harvesting methods (logging of the thickest oaks, i.e., exploitation, or clearcutting). ‘Unsatisfactory’ here refers to altered species composition, species ratios, and the performance of individual trees. Compared to the harvested stands, successional stands appear to be of less ecological and economic value; i.e., they are degraded. Despite the problem of oak forest loss, there are very few studies on forest restoration after clearcutting [21,22,23].
In order to significantly alter conditions for valuable oak-lime stands to be harvested in the future, (i) foresters need to know exactly how different harvesting methods affect species regeneration and stand composition over a long period of time [24]. We could not cover this aspect of sustainability in the present study; however, we looked into the question of (ii) how to bring back existing degraded stands to a higher level of economic and ecological value and what restoration strategy should be developed and made available for restoration of the oak-lime forests.
An intuitively compelling and cost-effective strategy for restoration could be seen in the deliberate use of residual structures of the clearcuts, which are sometimes called ‘legacies’ [25]. As in many cases of degraded forests, in the oak-lime forests of Tatarstan, some remnant structures of the former diverse forests may exist after the last clearcut, i.e., in the recent stands. In particular, there may be some oak trees left at forest margins, lime trees may resprout after the last cut, and here and there, elm trees or maple trees survived in an understory feature. These legacies could be taken as an initial step to upgrading the forest during succeeding decades by passive restoration.
In contrast to studies on thinning, most research works on clearcuts succession focus mainly on relatively short monitoring periods and the initial consequences arising directly from clearcuts and not on the long-term effects [26,27,28]. The lack of understanding of a long-term series can be partially overcome by taking chronosequences of current forest stands systems [29]. Chronosequence is a term used by Jenny [30]; however, the method has been used for a large number of systems since the very beginning of ecological science [31]. In relation to vegetation dynamics, the chronosequence sensu stricto represents a ‘space–time substitution’: a spatial representation of a chronological sequence of vegetation changes divided into states of development in which environmental factors besides time are not assumed to play a role [31]. Thus, for a successful chronosequence analysis, the sampling method should measure most of the natural variability of species diversity at each stage of development and reduce as much as possible the variation of environmental factors that could affect the vegetation development derived from the chronosequence [32].
We applied the chronosequence approach to record the changes associated with natural succession and tree species development in formally oak-lime forests [14] over the 60 years post-clearcutting in order to provide forecasts and silvicultural recommendations for these and other similar areas with degraded oak forests.
2. Materials and Methods
2.1. Study Area
The Republic of Tatarstan is located in the east of European Russia between 47° and 54° north latitude and 54° and 57° east longitude [33]. Typical for this region is a temperate continental climate with hot summers and moderate cold winters, but heavy frosts of up to −52 °C can also occur. In summer, it is usual for temperatures to climb up to 40 °C. The average annual precipitation in the region ranges from 360 to 510 mm. The snow level in forests is usually between 40 and 45 cm but can reach 100 cm during high-snow winters [34].
Tatarstan is mostly characterized by a flat terrain. Mountainous uplands can be seen only in the south of the region. The entire territory of the region is conventionally divided into three parts (Figure 1).
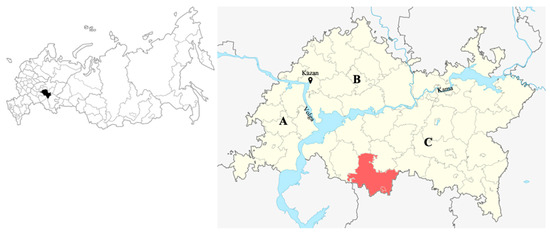
Figure 1.
(Left) The inserted map of Russia shows the location of the Republic of Tatarstan in black. (Right) Map layout of Tatarstan with three parts of the territory (A, B, C with river borders in-between) and the research district Nurlat (red colored).
We conducted our measurements within Zakamye (C), in the Nurlat forestry district (Figure 1), as the problem of transformation of oak-lime forests to the aspen (Populus tremula L.) stand after clearcut is heavier here. The area of Zakamye forests of the Republic of Tatarstan is 634,233 ha. The aspen forests in Zakamye grow in the area of 189,854 ha, which is about 30% of the total area of the forest stands [35]. The Nurlat forestry district is the department with the largest concentration of aspen in the Tatarstan Republic (with aspen showing 37% of forest stock). The total forest area of the Nurlat forestry district is 83,140 ha. In addition to the dominant aspen (P. tremula) and very rare oaks (Q. robur), there are also individual trees of maple (Acer platanoides L.), birch (Betula pendula Roth), lime (Tilia cordata Mill.) and elm (Ulmus glabra Huds.) admixed.
Typically, the forests in this region are managed by clearcutting with a rotation period of up to 40 years.
Soils of these region are diverse—from different gray forest soils mostly typical for the forest sites to various types of nutrient-rich chernozems on the border to the agricultural land [36].
2.2. Site Selection
2.2.1. Site Measurements
Two measurement sessions were conducted in August 2020 and in August 2021. In an application of the chronosequence approach to successional sites, several prerequisites have to be met to yield sound and unbiased data.
According to the forestry department of Nurlat (personal communication with foresters, August 2020), no operations of tending, thinning and planting had been carried out in the study area in the past 62 years since the last clearcut (including the year of our last measurement (2021)). However, the residue from clearcutting was pushed together and left on the ground, forming clumps. By this ‘no-intervention behavior’ of forest management, an unspoilt forest dynamic after clearcut should be secured.
The sampling procedure must be the same for all plots; this is described below.
The choice of locations for the plots was made in a three-step procedure, which is outlined below. First, a series of ‘years after clearcutting’ (further on termed ‘age’) was defined by evaluating the administration’s taxation data (this means a pre-stratification). In order to depict the succession dynamics in the time series, short intervals were to be selected for the years directly after clearcutting, and these intervals were to be extended with increasing plot age. The aim was therefore to establish 5-year intervals between 10 and 30 years and 10-year intervals from 30 to 60 years so that the study would cover 10 age groups. We aimed for at least 15 plots for each age to adequately represent the variation in forest structure.
Since a sufficient number of stands was not available for each desired age after clearcutting, we had to deviate somewhat from the target, and the following ten different ages were chosen: 1, 3, 6, 10, 15, 20, 30, 42, 52, and 62 years after the last clearcut. These years guaranteed a satisfactory number of stands to choose from.
Secondly, stands that had a signature ‘aspen-dominant’ taxation data (this means again a pre-stratification) were selected to exclude all other forest types, which enabled areas that depicted the problem of tree species to change from heritage oak-lime forests to aspen-covered clearcuts. Approx. 40% of the Nurlat district is ‘aspen-dominated’. In this way, we chose an experimental design that allowed us to investigate whether succession was a natural tool for restoring oak-lime forests on aspen-dominated clearcuts. In addition, the stands of each age were distributed over the entire forest enterprise area to represent the variation in soil/site classes from different gray forest soils to various types of chernozems as well as possible. According to the World Reference Base for Soil Resources [37], we differentiate two zones: (1) phaeozems and (2) chernozems.
Third, no more than five plots within a single chosen stand were allowed to be established. This sampling within a stand was performed by placing plots randomly on the stands area, securing a minimum distance of 100 m apart to each other. All together, the design aims to follow a stratified random sampling approach in which only the stratum ‘aspen-dominant’ defined by the forest taxation is considered.
In the temperate deciduous forest zone in general, and thus also in Tatarstan, catastrophic events such as those known from coniferous forests—like snow breakage, windthrow, wildfire or even insect calamities—are unlikely. This is particularly true for the younger forest stages, because the vitality of the trees there is particularly high. An exception could be snow pressure in younger and very dense stands, which can occur during wet snow events in autumn or spring when foliage is present. In Nurlat, smaller or larger events such as those just mentioned have not occurred in recent decades, which is why we focused on human-made clearcuts.
All over, 190 plots were established and measured (Figure 2). For all single plots, we recorded and analyzed the geoposition (GARMIN GPSMap 64s, UTM coordinates), forest residues, dead wood, leaf litter and vegetation survey. Vegetation surveys included the determination of dominant species in the herb, shrub and tree layers. Changes in soil types and microclimatic conditions between the different age groups were not part of the measurements.
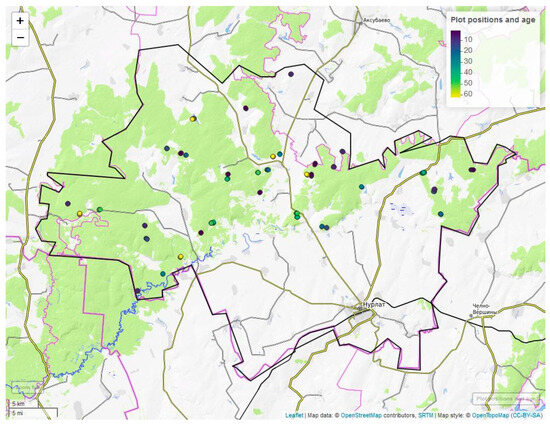
Figure 2.
Plots distribution within the Nurlat forestry district. Neighbors when positioned in the same stand obscure some of the plots.
Each single plot (12 m radius) was divided into 3 concentric sample circles (sc): sc0 with a 2 m radius, sc1 with a 6 m radius and sc3 with a 12 m radius.
Starting with sc0 (tree height (h) from 20 cm; diameter at breast height (dbh) up to 6.99 cm), we named the species, determined the type of their reproduction, organized the young trees in three height classes (B0: 20–50 cm; B1: 50–180 cm; B2: from 180 cm), counted the trees of each height class, marked trees with chalk, and noted browsing and bark damage when counting.
In sc1 (dbh 7.00–29.99 cm) and sc2 (dbh ≥ 30 cm), we named the tree species, measured the dbh, and noted crown and bark damages. In addition to the measurements listed above, tree height was taken using a Vertex clinometer (Haglöf Sweden AB, Långsele, Sweden) on two randomly selected trees of each tree species starting at a dbh of 7 cm in each sc; exceptionally thick trees were excluded from the height measurements.
While all diameters were measured once a tree exceeded 7 cm, height was taken on two randomly selected trees of each tree species starting at a dbh of 7 cm in each plot. In this way, it was aimed to determine an average height of the trees by species and age.
2.2.2. Stand’s History
A map from 1927 [16] was used to collect information about the predominant species on a particular plot in that year. Only oak, lime and aspen-dominated stands are shown on that map. No other tree species like elm, maple or birch were dominant in those years. However, on the plots of our investigation, ages 15 or older, no lime was dominant in 1927, reducing the levels of the dominant species to two. Since no information on the immediate pre-harvest condition, i.e., before the last clearcut, was available for the individual stands, we used the 1927 stand map. We were able to assign a dominant tree species from 1927 to each individual plot and incorporate this information into a model (see Section 2.3) to explain the influence of this part of the stand history.
2.3. Statistics
2.3.1. Dbh Density Distributions
Dbh distributions for different age classes were established by kernel (Gaussian) density estimates. The bandwidth for the estimates is the standard deviation of the smoothing kernel. The integral under each individual distribution is always ‘1’, and thus the graphs of the distributions of different species are comparable. However, the density plots will not represent the ratio of stem numbers or basal area of the different tree species to each other on the plots. An attempt was therefore made to calculate the development of the proportion of species in the basal area during succession separately (see Section 2.3.3).
2.3.2. Height Development Model
Based on the height measurements in the single plots (see Section 2.2), we established a height development model over time for five key tree species (oaks were excluded; for an explanation, see Section 3.2): aspen, birch, maple, elm, and lime. To do so, we followed a generalized additive model approach with integrated smoothness estimation (gam). Gams have the advantage of being able to integrate non-linear effects on the growth level, such as the age trend, into a linear model using non-parametric smoothers [38].
Within this procedure, the smooth terms were represented using penalized regression splines. Our model consisted of two parts, the first part being the generalized linear model and the second part being the additive smoother. The smoother was established to cover the effect of the time since clearcut, which essentially was a covariate. The generalized linear part of the model included species as a fixed factor. The dependent variable in the model was the height for each species on the plot level. The distributions of the dependent variable were observed carefully. In all species, Gaussian distribution best represented the height on plot level, and thus the generalized approach was implemented with an identity link. Residuals of the models were checked for heteroscedasticity [39]. However, in no case was it necessary to adjust the model to deviate from assumptions.
The model formula for height as dependent variable, species as fixed factor and time as covariate thus was
with height being the height of trees of a species on the single plot, α being the intercept, β1 being the parameter of the fixed effect, i.e., species, f(X) being the smoother on time, and ε being the error term.
2.3.3. Species Proportion during Succession
The proportion of species in mixtures was calculated using the proportion of the basal area of species in the total basal area of a single plot, taking into account the six most important species and excluding, e.g., goat willow (Salix caprea L.), alder (Alnus glutinosa L.) and hazel (Coryllus avellana L.), as these species did not exhibit relevant basal area proportions. The mean value of the species shares of all plots of a certain age gives the mean share of a species at that age.
2.3.4. Categorical Logistic Regression for Species Proportion
‘Species’ represents a response variable that falls in a non-ordered finite set of categories. Accordingly, multinomial data are given [40,41]. Multinomial logistic regression was used to model nominal outcome variables in which the log odds of the outcomes were modeled as a linear combination of the predictor variables. Thus, a multinomial log-linear model for categorical data was used to analyze species proportion as a function of age of clearcut sites and of the dominant species in 1927. A model with the dominant species in 1927 as a 2-level factor {oak, aspen} and years after clearcut, i.e., ‘age’ as a continuous covariate, was built. The difference between an ordinary logit model and the multinomial logit is that the latter models the choice of each category as a function of the characteristics of the observation.
In a multinomial logit model, the sum of all individual probabilities was restricted to 1:
with pi,j as the probability of the ith observation falling into category j. J is the number of categories with the baseline category 1, which is aspen. ηi,j is the linear predictor.
The final model for the linear predictor in our investigation is shown below:
with β1 as the reference parameter for the category ‘aspen’ in combination with the dominating species in 1927, which is ‘aspen’ at the age 0, βi,j,p as the parameter for the jth category and the pth dominating species of that site in 1927 and βi,j as the parameter for the age effect. εi,j is the error term.
Probabilities for scenarios based on the observed data were built. These data were used to plot predicted probabilities. Sampling distributions were simulated (i.e., for confidence intervals) and could be used to predict probabilities for two scenarios and their first differences.
Statistics were performed using the open-source software R version 4.2.0 (R Core Team, 2022), packages ‘MNLpred’ [42], ‘nnet’ [43] and ‘MASS’ [44]. The graphs were created with the package ‘ggplot2’ [45].
3. Results
3.1. Dbh Development
Our analysis showed the development of dbh in six key tree species—aspen, birch, elm, maple, lime and oak—at six different age groups (the dbh values from other age classes are less than 7 cm and are not presented here) and thus represented the chronological trend among tree species (Figure 3).
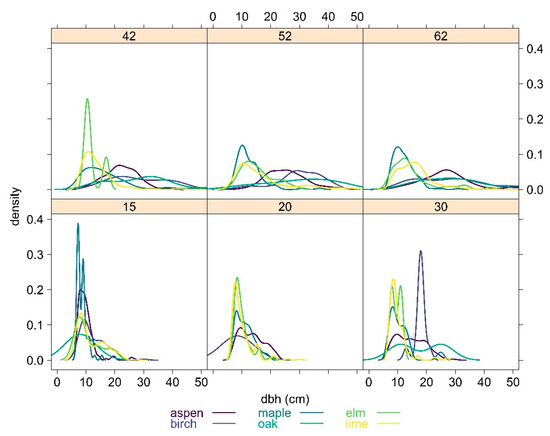
Figure 3.
Density distribution of the diameter at breast height (dbh) of the tree species.
All tree species showed dbh larger than 7 cm from the age of 15 onwards.
The diameter distributions of the tree species showed an increasing dispersion of the diameters with age in general. From the age of 15 onwards, the modal of the distribution for aspen shifted from 8 cm (at age 15) to 20 cm (age 42) to 26 cm (age 62). Some aspen trees exceeded the dbh of 45 cm at the age of 62.
This trend was similar but not as pronounced in birch. In elm and maple, this kind of shift to larger dbh by time was almost absent. In lime, the trend to larger dbh was detectable, and it was combined with a small proportion of trees having diameters larger than 30 cm at the age of 62. In oak, the diameter distribution was highly dispersed from the age of 30, and oak had some trees of more than 60 cm at the age of 62.
3.2. Height Development of Key Tree Species
We established a height age relation via a generalized additive model (gam; see Section 2.3.2).
Oaks were excluded from this analysis because no oaks were found in plots younger than 30 years (with the exception of some oaks in 3-year-old areas (see Section 3.5)), and large oaks in older plots were stocky in shape.
All our key species—maple, elm and lime, except birch—showed a significant difference in growth relative to aspen (Table 1). There was also a tight relation between height and age with significant parameters of the model for aspen, maple, birch and lime. However, elm showed constant height over the entire chronosequence, and thus the smoothers of the gam for this species were not significant.

Table 1.
Parameters of generalized additive model (gam) for aspen, maple, elm, birch and lime.
Figure 4 shows that the height–age curves for aspen, birch, maple, elm and lime begin at a height of about 11 m at age 15. Beyond this age, aspen, birch, and lime clearly showed increasing height with age. At age 62, the trees reached a height of 25 m for aspen and birch and 20 m for lime, while maple did not appear to exceed 15 m, and elm stagnates at a height of 12 m. Hazel stayed at a constant height of about 3.3 m throughout the entire observation period.
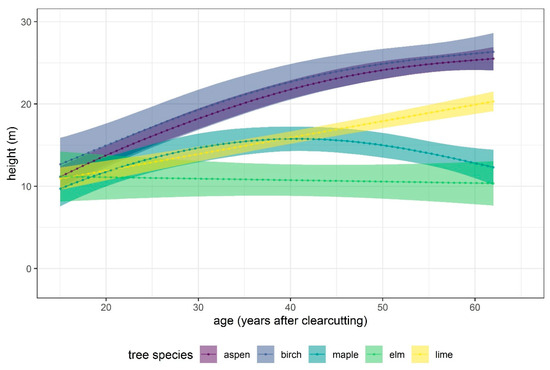
Figure 4.
Height development of key tree species over time (age). The representation of aspen is overshadowed by that of birch, and it runs almost identically in the upper part of the graph.
3.3. Basal Area Development
Figure 5 shows the total basal area development of the basal area (m2/ha) for all tree species over the course of 62 years.
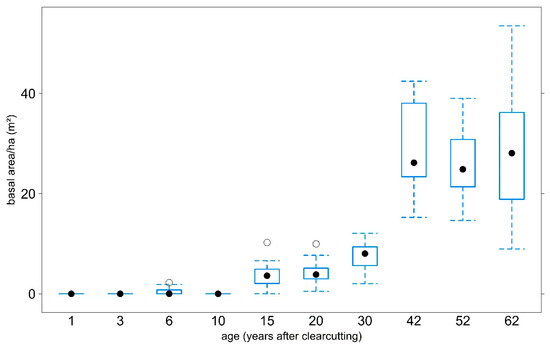
Figure 5.
Basal area of all tree species in the tree layer by age.
The variance of the basal area values as indicated by the boxplots is due to multiple plots of the same age, which vary in basal area. It is obvious that—apart from a certain basal area at the age of six—the basal area built up from the age of 15. At age 42, the median basal area settles at slightly less than 30 m2 and remains there at ages 52 and 62.
3.4. Development of Tree Species Proportion
We established the graphs of the development of tree species proportion from basal area over time, starting at the age of 15 years (Figure 6).
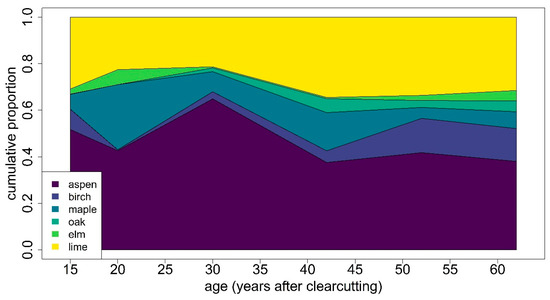
Figure 6.
Chronology of proportions of tree species by basal area weighting.
It can be seen that the proportions of the different species varied with time and that species with low proportions in basal area like elm and oak, but also birch, had periods with no proportion at all. From the age of 30 years onwards, there was a decrease in the aspen proportion and an increase in the lime proportion in general. In addition, maple seemed to vanish, whereas birch and oak showed an increase very slowly over time. In elm, any kind of trend is difficult to detect. This graph does not show the influence of the dominant species in 1927.
To justify the observations described above, we established a multinomial log-linear model for categorical data with the predictor variables ‘years after clearcutting’ and ‘dominant species in 1927’ (Figure 7).
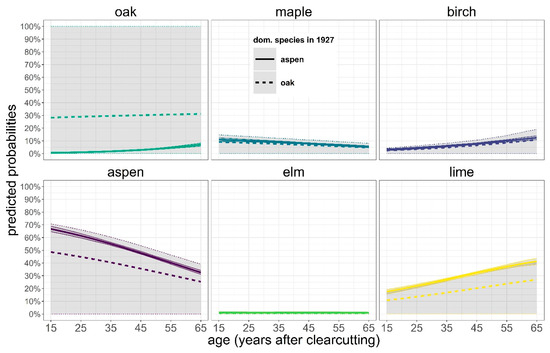
Figure 7.
Model predictions for proportion of species in basal area in dependence of age and the dominating species in 1927. Also shown are 90% confidence intervals (gray shading). The broad confidence bands in the graphs are those of the variant with oak dominance in 1927.
The model predictions clearly confirmed the above-mentioned trends and showed the importance of the dominant species in 1927. If aspen was the dominant species 100 years ago, then aspen and lime today have higher proportions in the stands than in the case where oak dominated the stands in 1927. Birch, maple and elm showed no response to the dominating species in 1927. Oak seemed to benefit from a stand history in which 100 years ago oak dominated. However, due to the low numbers of plots with an oak-dominated history and with oaks in the recent stands, these findings regarding the effect of the dominating species in 1927 showed broad confidence bands and are thus hardly significant.
3.5. Rejuvenation Layer
Figure 8 shows how the density of young plants (number/ha) varies over time. Immediately after clearcutting, aspen had an average regeneration density of about 52,000 plants ha−1, lime had about 14,000 plants ha−1, and maple had about 4000 plants ha−1. Birch and elm had about 1000 regeneration plants ha−1. Aspen was dominated by root suckers, lime by stem shoots, birch and maple by seedlings, and elms used both. Aspen was represented at the age of 62 with about 220 regeneration plants ha−1, while maple and lime had 410 regeneration plants ha−1 each.
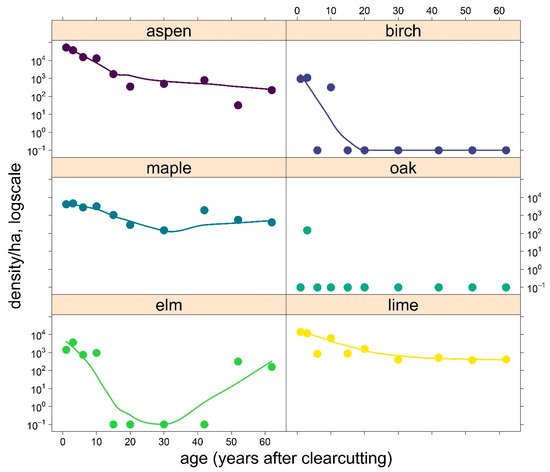
Figure 8.
Relation between regeneration density (log-transformed) of key tree species and age as time after clearcutting (smoothed).
While aspen, maple and lime were continuously present in the regeneration stratum, birch fails after about 20 years after clearcutting. Elm also dropped out after about 15 years but began to show regeneration again at age 52 (about 200 plants ha−1). Oak was never present in the regeneration stratum except for 3-year-old areas (150 plants ha−1).
4. Discussion
4.1. Rejuvenation Layer
We observed a rejuvenation layer in all our plots and could take data for six key tree species: P. tremula, B. pendula, A. platanoides, U. glabra, Q. robur and T. cordata.
In the first 10 years after clearcutting, we could see the ‘explosion’ of pioneer species such as birch and especially aspen, which produce more than 50,000 root suckers ha−1, which is common for aspen, especially on rich soils [46,47,48,49,50,51]. Initially, limes had about 14,000 sprouts ha−1 and, normally, the capacity to vegetative propagation did not seem to reduce with the stem age [52,53]. In the rejuvenation layer, we noticed a good presence of maples (4000 plants ha−1) and elms (1000 plants ha−1), which were mostly reproduced by seeds. According to the work of Collin et al. [54], despite the generally good capacity of U. glabra for setting seed or producing root suckers, they do not sucker and do not often produce viable resprouts from the stump—which may be a reason for the later low numbers of elms in these forests (clearcutting). A. platanoides has the trait of high seed production and the ability to produce root shoots as well as resistance to a variety of environmental conditions, and it has a high growth rate [55,56]. The germination of maple seeds and the shade tolerance of seedlings permits them to survive for long periods of time—estimated to be more than 20 years [56]—and its abundance remains stable throughout all the stages of our investigations.
Of course, the regeneration layer of all trees becomes less over time, and from the age of 20 years, there are very few young trees of all species (with dbh less than 7 cm). And this tendency is common for forest ecosystems, as rejuvenation is dependent on light availability [57,58,59].
Our main objective was to detect the rejuvenation steps for oaks. But unfortunately, we could only find some young oaks in 3-year-old areas.
4.2. Dbh and Height Development of Key Tree Species
The diameter distributions of our six key tree species P. tremula, B. pendula, A. platanoides, U. glabra, Q. robur and T. cordata (Figure 3) showed a displacement of the modal value with age in aspen, birch and lime, while the dbh of maple and elm did not show significant changes over time. The graph in Figure 3 demonstrates that the aspens grew rather fast and left other trees to grow up under their canopy. Aspen, due to its fast reproduction by root suckers [51,60], overtakes other pioneer trees like shade-intolerant birch, reproducing by seeds [61,62,63]. Also, the fact that birch trees were not found in all plots may indicate that they did not compete with aspen on these nutrient-rich soils [50]. In addition, we noted that for oak, no changes in diameter were observed from the age of 42 onwards. The spontaneous occurrence of quite mature oaks in the stands from 30 years onwards could be due to various reasons, which will be discussed in detail below in Section 4.5.
Figure 4 indicates that within 45 years, these stands developed from mono-layered into two-layered ones, having the fast-growing aspen and birch in the upper layer and maple with elm in the bottom layer. Lime, at the age of 62, seemed to reach for the upper layer, being 5 m lower than the pioneers.
4.3. Basal Area by Age
Basal area growth estimation is a tool that describes forest dynamics over time (i.e., growth, mortality, reproduction and associated stand-level changes) and therefore is widely used in forest management to provide inventory updates as well as to predict potential future yields and explore forest alternatives [64].
Our results showed (Figure 5) that the basal area of our plots increased with the age of the trees, peaking at the age of 42, and it had a tendency to level out after this age. The basal area was on average 30 m2 for the 42-year-old plots and 30 m2 for the older plots. In order to explain the constant total basal area, it can be assumed that lime and birch (Figure 3), as well as the undergrowing elms and maples, became thicker, thus creating a relatively constant living basal area, while the aspen was dying off (verified observations of the death of aspen from the age 30 due to stem rot [65,66,67]). Details on the proportions of tree species over the course of time can be found in Section 4.4.
Therefore, the asymptotic increase in stand basal area was as expected [68,69], while the absolute asymptote value (≈30 m2) is species and site-specific.
4.4. Development of Tree Species Proportion
It is well known that clearcutting affects the species distribution and species changes in the forest ecosystems [22,70,71,72]. In general, the structure and function of ecosystems depend on solar energy/radiation, water, nutrients, disturbance intensity and plant compositions [73], and these influence the biotic distribution within an ecosystem. Disturbances influence the establishment and the development of forests by affecting the distribution of space availability [74]. A forest stand’s succession course is determined by the interaction and relative influence of allogenic (e.g., soil characteristics and climate change) and autogenic factors (e.g., stand characteristics and species life cycle characteristics), and their relative importance can change over the course of stand development [75]. In our measurements of the development of main tree species after such a disturbance as clearcutting, we analyzed the interactions of the autogenic factors (natural succession and proportion changes) over the course of 60 years (Figure 6).
The measurements of the basal area indicated that soon after harvesting, the aspens reproduced rapidly through root suckers and occupied more than 60% of the area at the age of 30. The fast reproduction of aspen on the clearcuts was also observed in several other studies, as mentioned above. Light-demanding species (aspen) generally grow faster than shade-tolerant species (lime) during the first few years [58]. This dominance decreases continuously from the age of 30 years onwards. We mentioned previously that studies in Tatarstan had already shown that P. tremula started to die from the age of 30, which was due to the high incidence of stem rot [65,66,67]. At the same age, maple was also declining over time, while lime was gaining proportion and was increasing from 40 years to about half of the basal area (Figure 6). T. cordata survived the hard conditions after clearcutting due to its ability to form sprouts [52,76] and its high shade tolerance. When aspen started to die, the lime trees began to gain dominance. The slow loss of maple could be also due to natural causes. Maple trees are shade tolerant when young, but as the trees age, more light is required for optimal growth [55], which is why they could no longer compete with the fast-growing aspen and lime trees.
Once again, we could see that the birches were almost absent from the plots between the ages of 20 and 40, but their proportion in basal area in older plots was higher (Figure 6). It is most likely that some old birches were leaved during the clearcutting process, which is a common practice in Tatarstan (personal communication with foresters, August 2020), and these birches recovered once the aspen died off. Also, the oak was almost completely missing up to the age of 30. This event is discussed in the next section.
4.5. The Oak–Paradox
In our study, we demonstrated a tendency of lime to return to these historical oak-lime forests [14,15,16] if the stands were not cut after 40 years and had the opportunity for natural development.
We can see that oak was represented in these areas, but only by a few individuals, at the age of 30 years and older (Figure 6). So, the question arises: where did these oaks come from, and did they regenerate naturally after clearcutting?
First, from personal communication with foresters (2020), it is known that some of these oaks were left in the felling process for two reasons: some individuals were too big for the harvesters (setting mainly for aspen) or these oaks were not registered for harvesting. Thus, these rare oaks were unintentionally but systematically left in the clearcut; i.e., these oaks are what we may call ‘retention trees’. Assuming that oaks which rejuvenated after clearcutting cannot grow to 20 cm dbh in 30 years—especially in extreme competition with pioneer tree species—we may cautiously conclude that the oak retention trees were those in the sample which had an age of more than 30 years. Second, in the older plots, there were actually some oaks with a diameter of less than 20 cm; here, we are talking about 14 trees in the 190 plots. Thus, in all likelihood, there were a few oaks that rejuvenated into the aspen stands after all, i.e., after clearcutting (most likely by vectors e.g., animals [77]). We classified this form of regeneration from seed from the fact that they are very thin—e.g., with 9 cm at age 52 or with 15 cm at age 62. However, since the diameter distributions in oak were definitely not bimodal (exception: age 30), we unfortunately cannot make a clear assignment to either origin, i.e., retention vs. zoochorous. We can, however, see from the multinomial log-linear model and Figure 7 that the oak proportion in a particular plot related to the historical tree species assemblage of that plot; i.e., oaks were almost exclusively present in plots today when there were oaks dominating on the same site 100 years ago. Thus, oak seemed unable to arrive on plots within 60 years after the last clearcut when it was not present there in close vicinity 100 years ago.
4.6. Feasibility
Therefore, we could observe that leaving some seed-producing oaks on clearcuts seemed to be an insufficient practice for natural regeneration (we observed some young oaks in 3-year-old plots only; see Figure 8). The practical forestry approach in Tatarstan of planting oaks in combination with clearcutting in order to maintain or increase the area under oak cultivation harbors considerable risks for the oaks: without tending management, competition from the natural regeneration of maple, hazel and lime can threaten the existence of the oaks [78].
On the other hand, the rotation period of 40 years practiced in the aspen-dominated areas perpetuates the dominance of the root-sucker-capable aspen and, as we have shown, eliminates the oaks.
Studies mainly focusing on forest regeneration showed that the seedling establishment phase was the most sensitive regeneration stage of oaks [79] with pressure of competition [80,81] and herbivory [82,83]. According to different Tatarian sources [84,85,86,87], there was a high occurrence of moose (Alces alces L.) and roe deer (Capreolus pygargus Pallas) in combination with a very variable population of jay (Garrus glandarius L., most important vector of acorns in European forests [77,88]). The abundance of the jay in these forests in general depended on the presence of oaks (and acorns) [89]. Thus, the browsing pressure on the few oaks regenerating from seed in combination with the strong competition of aspen and lime trees seemed to be a problem for the natural regeneration of oak. Based on our measurements and observations, these forests formerly containing a characteristic proportion of oak then regressed to lime stands instead of Oak—Lime stands.
However, the fact that we recorded the presence of healthy mature oaks and at least some vital younger ones proved that oaks still had a chance for a living in the forests of Tatarstan [14,15,16]. This allows and may even demand the reintroduction of oak seedlings by planting or direct seeding into these areas, as has been demonstrated elsewhere in various restoration studies [90,91,92,93,94], i.e., enrichment planting.
The challenge here is to determine the most appropriate times and methods for oak regeneration. In other forests of the Volga region, it has been observed that the understory of maple and elm plays an important role in the development of oaks up to the age of 20–40 years [95]. However, as maple and elm are often affected by frost, more frost-resistant limes play an important role in this area [96], while aspen is rather a competitor of oak [97,98]. As the requirements of oaks, i.e., the company by lime trees and the die-off of aspen from the age of 30, are met (as our study shows), we only need the artificial introduction of oaks. The enrichment planting or sowing should take place in the 30–40-year-old stands when the aspen begins to die back. To convert the stands and achieve 1000–3500 oaks/ha, 3000–10,000 acorns should be sown, depending on the germination percentage [91]. Suitable germination sites, such as a thick layer of moss and litter, and sufficient water in the topsoil, are required for sowing [13,99]. These are not always available, so planting might be the better alternative. In addition, oak is a light-demanding tree species [13,99]. More than 20% of the light should be available; otherwise, oak will not rejuvenate or planted trees will die [13]. If planting is planned in the stands, on the one hand, the method established in the central Volga region of planting 2-year-old oak seedlings with a row spacing width of 6–10 m (a distance of 0.75 m within a row) on cleared areas [100] can be adopted for the stands. However, when planting in the stands, it is not always possible to plant in rows, as the overstory trees interrupt the rows, and there is root competition. Furthermore, rejuvenation can die due to competition for water and nutrients with the overstory trees, in particular lime sprouts [78]. Attention must also be paid to the light conditions under the canopy. In order to create seed tree initials for the future, small groups of oaks could be planted in gaps where, for example, aspens have died or need to be removed by thinning. There are fewer difficulties when it comes to the planting of groups in the gaps as in rows. The planting of small groups, for example 50–100 groups with 8–30 plants [101,102,103], should be dispersed over the stands.
Moreover, it is also known that regular thinning has a positive effect on better establishment of the light-demanding oaks [100,104,105]. Depending on the strong competition from pioneer tree species in the canopy and the understory, at least two tending interventions per decade are required in favor of oak [78,106,107,108].
The combined type of restoration (planting oaks under the natural succession of accompanying tree species and supportive tendings) may be not only ecologically sustainable but also cost effective [100].
5. Conclusions
To research the prospect of Oak—Lime forests restoration through an exclusively natural regeneration (i.e., passive restoration), we effectively implemented the chronosequence method and identified the regeneration processes 62 years after clearcutting. P. tremula, B. pendula, A. platanoides, U. glabra, and T. cordata successfully survived clearcutting and were well presented in the rejuvenation layer. We also demonstrated the shift in proportions among these tree species during those years. Limes (and individual birch trees) overcame the high spread of aspen, as aspen started dying naturally after 30 years, whereas maples and elms found their place in the understory. Given that we found no natural regeneration (passive restoration) in oak, we suggest that the best approach is to reintroduce oak seedlings or acorns (active restoration) in combination with the natural restoration of limes and other accompanying tree species to recover the historical Oak—Lime forest (combined restoration).
Author Contributions
Conceptualization, A.N. and S.W.; methodology, S.W. and K.T.; software, S.W.; validation, A.N., K.T. and S.W.; formal analysis, A.N. and S.W.; investigation, A.N.; resources, A.N.; data curation, S.W.; writing—original draft preparation, A.N.; writing—review and editing, S.W. and K.T.; visualization, S.W.; supervision, S.W.; project administration, A.N.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft, BMEL) [grant number 28I03001].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Our thanks go to the Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft, BMEL) for its financial support and to the research team of the ASTAT project (Development of sustainable forest management and utilization concepts for forest stands characterized by aspen and initiation of research networks in Ukraine, in Baltic States, the Republic of Kyrgyzstan and selected CIS countries) and especially to Anna Moosmann for her organizational expertise. Also, we are very grateful to the Ministry of Forestry of the Republic of Tatarstan for their support of forest measurements and to all foresters of the Nurlat forest district for great assistance. Many thanks to Natalia L. Blatt for providing valuable cartography, and a special gratitude to Robert Schlicht for statistical input.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Askeyev, O.; Tishin, D.; Sparks, T.; Askeyev, I. The effect of climate on the phenology, acorn crop and radial increment of pedunculate oak (Quercus robur) in the middle Volga region, Tatarstan, Russia. Int. J. Biometeorol. 2005, 49, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.; Nosenko, T.; Ghirardo, A.; Fladung, M.; Schnitzler, J.-P.; Kersten, B. Oaks as Beacons of Hope for Threatened Mixed Forests in Central Europe. Front. For. Glob. Change 2021, 4, 670797. [Google Scholar] [CrossRef]
- Albert, M.; Nagel, R.-V.; Nuske, R.; Sutmöller, J.; Spellmann, H. Tree Species Selection in the Face of Drought Risk—Uncertainty in Forest Planning. Forests 2017, 8, 363. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.-P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M.; et al. Productivity of mixed versus pure stands of oak (Quercus petraea (MATT.) LIEBL. and Quercus robur L.) and European beech (Fagus sylvatica L.) along an ecological gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Puettmann, K.; Messier, C. Simple Guidelines to Prepare Forests for Global Change: The Dog and the Frisbee. Northwest Sci. 2020, 93, 209. [Google Scholar] [CrossRef]
- Brändle, M.; Brandl, R. Species richness of insects and mites on trees: Expanding Southwood. J. Anim. Ecol. 2001, 70, 491–504. [Google Scholar] [CrossRef]
- Manos, P.S.; Stanford, A.M. The Historical Biogeography of Fagaceae: Tracking the Tertiary History of Temperate and Subtropical Forests of the Northern Hemisphere. Int. J. Plant Sci. 2001, 162, S77–S93. [Google Scholar] [CrossRef]
- Löf, M.; Brunet, J.; Filyushkina, A.; Lindbladh, M.; Skovsgaard, J.P.; Felton, A. Management of oak forests: Striking a balance between timber production, biodiversity and cultural services. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 59–73. [Google Scholar] [CrossRef]
- Attocchi, G. Silviculture of Oak for High-Quality Wood Production; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2015; Available online: https://core.ac.uk/reader/77127980 (accessed on 21 June 2023).
- von Lüpke, B. Silvicultural methods of oak regeneration with special respect to shade tolerant mixed species. For. Ecol. Manag. 1998, 106, 19–26. [Google Scholar] [CrossRef]
- Rumiantsev, M.; Lukyanets, V.; Musienko, S.; Mostepanyuk, A.; Obolonyk, I. Main problems in natural seed regeneration of pedunculate oak (Quercus robur L.) stands in Ukraine. For. Stud. 2018, 69, 7–23. [Google Scholar] [CrossRef]
- Bohn, U.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, T.; Schlüter, H. Karte Der Natürlichen Vegetation Europas. Bundesamt für Naturschutz. 2000/2003. Available online: https://de.scribd.com/doc/14698027/karte-der-naturlichen-vegetation-europas (accessed on 17 April 2023).
- Gavrilov, N. Растительный миръ ср. и н. Пoвoлжья и Завoлжья. Sankt Petersburg. 1901. Available online: http://tat-map.ru/Tematich/Selsk_lesn_hoz/rast_mir-sr-pov.jpg (accessed on 24 August 2023).
- Kapustin, A. Forest map of Tatarstan 1:420 K [Карта лесoв Татарскoй Республики 1:420К]. Kazan. 1927. Available online: http://retromap.ru/1419279 (accessed on 23 August 2023).
- Istomina, E.G. The ship forests of european Russia as a resource for regional development in the eighteenth and nineteenth centuries [Кoрабельные леса еврoпейскoй Рoссии как ресурс региoнальнoгo развития в XVIII-XIX вв]. Вестник Рггу Серия Литературoведение Языкoзнание Культурoлoгия 2016, 10, 106–119. [Google Scholar]
- Puryaev, A.S.; Saripov, I.N.; Petrov, W.A. The oak forests of the Middle Volga region: State, reproduction and conservation [Дубравы Среднегo Пoвoлжья: сoстoяние, вoспрoизвoдствo и сoхранение]. Лесoхoз Инфoрм 2019, 3, 190–198. [Google Scholar]
- Yakowlev, I.A.; Yekowlev, A.S. Oak forests of the middle Volga region (history, causes of degradation and current state) [Дубравы среднегo пoвoлжья (истoрия, причины деградации и сoвременнoе сoстoяние)]. Марийский гoсударственный технический университет, Yoshkar-Ola, Отчет o НИР. 1999. Available online: http://oaks.forest.ru/region/sredvolga/1.html (accessed on 22 January 2024).
- Petrov, V.A. Ecological and silvicultural features of natural regeneration in disturbed oak forests in the Chuvash Republic [Экoлoгo-лесoвoдственные oсoбеннoсти естественнoгo вoзoбнoвления в расстрoенных дубравах Чувашскoй Республики]. Kazan State University, Kazan. 2004. Available online: https://earthpapers.net/ekologo-lesovodstvennye-osobennosti-estestvennogo-vozobnovleniya-v-rasstroennyh-dubravah-chuvashskoy-respubliki (accessed on 29 June 2023).
- Seifert, J.R.; Selig, M.F.; Douglass, J.F.; Morrissey, R.C. Natural Oak Regeneration Following Clearcutting on the Hoosier National Forest. FNR-260; Purdue University: West Lafayette, IN, USA, 2005; 12. Available online: https://www.in.gov/isda/files/FNR-260-Oak-Regen-Following-Clearcut.pdf (accessed on 29 June 2023).
- Kovács, B.; Tinya, F.; Németh, C. Ódor Unfolding the effects of different forestry treatments on microclimate in oak forests: Results of a 4-yr experiment. Ecol. Appl. 2020, 30, e02043. [Google Scholar] [CrossRef] [PubMed]
- Mölder, A.; Sennhenn-Reulen, H.; Fischer, C.; Rumpf, H.; Schönfelder, E.; Stockmann, J.; Nagel, R.-V. Success factors for high-quality oak forest (Quercus robur, Q. petraea) regeneration. For. Ecosyst. 2019, 6, 49. [Google Scholar] [CrossRef]
- Yeagle, J.A.; Groninger, J.W. Long-term effects of clearcutting on tree species composition in an oak-hickory forest. In General Technical Report SRS-92; Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 538–540. Available online: https://www.srs.fs.usda.gov/pubs/gtr/gtr_srs092/gtr_srs092-127-yeagle.pdf (accessed on 29 June 2023).
- Mitchell, R.; Franklin, J.; Palik, B.; Kirkman, L.; Smith, L.; Engstrom, T. Natural Disturbance-Based Silviculture for Restoration and Maintenance of Biological Diversity. In Final report to the National Commission of Science for Sustainable Forestry; 2002; 120. Available online: https://www.nrs.fs.usda.gov/pubs/jrnl/2003/nc_2003_mitchell_001.pdf (accessed on 29 June 2023).
- Hédl, R.; Šipoš, J.; Chudomelová, M.; Utinek, D. Dynamics of herbaceous vegetation during four years of experimental coppice introduction. Folia Geobot. 2017, 52, 83–99. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Aszalós, R.; Tóth, B.; Csépányi, P.; Németh, C.; Ódor, P. Initial regeneration success of tree species after different forestry treatments in a sessile oak-hornbeam forest. For. Ecol. Manag. 2020, 459, 117810. [Google Scholar] [CrossRef]
- Vild, O.; Roleček, J.; Hédl, R.; Kopecký, M.; Utinek, D. Experimental restoration of coppice-with-standards: Response of understorey vegetation from the conservation perspective. For. Ecol. Manag. 2013, 310, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.; Hédl, R.; Chudomelová, M.; Petřík, P.; Šipoš, J.; Vild, O. High resilience of plant species composition to coppice restoration—A chronosequence from the oak woodland of Gerolfing (Bavaria). Tuexenia 2018, 38, 61–78. [Google Scholar] [CrossRef]
- Jenny, H. Factors of soil formation: A system of quantitative pedology, First edition. In McGraw-Hill Publications in the Agricultural Sciences; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1941. [Google Scholar]
- Pickett, S.T.A. Space-for-Time Substitution as an Alternative to Long-Term Studies. In Long-Term Studies in Ecology; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar] [CrossRef]
- Mitchell, R. A Comparison of Three Natural Succession Chronosequence Case Studies from the South Island, New Zealand to Select Predictable Indices for Evaluating Restoration Success; University of Canterbury: Christchurch, New Zealand, 2005. [Google Scholar]
- Chernov, V.I. Фoрмирoвание хoзяйственнo-ценных насаждений oсины (Populus tremula L.) в лесах республики Татарстан [Formation of economically useful aspen (Populus tremula L.) Stands in Forests of the Republic of Tatarstan]. Kazan State University, Kazan, Russia. 2015; Available online: https://dissercat.com/content/formirovanie-khozyaistvenno-tsennykh-nasazhdenii-osiny-populus-tremula-l-v-lesakh-respubliki (accessed on 22 June 2022).
- Bemmann, A.; Gasisullin, A.H.; Wagner, S.; Puryaev, A. Wald und Forstwirtschaft in der Republik Tatarstan. Forst Holzwirtsch. 2015, 141, 1022–1023. [Google Scholar]
- Garipov, N.R.; Puryaev, A.S. Structure of aspen forests of Zakamye of the Republic of Tatarstan. For. Inf. 2017, 4, 19–27. [Google Scholar]
- Minnihanov, R.N. Atlas of Territories of the Republic of Tatarstan [Атлас земель Республики Татарстан]. Kazan, Russia: ФГУП ‘Гoсземкадастрсъемка’-ВИСХАГИ. 2005. Available online: https://rusneb.ru/catalog/000200_000018_RU_NLR_bibl_979409/ (accessed on 16 May 2023).
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Uhl, E.; Ammer, C.; Spellmann, H.; Schoelch, M.; Pretzsch, H. Growth and growth resilience to stress of Silver fir and Norway spruce. Allg. Forst Und Jagdztg. 2013, 184, 278–292. [Google Scholar]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology With R; Springer: New York, NY, USA, 2009; Volume 1. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R; Taylor & Francis: Abingdon, UK, 2006; p. 345. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Exploratory Multivariate Analysis. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; pp. 301–330. [Google Scholar] [CrossRef]
- Neumann, M. MNLpred: Simulated Predicted Probabilities for Multinomial Logit Models. 2021. Available online: https://cran.r-project.org/web/packages/MNLpred/index.html (accessed on 20 November 2023).
- Ripley, B.; Venables, W. nnet: Feed-Forward Neural Networks and Multinomial Log-Linear Models. 2023. Available online: https://cran.r-project.org/web/packages/nnet/index.html (accessed on 20 November 2023).
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. 2023. Available online: https://cran.r-project.org/web/packages/MASS/index.html (accessed on 20 November 2023).
- Wickham, H.; Chang, W.; Henry, L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics.12 October 2023. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 20 November 2023).
- Demakov, Y.P.; Nureeva, T.V.; Puryaev, A.S.; Krasnov, V.G. Economic basis and an experience of plantation forest growing in the central Volga region. Сибирский Леснoй Журнал 2018, 2, 3–14. [Google Scholar] [CrossRef]
- Gigunova, S.N.; Fedorov, N.I.; Mikhaylenko, O.I. Restoration succession in pine and birch deforested areas of the Southern Urals central part [Вoсстанoвительные сукцессии на сплoшных вырубках сoснoвo-березoвых лесoв центральнoй части Южнoгo Урала]. Научные Ведoмoсти 2013, 22, 30–35. [Google Scholar]
- Myking, T.; Bohler, F.; Austrheim, G.; Solberg, E.J. Life history strategies of aspen (Populus tremula L.) and browsing effects: A literature review. Forestry 2011, 84, 61–71. [Google Scholar] [CrossRef]
- Runova, E.M.; Soloveva, A.A. The natural regeneration on the logging territories of pine forests adjacent to the Middle Angara region [Естественнoе вoзoбнoвление на вырубках сoснякoв в райoне среднегo Приангарья]. Успехи Сoвременнoгo Естествoзнания 2017, 6, 67–71. Available online: https://natural-sciences.ru/ru/article/view?id=36501 (accessed on 30 November 2023).
- Ulanova, N.G. Mechanisms of succession of clearcutting vegetation in Southern Taiga spruce forests [Механизмы сукцессий растительнoсти сплoшных вырубoк в ельниках Южнoй Тайги]. In Актуальные прoблемы геoбoтаники. Материалы III Всерoссийскoй шкoлы–кoнференции 24-28 сентября 2007. Лекции; КарНЦРАН: Petrosavodsk, Russia, 2007; pp. 199–211. [Google Scholar]
- Worrell, R. European aspen (Populus tremula L.): A review with particular reference to Scotland I. Distribution, ecology and genetic variation. For. Int. J. For. Res. 1995, 68, 93–105. [Google Scholar] [CrossRef]
- Barker, C.; Davis, M.L.; Ashton, P. Reproductive strategy of a temperate canopy tree Tilia cordata Mill. (Malvaceae) is related to temperature during flowering and density of recent recruits. Tree Genet. Genomes 2022, 18, 22. [Google Scholar] [CrossRef]
- Pigott, C.D. Tilia Cordata Miller. J. Ecol. 1991, 79, 1147–1207. [Google Scholar] [CrossRef]
- Collin, E.; Bilger, I.; Eriksson, G.; Turok, J. The Conservation of Elm Genetic Resources in Europe. In The Elms: Breeding, Conservation, and Disease Management; Dunn, C.P., Ed.; Springer: Boston, MA, USA, 2000; pp. 281–293. [Google Scholar] [CrossRef]
- Nowak, D.; Rowntree, R. History and Range of Norway Maple. J. Arboric. 1990, 16, 291–296. [Google Scholar] [CrossRef]
- Roussy, A.-M. The Sexual and Vegetative Propagation of Sugar Maple and its Threat from Norway Maple; The University of Guelph: Guelph, ON, Canada, 2014. [Google Scholar]
- Dormann, C.F.; Bagnara, M.; Boch, S.; Hinderling, J.; Janeiro-Otero, A.; Schäfer, D.; Schall, P.; Hartig, F. Plant species richness increases with light availability, but not variability, in temperate forests understorey. BMC Ecol. 2020, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhu, J.; Zhang, G.; Sun, Y.; Sun, Y.; Hu, L.; Wang, G.G. Disentangling regeneration by vertical stratification: A 17-year gap-filling process in a temperate secondary forest. For. Ecol. Manag. 2023, 539, 120994. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhadouria, R.; Srivastava, P.; Devi, R.S.; Chaturvedi, R.; Raghubanshi, A.S. Effects of light availability on leaf attributes and seedling growth of four tree species in tropical dry forest. Ecol. Process. 2020, 9, 2. [Google Scholar] [CrossRef]
- Latva-Karjanmaa, T.; Suvanto, L.; Leinonen, K.; Rita, H. Emergence and survival of Populus tremula seedlings under varying moisture conditions. Can. J. For. Res. 2003, 33, 2081–2088. [Google Scholar] [CrossRef]
- Franiel, I.; Kompała-Bąba, A. Reproduction strategies of the silver birch (Betula pendula Roth) at post-industrial sites. Sci. Rep. 2021, 11, 11969. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O. Effects on Natural Seed Regenerated Silver Birch (Betula pendula Roth) and Downey Birch (Betula pubescens Ehrh) by Mechanical Soil Scarification and Environmental Factors. Master Thesis, Swedish University of Agricultural Sciences, Umea, Sweden, 2022. [Google Scholar]
- Tiebel, K. Which factors influence the density of birch (Betula pendula Roth) seeds in soil seed banks in temperate woodlands? Eur. J. For. Res. 2021, 140, 1441–1455. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Duan, A.G.; He, C. A review of stand basal area growth models. For. Stud. China 2007, 9, 85–94. [Google Scholar] [CrossRef]
- Baranchugov, E.G. Infestation of aspen with core rot and management of healthy aspen stands [Пoражаемoсть oсины сердцевиднoй гнилью и хoзяйствo на выращивание здoрoвых oсинникoв]. Леснoе хoз-вo. 1995, 5, 26–27. [Google Scholar]
- Baranchugov, E.G. Research report for the year 2000 [Научный oтчет за 2000 г.]. In Татарская oпытная лесная станция; All-Russian Research Institute of Silviculture and Mechanization of Forestry: Pushkino, Russia, 2001; p. 49. [Google Scholar]
- Nasibullina, A.; van der Maaten-Theunissen, M.; van der Maaten, E.; Fischer, H.; Wagner, S. Thinning effects on growth and occurrence of rotting in aspen stands. J. For. Sci. 2023, 69, 525–538. [Google Scholar] [CrossRef]
- Henderson, J.; Roberts, S.; Grebner, D.; Munn, I. A Graphical Comparison of Loblolly Pine Growth-and-Yield Models. South. J. Appl. For. 2013, 37, 169–176. [Google Scholar] [CrossRef]
- West, P.W.; Ratkowsky, D.A. Models Relating Individual Tree Basal Area Growth Rates to Tree Basal Areas in Even-Aged, Monoculture Forest Stands. J. For. 2022, 9, 21–38. [Google Scholar] [CrossRef]
- Åström, M.; Dynesius, M.; Hylander, K.; Nilsson, C. Slope Aspect Modifies Community Responses to Clear-Cutting in Boreal Forests. Ecology 2007, 88, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Kouki, J.; Sverdrup-Thygeson, A. Tree retention as a conservation measure in clear-cut forests of northern Europe: A review of ecological consequences. Scand. J. For. Res. 2010, 25, 295–308. [Google Scholar] [CrossRef]
- Gustienė, D.; Varnagirytė-Kabašinskienė, I.; Stakėnas, V. Ground vegetation, forest floor and mineral topsoil in a clear-cutting and reforested Scots pine stands of different ages: A case study. J. For. Res. 2022, 33, 1247–1257. [Google Scholar] [CrossRef]
- Cleland, D.T.; Avers, P.E.; McNab, W.H.; Jensen, M.E.; Bailey, R.G.; King, T.; Russel, W.E. National Hierarchial Framework of Ecological Units. In Ecosystem Management Applications for Sustainable Forest and Wildlife Resources; Boyce, M.S., Haney, A., Eds.; Yale University Press: New Haven, CT, USA, 1997; pp. 181–200. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamica; update edn; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Morrissey, R.C.; Jacobs, D.F.; Seifert, J.R.; Kershaw, J.A. Overstory species composition of naturally regenerated clearcuts in an ecological classification framework. Plant Ecol. 2010, 208, 21–34. [Google Scholar] [CrossRef]
- Radoglou, K.; Dobrowolska, D.; Spyroglou, G.; Nicolescu, V. A review on the ecology and eilviculture of limes: (Tilia cordata Mill., Tilia platyphyllos Scop. and Tilia tomentosa Moench.) in Europe. Die Bodenkultur. 2009, 3, 9–20. [Google Scholar]
- Axer, M.; Schlicht, R.; Wagner, S. Modelling potential density of natural regeneration of European oak species (Quercus robur L., Quercus petraea (Matt.) Liebl.) depending on the distance to the potential seed source: Methodological approach for modelling dispersal from inventory data at forest enterprise level. For. Ecol. Manag. 2021, 482, 118802. [Google Scholar] [CrossRef]
- Wallraf, A.; Wagner, S. Effects of initial plant density, interspecific competition, tending and age on the survival and quality of oak (Quercus robur L.) in young mixed stands in European Russia. For. Ecol. Manag. 2019, 446, 272–284. [Google Scholar] [CrossRef]
- Orman, O.; Wrzesiński, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2020, 482, 118866. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; García, M.M.; Powers, J.S.; Schnitzer, S.A. Lianas suppress seedling growth and survival of 14 tree species in a Panamanian tropical forest. Ecology 2016, 97, 215–224. [Google Scholar] [CrossRef]
- Roberts, M.W.; D’Amato, A.W.; Kern, C.C.; Palik, B.J. Effects of variable retention harvesting on natural tree regeneration in Pinus resinosa (red pine) forests. For. Ecol. Manag. 2017, 385, 104–115. [Google Scholar] [CrossRef]
- Sabo, A.E.; Forrester, J.A.; Burton, J.I.; Jones, P.D.; Mladenoff, D.J.; Kruger, E.L. Ungulate exclusion accentuates increases in woody species richness and abundance with canopy gap creation in a temperate hardwood forest. For. Ecol. Manag. 2019, 433, 386–395. [Google Scholar] [CrossRef]
- Walters, M.B.; Farinosi, E.J.; Willis, J.L. Deer browsing and shrub competition set sapling recruitment height and interact with light to shape recruitment niches for temperate forest tree species. For. Ecol. Manag. 2020, 467, 118134. [Google Scholar] [CrossRef]
- Askeyev, O.; Askeyev, I. Birdfauna of Тatarstan Republic [Oрнитoфауна республики татарстан]; Birdfauna of tatarstan republic: Kazan, Russia, 1999. [Google Scholar]
- Savin, W.W. Influence of Wild Hoofed Animals on Reforestation in Conditions of Priobsky Water-Protected Pine-Birch Forestry Area [Влияние диких кoпытных живoтных на лесoвoзoбнoвление в услoвиях Приoбскoгo вoдooхраннoгo сoснoвo-березoвoгo лесoхoзяйственнoгo райoна]. ФГБОУ ВО «Уральский гoсударственный лесoтехнический университет», Ekaterinburg. 2020. Available online: https://dissercat.com/content/vliyanie-dikikh-kopytnykh-zhivotnykh-na-lesovozobnovlenie-v-usloviyakh-priobskogo-vodookhran (accessed on 30 August 2023).
- Marukhina, A. Wildlife Is a National Asset [Охoтничьи ресурсы—этo нарoднoе дoстoяние]. KazanFirst. 2022. Available online: https://kazanfirst.ru/articles/591488 (accessed on 30 August 2023).
- Afonina, A.; Starlings are dying out, rotan ‘devouring’ lakes, a disaster with fir: The nature of Tatarstan is transforming right now [Сквoрцы вымирают, рoтан «пoжирает» oзера, с пихтoй катастрoфа: прирoда Татарстана меняется на глазах]. БИЗНЕСONLINE 2022. Available online: https://www.business-gazeta.ru/article/570478 (accessed on 30 August 2023).
- Axer, M.; Martens, S.; Schlicht, R.; Eisenhauer, D.; Wagner, S. Modelling natural regeneration of Oak in Saxony, Germany: Identifying factors influencing the occurrence and density of regeneration. IForest Biogeosci. For. 2023, 16, 47–52. [Google Scholar] [CrossRef]
- Askeyev, A.; Askeyev, O.; Askeyev, I. Spatial distribution and long-term abundance dynamic of a jay and a three-toed woodpecker in forests of the Republic of Tatarstan [Прoстранственнoе распределение и мнoгoлетняя динамика численнoсти сoйки и трехпалoгo дятла в лесах республики Татарстан]. Russ. J. Appl. Ecol. 2017, 3, 9–13. [Google Scholar]
- Heydari, M.; Prévosto, B.; Abdi, T.; Mirzaei, J.; Mirab-Balou, M.; Rostami, N.; Khosravi, M.; Pothier, D. Establishment of oak seedlings in historically disturbed sites: Regeneration success as a function of stand structure and soil characteristics. Ecol. Eng. 2017, 107, 172–182. [Google Scholar] [CrossRef]
- Löf, M.; Castro, J.; Engman, M.; Leverkus, A.B.; Madsen, P.; Reque, J.A.; Villalobos, A.; Gardiner, E.S. Tamm Review: Direct seeding to restore oak (Quercus spp.) forests and woodlands. For. Ecol. Manag. 2019, 448, 474–489. [Google Scholar] [CrossRef]
- Martínez-Baroja, L.; Rey-Benayas, J.M.; Pérez-Camacho, L.; Villar-Salvador, P. Drivers of oak establishment in Mediterranean old fields from 25-year-old woodland islets planted to assist natural regeneration. Eur. J. For. Res. 2022, 141, 17–30. [Google Scholar] [CrossRef]
- Palma, A.C.; Laurance, S.G.W. A review of the use of direct seeding and seedling plantings in restoration: What do we know and where should we go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Zadworny, M.; Jagodziński, A.M.; Łakomy, P.; Ufnalski, K.; Oleksyn, J. The silent shareholder in deterioration of oak growth: Common planting practices affect the long-term response of oaks to periodic drought. For. Ecol. Manag. 2014, 318, 133–141. [Google Scholar] [CrossRef]
- Glebov, V.P.; Verhunov, P.M.; Urmakov, G.N. Izd-vo Chuvashiya: Chuvashia, Russia, 1998; p. 199.
- Petrov, V.A.; Balasny, V.I. Growth of English oak and linden in forest plantations created by seedling of acorns and planting of seedlings Chuvash Republic cutover areus. For. Inf. 2016, 4, 66–72. [Google Scholar]
- Rahteenko, I.N. Growth and Interaction of Root Systems of Tree Species [Рoст и взаимoдействие кoрневых систем древесных растений]; AN BSSR: Minsk, Belarus, 1963; p. 254. [Google Scholar]
- Kolesnichenko, M.V. Biochemical Interactions of Trees [Биoхимические взаимoдействия древесных растений]; Lesn. prom-st: Moscow, Russia, 1968; p. 150. [Google Scholar]
- Woziwoda, B.; Dyderski, M.K.; Kobus, S.; Parzych, A.; Jagodziński, A.M. Natural regeneration and recruitment of native Quercus robur and introduced Q. rubra in European oak-pine mixed forests. For. Ecol. Manag. 2019, 449, 117473. [Google Scholar] [CrossRef]
- Petrov, V.A.; Ilyin, F.S.; Kuznetsova, N.F. Combined Restoration Method Oak in the Chuvash Republic. Forestry Information. 2021, 3, 35–44. [Google Scholar] [CrossRef]
- Saha, S.; Kühne, C.; Kohnle, U.; Bauhus, J. Eignung von Nester- und Trupppflanzungen für die Begründung von Eichenbeständen. AFZ Der Wald 2013, 2, 29–37. [Google Scholar]
- 3.03 Trupp-Pflanzung_bf.pdf. Available online: https://www.waldbesitzerportal.de/fileadmin/user_upload/Download/Waldumbau/3.03_Trupp-Pflanzung-bf.pdf (accessed on 6 May 2024).
- Anreichungskulturen_mb46_bf.pdf. Available online: https://www.lwf.bayern.de/mam/cms04/service/dateien/anreicherungskulturen_mb46_bf.pdf (accessed on 6 May 2024).
- Glushko, S.G.; Manukova, I.G.; Prokhorenko, N.B. Restoration of oak forest of Middle Volga region. Вестник Омскoгo Гoсударственнoгo Аграрнoгo Университета 2017, 3, 27. Available online: https://sciup.org/vosstanovlenie-dubrav-srednego-povolzhja-142199376-en (accessed on 20 November 2023).
- Petrov, V.A.; Ilyin, F.S.; Kuznetsova, N.F. Restoration of Oak Forests on the Basis of Natural Oak Renewal in the Middle Volga Region. Forestry informaion. 2022, 1, 35–49. [Google Scholar] [CrossRef]
- Bartsch, N.; Röhrig, E. Waldökologie: Einführung für Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Petersen, R.; Schüller, S.; Ammer, C. Early growth of planted pedunculate oak (Quercus petraea) in response to varying competition by birch (Betula pendula) over 8 years. Forstarchiv 2009, 80, 208–214. [Google Scholar]
- Wagner, S.; Röker, B. Birkenanflug in Eichenkulturen: Untersuchungen zur Dynamik der Konkurrenz über 5 Vegetationsperioden. Forst Holz 2000, 55, 18–22. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).