Abstract
Xylaria is a widely distributed genus in the Ascomycota phylum that can decompose wood. It is an essential decomposer in ecosystems and a source of bioactive secondary metabolites. Based on morphological characteristics and molecular evidence, this article thoroughly describes two new species discovered on the fallen leaves in Hainan Tropical Rainforest National Park, along with illustrations and comparisons with similar species. Xylaria diaoluoshanensis is characterized by filamentous stromata with long infertile apexes, ascospores sometimes with non-cellular appendages. Xylaria fulvotomentosa differentiates itself from other Xylaria species that grow on fallen leaves by its stroma surface, being yellow tomentose. These two new species of the genus Xylaria were found by phylogenetic analysis using the ITS-β-tubulin-RPB2 sequence dataset. Furthermore, a species first discovered in China, X. petchii, is described. Finally, a search table for 44 species related to fallen leaves and petioles in the world is established.
1. Introduction
Xylaria Hill ex Schrank is the largest genus in Xylariaceae [1,2]. Over 300 Xylaria species have been reported worldwide [3], and there are 879 records related to Xylaria in Index Fungorum (http://www.indexfungorum.org/, accessed on 21 December 2023). Many species in this genus exhibit significant changes in their stromata morphology at different development stages [4]. They are generally characterized by cylindrical or filamentous upright stromata with a pale interior, asci with amyloid apical rings that turn blue in an iodine solution, eight brown unicellular ascospores with germ slit, and geniculosporium-like conidiophores [2,5,6,7]. To date, most reported Xylaria species grow on wood and branches, few grow on fallen fruits and seeds, termite nests and soil, and fallen leaves and petioles [7,8,9,10,11,12,13]. Fallen leaves and petioles are one of the growth substrates of Xylaria species. However, species growing on fallen leaves and petioles are usually overlooked due to their fragile and tiny stromata [14]. Generally, they have a small number of stromata, and different species may grow on the same leaf [14]. This challenges identification and makes the research on this group more difficult than that on other substrate groups. Ju and Hsieh [14] systematically combed global Xylaria species related to fallen leaves and petioles and profoundly promoted research on this group of substrates. However, the specimens collected of most of the species in that article were insufficient, making it difficult to determine the existence of substrate-specific species. For instance, there is only one specimen for X. allima Y.M. Ju & H.M. Hsieh, X. hispidipes Y.-M. Ju & H.-M. Hsieh, X. neblinensis Y.-M. Ju & H.-M. Hsieh, and X. noduliformis Y.-M. Ju & H.-M. Hsieh [14]. However, some species are not restricted to fallen leaves and petioles, they can also grow on woods, such as X. meliacearum Læssøe and X. petchii Lloyd [14]. Xylaria clusiae K.F. Rodrigues, J.D. Rogers & Samuels, X. duranii F. San Martín & Vanoye, and X. heloidea Penz. & Sacc. can be found on fallen leaves and fallen fruits or seeds [7]. Therefore, continuing to explore Xylaria species that grow on fallen leaves and petioles is of great ecological significance.
Xylaria species related to fallen leaves and petioles are mainly distributed in tropical and subtropical regions. The Hainan Tropical Rainforest National Park is located in the middle-south of Hainan Island, China, at latitude 18°33′16″–19°14′16″ N and longitude 108°44′32″–110°04′43″ E (https://www.hntrnp.com/, accessed on 21 December 2023). It belongs to the tropical island monsoon climate, with sufficient hydrothermal resources and abundant plant and fungal resources, and contains numerous endemic species. [4,15]. In this article, through morphological comparison and molecular investigation, three Xylaria species that grow on fallen leaves found in China are identified for the first time, including two new species and one first record in China. Finally, this paper establishes a key to Xylaria species related to fallen leaves and petioles worldwide.
2. Materials and Methods
2.1. Sample Source
The samples were collected at the Diaoluoshan Management Bureau of Hainan Tropical Rainforest National Park in February and June 2023 and stored in the Forest Resource Institute of Hainan Academy of Forestry.
2.2. Morphological Investigation
The habitat photos of the specimens were taken using a Canon D3 (Canon Corporation, Tokyo, Japan) and a Huawei Mate 50 (Huawei Corporation, Shenzhen, China). Fresh specimens were dried with a portable dryer (made in China). The dried specimens were marked and stored at minus 80 °C for morphological and molecular examination. The macroscopic morphology of the specimens was observed with a VHX-5000 digital microscope (Keyence Corporation, Osaka, Japan), focusing on the surfaces of the stromata, ostioles, and perithecia. Microscopic characteristics were observed and measured using three aqueous solution agents, water, Melzer’s reagent, and 1% Sodium Dodecyl Sulfate (SDS), under a full-automatic optical microscope DM6B (Leica Corporation, Wetzlar, Germany) [13]. In this study, N represented the observed and measured number of ascospores, and M denoted the average size of ascospores.
2.3. Molecular Research
The total DNA of the specimens was extracted using cetyltrimethylammonium bromide (CTAB) plant genome rapid extraction kits (Aidlab Biotechnology, Beijing, China). The gene sequences at three sites, ITS, RPB2, and β-tubulin, were amplified. The 40 µL system was employed in all PCR reactions (ddH2O 16 µL, 2 × HS™ Mix 20 µL, forward primer 1 µL, reverse primer 1 µL, and DNA template 2 µL). ITS was amplified using the primers ITS4/ITS5 [16]. The PCR program was as follows: initial denaturation at 95 °C for 3 min; 30 cycles at 94 °C for 40 s, 55.8 °C for 45 s, and 72 °C for 1 min; and a final extension at 72 °C for 10 min [4]. The amplification primers for RPB2 and β-tubulin were 7CR/5F [17] and T1/T22 [18], respectively. The PCR programs for these two sites were: initial denaturation at 95 °C for 3 min, followed by 35 cycles at 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 1.5 min, and a final extension at 72 °C for 10 min [19]. The PCR products were sent to the Tianyi Huiyuan Gene Technology Co., Ltd. (Wuhan, China) for sequencing. Serial numbers were obtained after the sequences were submitted to GenBank.
2.4. Phylogenetic Analyses
The newly obtained sequences and the Xylariaceae and Graphromataceae sequences collected from the National Center for Biotechnology Information (NCBI) were used to construct phylogenetic trees based on the sequence dataset ITS-β-tubulin-RPB2 (Table 1). Hypoxylon fragiforme (Pers.) J. Kickx f. and Camillea obularia (Fr.) Læssøe, J.D. Rogers & Lodge were selected as outgroups. Sequences were verified and adjusted in MAFFT v.7 (http://mafft.cbrc.jp/alignment/server/, accessed on 7 November 2023). BioEdit v.7.0.5.2 was adopted for manual cropping and optimization [20]. MEGA v.6.0 was used in site splicing [21,22]. Phylogenetic analyses were performed based on the Maximum Likelihood (ML) and Bayesian Inference (BI) methods. The ML analysis was conducted in RAxML v.8.2.10 [23] using the GTRGAMMA substitution model and 1000 bootstrap inferences. The BI analysis was carried out with MrBayes v.3.2.6 [24]. The applicable model was automatically selected according to the Bayesian information criterion (BIC) with initial generations set to be 1,000,000 [24,25]. The phylogenetic trees were inspected and adjusted in FigTree v.1.4.3, using Adobe Photoshop CS6 to add background colors.

Table 1.
Sequences and species used in the phylogenetic analysis, including growth substrate, origin, specimen number, GenBank access numbers, and references. Type specimens are labeled with HT. The new sequences from this study are in bold. NA: not available.
3. Results
3.1. Molecular Phylogeny
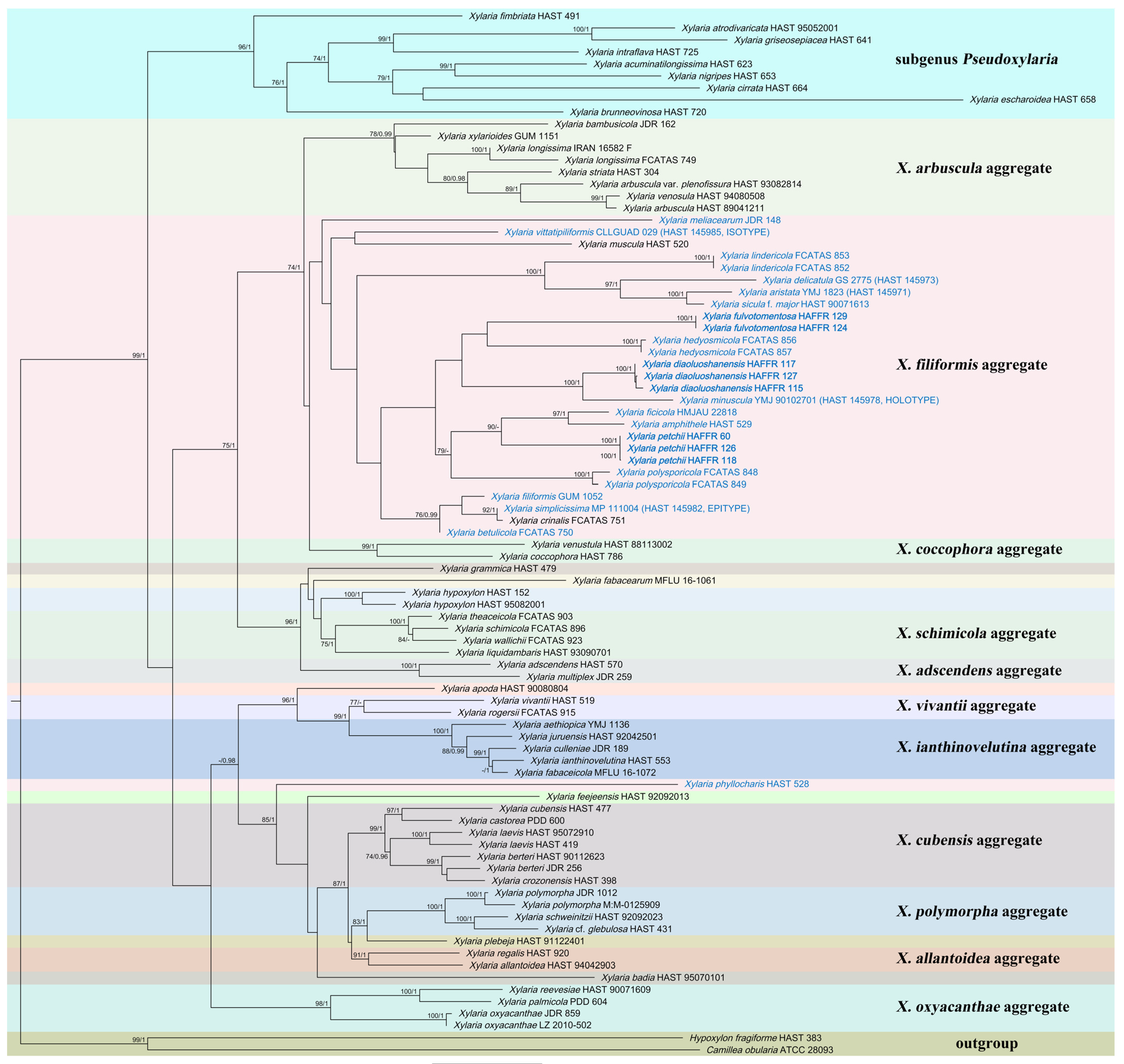
This paper used 86 ITS sequences, 72 β-tubulin sequences, and 68 RPB2 sequences for the phylogenetic analyses. Among them, 205 came from the NCBI database and 21 were obtained for this article, including 224 Xylaria species, one Hypoxylon, and one Camillea species. The sequence length of ITS was 796 character positions, β-tubulin 2241, and RPB2 1240. After cropping, the remaining character positions of ITS, β-tubulin, and RPB2 were 530, 1444, and 907, respectively. The complete dataset had a length of 2881 characters, containing 1217 parsimony-informative. The results of phylogenetic analyses showed no significant differences between the ML and BI trees. RAxML bootstrap values (≥70%) and Bayesian posterior probability (≥0.95) were labeled on the phylogenetic trees, respectively (Figure 1). The phylogenetic tree revealed that X. diaoluoshanensis sp. nov. clustered with X. minuscula Y.M. Ju & H.M. Hsieh. Xylaria fulvotomentosa sp. nov. and X. hedyosmicola Hai X. Ma & X.Y. Pan clustered together. Xylaria petchii is closely related to X. amphithele F. San Martín & J.D. Rogers and X. ficicola Hai X. Ma, Lar.N. Vassiljeva & Yu Li.
Figure 1.
The ML phylogenetic tree of Xylaria constructed with ITS-β-tubulin-RPB2 sequences. Support values of ML and BI analyses (bootstrap supports ≥ 70% and posterior probability values ≥ 0.95) are indicated above or below the branches. Species related to fallen leaves and petioles are in blue, and those described in this article are in bold.
3.2. Taxonomy
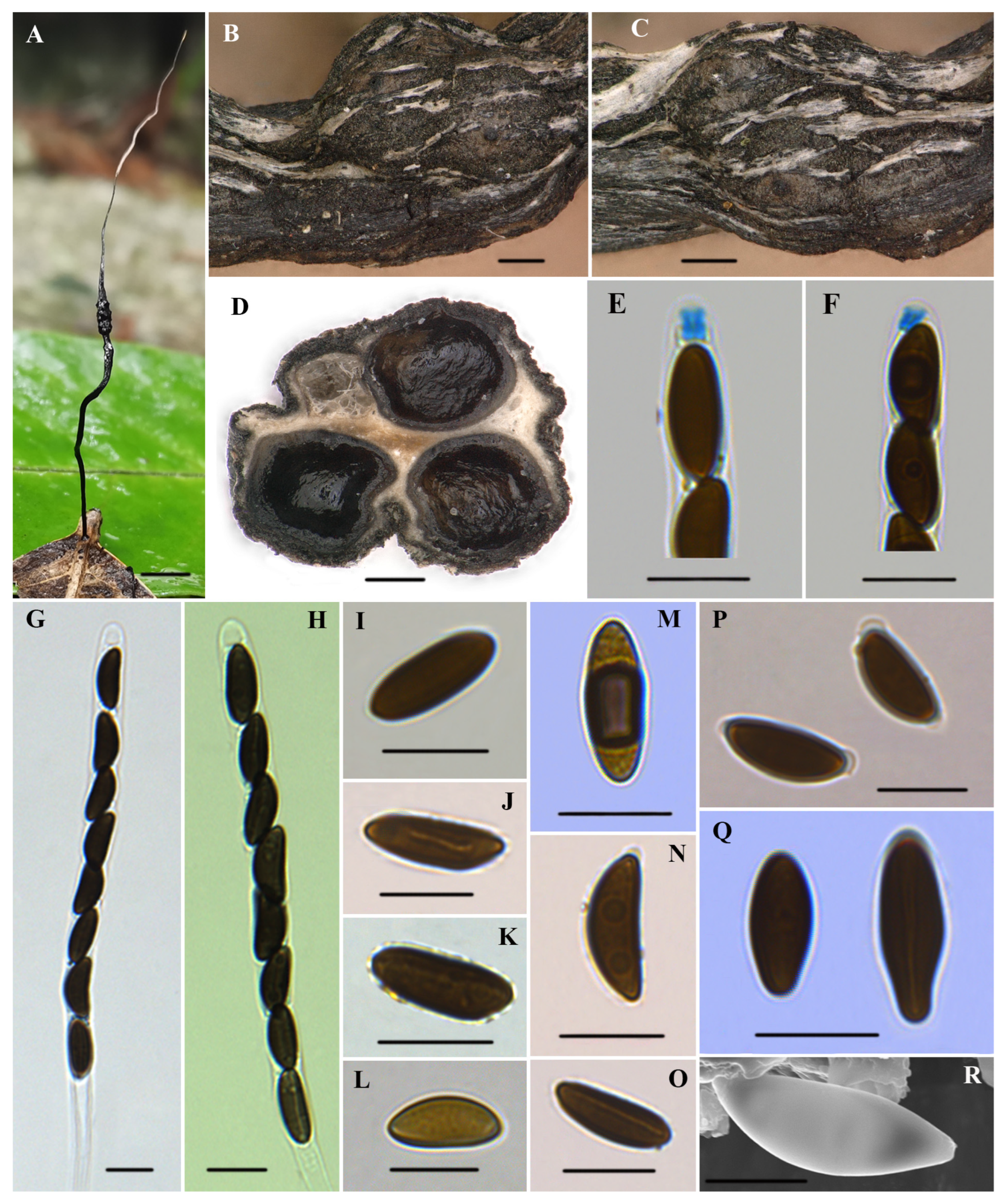
Xylaria diaoluoshanensis Xiao Y. Pan, sp. nov. Figure 2.
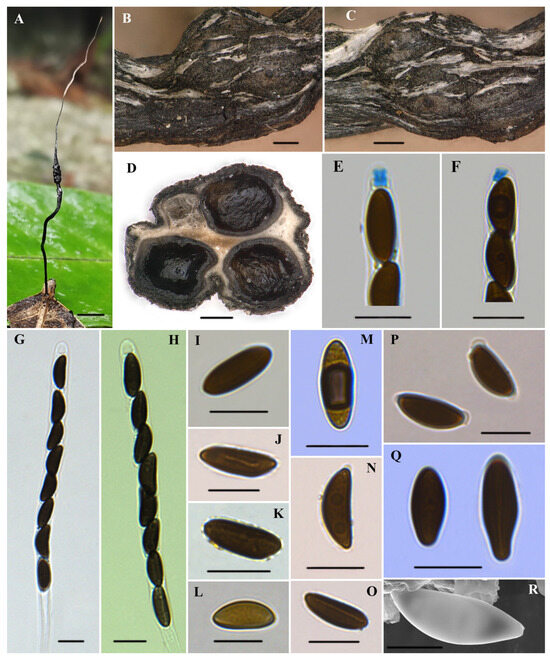
Figure 2.
Xylaria diaoluoshanensis (HAFFR 117). (A) Stromata on leaves; (B,C) stromatal surface and ostioles; (D) section through stroma, showing perithecia; (E,F) ascal apical apparatus in Melzer’s reagent; (G,H) asci in 1% SDS; (I,L,M,Q) ascospores in Melzer’s reagent; (J) ascospore in 1% SDS, with a slightly curved germ slit along almost half of the spore-length; (K) ascospore in water; (N) ascospore in 1% SDS; (O) ascospore in 1% SDS, with nearly spore-length germ slit; (P) ascospores in 1% SDS, presenting non-cellular appendages; (R) ascospore under SEM; scale bars: (A) = 0.5 cm; (B–D) = 200 µm; (E–Q) = 10 µm; (R) = 5 µm.
MycoBank: 851069
Diagnosis. It is differentiated from X. minuscula by its stromata lacking peeling layers and smaller ascospores. Differences from X. vittatipiliformis by its stromata lacking peeling layers and longer and thinner ascospores. Differences from X. vermiformis by its stromata surfaces with flatter perithecial contours and ostioles and larger ascospores.
Etymology. Dedicated to the place where the type specimen was collected, the Diaoluoshan Management Bureau.
Holotype. CHINA: Hainan Province, Hainan Tropical Rainforest National Park, Diaoluoshan Management Bureau, on fallen leaves, 18 June 2023, Xiaoyan Pan (HAFFR 117).
Teleomorph. Stromata were solitary, upright, filiform, unbranched, 30–65 mm total height; sterile filiform apexes were 10–35 mm long; fertile parts were 2–10 × 1–3 mm, cylindrical, consisting of closely packed perithecia; stipes were glabrous, 15–35 × 0.2–1 mm, with longitudinal wrinkles, slightly enlarged base; surface of sterile apex, fertile part (with slightly to half-exposed perithecial contours) and stipe were all roughened, white to cream-colored at the young stage and black at the mature stage; interior was white; consistency was soft. Perithecia were subglobose to depressed-spherical, 250–600 µm in diameter. Ostioles were slightly papillate. Asci were cylindrical, with eight uniseriate ascospores, were 110–165 µm long in total, spore-bearing parts were 75–105 × 6.5–7.7(–8.8) µm, stipes were 25–75 µm long, with apical rings turning blue in Melzer’s reagent, which were tubular to slightly urn-shaped, and (2.1–)2.5–3.5(–4.5) × 1.5–3 µm. Ascospores were brown to dark brown, unicellular, ellipsoid to fusiform, inequilateral, with slightly narrowly to broadly rounded ends, one end occasionally squeezed, smooth, (10.3–)11.5–14(–16.5) × (4.1–)4.6–5.7(–6.8) µm (M = 12.7 × 5.2 µm, N = 60), germ slit was mostly straight in nearly the spore-length, few slightly curved germ slit with nearly half of the spore-length, some with a hyaline sheath slightly swelling at both ends to form non-cellular appendages in 1% SDS.
Additional specimens examined. CHINA: Hainan Province, Hainan Tropical Rainforest National Park, Diaoluoshan Management Bureau, on fallen leaves, 18 June 2023, Xiaoyan Pan (HAFFR 115 and 127).
Xylaria fulvotomentosa Xiao Y. Pan, sp. nov. Figure 3.
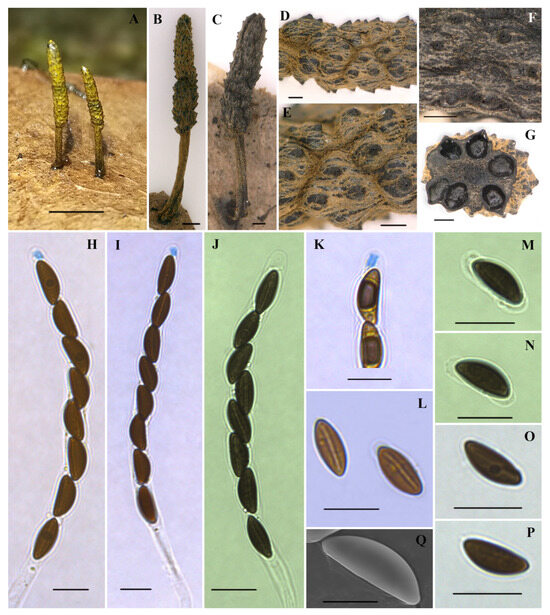
Figure 3.
Xylaria fulvotomentosa (holotype HAFFR 124). (A–C) Stromata on leaves ((C), HAFFR 129); (D–F) stromatal tomentose surface and ostioles ((F), HAFFR 129); (G) section through stroma, showing perithecia; (H,I) asci in Melzer’s reagent; (J) ascus in 1% SDS; (K) ascal apical ring in Melzer’s reagent; (L) ascospores in 1% SDS, showing germ slit; (M,N) ascospores in 1% SDS, with non-cellular appendages; (O,P) ascospores in water; (Q) ascospore under SEM; scale bars: (A) = 0.5 cm; (B–G,I) = 200 µm; (H,J–P) = 10 µm; (Q) = 5 µm.
MycoBank: 851070
Diagnosis. It can be distinguished from most Xylaria species by its yellow tomentose stromatal surface. It differs from X. appendiculata in that X. fulvotomentosa has evident perithecial mounds on its stromata and smaller ascospores.
Etymology. fulvotomentosa (lat.) denotes the primary features of yellow tomentose stromatal surfaces.
Holotype. CHINA: Hainan Province, Hainan Tropical Rainforest National Park, Diaoluoshan Management Bureau, on fallen leaves, 18 June 2023, Xiaoyan Pan (HAFFR 124).
Teleomorph. Stromata were solitary to scattered, upright, cylindrical, unbranched, 3–13 mm total height; fertile parts were 1.5–8 × 1–1.5 mm, cylindrical, composed of tightly arranged perithecia, apices attenuated or broadly rounded; stipes were 1.5–5 × 0.2–0.6 mm, with longitudinally fine stripes slightly expanded at bases; surface of fertile part (with slightly to half-exposed perithecial contours) and stipe were all roughened, except for the black upper apexes of protuberant perithecium, other parts were densely covered by yellow tomentum that gradually faded away with age and turned greyish brown, especially in fertile parts; interior was dark brown; consistency was hard. Perithecia were subglobose, 160–400 µm in diameter. Ostioles were papillate, up to 18 µm long. Asci were cylindrical, with eight uniseriate ascospores, were 80–115 µm long in total, spore-bearing parts were 60–80 × 6–7.8 µm, stipes were 15–38 µm long, with apical apparatuses turning blue in Melzer’s reagent, were tubular to urn-shaped, and 2.3–3.4 × 1.4–2.5(–3) µm. Ascospores brown, unicellular, elliptical, inequilateral, with narrowly rounded ends, smooth, 9.2–10.8(–11.2) × 3.7–4.9(–5.3) µm (M = 10.1 × 4.3 µm, N = 60), straight germ slit nearly spore-length, with a hyaline sheath swelling at both ends to form non-cellular appendages in 1% SDS.
Additional specimen examined. CHINA: Hainan Province, Hainan Tropical Rainforest National Park, Diaoluoshan Management Bureau, on fallen leaves, 18 June 2023, Xiaoyan Pan (HAFFR 129).
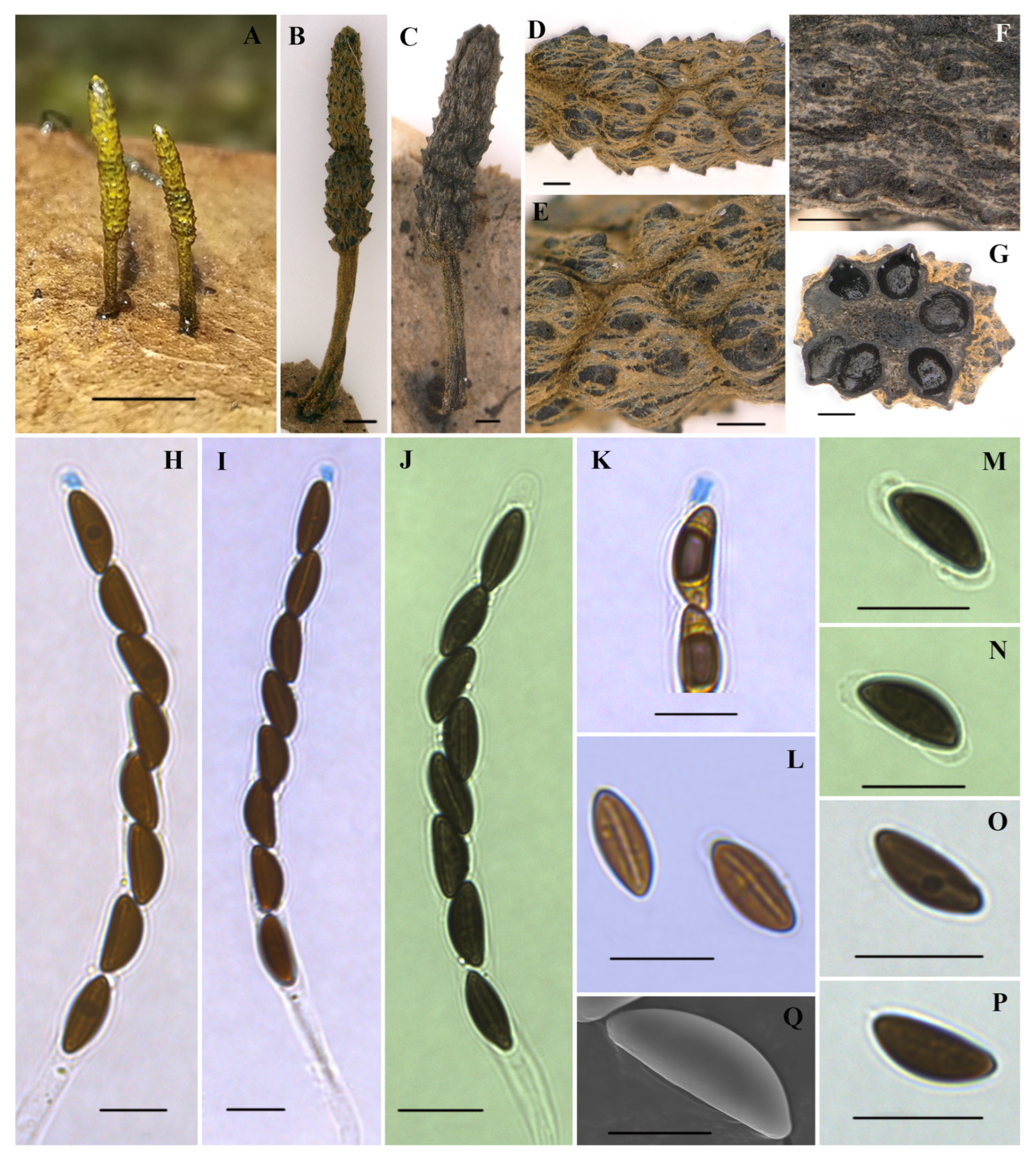
Xylaria petchii C. -G. Lloyd, Mycol. Writings 7: 1310. 1924. Figure 4.

Figure 4.
Xylaria petchii (HAFFR 118). (A,B) Stromata on leaves ((B), HAFFR 57); (C) stromata on branches (HAFFR 60); (D) stromatal surface; (E) ostioles; (F,G) section through stroma, showing perithecia; (H) ascal apical apparatus in Melzer’s reagent; (I,J) asci with apical apparatus in Melzer’s reagent; (K) asci in water; (L) ascospores in 1% SDS, presenting non-cellular appendages; (M) ascospores in Melzer’s reagent; (N) ascospore with a spore-length germ slit in water; (O) ascospore in water; (P) ascospore in Melzer’s reagent, showing a slightly sigmoid germ slit; (Q) ascospore under SEM; scale bars: (A–C) = 0.5 cm; (D–G) = 200 µm; (H,I,L–P) = 10 µm; (J,K) = 25 µm; (O) = 5 µm.
Teleomorph. Stromata were solitary to scattered, upright, cylindrical, and unbranched or occasionally branched once at stipe, 5–21 mm long in total; acute sterile apexes were 0.1–1 mm long; fertile parts were 1–10 × 0.5–2 mm, cylindrical or conical to subglobose, usually composed of clusters of perithecium near the top of the stromata, with a few occasionally scattered below; stipes were glabrous, 3–12 × 0.1–1.5 mm, with longitudinally fine stripes slightly swollen at bases; surface of sterile apex, fertile part (with conspicuous to fully exposed perithecial contours), and stipe were all roughened, black, without outer layer; interior was white; consistency was soft. Perithecia were subglobose, 250–550 µm in diameter. Ostioles were papillate. Asci were cylindrical, with eight uniseriate ascospores, a total length of 95–160 µm, spore-bearing parts of 60–110 × 7.5–11 µm, stipes that were 30–80 µm long, with apical apparatus turning blue in Melzer’s reagent, which were tubular to slightly urn-shaped, 2.5–5 × 2–4 µm. Ascospores were brown to blackish brown, unicellular, elliptical, inequilateral, with narrowly to broadly rounded ends, smooth, (8.5–)10–12.5(–15) × (4.5–) 5–6.5(–7) µm (M = 11.3 × 5.7 µm, N = 60), with a straight to slightly sigmoid germ slit that was nearly spore-length, with a hyaline sheath swelling at both ends to form non-cellular appendages in 1% SDS.
Specimens examined. CHINA: Hainan Province, Hainan Tropical Rainforest National Park, Diaoluoshan Management Bureau, on fallen branches, 26 February 2023, Xiaoyan Pan (HAFFR 60); on fallen leaves of Daphniphyllum paxianum, 18 June 2023, Xiaoyan Pan (HAFFR 118 and 126).
4. Discussion
Nine new species have been described in the Hainan Tropical Rainforest National Park [4,32,33], indicating the abundant species diversity in this region. This article combines morphological features and molecular evidence to continue species description in this region, with two new species (X. diaoluoshanensis and X. fulvotomentosa) and one species first recorded in China (X. petchii).
In the phylogenetic tree, X. diaoluoshanensis and X. minuscula cluster together, sharing somewhat similar stromal morphology. By comparison, X. minuscula has smaller stromata (3–14 mm total length), dull grayish brown, with peeling layers that split into narrow or thread-like stripes, and larger ascospores [(13.5–)14–15(–17) × (4.5–)5–6(–7) µm (M = 14.5 × 5.7 µm)] [14]. Xylaria vittatipiliformis Y.-M. Ju, H.-M. Hsieh & Fournier and X. vermiformis Y.-M. Ju & H.-M. Hsieh are also similar to X. diaoluoshanensis in stroma morphology. Xylaria vittatipiliformis has the stromata outer layer peeling and splitting into band-like stripes, and the ascospores are shorter and wider, (10–)11–12 (–12.5) × (5.5–) 6–7 (–7.5) µm (M = 11.4 × 6.5 µm) [14]. Xylaria vermiformis is distinguished from X. diaoluoshanensis by its more prominent perithecial mounds (half-exposed) and its sharper ostioles (conical-papillate) on the surface of the stromata and smaller ascospores [(9–)9.5–10.5(–11) × (3.5–)4–4.5(–5) µm (M = 10.1 × 4.4 μm)], without non-cellular appendages [14]. Xylaria appendiculatoides Y.M. Ju & H.M. Hsieh is somewhat similar to X. diaoluoshanensis in stroma morphology. Xylaria appendiculatoides is separated from X. diaoluoshanensis by its stromata surface color (blackish brown to black), sharper ostioles (conical-papillate), and larger ascospores [ (14–)15–16(–17) × (6.5–)7.0–7.5(–8) µm] [14]. Xylaria betulicola Hai X. Ma, Lar.N. Vassiljeva & Yu Li, X. crinalis Hai X. Ma, Lar.N. Vassiljeva & Yu Li, X. eugeniae F. San Martín, Vanoye & P. Lavín, X. filiformis (Alb. & Schwein.) Fr., and X. hedyosmicola all have filamentous stromata [13,14,15,34], slightly similar to X. diaoluoshanensis. However, X. eugeniae has smaller stromata (15–20 mm total length) with more conspicuous perithecial mounds (half to fully exposed) and smaller ascospores (12–13.5 × 4–5) [34]. Xylaria betulicola, X. crinalis, X. filiformis, and X. hedyosmicola can be clearly separated from X. diaoluoshanensis in the phylogenetic analyses.
Xylaria fulvotomentosa and X. hedyosmicola cluster together in the system analysis (Figure 1). However, a significant difference in morphology between the two is the long sterile filiform apexes and hairless stipes of the filamentous stromata of X. hedyosmicola [13]. Morphologically, X. appendiculata Ferd. & Winge and X. fulvotomentosa share cylindrical fertile parts growing on hairy stipes and the colors of their stromata are somewhat similar. However, X. appendiculata has smoother stromata without obvious perithecial mounds and larger ascospores, (11.5–)12.5–14(–15) × (6–)6.5–7.5(–9) µm (M = 13.1 × 7.2 μm) [14]. Xylaria friabilis J. Fourn. & Lechat is similar to X. fulvotomentosa in the color of stromata. However, X. friabilis has stromata with hairless stalks and ascospores lacking appendages [10].
Xylaria petchii was originally collected by Petch on fallen leaves in Sri Lanka and described by Lloyd in 1924 [14,35]. Hladki and Romero (2010) published X. filiformoidea Hladki & A.I. Romero collected in Argentina [36]. Ju and Hsieh (2023) believed that X. petchii and X. filiformoidea belonged to the same species [14]. The major characteristic of X. petchii is the diverse morphology of the fertile parts of stromata, with most perithecium clustered near the top of the stromata and a few scattered below. Ju and Hsieh (2023) observed X. petchii collected from Sri Lanka. Compared with specimens gathered in China, except for their larger ascus [(95–160 × 7.5–11 µm vs. 95–125 × 6–7 μm)] and ascospores [(8.5–)10–12.5(–15) × (4.5–)5–6.5(–7) µm vs. (7.5–)8.5–9.5(–10) × (3.5–)4–4.5(–5) µm)], other features are basically in accordance with the observation results of Ju and Hsieh (2023) [14]. Therefore, here they were identified as the same species. Unfortunately, since no gene sequences related to X. petchii were found in the NCBI, the Chinese sequences could not be compared with them. On the other hand, our sequences did not group with others included in this analysis. This study is the first to provide the gene sequence of X. petchii and determine the phylogenetic relationship between it and other Xylaria species. The results of the phylogenetic analyses showed that X. amphithele and X. ficicola are closely related to X. petchii. Moreover, they have some similarities in morphology. Xylaria amphithele differs from X. petchii mainly in its smaller stromata (≤50 mm) with conspicuous to half-exposed perithecial mounds and larger ascospores [(12–)12.5–15.5(–17) × (5–)6–7.5(–8) µm (M = 14.0 × 6.7 μm)] [14]. Xylaria ficicola is distinguished from X. petchi by larger ascospores [(16–)17.5–21(–22.7) × 6.5–8.5 µm] and larger apical apparatuses [5–6.5(–7.5) × 3–3.5 µm] [37]. Xylaria filiformis, placed in another clade (Figure 1), has similar stromata to X. petchii. However, there is an apparent difference in the ascospores of the two. In the former, the ascospores are short fusoid and light brown [14], while the latter has elliptical brown to blackish brown ascospores.
After carefully examining all Xylaria species that were lacking molecular data, these species were separated from the two new species described in this article primarily based on stromata morphology, lack of yellow tomentum on the stromal surface, perithecia, ostioles, ascospore size, germ slit, and appendage. The research results show that X. diaoluoshanensis has filamentous stromata with long sterile apexes, X. fulvotomentosa possesses cylindrical stromata with yellow tomentum, and X. petchii has cylindrical stromata with variable fertile parts, implying diversity in their stroma morphology. Meanwhile, the three species exhibit a degree of unity, such as having a few tiny and fragile stromata, papillate ostioles, and brown ascospores. According to the observations of Ju and Hsieh (2023), this kind of uniformity generally appears in Xylaria species related to fallen leaves and petioles [14]. This study collected gene sequences of 18 Xylaria species related to fallen leaves and petioles, of which 17 species clustered in the same branch of the phylogenetic tree. Xylaria phyllocharis Mont. is separated from other species related to fallen leaves and petioles and clusters with wood-inhabiting Xylaria species (Figure 1). It is not notably different from the findings of Hsieh et al. (2010) and Pan et al. (2022) [5,13], verifying that the systematic analysis results of this paper are not paradoxical. Xylaria diaoluoshanensis, X. fulvotomentosa, and X. petchii cluster in the different sub-branches of X. filiformis aggregate (Figure 1). In contrast with the Xylaria species on other substrates, they are highly correlated but also clearly separated. In X. filiformis aggregate, 19 species are gathered. Excluding two wood-inhabiting species (X. muscula Lloyd and X. crinalis), the remaining 17 are related to fallen leaves and petioles, demonstrating that genes of the species growing on this substance are more similar and may be evolving into a distinctive taxon in Xylaria. Moreover, X. clusiae, X. hedyosmicola, X. pisoniae D. Scott, J.D. Rogers & Y.M. Ju, and X. polysporicola Hai X. Ma & X.Y. Pan were all named after their hosts [13,38,39]. However, it is currently unclear whether these four species and most other Xylaria species that grow on fallen leaves and petioles have host specificity. Therefore, in-depth research is critical to unravel the above mystery.
Ju et al. (2018) argued that X. ficicola and X. heloidea were the same species [7]. Ju and Hsieh (2023) summarized Xylaria species related to fallen leaves and petioles in the world and reclassified many species [14]. For example, they believed that X. crinalis was X. simplicissima (Pers.) Y.M. Ju & H.M. Hsieh, X. hainanensis Y.F. Zhu & L. Guo was X. aristata var. aristata Mont. Based on the above studies, 42 Xylaria species growing on fallen leaves and petioles have been officially published worldwide. This paper describes two new species. A search table for 44 Xylaria species related to fallen leaves and petioles is established [7,13,34,38,40,41,42], as shown below.
The key to the species of Xylaria related to fallen leaves and petioles worldwide
- 1 Stromata branched, long stipes that bear one to three clavae on each terminal branch
- .....................................................................................................................................X. luxurians
- 1 Stromata unbranched to occasionally branched.....................................................................2
- 2 Stipes tomentose or glabrous to tomentose.............................................................................3
- 2 Stipes glabrous...........................................................................................................................15
- 3 Fertile parts filiform.....................................................................................................X. duranii
- 3 Fertile parts not filiform.............................................................................................................4
- 4 Fertile parts cylindrical...............................................................................................................5
- 4 Fertile parts capitate..................................................................................................................11
- 5 Fertile parts overlain by dark long spikes...............................................................X. asperata
- 5 Fertile parts without dark long spikes.....................................................................................6
- 6 Fertile parts glabrous.........................................................................................X. appendiculata
- 6 Fertile parts overlain by tomentum..........................................................................................7
- 7 Fertile parts densely covered by yellow tomentum...................................X. fulvotomentosa
- 7 Fertile parts with non-yellow tomentum.................................................................................8
- 8 Ascospores with non-cellular appendages..............................................................................9
- 8 Ascospores without non-cellular appendages......................................................................10
- 9 Ascospores (14.5–)15–16.5(–17) × (8–)8.5–9.5(–10) µm..............................................X. allima
- 9 Ascospores (10–)10.5–12(–14) × (5–)6–7(–7.5) µm.........................................................X. lima
- 10 Surface of fertile parts with half-exposed to fully exposed perithecial contours...............
- ...........................................................................................................................................X. castilloi
- 10 Surface of fertile parts lacking perithecial mounds or with slight perithecial mounds
- .......................................................................................................................................X. maitlandii
- 11 Stromata with an acute apex.................................................................................................12
- 11 Stromata with a rounded apex..............................................................................................14
- 12 Ascospores with non-cellular appendages..............................................................X. axifera
- 12 Ascospores without non-cellular appendages....................................................................13
- 13 Consistency fragile, ascospores (10–)10.5–12.5(–14) × (5.5–)6–7(–7.5) µm............................
- .......................................................................................................................X. aristata var. aristata
- 13 Consistency soft, ascospores (15–)15.5–17(–18) × (6.5–)7.5–9(–9.5) µm..........X. hispidipes
- 14 Ascospores (13.5–)14–16(–17) × (5.5–)6–7(–7.5) µm...........................X. aristata var. hirsuta
- 14 Ascospores 8–9(–9.5) × 4–4.5(–6.6) µm................................................................X. imminuta
- 15 Fertile parts cylindrical or conical to subglobose, most perithecia gather near the top of
- the stromata, with several occasionally scattered below.............................................X. petchii
- 15 Fertile parts with uniform morphology, perithecia lacking the above cluster patterns
- .......................................................................................................................................................16
- 16 Fertile parts cylindrical...........................................................................................................17
- 16 Fertile parts not cylindrical....................................................................................................30
- 17 Ascospores with non-cellular appendages..........................................................................18
- 17 Ascospores without non-cellular appendages....................................................................25
- 18 Stromata with an outer peeling layer split into band-like stripes, perithecia 150–200 µm
- .......................................................................................................................................X. vittiformis
- 18 Stromata without an outer peeling layer or the outer peeling layer without band-like
- stripes, perithecia greater than 250 µm.....................................................................................19
- 19 Surface of fertile parts lacking perithecial mounds, ascospores (22–)23.5–27(–28) × (8.5–)
- 9–10.5(–11) µm................................................................................................X. spiculaticlavata
- 19 Surface of fertile parts with conspicuous perithecial mounds, ascospores length less
- than 19 µm and width nearly less than 9 µm.......................................................................20
- 20 Stromata with an outer peeling layer split into narrow or thread-like stripes, ascospores
- (13.5–)14–15(–17) × (4.5–)5–6(–7) µm........................................................................X. minuscula
- 20 Stromata without an outer peeling layer or the outer peeling layer without narrow or
- thread-like stripes.........................................................................................................................21
- 21 Stromata with a blunt apex, ascospores 8–10 × 4–6 μm........................................X. kamatii
- 21 Stromata with an acute apex, ascospores length greater than 10.3 μm..........................22
- 22 Ostioles conic-papillate, tilting upwards, 120–150 μm broad at base............................
- ...................................................................................................................X. appendiculatoides
- 22 Ostioles papillate or slightly papillate, less than 80 μm broad at base...........................23
- 23 Stromata surface blackish brown, ascospores (15–)16.5–18(–19) × (7.5–)8–9(–9.5) µm
- .............................................................................................................................X. phyllophila
- 23 Stromata surface black, ascospores length less than 16.5 µm and width nearly less than
- 7.5 µm...........................................................................................................................................24
- 24 Stromata filiform, with long sterile filiform apexes up to 10–35 mm, ascospores
- ellipsoid to fusiform, (10.3–)11.5–14(–16.5) × (4.1–)4.6–5.7(–6.8) μm........X. diaoluoshanensis
- 24 Stromata cylindrical, with a mucronate apex 2 mm, ascospores ellipsoid, (11.5–)12.5–
- 14.5(–15) × 5.5–8 μm............................................................................................X. polysporicola
- 25 Stromata surface dark vinaceous brown, ascospores strongly inequilateral...............
- .................................................................................................................................X. phyllocharis
- 25 Stromata surface not dark vinaceous brown, ascospores inequilateral..........................26
- 26 Ascospores light brown to brown, (5.5–)6–7 × 3–3.5(–4) µm............................X. diminuta
- 26 Ascospores brown to blackish brown, length greater than 8.5 µm and width larger than
- 4 µm...........................................................................................................................................27
- 27 Stromata without an outer layer.........................................................................X. neblinensis
- 27 Stromata with an outer layer.................................................................................................28
- 28 Stromata with an acuminate or mucronate apex, ascospores (9–)9.5–10.5(–11) × (5.5–)6–
- 6.5(–7) µm............................................................................................................X. noduliformis
- 28 Stromata with a long acicular apex......................................................................................29
- 29 Stromata 23–35 mm total length, overlain by an outer peeling layer split into narrow or
- thread-like stripes, ascospores (8.5–)9–11 × 4–6 μm.................................................X. foliicola
- 29 Stromata 61–78 mm total length, overlain by an outer peeling layer split into band-like
- stripes, ascospores (10–)11–12(–12.5) × (5.5–)6–7(–7.5) µm........X. vittatipiliformis
- 30 Fertile parts filiform................................................................................................................31
- 30 Fertile parts not filiform.........................................................................................................36
- 31 Ascospores with spiral germ slit.....................................................................X. meliacearum
- 31 Ascospores with straight germ slit.......................................................................................32
- 32 Consistency fragile, ascospores (15–)16.5–19(–21.5) × (5–)5.5–6.5(–7.5) µm.....................
- ..................................................................................................................................X. simplicissima
- 32 Consistency soft, ascospores length less than 14.5 µm.......................................................33
- 33 Ascospores with non-cellular appendages..........................................................................34
- 33 Ascospores without non-cellular appendages....................................................................35
- 34 Ascospores light brown, short fusoid....................................................................X. fliformis
- 34 Ascospores brown to dark brown, ellipsoid...........................................................X. vagans
- 35 Stromata 15–20 mm total length, ascospores ellipsoid, 12–13.5 × 4–5 μm.......X. eugeniae
- 35 Stromata 35–83 mm total length, ascospores ellipsoid to shortly fusoid, (9–)9.5–10.5(–
- 11) × (3.5–)4–4.5(–5) µm..................................................................X. vermiformis
- 36 Ascospores with non-cellular appendages..........................................................................37
- 36 Ascospores without non-cellular appendages....................................................................43
- 37 Surface of fertile parts with conspicuous perithecial mounds.........................................38
- 37 Surface of fertile parts lacking perithecial mounds...........................................................42
- 38 Ascospores with cellular appendage on one end...............................................................39
- 38 Ascospores without cellular appendage..............................................................................40
- 39 Stromata 50 mm total length, without a long apex, ostioles slightly papillate, ascospores
- (12–)12.5–15.5(–17) × (5–)6–7.5(–8) µm...............................................................X. amphithele
- 39 Stromata 91–147 mm total length, with a long apex, ostioles coarsely papillate,
- ascospores (10.5–)11.5–13.5(–15) × (5–)5.5–6.5(–7.5) µm...................................X. nainitalensis
- 40 Stromata without an acute apex, ascospores (14.5–)15.5–18(–19) × (5–)5.5–6.5(–7) μm
- ...........................................................................................................................................X. heloidea
- 40 Stromata with an acute apex.................................................................................................41
- 41 Stromata with a mucronate apex, ascospores (10–)10.5–12(–12.5) × (5–)5.5–6(–6.5) µm
- .....................................................................................................................................X. pisoniae
- 41 Stromata with a long acicular apex, much longer than the fertile part, ascospores (8.5–)
- 9.5–11(–12) × (4–)4.5–6(–6.5) µm........................................................................................X. sicula
- 42 Stromata surface dull grayish brown, overlain with a thin pellicle cracked reticulately
- into plaques 100–200 µm broad, fertile parts capitate...........................................X. hypsipoda
- 42 Stromata surface dark brown to blackish, without the above plaques, fertile parts peltate
- .............................................................................................................................................X. memecyli
- 43 Ascospores dark brown, nearly semicircular to broadly ellipsoid, (10–)10.5–12.5(–14) ×
- (5.5–)6–7(–7.5) µm........................................................................................................X. delicatula
- 43 Ascospores dark brown to blackish brown, ellipsoid, (12.5–)13–15(–16) × (7.5–)8–9(–10) µm
- ..................................................................................................................................................X. clusiae
Author Contributions
Conceptualization, Y.C.; Data curation, X.P. and J.L.; Formal analysis, J.L. and X.C.; Funding acquisition, Z.C. and Y.C.; Investigation, X.P., T.W. and Y.L.; Methodology, X.P. and Z.C.; Project administration, Z.C. and Y.C.; Visualization, X.P. and Z.C.; Writing—original draft, X.P.; Writing—review and editing, X.P. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the Technical Innovation Project of Hainan Provincial Research Institute (SQKY2022-0036) and the Forestry and Grassland Ecological Protection and Restoration Fund (National Park Subsidy) Project (HDZB-2023-071).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets analyzed in this study are publicly available. All the gene sequences obtained in this article can be searched in GenBank (https://www.ncbi.nlm.nih.gov/genbank/; Table 1). All new taxa are saved in MycoBank (https://www.mycobank.org/).
Acknowledgments
We would like to thank the Hainan Tropical Rainforest National Park Service for their assistance during the specimen collection process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Husbands, D.R.; Urbina, H.; Lewis, S.M.; Aime, M.C. Xylaria karyophthora: A new seed-inhabiting fungus of Greenheart from Guyana. Mycologia 2018, 110, 434–447. [Google Scholar] [CrossRef]
- Wangsawat, N.; Ju, Y.M.; Phosri, C.; Whalley, A.J.S.; Suwannasai, N. Twelve new taxa of Xylaria associated with termite nests and soil from Northeast Thailand. Biology 2021, 10, 575. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; p. 771. [Google Scholar]
- Ma, H.X.; Song, Z.K.; Pan, X.Y.; Li, Y.; Yang, Z.N.; Qu, Z. Multi-gene phylogeny and taxonomy of Hypoxylon (Hypoxylaceae, Ascomycota) from China. Diversity 2022, 14, 37. [Google Scholar] [CrossRef]
- Hsieh, H.M.; Lin, C.R.; Fang, M.J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenet. Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Kim, C.S.; Jo, G.W.; Kwag, Y.N.; Oh, S.O.; Lee, S.G.; Sung, G.H.; Oh, G.; Shrestha, B.; Kim, S.Y.; Shin, C.H.; et al. New records of Xylaria species in Korea: X. ripicola sp. nov. and X. tentaculata. Mycobiology 2016, 44, 21–28. [Google Scholar] [CrossRef]
- Ju, Y.M.; Rogers, J.D.; Hsieh, H.M. Xylaria species associated with fallen fruits and seeds. Mycologia 2018, 110, 726–749. [Google Scholar] [CrossRef]
- Rogers, J.D.; Ju, Y.M.; Lehmann, J. Some Xylaria species on termite nests. Mycologia 2005, 97, 914–923. [Google Scholar] [CrossRef]
- Ju, Y.M.; Hsieh, H.M. Xylaria species associated with nests of Odontotermes formosanus in Taiwan. Mycologia 2007, 99, 936–957. [Google Scholar] [CrossRef]
- Fournier, J.; Lechat, C.; Courtecuisse, R. The genus Xylaria sensu lato (Xylariaceae) in Guadeloupe and Martinique (French West Indies) III. Taxa with slender upright stromata. Ascomycete.org 2020, 12, 81–164. [Google Scholar] [CrossRef]
- Hsieh, H.M.; Ju, Y.M.; Lechat, C.; Fournier, J.; Huart, D. New ecological, morphological, cultural and molecular phylogenetic insights into Xylaria guepini (Xylariaceae). Ascomycete.org 2022, 14, 177–184. [Google Scholar]
- Ju, Y.M.; Hsieh, H.M.; He, X.S. Wulingshen, the massive Xylaria sclerotia used as traditional Chinese medicine, is produced by multiple species. Mycologia 2022, 114, 175–189. [Google Scholar] [CrossRef]
- Pan, X.Y.; Song, Z.K.; Qu, Z.; Liu, T.D.; Ma, H.X. Three new Xylaria species (Xylariaceae, Xylariales) on fallen leaves from Hainan Tropical Rainforest National Park. MycoKeys 2022, 86, 47–63. [Google Scholar] [CrossRef]
- Ju, Y.M.; Hsieh, H.M. Xylaria species associated with fallen leaves and petioles. Bot. Stud. 2023, 64, 19. [Google Scholar] [CrossRef]
- Ma, H.X.; Li, Y. Xylaria crinalis and X. betulicola from China—Two new species with thread-like stromata. Sydowia 2018, 70, 37–49. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Hsieh, H.M.; Ju, Y.M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Fournier, J.; Ju, Y.M.; Hsieh, H.M.; Lindermann, U. Xylaria aethiopica sp. nov.—A new pod-inhabiting species of Xylaria (Xylariaceae) from Ethiopia. Ascomycete.org 2018, 10, 209–215. [Google Scholar]
- Perera, R.H.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Jones, E.B.G.; McKenzie, E.H.C.; Stadler, M.; Lee, H.B.; Samarakoon, M.C.; Ekanayaka, A.H.; Camporesi, E.; et al. Fungi on wild seeds and fruits. Mycosphere 2020, 11, 2108–2480. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Zare, R.; Khodaparast, S.A.; Elahinia, S.A. A new Xylaria species from Iran. Mycol. Iran. 2015, 2, 1–10. [Google Scholar]
- Roensch, P.; Roensch, S.; Reiher, A.; Otto, P. Investigations on the fructicolous Xylaria delitschii and Xylaria Oxyacanthae. Boletus 2010, 32, 106–122. [Google Scholar]
- Persoh, D.; Melcher, M.; Graf, K.; Fournier, J.; Stadler, M.; Rambold, G. Molecular and morphological evidence for the delimitation of Xylaria hypoxylon. Mycologia 2009, 101, 256–268. [Google Scholar] [CrossRef]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsaard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from amultigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Song, Z.K.; Pan, X.Y.; Li, C.T.; Ma, H.X.; Li, Y. Two new species of Hypoxylon (Hypoxylaceae) from China based on morphological and DNA sequence data analyses. Phytotaxa 2022, 538, 213–224. [Google Scholar] [CrossRef]
- Deng, H.; Wang, Y.; Lei, J.R.; Chen, Z.Z.; Liang, Z.Q.; Zeng, N.K. Four New Species of Strobilomyces (Boletaceae, Boletales) from Hainan Island, Tropical China. J. Fungi 2023, 9, 1128. [Google Scholar] [CrossRef]
- San Martín, F.; Rogers, J.D.; Lavín, P. Algunas especies de Xylaria (Pyrenomycetes, Sphaeriales) habitantes en hojarasca de bosques Mexicanos. Rev. Mex. Micol. 1997, 13, 58–69. [Google Scholar]
- Lloyd, C.G. Mycological notes no. 73. Mycol. Writ. 1924, 7, 1301–1332. [Google Scholar]
- Hladki, A.I.; Romero, A.I. A preliminary account of Xylaria in the Tucuman Province, Argentina, with a key to the known species from the Northern Provinces. Fungal Divers. 2010, 42, 79–96. [Google Scholar] [CrossRef]
- Ma, H.X.; Vasilyeva, L.; Li, Y. A new species of Xylaria from China. Mycotaxon 2011, 116, 151–155. [Google Scholar] [CrossRef]
- Samuels, G.J.; Rogerson, C.T. New Ascomycetes from the Guayana Highland. Mem. N. Y. Bot. Garden 1990, 64, 165–183. [Google Scholar]
- Rogers, J.D.; Scott, D.; Ju, Y.M. Xylaria pisoniae sp. nov. from Pisonia leaves in Hawaii. Harv. Pap. Bot. 2001, 6, 189–191. [Google Scholar]
- Pande, A. Contribution to the Xylariaceae of Western India II. Nova Hedwig. 1973, 24, 13–16. [Google Scholar]
- San Martín, F.; Lavín, P.; Rogers, J.D. Some species of Xylaria (Hymenoascomycetes, Xylariaceae) associated with oaks in México. Mycotaxon 2001, 79, 337–360. [Google Scholar]
- Huang, G.; Guo, L.; Liu, N. Two new species of Xylaria and X. diminuta new to China. Mycotaxon 2014, 129, 149–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).